Abstract
Significant interest in 44Sc as a radioactive synthon to label small molecules for positron emission tomography (PET) imaging has been recently observed. Despite the efforts of several research groups, the ideal 44Sc production and separation method remains elusive. Herein, we propose a novel separation method to obtain 44Sc from the proton irradiation of calcium targets based on extraction chromatography, which promises to greatly simplify current production methodologies. Using the commercially available Uranium and Tetravalent Actinides (UTEVA) extraction resin we were able to rapidly (< 20 min) recover > 80% of the activity generated at end of bombardment (EoB) in small ~1 M HCl fractions (400 μL). The chemical purity of the 44Sc eluates was evaluated through chelation with DOTA and DTPA, and by trace metal analysis using microwave induced plasma atomic emission spectrometry. The distribution coefficients (Kd) of Sc(III) and Ca(II) in UTEVA were determined in HCl medium in a range of concentrations from zero to 12.1 M The 44Sc obtained with our method proved to be suitable for the direct labeling of small biomolecules for PET imaging, with excellent specific activities and radiochemical purity.
Keywords: 44Sc, Scandium-44, PET, Radioisotope production, Radiochemical separation, Radiometal, Radiolabeling
1. Introduction
Due to its favorable nuclear properties and amenable chemistry, 44Sc (t1/2=3.927 h, 94.3% β+, Emax=1474 ke V)1 has been recently recognized as a radiometal holding great potential for PET applications. In spite of this, only a handful of small molecules have been radiolabeled with 44Sc, and even less tested in a preclinical setting (Hernandez et al., 2014; Koumarianou et al., 2012; Koumarianou et al., 2011; Muller et al., 2013; Pruszynski et al., 2012). This is a result of the non-optimal current production and separation methods, which have limited the broad availability of this isotope. Currently, 44Sc can be produced from a 44Ti/44Sc generator (Filosofov et al., 2010) and from biomedical cyclotrons via the 44Ca(p,n)44Sc route (Hoehr et al., 2014; Krajewski et al., 2013; Muller et al., 2013; Severin et al., 2012a; Severin et al., 2012b). However, several issues plague these methodologies. For instance, the difficult production of the parent isotope (44Ti) together with the required extensive post-elution purification of the 44Sc eluate, limit the applicability of generator-based 44Sc (Pruszynski et al., 2010). On the other hand, cyclotron production provides a more efficient method to produce significantly larger 44Sc activities. Nevertheless, a simple yet efficient separation method to isolate 44Sc from the irradiated calcium target remains inexistent. In this study, we describe a novel separation method to obtain 44Sc from irradiated natural calcium targets using extraction chromatography.
Tri-n-butylphosphate (TBP), shown in Fig. 1a, is one of the most common organophosphorus extractants, for which extensive data on distribution coefficients of most metals is available (Braun and Ghersini, 1975). Based on this data the separation of Sc(III) from bulk calcium can be effectively achieved in a TBP–HCl extraction system, since Sc(III) and Ca(II) have more than 1000-fold difference in distribution coefficients when the concentration of the acid is ≥ 9 M. Hence, given the striking structural similarities between TBP and dipentyl pentylphosphonate (DP[PP]), shown in Fig. 1b, we hypothesized that separation of radioactive scandium from calcium could be achieved using UTEVA, a commercially available resin functionalized with DP[PP].
Fig. 1.
(a) Tri-n-butylphosphate (TBP) and (b) Dipentyl pentylphosphonate (DP[PP]), the active extractant in the UTEVA resin.
Herein, we report the successful production and facile UTEVA-based separation of 44Sc from proton irradiated natural calcium targets with excellent yield and chemical purity, in a chemical form amenable for radiolabeling. Additionally, we determined the distribution coefficients of Sc(III) and Ca(II) between UTEVA and HCl to confirm the optimal conditions for their separation. We compare this novel and simple separation method with those from (Krajewski et al., 2013; Muller et al., 2013; Severin et al., 2012a, 2012b), which are based on the difference in binding affinity of scandium and calcium to the chelating resin Chelex 100, the difference in their distribution coefficients in the extraction resin N,N,N′,N′-tetra-n-octyldiglycolamide (DGA), their difference in solubility, and their difference in distribution coefficients in a hydroxamate-functionalized resin, respectively.
Finally, we believe that the implementation of our simple purification method promise to greatly simplify the cyclotron production of radioactive scandium using metallic calcium targets and allow for its readily automation.
2. Materials and methods
Optima grade HCl comes from Aristar Ultra, VWR, West Chester, PA. Natural calcium (natCa, 99.99%) in dendritic chunks comes from Sigma-Aldrich, St. Louis MO. UTEVA (100–150 μm) resin comes from Eichrom, Lisle IL. The cyclic chelating ligand1,4,7,10-tetraazacyclodo-decane-1,4,7,10-tetraacetic acid (DOTA) was purchased from Macrocyclics, Dallas TX. The acyclic chelating ligand diethylenetriamine pentaacetic acid (DTPA) was purchased from Acros Organics, Geel, Belgium. Sodium acetate (NaOAc) was purchased from Fisher Scientific, Pittsburg PA. Both chelating ligands and NaOAc were dissolved in 18 MΩ-cm deionized (DI) water and mixed with Chelex 100 from Sigma-Aldrich for trace metal purification. Scandium foil (99.9%) was purchased from Alfa Aesar, Ward Hill MA. A 50 ppm multi-element standard for calibration and Agilent’s 4200 Microwave Plasma Atomic Emission Spectroscopy (MP-AES) system come from Agilent Technologies, Santa Clara CA.
