Abstract
Background
We hypothesized that targeting two mechanisms of epigenetic silencing would be additive or synergistic with regard to expression of specific target genes. The primary objective of the study was to establish the maximum tolerated dose (MTD) of belinostat in combination with a fixed dose of azacitidine (AZA).
Methods
In Part A of the study, patients received a fixed dose of AZA, with escalating doses of belinostat given on the same days 1–5, in a 28 day cycle. Part B was designed to evaluate the relative contribution of belinostat to the combination based on analysis of pharmacodynamic markers, and incorporated a design in which patients were randomized during cycle 1 to AZA alone, or the combination, at the maximally tolerated dose of belinostat.
Results
56 patients with myeloid neoplasia were enrolled. Dose escalation was feasible in part A, up to 1000 mg/m2 dose level of belinostat. In Part B, 18 patients were assessable for quantitative analysis of specific target genes. At day 5 of therapy, MDR1 was significantly up-regulated in the belinostat/AZA arm compared with AZA alone arm (p=0.0023). There were 18 responses among the 56 patients.
Conclusions
The combination of belinostat and AZA is feasible and associated with clinical activity. The recommended phase II dose is 1000 mg/m2 of belinostat plus 75 mg/m2 of AZA on days 1–5, every 28 days. Upregulation in MDR1 was observed in the combination arm at day 5 compared with the AZA alone arm, suggesting a relative biologic contribution of belinostat to the combination.
Introduction
Myeloid neoplasms are characterized by gene mutations and epigenetic alterations that result in deregulation of cellular proliferation and survival pathways [1]. Epigenetic silencing via aberrant DNA methylation has been implicated in leukemogenesis, and this phenomenon also involves the recruitment of methyl binding proteins and histone deacetylases (HDACs) to transcriptional start sites [2]. Transcriptional repression via promoter DNA methylation and/or recruitment of HDACs can be potentially targeted by pharmacologic inhibitors of these enzymatic pathways [1,2].
Preclinical studies have demonstrated limited efficacy when HDAC inhibitors such as trichostatin A (TSA) are used as single agents in cancer cell lines where genes have been silenced by promoter-specific hypermethylation. However, when combined with DNA methyltransferase inhibitors in vitro, there is clear synergy with a more robust re-expression of previously silenced genes [3–7].
The DNA methyltransferase (DNMT) inhibitors decitabine and azacitidine are FDA-approved for myelodysplastic syndromes (MDS) based on the induction of objective responses, including trilineage hematologic improvement in approximately one-third of patients with these disorders. [8–10].
We and others have shown that single agent HDAC inhibitor use in myeloid neoplasms resulted, in general, in limited clinical benefit[11–14]. Recent reports of early phase studies of combined HDAC and DNMT inhibitor use in MDS and acute myeloid leukemia (AML) have demonstrated the feasibility of this approach [15–17] but the relative contribution of the HDAC inhibitor to these combinations is relatively undefined.
Belinostat (PXD 101; N-hydroxy-3-[phenylsulphamoylphenyl] acryl amide) is a potent low molecular weight inhibitor of class I and II HDACs, with a zinc chelating hydroxamic acid moiety similar to TSA, and demonstrable antitumor activity in both T cell and myeloid leukemia cell lines as well as in xenograft models. Tolerability has been demonstrated up to the 1000mg/m2 dose level in Phase I trials in solid tumors[18]. The agent recently received accelerated FDA approval for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma.
We conducted a phase I trial with belinostat in combination with azacitidine in adults with advanced and/or high risk myeloid neoplasia. The primary objective of the study was to determine the dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) of belinostat when combined with a fixed dose of azacitidine. The secondary objective was to determine the relative contribution of belinostat to the combination, based on an evaluation of pharmacodynamic markers. The trial therefore included a dose escalation phase and a randomized phase.
