Abstract
BACKGROUND
Hernia repair failure may occur due to suboptimal mesh fixation by mechanical constructs before mesh integration. Construct design and acute penetration angle may alter mesh-tissue fixation strength. We compared acute fixation strengths of absorbable fixation devices at various deployment angles, directions of loading, and construct orientations.
METHODS
Porcine abdominal walls were sectioned. Constructs were deployed at 30, 45, 60, and 90 degree angles to fix mesh to the tissue specimens. Lap-shear testing was performed in upward, downward, and lateral directions in relation to the abdominal wall cranial-caudal axis to evaluate fixation. Absorbatack™ (AT), SorbaFix™ (SF), and SecureStrap™ in vertical (SSV) and horizontal (SSH) orientations in relation to the abdominal wall cranial-caudal axis were tested. Ten tests were performed for each combination of device, angle, and loading direction. Failure types and strength data were recorded. ANOVA with Tukey-Kramer adjustments for multiple comparisons and chi-square tests were performed as appropriate (p<0.05 considered significant).
RESULTS
At 30 degrees, SSH and SSV had greater fixation strengths (12.95 N, 12.98 N, respectively) than SF (5.70 N; p=0.0057, p=0.0053, respectively). At 45 degrees, mean fixation strength of SSH was significantly greater than SF (18.14 N, 11.40 N; p=0.0002). No differences in strength were identified at 60 or 90 degrees. No differences in strength were noted between SSV and SSH with different directions of loading. No differences were noted between SS and AT at any angle. Immediate failure was associated with SF (p<0.0001) and the 30 degree tacking angle (p<0.01).
CONCLUSIONS
Mesh-tissue fixation was stronger at acute deployment angles with SS compared to SF constructs. The 30 degree angle and the SF device were associated with increased immediate failures. Varying construct and loading direction did not generate statistically significant differences in the fixation strength of absorbable fixation devices in this study.
Keywords: Abdominal Wall, Hernia, Hernia Repair, Laparoscopic Hernia Repair, Fixation Device, Absorbable Tack, Mechanical Strength, Deployment Angle, Mesh
Introduction
Laparoscopic repair has become a widely adopted technique for a variety of abdominal wall hernias[1–3]. These procedures are safe and effective in many clinical scenarios, and may produce favorable outcomes including significantly lower wound morbidity and recurrence rates when compared to traditional open hernia repair procedures[4, 5]. Most commonly performed laparoscopic hernia repairs involve a tension-free technique that employs mesh to cover the hernia defect, and fixation devices to affix the mesh to the abdominal wall[6]. Commonly used fixation devices include sutures, permanent tacks, and a variety of absorbable fixation devices that have recently been introduced.
Mesh fixation prevents slippage and migration of mesh, and it is thought that failure of fixation can contribute to hernia recurrence[7]. Failures of mesh fixation are thought to occur for a variety of reasons including poor construct design and inadequate tissue penetration due to a suboptimal deployment angle. Most tackers are designed to optimally place a fixation device when the tacker is held at a 90 degree angle relative to the mesh and abdominal wall. For operating surgeons, it is often difficult to deploy tacks at an optimal angle because of the curvature of the insufflated abdominal wall and working space limitations associated with laparoscopy.
Among absorbable fixation devices, a unique construct employing a “strap” design has been hypothesized to offer greater tissue penetration at suboptimal deployment angles when compared to absorbable screw fasteners[8]. This “strap” construct has two points of fixation, which may lead to stronger fixation at acute deployment angles. To date, however, there have been few studies of the fixation strength of absorbable fixation devices at acute deployment angles to substantiate these claims. A recent report by Sadava et al. examining various tacks at 30 and 90 degree firing angles showed a greater mean strength of fixation at 30 degrees in an absorbable construct with a “strap” design compared to absorbable screw fasteners, though these differences did not reach statistical significance[9]. A “strap” construct also may perform differently depending on the orientation of the long axis of the construct in relation to the loads that are applied to the construct. Loads perpendicular to the long axis of the construct may be distributed more evenly to both fixation points while those applied parallel to the long axis of the construct may act on one fixation point more than the other. This potential difference may influence surgical decision-making when surgeons place these constructs at fixation points where the loading direction may be anticipated. To date, the effect of construct orientation on fixation strength has not been examined.
In this study we aimed to evaluate fixation strength of absorbable fixation devices at various tacking angles, construct orientations, and directions of loading. We hypothesized that absorbable constructs with a “strap” design will have significantly greater fixation strength than absorbable screw fasteners at acute tacking angles. We further hypothesized that “strap” constructs would have significantly greater fixation strengths when loaded perpendicular to their long axes compared to their fixation strength when loaded parallel to their long axes, whereas loading direction would have no significant impact on the fixation strength of absorbable screw fasteners due to their biaxial symmetry.
Materials & Methods
Abdominal Wall Harvesting and Preparation
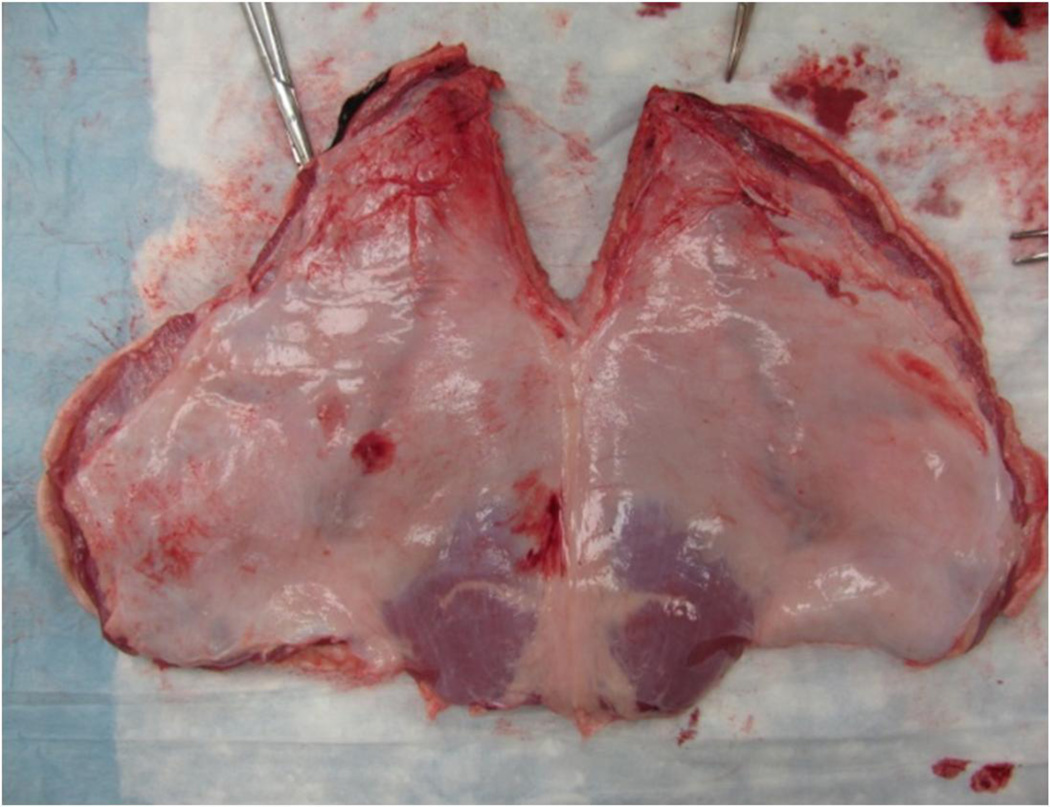
Under an Institutional Animal Care and Use Committee (IACUC) approved protocol at Washington University in Saint Louis (protocol #2013-0004), 32 porcine abdominal walls were harvested from adult female domestic pigs weighing 80–100 lbs. The abdominal walls were prepared by removing the skin, the subcutaneous fat, and the cutaneous maximus muscle, and transported to the laboratory in 0.9% saline solution at 4 degrees Celsius for immediate use (Figure 1). Abdominal walls were laid in anatomic orientation, and marked to identify the borders and the cranial-caudal axes of the specimens. The 3.5 cm × 5.0 cm specimens were then cut from the abdominal wall tissue. The linea alba and any tissue without complete peritoneal coverage was excluded. Each specimen was suspended using a clip secured to a mounting bracket attached to a metal backing plate on the lab bench. A 168 gram weight was clipped to the lower edge of the specimen to simulate the surface tension of an abdominal wall insufflated to 12 mmHg [10](Figure 2).
Figure 1.
Prepared porcine abdominal wall (peritoneal surface shown). Regions without peritoneal coverage or within 2 mm of the linea alba were excluded. The remainder of the abdominal walls was sectioned into 3.5 cm×5 cm myofascial specimens with associated peritoneum, and used for tacking.
Figure 2.
Mesh fixation apparatus. Mesh fixation devices were oriented at specific angles to the tissue using a bracket that fixed the shaft at the desired angle (A). The abdominal wall specimen was fixed superiorly to a clip attached to the metal backing plate (B), and inferiorly to a 168 g weight providing tension equal to an insufflated abdominal wall (C). The tip of the mechanical fixation device was attached to a preload force measuring device to ensure that constructs were applied with a preload force between 1.25 and 1.90 lbs (D).
Fixation Device Deployment
Absorbable barrier-coated PROCEED™ surgical mesh (Ethicon® Inc.; Somerville, NJ) was cut into 1.5 cm×3.0 cm pieces and hydrated in 0.9% sterile saline for at least 15 minutes before use. Each 1.5 cm×3.0 cm piece of mesh was tacked to the peritoneal surface in the center of each tissue specimen. Mesh fixation devices were oriented at 30, 45, 60, or 90 degrees in relation to tissue specimens using a metal bracket that held the shaft of each tacking device at the desired angle. A fixture for measuring preload force was applied to the tip of each deployment device to ensure that the fixation devices were applied with a preload force between 1.25 and 1.9 lbs (Figure 2). Higher preloads are associated with puckering of the tissue, and potential normalizing of an acute tacking angle to 90 degrees. A 0.9525 cm thick piece of foam, (Limbs and Things®, LTD; Savannah, GA) was placed between the tissue and the metal backing plate to simulate a realistic tissue backing behind each specimen.