Patients, Materials and Methods
Patient Selection
Patients were eligible for the study if they were 18 years or older and had relapsed or refractory AML, secondary AML, or newly diagnosed AML if older than 60 years of age and not candidates for, or who had refused conventional chemotherapy. Patients with MDS, including chronic myelomonocytic leukemia (CMML), were eligible if they had intermediate or high risk IPSS scores or one or more of the following criteria: hemoglobin < 10 g/dL or red cell transfusion dependence, platelets < 50,000/uL, or absolute neutrophil count <1,000/uL. Patients with advanced phases of Philadelphia chromosome negative myeloproliferative diseases were also eligible. In the randomized phase of the study, eligibility was limited to those patients with AML in the categories defined above with dysplasia on bone marrow cytology, and the MDS/CMML categories defined above. There was no limit to the number or types of prior regimens received. Prior therapy with azacitidine was permitted. No cytotoxic therapy or radiation was permitted within 2 weeks prior to enrollment. The exception was hydroxyurea, which was permitted up to 24 hours prior to starting therapy to control hyperleukocytosis. Patients were required to have a Karnofsky performance status ≥ 60% and adequate hepatic and renal function. Because a class effect of HDAC inhibitors is the induction of QT prolongation, patients with baseline prolongation of the QTc interval of >500 msec were excluded. The protocol was reviewed and approved at each institution’s institutional review board, and all subjects enrolled gave written informed consent.
Study Design and Treatment Plan
This was a multicenter, phase 1 study. The dose escalation phase (Part A) was conducted solely at the University of Chicago, whereas the randomized phase of the study was conducted in collaboration with Princess Margaret Hospital, Toronto, and University of Wisconsin, Madison. In Part A, azacitidine was administered at a fixed dose of 75 mg/m2/day subcutaneously on days 1–5, with escalating doses of belinostat administered as a 30 minute intravenous infusion on the same days (1–5) every 28 days, following a 3+3 phase I design to determine the MTD.
A DLT was defined as a grade ≥3 non-hematologic toxicity that was probably or definitely drug related (except transient liver function abnormalities, transient nausea and vomiting, diarrhea, alopecia and culture-negative neutropenic fever), or a grade 4 hematologic toxicity persisting beyond Day 42 in the absence of bone marrow involvement with disease. The incidence and severity of infectious complications were assessed but not used to define DLT in this patient population with pre-existing bone marrow failure. The MTD was defined as the dose level at which fewer than two of six patients experienced first-course DLT. Dose escalation was designed to proceed until the MTD or the 1000 mg/m2 dose level of belinostat was reached. Toxicities and Adverse Events (AE) were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, versions 3.0 and 4.0 (after January 1, 2011).
In the randomized phase (Part B) of the study, 18 evaluable patients were randomized in cycle 1 to treatment with either azacitidine alone or the combination at the established MTD of belinostat. Subsequently, in cycle 2 and beyond, all patients received treatment with the combination. The objective of Part B was to assess whether there were any additive or synergistic effects of the combination based on an analysis of pharmacodynamic biomarkers in cycle 1.
Treatment could continue on the study in the absence of unacceptable adverse events or disease progression. Belinostat was supplied by CuraGen/Topotarget Corporation and distributed by CTEP, Division of Cancer Treatment and Diagnosis (DCTD), NCI. Azacitidine was supplied by Pharmion and distributed by CTEP, DCTD, NCI under a Clinical Trials Agreement (CTA) between Pharmion Corporation and the DCTD, NCI.
An ECG was obtained at the end of the final belinostat infusion on day 5 of each cycle of treatment. A follow-up bone marrow aspirate and biopsy was performed after two cycles of therapy, after four cycles of therapy, and thereafter as clinically indicated to document response. Efficacy was assessed according to the published response criteria for patients with AML/chronic myeloproliferative diseases in accelerated or blast phase [19] or MDS and CMML [20]. International Working Group (IWG) MDS criteria for hematologic improvement (HI) were also assessed as appropriate for patients with AML and chronic myeloproliferative diseases.
Pharmacodynamic Studies
Bone marrow aspirate samples were obtained pretreatment and on day 5 of cycle 1 on patients enrolled in the randomized phase.
Gene expression analysis- p15INK4B, p21, MDR1
Target genes of interest included p15INK4B, p21, MDR1. Quantitative real-time PCR (qRT-PCR) was optimized for each gene using a transcript specific primer set (0.2–0.5uM) and consensus probes (0.1–0.2uM) from the Universal Probe Library (Roche Applied Science), with the exception of p15INK4B, which was amplified using a specifically designed primer-probe set (Biosearch Inc, Novato, CA). Patient samples and no template controls were assayed in triplicate using the LightCycler 480II (Roche). All transcripts were determined by reference to standard curves. All standard curves were generated from 5-fold serial dilutions of cell line cDNA (0.08–250ng). The absolute transcript copy number was normalized to the endogenous control gene, ABL1.
Statistical considerations
Changes in the pharmacodynamic variables (target gene expression) during cycle 1 (baseline to day 5), were compared between the two groups in the randomized phase of the study (azacitidine alone versus the combination) using two-sample t tests. Patients were considered evaluable for this analysis if they had sufficient material from bone marrow aspirates obtained at baseline and day 5 for gene expression analysis. A total of 18 evaluable patients (9 per arm) provided 80% power to detect a 1.3 SD difference between the groups, where SD was the standard deviation of the within-subject changes. Given the small sample size, if the pharmacodynamic data did not appear to be normally distributed, the data were log-transformed or a Wilcoxon rank sum test was used in place of the t-test. Normality was assessed using graphical techniques, specifically normality probability plots.
To assess for evidence of clinical activity, each patient’s best response was recorded and tabulated for both the dose escalation and randomized cohorts.
Results
Patient characteristics
Fifty-six patients were enrolled on this study between July 2006 and August 2010. Twenty-four patients were enrolled in part A (dose escalation phase) and 32 in part B (the randomization phase). Patient characteristics are shown in Table 1.
Table 1.
Baseline Patient Characteristics
| Characteristic | No. (%) |
|---|---|
|
| |
| Total No. Patients | 56 |
|
| |
| Median age, y (range) | 68 (42–83) |
| Age < 60 | 12 (21) |
| Age ≥ 60 | 44 (79) |
|
| |
| Sex | |
| Male | 40 (71) |
| Female | 16 (29) |
|
| |
| Performance Status | |
| 0 | 25 (45) |
| 1 | 28 (50) |
| 2 | 2 (3) |
| Not available | 1 (2) |
|
| |
| Diagnosis | |
| AML | 28 (50) |
| MDS | 12 (21) |
| CMML | 4 (7) |
| t-MN | 11 (20) |
| PMF | 1 (2) |
|
| |
| Stage of Disease | |
| Previously untreated | 16 (29) |
| Primary refractory | 22 (39) |
| Relapsed/refractory | 18 (32) |
|
| |
| Median no. Prior Therapies (range) | 1 (0–7) |
|
| |
| Prior Therapies | |
| Untreated | 16 (29) |
| Cytarabine based | 23 (41) |
| Hypomethylating agent | 14 (25) |
| Allogeneic stem cell transplant | 9 (16) |
| Autologous stem cell transplant | 1 (2) |
|
| |
| Karyotypic Risk Group | |
| ∞ Favorable | 11 (20) |
| Intermediate | 19 (34) |
| Unfavorable | 23 (41) |
| Not available | 3 (5) |
t-MN= therapy related myeloid neoplasm, PMF= primary myelofibrosis.
denotes 11 patients with MDS or CMML with good risk cytogenetics including 3 with a del 20q clone, 2 with del 5q clone and 6 with normal karyotype.
Toxicities
The most common drug related toxicities included nausea, vomiting, anorexia, and fatigue, which were mild (grade 1–2), and not dose-limiting (Table 2). Grade 1/2 QTc prolongation, a recognized class effect of HDAC inhibition, was observed relatively frequently (4 of 6 patients in dose level 1) early in the study, but the incidence reduced significantly when patients were instructed to hold concomitant medications known to prolong the QT interval (e.g., fluoroquinolones and azoles) on the days of treatment with belinostat. Grade 3/4 neutropenia and/or thrombocytopenia that were deemed possibly drug related, occurred in patients with pre-existing grade 1/2 cytopenias, and did not constitute DLT. Grade 3 infections occurring in part A included pneumonia (2 cases), bacteremia, catheter related infection, cellulitis, and culture negative febrile neutropenia (1 case each). In general, these patients had relapsed/refractory AML and/or absolute neutrophil counts <1000/uL at baseline, and these events were not dose- limiting. There were two deaths on study in Part A due to disease progression. Both patients had AML and were over the age of 60.
Table 2.