The absorbable fixation devices evaluated in this study (Figure 3) were: Absorbatack™ (AT; Covidien®, Inc.; Mansfield, MA), a tapered solid spiral screw fastener comprised of poly(glycolide-co-L-lactide) (PGLA); SorbaFix™ (SF; Davol®, Inc.; Warwick, Rl), a hollow spiral screw fastener comprised of poly(D, L)-lactide (PLA); and SecureStrap™ (SS; Ethicon®, Inc.; Somerville, NJ), a “strap” construct comprised of polydioxanone and L(-)-lactide/glycolide copolymer. SecureStrap™ was tested in both the vertical orientation in which the long axis of the construct was oriented parallel to the cranial-caudal axis of the abdominal wall (SSV), and in the horizontal orientation in which the long axis of the construct was oriented perpendicular to the cranial-caudal axis of the abdominal wall (SSH).
Figure 3.
Mechanical fixation devices tested (from left to right): Davol®, Inc. SorbaFix™; Covidien®, Inc. AbsorbaTack™; and Ethicon®, Inc. SecureStrap™.
At the time of tacking, constructs were evaluated by inspection to assess how well the tacks deployed relative to the mesh surface. Constructs were described as “flush” (construct that was fully seated into the tissue)"high-seated” (construct that was only partially seated into the tissue)"unseated” (construct that dislodged from the tissue immediately after application), or “no penetration” (no significant mesh or tissue penetration).
Mechanical Testing
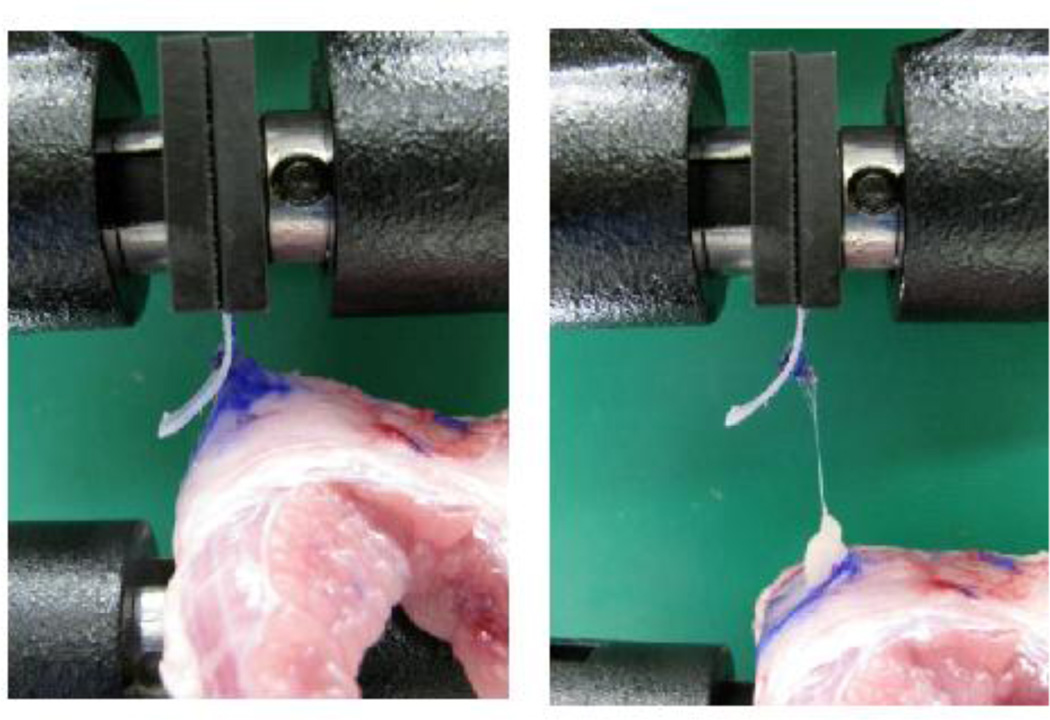
Fixation strength was assessed by lap-shear testing using an Instron® Series 5542 Materials Testing Machine (Instron®; Norwood, MA). The free edge of the mesh tacked to each specimen was secured in one pneumatic grip of the machine set to a pressure of 60 psi, while the free edge of the abdominal wall tissue was similarly secured in the other pneumatic grip of the machine. The fixation interface was tested under tension at a constant rate of displacement of 0.42mm/sec until failure (Figure 4). The maximum load sustained by the construct before failure was recorded as the fixation strength in units of Newtons (N). Failure types (tack failure, mesh failure, or tissue failure) were recorded, based on the component of the construct at which mechanical failure occurred. Constructs in which the tack became dislodged during manipulation before being secured in the machine, or immediately upon the initiation of lap-shear testing were recorded as immediate tack failures (0 N fixation strength). Testing was performed on each construct in three directions (upward and downward in parallel orientation to the cranial-caudal axis of the abdominal wall, and laterally in perpendicular orientation to the cranial caudal axis of the abdominal wall).
Figure 4.
Fixation strength testing. The free edge of the mesh tacked to each specimen was secured in one pneumatic grip of the Instron® Series 5542 Materials Testing Machine, while the free edge of the abdominal wall tissue was secured in the other pneumatic grip. The fixation interface was tested under tension at a constant rate of displacement of 0.42mm/sec until failure. The maximum load sustained by the construct before failure was recorded as the fixation strength in Newtons (N).