Drug related toxicities: dose escalation phase: AZA/belinostat
| Toxicity | 75/150 (n=6) | 75/300 (n=5) | 75/600 (n=6) | 75/1000 (N=7) |
|---|---|---|---|---|
|
| ||||
| Hematologic | ||||
| Grade 1/2 | 0 | 1 | 3 | 1 |
| Grade 3/4 | 1 | 1 | 1 | 2 |
|
| ||||
| Fatigue | ||||
| Grade 1/2 | 3 | 1 | 4 | 2 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Injection Site Reaction | ||||
| Grade 1/2 | 2 | 3 | 4 | 3 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Flushing | ||||
| Grade 1/2 | 0 | 0 | 1 | 1 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Nausea/Vomiting | ||||
| Grade 1/2 | 3 | 3 | 5 | 5 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Constipation | ||||
| Grade 1/2 | 2 | 1 | 3 | 1 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Anorexia | ||||
| Grade 1/2 | 1 | 1 | 1 | 4 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Diarrhea | ||||
| Grade 1/2 | 2 | 0 | 1 | 1 |
| Grade 3/4 | 0 | 1 | 0 | 0 |
|
| ||||
| Neuropathy | ||||
| Grade 1/2 | 0 | 1 | 1 | 1 |
| Grade 3/4 | 0 | 0 | 0 | 0 |
|
| ||||
| Prolonged QT | ||||
| Grade 1/2 | 4 | 0 | 1 | 0 |
| Grade 3/4 | 0 | 1 | 0 | 0 |
In the absence of DLT, dose escalation proceeded to the maximum dose of belinostat explored- 1000 mg/m2, given in combination with azacitidine at 75 mg/m2/day. This dose was utilized in part B of the study, to which 32 patients were accrued. The spectrum of toxicities observed in part B of the study (Table 3) was similar to that seen in part A. The addition of belinostat did not appear to increase the incidence or spectrum of toxicities observed, when compared to azacitidine alone in cycle 1 (Table 3). Four patients in this phase of the study had culture negative febrile neutropenia, and an additional four patients had documented infections, including two patients with pneumonia. All of these patients also had relapsed/refractory AML and/or evidence of pre-existing marrow failure with absolute neutrophil counts < than 1,000/uL. There were six deaths on study (or within 30 days of drug administration) in this phase. Four of these (including two on the azacitidine alone arm) occurred within the first cycle of therapy and all were related to disease progression and/or existing marrow failure.
Table 3.
Drug related toxicities: randomization phase
| Toxicity | AZA alone n=18 |
AZA/belinostat n=14 |
|---|---|---|
|
| ||
| Hematologic | ||
| Grade 1/2 | 2 | 1 |
| Grade 3/4 | 4 | 3 |
|
| ||
| Fatigue | ||
| Grade 1/2 | 10 | 9 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Injection Site Reaction | ||
| Grade 1/2 | 7 | 7 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Nausea/Vomiting | ||
| Grade 1/2 | 13 | 11 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Anorexia | ||
| Grade 1/2 | 4 | 3 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Diarrhea | ||
| Grade 1/2 | 4 | 4 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Prolonged QT | ||
| Grade 1/2 | 2 | 3 |
| Grade 3/4 | 0 | 1 |
|
| ||
| Dizziness | ||
| Grade 1/2 | 5 | 1 |
| Grade 3/4 | 0 | 0 |
|
| ||
| LFT abnormality | ||
| Grade 1/2 | 2 | 0 |
| Grade 3/4 | 0 | 1 |
|
| ||
| Hypotension | ||
| Grade 1/2 | 4 | 1 |
| Grade 3/4 | 0 | 0 |
|
| ||
| Mucositis | ||
| Grade 1/2 | 2 | 1 |
| Grade 3/4 | 0 | 0 |
The overall experience reflected that the regimen was well tolerated. Patients were able to receive repeated cycles of therapy in both parts of the trial. The median number of cycles administered in part A was 4.5 (range 1–64), and in part B was 4 (range 1–28).
Clinical Activity
All patients enrolled on the study were considered evaluable for evidence of clinical activity. In part A, responses occurred at virtually all dose levels (Table 4). Nine of the 24 patients enrolled in this part of the study had evidence of a response, including three complete responses. In part B of the study, 9 of 32 patients achieved a response, including four complete responses (Table 5). Of the 21 patients who were treated at the 1000mg/m2 dose level of belinostat from cycle 1 (7 in part A and 14 in part B), there were 10 responders.
Table 4.