Data Analysis
A power analysis was performed for our primary outcome measure (mean fixation strength for each device at a given deployment angle). Based on preliminary investigations[10] and a recent report that did not detect differences between absorbable fixation devices when powered to detect 50% differences in fixation strength[9], we used a power of 0.80, an α of 0.05, and considered a difference of 25% between group means to be significant, yielding a sample size of 30 per group. This level of power is achieved for each device at a given deployment angle by testing in three directions (upward, downward, and laterally) with 10 testing repetitions performed in each direction, and pooling of the results. This pooling presupposes insignificant or no two-way statistical interaction between the deployment angle and the loading direction variables, or three way statistical interaction between the deployment angle, the loading direction, and the device variables. Based on this power analysis, a total of 480 tests were performed (10 tests per combination of device, deployment angle, and loading direction).
To perform pairwise comparative analyses between mean fixation strengths, independent-sample Student’s t-tests were performed following significant analysis of variance results. We applied Tukey-Kramer adjustments to all pairwise comparisons to correct for multiple comparisons. A p-value of 0.05 was considered the threshold of statistical significance for each comparison. To evaluate failure types, we performed chi-square analyses of failure type by device, deployment angle, and loading direction. As a failure type with particular clinical relevance, we separately evaluated immediate device failures by performing chi-square analysis on the occurrence of immediate failure by device, deployment angle, and loading direction. For each chi-square analysis, we considered a p-value of 0.05 to be the threshold of statistical significance.
To evaluate construct deployment data, we considered deployment categories of “flush” and “suboptimal” (any deployment other than flush as determined by inspection). Chi-square analysis of deployment was performed by device, deployment angle, and loading direction. For each chi-square analysis, we considered a p-value of 0.05 to be the threshold of statistical significance.
All analyses were performed with the SAS statistical package, version 9.3 for the Windows platform (SAS Institute Inc., Cary, NC).
Results
Comparison of Absorbable Fixation Devices at Various Deployment Angles
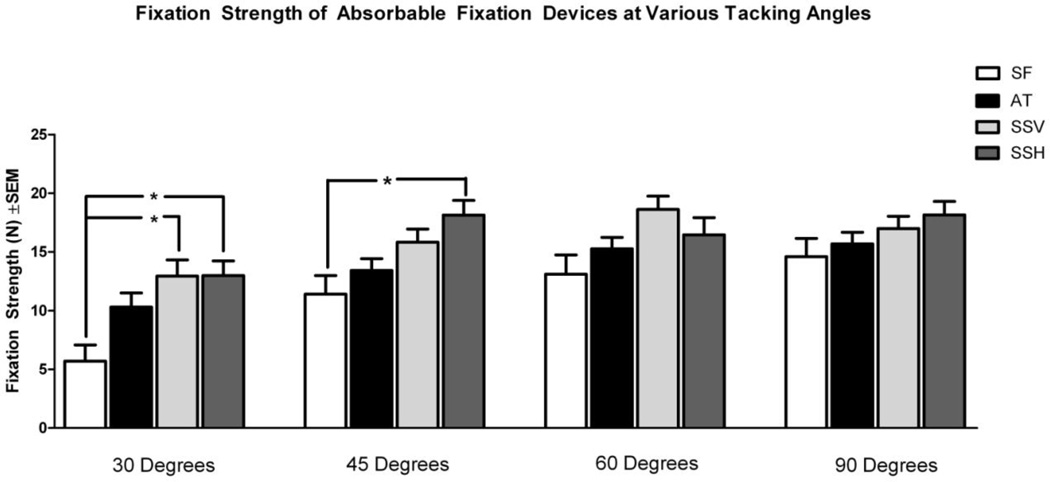
At a 30 degree deployment angle (Figure 5), the SS device in both vertical (SSV) and horizontal (SSH) orientations had significantly greater fixation strengths (12.95 N and 12.98 N, respectively) compared to the SF device (5.70 N; p=0.0057 and 0.0053, respectively). At a 45 degree tacking angle, the mean fixation strength of SSH (18.14 N) was significantly greater than the mean fixation strength of SF (11.40 N; p=0.0002). At tacking angles of 60 and 90 degrees, there were no statistically significant differences in fixation strength detected in any pairwise comparisons between devices. We also performed comparisons of each device to itself at the four deployment angles (comparisons not shown). SF at 30 degrees (5.70 N) had significantly lower fixation strength compared to SF at 60 degrees (13.10 N; p=0.004) and 90 degrees (14.60 N; p<0.0001). Comparisons within the AT, SSV, and SSH groups at each of the four deployment angles did not demonstrate any other statistically significant differences.
Figure 5.
Comparison of mean fixation strength of absorbable fixation devices at various tacking angles. SF: SorbaFix™; AT: AbsorbaTack™; SSV: SecureStrap™ in the vertical orientation, parallel to the abdominal wall cranial-caudal axis; SSH: SecureStrap™ in the horizontal orientation, perpendicular to the abdominal wall cranial-caudal axis. *p<0.05
Comparison of Absorbable Fixation Devices in Various Loading Directions
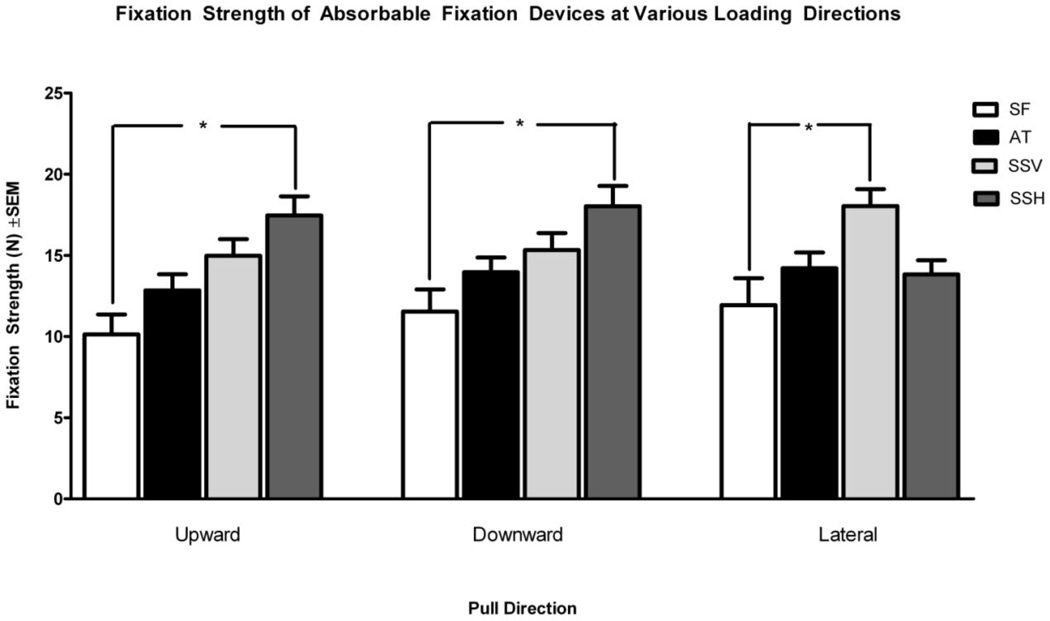
When tested in the upward direction and loaded parallel to the cranial-caudal axis of the abdominal wall (Figure 6), SSH had a significantly greater fixation strength (17.46 N) when compared to SF (10.133 N; p=0.0002). When tested in the downward direction and loaded parallel to the cranial-caudal axis of the abdominal wall, SSH again had a significantly greater fixation strength (18.03 N) when compared to SF (11.54 N; p=0.0019). When lateral loading was applied, SSV had a greater mean fixation strength (18.04 N) compared to SF (11.93 N; p=0.005). No other significant differences were noted when comparing devices at a given direction of loading. There were also no statistically significant differences when comparing fixation strengths of each device to itself at different loading directions.
Figure 6.
Comparison of mean fixation strength of absorbable fixation devices undergoing shear force in various loading directions. SF: SorbaFix™; AT: AbsorbaTack™; SSV: SecureStrap™ in the vertical orientation, parallel to the abdominal wall cranial-caudal axis; SSH: SecureStrap™ in the horizontal orientation, perpendicular to the abdominal wall cranial-caudal axis. *p<0.05
Analysis of Fixation Device Failures
Chi-square analysis of failure types by device (Table 1) demonstrated a statistically significant deviation from expected failure frequencies (p<0.0001). No significant deviations from expected failure frequencies were detected when evaluating failure type by loading direction (p=0.706, results not shown) or deployment angle (p=0.095, results not shown). For immediate tack failures (Table 2), chi-square analyses comparing the SF device to pooled data from the other tested devices demonstrated a significant deviation from expected failure frequencies (p<0.0001). Immediate tack failures within the non-SF devices followed expected chi-square distributions (p=0.8753). Chi-square analyses comparing immediate tack failures of all devices between the 30 degree deployment angle and pooled data from all other tested tacking angles demonstrated a statistically significant deviation from expected failure frequencies (p=0.0012). Immediate device failures at deployment angles greater than 30 degrees followed expected chi-square distributions (p=0.4447).
Table 1.
Analysis of mechanical failures by device. SF: SorbaFix™; AT: AbsorbaTack™; SSV: SecureStrap™ in the vertical orientation, parallel to the abdominal wall cranial-caudal axis; SSH: SecureStrap™ in the horizontal orientation, perpendicular to the abdominal wall cranial-caudal axis.
| Fixation Device* | ||||
|---|---|---|---|---|
| Failure Type | SF | AT | SSV | SSH |
| Immediate Failure | 25 | 3 | 3 | 3 |
| Tack Failure | 0 | 1 | 9 | 10 |
| Mesh Failure | 6 | 2 | 6 | 14 |
| Tissue Failure | 89 | 115 | 102 | 95 |
Significant deviation from expected chi-square distribution (p<0.05).
Table 2.