Nine responders in Dose Escalation Phase (n=24)
| ID # | Age | Diagnosis | Stage of Disease | Cytogenetic Risk Group | No. Prior Regimens | Dose BEL | ±No. Cycles | Best Responses | Time to Initial Response (days) | Response Duration (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 49 | AML | Relapsed | Intermediate | 5§ | 150 | 9 | HI-N | 102 | 147 |
| 3 | 75 | CMML-1 | Refractory | Favorable | 1 | 150 | 64 | PR | 27 | 1860 |
| 9 | 54 | MDS-RCMD | Relapsed | Favorable | 4§* | 300 | 11 | HI-P | 28 | 279 |
| 13 | 56 | AML | Relapsed | Unfavorable | 2§ | 300 | 4 | HI-N | 59 | 41 |
| 14 | 67 | AML | Refractory | Intermediate | 2 | 300 | 6 | CR^ | 49 | 239 |
| 15 | 67 | PMF | Refractory | Intermediate | 1* | 1000 | 2 | HI-P | 21 | 35 |
| 17 | 70 | MDS-RAEB-1 | Relapsed | Unfavorable | 2§ | 1000 | 6 | HI-P | 86 | 42 |
| 22 | 76 | t-MN | Prev. untreated | Unfavorable | 0 | 1000 | 4 | CR^ | 21 | 399 |
| 24 | 68 | MDS-RAEB-2 | Prev. untreated | Favorable | 1 | 1000 | 15 | CR^ | 245 | 534 |
Prior therapy included hypomethylating agent
Prior therapy included allogeneic stem cell transplant
Number of cycles administered
Response was ongoing at the time of discontinuation of study treatment; HI-N, HI-P denote hematologic improvement in neutrophils or platelets
Table 5.
Nine responders in Randomized Phase (n=32)
| ID # | Age | Diagnosis | Stage of Disease | Cytogenetic Risk Group | No. Prior Regimens | Randomization Arm (Cycle 1) | ±No. Cycles | Best Response | Time to Initial Response (days) | Response Duration (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 31 | 57 | MDS: RAEB-2 | Refractory | Intermediate | 1* | 0 | 14 | CR-marr | 50 | 349 |
| 34 | 74 | t-MN | Prev. Untreated | Unfavorable | 0 | 1000 | 7 | CR | 59 | 161 |
| 36 | 63 | AML | Relapsed | Intermediate | 1 | 1000 | 5 | CR^ | 98 | 59 |
| 48 | 69 | CMML | Prev. Untreated | Intermediate | 0 | 1000 | 6 | HI-P/HI-E | 28 | 141 |
| 49 | 77 | MDS: RAEB-2 | Refractory | Favorable | 2* | 1000 | 28 | HI-E^ | 161 | 682 |
| 50 | 72 | t-MN | Prev. Untreated | Unfavorable | 0 | 1000 | 28 | CR | 41 | 753 |
| 51 | 53 | AML | Relapsed | Intermediate | 3§ | 0 | 5 | CR^ | 44 | 99 |
| 54 | 64 | MDS | Refractory | Unfavorable | 1 | 1000 | 6 | HI-P | 91 | 56 |
| 55 | 79 | MDS | Relapsed | Unfavorable | 1 | 0 | 5 | HI-P | 28 | 91 |
Prior therapy included hypomethylating agent
Prior therapy included allogeneic stem cell transplant
Number of cycles administered
Response was ongoing at the time of discontinuation of study treatment; CR-marr denotes complete response in the marrow HI-N, HI-P, HI-E denote hematologic improvement in neutrophils, platelets or erythroid lineage
Pharmacodynamic Studies
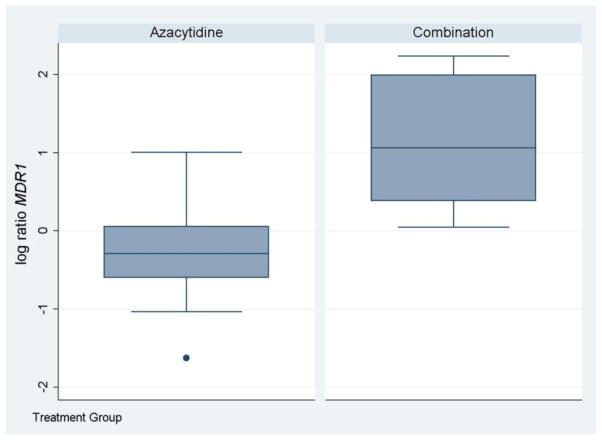
We evaluated prospectively, the change in transcript levels of the cell cycle regulatory genes p15, p21 and the multidrug resistance gene MDR1 in these samples by quantitative RT-PCR (q-RT-PCR), since these genes have been demonstrated previously to be upregulated by HDAC inhibitors and/or DNA methyltransferase inhibitors [3,21,22]. Eighteen patients (nine in each arm) had sufficient material from bone marrow aspirates obtained at baseline and day 5 for gene expression analysis, and were therefore evaluable for these studies. Samples were analyzed by q-RT-PCR for p15, p21, MDR1. At day 5, MDR1 was significantly up-regulated in the combination arm (3.1 fold increase in day 5 level) when compared with the azacitidine alone arm (p=0.0023) (Figure 1). The change in expression levels of the other genes analyzed by RT-PCR was not significantly different between the two arms.