Analysis of immediate failures. SF: SorbaFix™; Non-SF: AbsorbaTack™, SecureStrap™ in the vertical orientation, parallel to the abdominal wall cranial-caudal axis, and SecureStrap™ in the horizontal orientation, perpendicular to the abdominal wall cranial-caudal axis.
| Device* | Angle‡ | |||
|---|---|---|---|---|
| Failure Type | SF | Other Devices | 30 Degrees | Other Angles |
| Immediate Failure | 25 | 9 | 15 | 19 |
| Other Failures | 95 | 354 | 105 | 344 |
Comparison of immediate failures in the SF and non-SF device groups deviated from chi-square distribution (*p<0.0001).
Comparison of immediate failures at the 30 degree tacking angle compared to all other angles deviated from chi-square distribution (‡p=0.0012).
Analysis of Fixation Device Deployment Characteristics
Chi-square analysis of deployment data by device (Table 3) demonstrated a statistically significant deviation from expected values of suboptimal deployments (high seated, unseated, and no penetration deployments) (p<0.0001). The SF device demonstrated more suboptimal deployments than would be under the null hypothesis of no difference (44 observed, 24 expected). All other devices had fewer suboptimal deployments than would be expected. Chi-square analysis of deployment data by deployment angle (Table 3) demonstrated a statistically significant deviation from expected values of suboptimal deployments (p<0.0001). The 30 degree deployment angle demonstrated a statistically significant greater frequency of suboptimal deployments than would be expected based on a chi-square distribution (54 observed, 24 expected). Each of the other deployment angles had fewer suboptimal deployments than would be expected. Chi-square analysis of deployment data by loading direction (Table 3) did not reveal any statistically significant deviations from expected values of suboptimal deployments (p=0.766).
Table 3.
Analysis of construct deployment. SF: SorbaFix™; AT: AbsorbaTack™; SSV: SecureStrap™ in the vertical orientation, parallel to the abdominal wall cranial-caudal axis; SSH: SecureStrap™ in the horizontal orientation, perpendicular to the abdominal wall cranial-caudal axis.
| Device* | Angle* | Direction of Stress | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Device Deployment |
SF | AT | SSV | SSH | 30 | 45 | 60 | 90 | Upward | Downward | Lateral |
| Flush | 76 | 108 | 105 | 98 | 66 | 100 | 110 | 111 | 128 | 132 | 127 |
| Suboptimal | 44 | 13 | 17 | 22 | 54 | 21 | 10 | 11 | 34 | 29 | 33 |
Significant deviation from expected chi-square distribution (p<0.05).
Discussion
In recent years, absorbable fixation devices have become more widely adopted by surgeons for use in laparoscopic abdominal hernia repair. These devices have the potential to decrease postoperative pain compared to nonabsorbable fixation devices and sutures while producing a comparable recurrence rate, though significant controversy over this point persists [11–14]. Absorbable devices were designed to minimize the development of intra-abdominal adhesions, and the erosion of tacks through the abdominal wall or into visceral structures [15–17]. Mechanical fixation devices can be deployed quickly and easily, reducing operative time[5]. Absorbable fixation constructs can also be used in conjunction with absorbable mesh prostheses in clinical situations where permanent implants should be avoided. A variety of absorbable fixation devices with possible mechanical advantages have been introduced recently, including “strap” constructs [5].
While absorbable mesh fixation options for clinical use have proliferated, there is a paucity of pre-clinical data examining the fixation strengths of these absorbable constructs deployed at acute angles. Our group recently examined the fixation strength of fibrin sealant, transabdominal sutures, permanent metallic tacks and absorbable devices applied at a 90 degree angle to the abdominal wall[18]. We found that transabdominal polypropylene sutures demonstrated greater fixation strengths compared to all other fixation devices tested, but we did not detect statistically significant differences between the metallic and absorbable tack groups. More recently, Sadava et al. published the first comparison of fixation devices at different deployment angles[9]. They reported statistically significant greater fixation strengths for a titanium helical tack compared to all absorbable constructs at both 30 and 90 degree tacking angles, but were also unable to detect statistically significant differences between the types of absorbable fixation devices. In this study, we attempted to perform an appropriately-powered analysis of absorbable fixation devices at various orientations, deployment angles, and directions of loading.
The data from this study demonstrate that at the most acute tacking angle (30 degrees), the “strap” construct in both vertical and horizontal orientations displays greater fixation strength compared to the hollow spiral screw fastener. The “strap” construct in the horizontal orientation demonstrated greater fixation strength compared to the hollow screw fastener at the 45 degree deployment angle, while at the 60 degree and 90 degree deployment angles, no statistically significant differences are detected. These findings suggest that the “strap” construct design achieves greater fixation strength compared to the hollow screw fastener at deployment angles less than or equal to 45 degrees. The strap construct did not have higher fixation strengths in either the vertical or horizontal orientation compared to the tapered solid spiral screw fastener at any tacking angle. These fixation strength data are supported by our analyses of the device failure and device deployment data, which demonstrate that both the hollow spiral screw fastener and the 30 degree deployment angle were associated with significantly higher occurrences of immediate tack failure. In addition, the hollow spiral screw fastener and the 30 degree deployment angle were associated with a greater frequency of suboptimal deployments than would be expected by chance. In comparison, the tapered solid spiral screw fastener performed as expected at suboptimal angles and did not demonstrate a statistically significant difference in the rate of immediate tack failures compared to the hollow spiral screw fastener (data not shown). This may be due to the fact that these solid spiral screw fasteners are tapered whereas the hollow spiral screw fasteners are blunt leading to poorer tissue purchase for the blunt-tip hollow spiral screw fasteners at acute deployment angles. It is known that poor mesh fixation and mesh detachment can contribute to subsequent hernia recurrence, and this finding may therefore be of particular clinical relevance[7].
The fixation strengths of each device were evaluated at different directions of loading. The “strap” construct demonstrated greater fixation strength compared to the hollow spiral screw fastener when loading was applied perpendicular to the long axis of the “strap” construct. There were no statistically significant differences detected between the vertical and horizontal orientations of the “strap” construct when loading in any of the three tested directions was applied. These data suggests that the “strap” construct may be subjected to loading in the upward, downward, or lateral directions when deployed in either the vertical or the horizontal orientation without significant change in fixation strength.
This study is limited by several factors. We elected to examine absorbable fixation devices and therefore did not test permanent tacks, transabdominal sutures, sealants, or any other fixation options for direct comparison. While it is known that permanent metallic tacks and transabdominal sutures tend to have greater fixation strengths compared to some absorbable fixation devices at acute deployment angles, little is known about the performance of fibrin sealants under suboptimal fixation conditions. In addition, we did not randomize abdominal wall segments based on their location on the abdominal wall for use in our study. There is a theoretical possibility that different regions of the porcine abdominal wall, or the abdominal walls of individual pigs, may exhibit different properties with regard to intrinsic tissue strength and tack purchase, and that this may have interacted significantly with other variables in our experimental model. Furthermore, while the normal intra-abdominal pressure has been detailed[19], the threshold fixation strength needed for a fixation device to perform adequately in the clinical setting is unknown; therefore, conclusions of clinical relevance cannot be drawn from these and other ex vivo fixation strength data. A recent publication by Reynvoet et al. examining the fixation strengths of tacks in a porcine model suggested that the significant differences in fixation strength noted between the titanium spiral tack and the hollow spiral screw fastener at 2 weeks following implantation were largely mitigated at 6 months following implantation[17]. Future in vivo studies using animal hernia models are needed to examine the performance over time of various absorbable fixation devices deployed at different angles.
Conclusion
An absorbable “strap” device provides greater mesh-tissue fixation strength at acute deployment angles compared to an absorbable blunt-tip hollow screw fastener device. The 30 degree deployment angle and the blunt-tip hollow screw fastener device were also associated with increased occurrence of immediate tack failure. These findings suggest that among absorbable fixation devices, the “strap” construct may provide an advantage in producing stronger mesh-tissue fixation compared to the blunt-tip hollow screw fastener. Construct orientation and loading direction did not appear to play significant roles in the fixation properties of the absorbable fixation devices evaluated. In vivo studies are required to examine the long-term fixation properties of absorbable fixation devices, and human studies are needed to correlate these findings to clinical outcomes.
Acknowledments
The authors thank Mr. JB for statistical analyses.
This study utilized the Research Electronic Data Capture® (REDCap®) application for data maintenance, which is supported at our institution by a Clinical and Translational Science Award (CTSA) (UL1TR000448), and a National Cancer Institute (NCI) Cancer Center Support Grant (P30CA091842). JC is supported by a KM1 Comparative Effectiveness Research (CER) Career Development Award (KM1CA156708) through the NCI of the National Institutes of Health (NIH); and our institutional CTSA program (UL1TR000448) through the National Center for Advancing Translational Sciences (NCATS) of the NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, the NCATS, or the NIH.
This study was supported by a research grant from Ethicon®, Inc. (Somerville, NJ).
AZ has received research grant funding for unrelated studies from the National Institutes of Health. JC has received research grant funding for unrelated studies from the National Institutes of Health, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), and the American Hernia Society in collaboration with Davol® Incorporated; and has served as a one-time consultant for Guidepoint Global® Incorporated. MF has received funding from Atrium Medical Corporation® and W. L. Gore and Associates® Incorporated for unrelated service contracts; and research grant funding for unrelated studies from the Foundation for Barnes-Jewish Hospital. BM has served on advisory boards for Musculoskeletal Transplant Foundation, Covidien® Incorporated, and Synthes® Incorporated; has served as a consultant for Atrium Medical Corporation®; has received speaking fess or honoraria from Atrium Medical Corporation®, Davol® Incorporated, Ethicon® Incorporated, W.L. Gore and Associates® Incorporated; has received payments for authorship of an unrelated publication from McMahon Group® Incorporated; has received research grant funding for unrelated research studies from Covidien® Incorporated, Ethicon® Incorporated, Karl Storz Endoscopy America® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, Synovis Surgical Innovations®, the Society of American Gastrointestinal and Endoscopic Surgeons, the National Institutes of Health, and the Foundation for Barnes-Jewish Hospital; and research funding for this study from Ethicon®, Incorporated. CD has served as a consultant for Atrium Medical Corporation® and Davol® Incorporated; has received speaking fess or honoraria from Atrium Medical Corporation®, Covidien® Incorporated and Musculoskeletal Transplant Foundation; has received research grant funding for unrelated research studies from Atrium Medical Corporation®, Covidien® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, OBI Biologics Incorporated®, and the Society of American Gastrointestinal and Endoscopic Surgeons; and research funding for this study from Ethicon®, Incorporated.