Figure 1. The combination of belinostat and azacitidine induced a significant upregulation of MDR1 compared with AZA alone.
Quantitative RT-PCR analysis of MDR1 at baseline and day 5 following treatment in cycle 1 revealed a relative change in MDR1 expression at day 5 (compared with baseline), that was significantly higher in the combination arm (p=0.0023) compared with the azacitidine alone arm.
Discussion
This phase I study demonstrates that the combination of belinostat and azacitidine is feasible and associated with clinical activity. The recommended phase II dose is 1000 mg/m2 of belinostat combined with 75 mg/m2/d of azacitidine, given for days 1 to 5 of a 28 day cycle. The incorporation of a novel randomized design in the context of this early phase trial enabled the detection of a significant upregulation of MDR1, a gene prospectively selected for evaluation in this study, in the combination arm which suggests a pharmacological effect of belinostat on gene expression. There were no unusual toxicities observed with the combination of belinostat and azacitidine, and full dose belinostat (as previously determined in solid tumor patients) was feasible. Although higher belinostat doses may be feasible, dose escalation was discontinued due to dose-dependent grade 1–2 gastrointestinal toxicity.
Prior published reports of dual pharmacologic targeting of DNMT and HDAC enzymes in early phase trials have demonstrated the feasibility of this approach, but in general, had not incorporated a randomized study design to investigate the relative biologic contribution of the HDAC inhibitor [15–17]. An exception to this is a recent intergroup trial in which patients with MDS were randomized to azacitidine versus azacitidine plus the HDAC inhibitor entinostat. The results of that trial suggested no advantage to the addition of the entinostat. In contrast however to belinostat and vorinostat which broadly target both nuclear and non-nuclear protein deacetylases, entinostat specifically targets nuclear deacetylases [23]. There is another ongoing trial in the intergroup setting in MDS, designed to investigate azacitidine combined with vorinostat in a randomized setting, based on early reports of encouraging clinical activity with that combination.
The significant increase in MDR1 observed in our study in the combination arm raises the possibility of up-regulation of MDR1 as a biomarker for HDAC inhibition. MDR1 is a target of hypermethylation and epigenetic silencing in various malignancies including both myeloid and lymphoid leukemia cells, and reversal of epigenetic silencing and upregulation of MDR1 has been demonstrated with the use of DNMT inhibitors [24–26], although there are also reports of MDR1 decrease with DNMT inhibitor exposure [27]. We and others have demonstrated that HDAC inhibitor use is associated with upregulation of MDR1 both in vitro and in vivo, and occurs in conjunction with global and promoter specific histone acetylation [28,24,12,22], and combined treatment with HDAC inhibitor and DNMT inhibitors has been shown to be synergistic for MDR1 reactivation in vitro [29,24,26].
The biologic consequence of upregulation of MDR1 in the context of clinical development of epigenetic modulators is largely unknown. There is a potential concern based on prior in vitro studies, that an MDR phenotype associated with clinically relevant drug resistance may be generated in vivo. This has, however, not yet been borne out in the clinical trial setting, even in clinical trials utilizing agents that were known MDR substrates [30–32]. In contrast to other HDAC inhibitors such as romidepsin, neither azacitidine nor belinostat are known substrates of MDR1. Therefore concerns regarding generation of drug resistance via MDR upregulation are less relevant with this combination.
In our study, neither p15 nor p21 was significantly different at day 5 between the two arms. A variety of reasons may account for this including tumor heterogeneity [33] and the relatively small sample size making the ability to detect a difference challenging. In contrast, there is a relatively strong signal with regard to MDR1 upregulation by HDAC inhibitors, a phenomenon that has been repeatedly observed in the literature [28,24,25,12,22]. It is also quite plausible of course, given the small sample size that the difference in MDR1 that was detected was an artifact of the study, occurring purely by chance, and as such these findings require confirmation in larger randomized trials.
Significant evidence of clinical activity was observed in this combination study across the spectrum of advanced myeloid neoplasia enrolled, including patients with multiply relapsed and/or refractory AML or MDS. Our results contrast with the limited single agent activity previously reported for HDAC inhibitors, including belinostat in myeloid neoplasms [13,14]. Single agent azacitidine is active in myeloid neoplasms including untreated AML with marrow blasts up to 30% [34,10], and has measurable clinical activityin the relapsed/refractory setting, largely based on retrospective experience[35–37]. The relative clinical contribution of belinostat to this combination is an open question, which cannot be addressed by the current study design, and requires investigation in the setting of a randomized Phase II study. This is warranted, and is currently in development, based on the encouraging signs of clinical activity observed in our advanced patient population.