Footnotes
AUTHOR CONTRIBUTIONS:
Study conception and design: BM and CD
Acquisition of data: AZ, JC, DT, NC, and CD
Analysis and interpretation of data: AZ, JC, BM, and CD
Drafting of manuscript: AZ, JC, DT, BM, and CD
Critical revision: AZ, JC, DT, NC, MF, BM, and CD
Disclosures
DT and NC have no conflicts of interest or financial ties to disclose.
References
- 1.Turner PL, Park AE. Laparoscopic repair of ventral incisional hernias: pros and cons. Surg Clin North Am. 2008;88(1):85–100. doi: 10.1016/j.suc.2007.11.003. viii. [DOI] [PubMed] [Google Scholar]
- 2.Stoikes N, Voeller GR. New Developments in Hernia Repair: A 2013 Update. Surg Technol Int. . :XXIII. [PubMed] [Google Scholar]
- 3.Sanchez LJ, Bencini L, Moretti R. Recurrences after laparoscopic ventral hernia repair: results and critical review. Hernia. 2004;8(2):138–143. doi: 10.1007/s10029-003-0195-0. [DOI] [PubMed] [Google Scholar]
- 4.Pierce RA, et al. Pooled data analysis of laparoscopic vs. open ventral hernia repair: 14 years of patient data accrual. . Surg Endosc. 2007;21(3):378–386. doi: 10.1007/s00464-006-9115-6. [DOI] [PubMed] [Google Scholar]
- 5.Ramshaw BJ, et al. Comparison of laparoscopic and open ventral herniorrhaphy. Am Surg. 1999;65(9):827–831. discussion 831–2. [PubMed] [Google Scholar]
- 6.Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362(9395):1561–1571. doi: 10.1016/S0140-6736(03)14746-0. [DOI] [PubMed] [Google Scholar]
- 7.Awad ZT, et al. Mechanisms of ventral hernia recurrence after mesh repair and a new proposed classification. J Am Coll Surg. 2005;201(1):132–140. doi: 10.1016/j.jamcollsurg.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Ethicon Endo-Surgery I. [cited 2013 6/6/2013];ETHICON SECURESTRAP™ Fixation Device. 2013 [Webpage], 6/5/2013, Available from: http://www.ethicon.com/healthcare-professionals/products/hernia-solutions/ethicon-securestrap-fixation-device.
- 9.Sadava EE, et al. Laparoscopic mechanical fixation devices: does firing angle matter? Surg Endosc. 27(6):2076–2081. doi: 10.1007/s00464-012-2713-6. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale Mea, et al. Comparison of Acute Holding Strength of an Absorbable Strap Fixation Device in Porcine Flank at Various Implantation Angles. (Report No. SEC-339-11-8/13). Ethicon. PDF File. 2011 [Google Scholar]
- 11.Carbonell AM, et al. Local injection for the treatment of suture site pain after laparoscopic ventral hernia repair. Am Surg. 2003;69(8):688–691. discussion 691-2. [PubMed] [Google Scholar]
- 12.Asencio F, et al. Open randomized clinical trial of laparoscopic versus open incisional hernia repair. Surg Endosc. 2009;23(7):1441–1448. doi: 10.1007/s00464-008-0230-4. [DOI] [PubMed] [Google Scholar]
- 13.Carbajo MA, et al. Laparoscopic treatment vs open surgery in the solution of major incisional and abdominal wall hernias with mesh. Surg Endosc. 1999;13(3):250–252. doi: 10.1007/s004649900956. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen JR, et al. Pain, quality of life and recovery after laparoscopic ventral hernia repair. Hernia. 2009;13(1):13–21. doi: 10.1007/s10029-008-0414-9. [DOI] [PubMed] [Google Scholar]
- 15.Byrd JF, et al. Evaluation of absorbable and permanent mesh fixation devices: adhesion formation and mechanical strength. Hernia. 15(5):553–558. doi: 10.1007/s10029-011-0826-9. [DOI] [PubMed] [Google Scholar]
- 16.Hollinsky C, et al. Tensile strength and adhesion formation of mesh fixation systems used in laparoscopic incisional hernia repair. Surg Endosc. 24(6):1318–1324. doi: 10.1007/s00464-009-0767-x. [DOI] [PubMed] [Google Scholar]
- 17.Reynvoet E, et al. Tensile strength testing for resorbable mesh fixation systems in laparoscopic ventral hernia repair. Surg Endosc. 26(9):2513–2520. doi: 10.1007/s00464-012-2224-5. [DOI] [PubMed] [Google Scholar]
- 18.Melman L, et al. Evaluation of acute fixation strength for mechanical tacking devices and fibrin sealant versus polypropylene suture for laparoscopic ventral hernia repair. Surg Innov. 17(4):285–290. doi: 10.1177/1553350610379427. [DOI] [PubMed] [Google Scholar]
- 19.Cobb WS, et al. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005;129(2):231–235. doi: 10.1016/j.jss.2005.06.015. [DOI] [PubMed] [Google Scholar]