Acknowledgments
This work was supported by: NCI (grant # U01-CA69852) in collaboration with Spectrum Pharmaceuticals.
Footnotes
Presented in abstract form at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, June, 2011
Conflict of Interest Statement:
Olatoyosi Odenike received research funding from Curagen/Topotarget, and has served on advisory boards convened by Spectrum pharmaceuticals, Suneisis, Sanofi-Aventis, Incyte and Algeta pharmaceuticals
Luc A. Godley has received research support from Pharmion/Celgene
Ryan J Mattison has served on advisory boards convened by Bristol Myers Squibb and Incyte pharmaceuticals
Karen Yee has served as a consultant for Celgene
Mark J. Ratain has served as a consultant for Onconova and Cyclacel
References
- 1.Chen J, Odenike O, Rowley JD. Leukaemogenesis: more than mutant genes. Nat Rev Cancer. 2010;10 (1):23–36. doi: 10.1038/nrc2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19 (2):187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 3.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21 (1):103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky SA, Grimes MJ, Picchi MA, Mitchell HD, Stidley CA, Tesfaigzi Y, Channell MM, Liu Y, Casero RA, Jr, Baylin SB, Reed MD, Tellez CS, March TH. Combination therapy with vidaza and entinostat suppresses tumor growth and reprograms the epigenome in an orthotopic lung cancer model. Cancer Res. 2011;71 (2):454–462. doi: 10.1158/0008-5472.CAN-10-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele N, Finn P, Brown R, Plumb JA. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer. 2009;100 (5):758–763. doi: 10.1038/sj.bjc.6604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31 (2):141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 7.Klisovic MI, Maghraby EA, Parthun MR, Guimond M, Sklenar AR, Whitman SP, Chan KK, Murphy T, Anon J, Archer KJ, Rush LJ, Plass C, Grever MR, Byrd JC, Marcucci G. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia. 2003;17 (2):350–358. doi: 10.1038/sj.leu.2402776. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106 (8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 9.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20 (10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 10.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, Larson RA. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24 (24):3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M, Lin TS, Liu S, Sklenar AR, Davis ME, Lucas DM, Fischer B, Shank R, Tejaswi SL, Binkley P, Wright J, Chan KK, Grever MR. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105 (3):959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 12.Odenike OM, Alkan S, Sher D, Godwin JE, Huo D, Brandt SJ, Green M, Xie J, Zhang Y, Vesole DH, Stiff P, Wright J, Larson RA, Stock W. Histone deacetylase inhibitor romidepsin has differential activity in core binding factor acute myeloid leukemia. Clin Cancer Res. 2008;14 (21):7095–7101. doi: 10.1158/1078-0432.CCR-08-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cashen A, Juckett M, Jumonville A, Litzow M, Flynn PJ, Eckardt J, LaPlant B, Laumann K, Erlichman C, DiPersio J. Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS) Ann Hematol. 2012;91 (1):33–38. doi: 10.1007/s00277-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschbaum MH, Foon KA, Frankel P, Ruel C, Pulone B, Tuscano JM, Newman EM. A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium Study. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2013.877134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci G. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25 (25):3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Xiao L, Faderl S, Estrov Z, Cortes J, O’Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa JP. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108 (10):3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, Rudek MA, Zhao M, Smith BD, Manning J, Jiemjit A, Dover G, Mays A, Zwiebel J, Murgo A, Weng LJ, Herman JG. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66 (12):6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 18.Steele NL, Plumb JA, Vidal L, Tjornelund J, Knoblauch P, Rasmussen A, Ooi CE, Buhl-Jensen P, Brown R, Evans TR, DeBono JS. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14 (3):804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21 (24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108 (2):419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 21.Jiemjit A, Fandy TE, Carraway H, Bailey KA, Baylin S, Herman JG, Gore SD. p21(WAF1/CIP1) induction by 5-azacytosine nucleosides requires DNA damage. Oncogene. 2008;27 (25):3615–3623. doi: 10.1038/sj.onc.1211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robey RW, Zhan Z, Piekarz RL, Kayastha GL, Fojo T, Bates SE. Increased MDR1 expression in normal and malignant peripheral blood mononuclear cells obtained from patients receiving depsipeptide (FR901228, FK228, NSC630176) Clin Cancer Res. 2006;12 (5):1547–1555. doi: 10.1158/1078-0432.CCR-05-1423. [DOI] [PubMed] [Google Scholar]
- 23.Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg PL, Herman J, Juckett M, Smith MR, Malick L, Paietta E, Czader M, Litzow M, Gabrilove J, Erba HP, Gore SD, Tallman MS. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. J Clin Oncol. 2014;32 (12):1242–1248. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henrique R, Oliveira AI, Costa VL, Baptista T, Martins AT, Morais A, Oliveira J, Jeronimo C. Epigenetic regulation of MDR1 gene through post-translational histone modifications in prostate cancer. BMC Genomics. 2013;14:898. doi: 10.1186/1471-2164-14-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantharidis P, El-Osta A, deSilva M, Wall DM, Hu XF, Slater A, Nadalin G, Parkin JD, Zalcberg JR. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin Cancer Res. 1997;3 (11):2025–2032. [PubMed] [Google Scholar]
- 26.Lee TB, Park JH, Min YD, Kim KJ, Choi CH. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008;8:33. doi: 10.1186/1471-230X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Efferth T, Futscher BW, Osieka R. 5-Azacytidine modulates the response of sensitive and multidrug-resistant K562 leukemic cells to cytostatic drugs. Blood Cells Mol Dis. 2001;27 (3):637–648. doi: 10.1006/bcmd.2001.0427. [DOI] [PubMed] [Google Scholar]
- 28.Hauswald S, Duque-Afonso J, Wagner MM, Schertl FM, Lubbert M, Peschel C, Keller U, Licht T. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15 (11):3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 29.David GL, Yegnasubramanian S, Kumar A, Marchi VL, De Marzo AM, Lin X, Nelson WG. MDR1 promoter hypermethylation in MCF-7 human breast cancer cells: changes in chromatin structure induced by treatment with 5-Aza-cytidine. Cancer Biol Ther. 2004;3 (6):540–548. doi: 10.4161/cbt.3.6.845. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Tambaro FP, Bekele NB, Yang H, Ravandi F, Jabbour E, Borthakur G, Kadia TM, Konopleva MY, Faderl S, Cortes JE, Brandt M, Hu Y, McCue D, Newsome WM, Pierce SR, de Lima M, Kantarjian HM. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol. 2012;30 (18):2204–2210. doi: 10.1200/JCO.2011.38.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gojo I, Tan M, Fang HB, Sadowska M, Lapidus R, Baer MR, Carrier F, Beumer JH, Anyang BN, Srivastava RK, Espinoza-Delgado I, Ross DD. Translational phase I trial of vorinostat (suberoylanilide hydroxamic acid) combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clin Cancer Res. 2013;19 (7):1838–1851. doi: 10.1158/1078-0432.CCR-12-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, Zain J, Prince HM, Leonard JP, Geskin LJ, Reeder C, Joske D, Figg WD, Gardner ER, Steinberg SM, Jaffe ES, Stetler-Stevenson M, Lade S, Fojo AT, Bates SE. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27 (32):5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klco JM, Spencer DH, Lamprecht TL, Sarkaria SM, Wylie T, Magrini V, Hundal J, Walker J, Varghese N, Erdmann-Gilmore P, Lichti CF, Meyer MR, Townsend RR, Wilson RK, Mardis ER, Ley TJ. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood. 2013;121 (9):1633–1643. doi: 10.1182/blood-2012-09-459313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28 (4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 35.Pleyer L, Burgstaller S, Girschikofsky M, Linkesch W, Stauder R, Pfeilstocker M, Schreder M, Tinchon C, Sliwa T, Lang A, Sperr WR, Krippl P, Geissler D, Voskova D, Schlick K, Thaler J, Machherndl-Spandl S, Theiler G, Eckmullner O, Greil R. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: results from the Austrian Azacitidine Registry of the AGMT-Study Group. Ann Hematol. 2014;93 (11):1825–1838. doi: 10.1007/s00277-014-2126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, Hoppe G, Niederwieser D. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53 (1):110–117. doi: 10.3109/10428194.2011.606382. [DOI] [PubMed] [Google Scholar]
- 37.Tawfik B, Sliesoraitis S, Lyerly S, Klepin HD, Lawrence J, Isom S, Ellis LR, Manuel M, Dralle S, Berenzon D, Powell BL, Pardee T. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML) Ann Hematol. 2014;93 (1):47–55. doi: 10.1007/s00277-013-1940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]