2.1. Cyclotron targetry and irradiations
The target system is very similar to that described in (Severin et al., 2012a). Briefly, 312 ± 19 mg (n=11) of natCa were pressed with a hydraulic press at > 400 kg/cm2 into an annular ring of 1.26 cm2, 2.2 mm deep made of aluminum. A 0.56 mm thick silver disk in direct contact with the pressed calcium separated it from water-jet cooling applied on the backside. A 25 μm molybdenum foil was placed over the irradiated face of the target to protect the cyclotron from vaporized calcium. Irradiations were performed on the UW-Madison PETtrace cyclotron using 16 MeV protons for 1 h with an average current of 25 μA.
2.2. Target yields, separation yields and radionuclidic contaminants
44Sc activities for separation yield quantification were measured with a Capintec CRC-15 (Capintec, Ramsey NJ) dose calibrator using the calibration setting 938 suggested by the manufacturer. However, the actual activity from 44Sc and other radionuclidic impurities was measured from the gamma intensities of 50 μL samples placed at distances with known efficiency calibration from a 60 cm3 high purity germanium (HPGe) detector (Canberra C1519) (FWHM=2.7 keV @1333 keV). Gamma-ray spectrum analysis software package, Maestro-32 MCA Emulator (Ortec, Oak Ridge TN), was used to collect and analyze the gamma-ray spectra. The gamma lines used to determine yields are listed in Table 1. The dead time was always kept below 10% and the acquisition time was set so that the statistical uncertainty from the number of counts per peak was kept below 1%, except for the 373 keV peak from 43Sc, for which the statistical uncertainty was 3%. From the accurate activity value of 44Sc it was confirmed that the Capintec measurement was within 10% of the HPGe result, thus this reading was used for the quantification of the separation yields.
Table 1.
Gamma emissions from the scandium radioisotopes used for determining yields.
| Nuclide | Gamma energies (keV) | Branching ratio |
|---|---|---|
| 43Sc | 373 | 0.23 |
| 44Sc | 1157 | 0.999 |
| 44mSc | 271 | 0.867 |
| 47Sc | 159 | 0.683 |
| 48Sc | 983.5 | 1.00 |
Theoretical activity yields at saturation for 43Sc, 44Sc and 44mSc at EoB were calculated using the cross-section data from (Levkovskij, 1991). However, this cross-section data was reduced by 20%, due to incorrect assessment of the excitation functions of the monitor reactions in the original publication (Takacs et al., 2002). The stopping power of protons in calcium was obtained from the SRIM software (Ziegler et al., 2013).
2.3. Radiochemical separation
The ~300 mg calcium target was pushed out of the target holder cavity into a 50 mL centrifuge tube, to which 5 to 15 mL of concentrated HCl were added to dissolve the target and maintain the H+ concentration > 9 M. This solution was then transferred manually to one of the three syringes that act as reservoirs connected to an automated module, similar to the one described in (Siikanen et al., 2012), which contains programmable dual pinch valves that control the access to the reservoirs. The module also includes two radiation detectors made in-house by encapsulating PIN photodiodes (HTV S8746-01, Hamamatsu Photonics, Japan) coupled with a CsI scintillator crystal. One detector monitors de activity in the syringe with the target solution and the other one the activity in the chromatography column. Before the activity was transferred onto the module, new syringes were connected and then filled with several milliliter of DI water to flush the tubing and remove metal impurities left from previous separations. Then, one of the reservoirs was filled with 5 mL of 10 M HCl for the washing step and another one with 400 μL of deionized water for the 44Sc release step. A peristaltic pump with a flow rate of 1.1 mL/min sent the solution to a 5 mm diameter column cartridge (SPE 1.5 mL reservoir, Grace Davison Discovery Sciences, Deerfield IL) filled with 52 ± 2 mg of UTEVA resin. This packed resin is previously equilibrated with 1 mL of 10 M HCl manually. We selected ~50 mg as the amount of resin for practical reasons such as establishing an appropriate resin bed in the column that permitted maximum contact with the mobile phase. Additionally, when we reduced the amount of resin by half (~25 mg, Table 5, row a) a significant drop on the trapping efficiency from 83% to 52% was observed, possibly due to an inefficient equilibration between mobile and stationary phases in the column.
Table 5.
Results of separation experiments.
| n | Calcium mass (mg) | mL of conc. HCl added for dissolution | Molarity of dissolutiona (mol/L) | UTEVA mass (mg) | % of activity trapped in UTEVA | % of total activity eluted in 1st fraction (400 μL DI) | % of total activity eluted in 2nd fraction (200 μL DI) | |
|---|---|---|---|---|---|---|---|---|
| a) | 1 | 295.7 | 5 | ~9.1 | 24.5 | 52 | 43 | 2.5 |
| b) | 3 | 313 ± 14 | 5–5.5 | ~9.1 | 52 ± 1 | 83 ± 5 | 68 ± 4 | 4.7 ± 1.6 |
| c) | 7 | 317 ± 22 | 10 | ~10.5 | 52 ± 3 | 89 ± 3 | 80 ± 4 | 5.1 ± 1. 3 |
| d) | 6 | 304 ± 8 | 15 | ~11.1 | 52 ± 1 | 92 ± 2 | 82 ± 2 | 5.6 ± 2.7 |
The molarity is estimated by subtracting the amount of moles of hydrogen released as H2 gas from the amount of moles of H+ ions in solution, and then diving this by the total volume of the dissolution. The amount of H2 that is released is estimated from the reaction equation Ca+2HCl→CaCl2+H2.
The trap and release sequence was as follows:
Load the target solution at varying HCl concentrations (9.1, 10.5, 11 M) to trap 44Sc.
Wash the column with 5 mL of 10 M HCl.
Elute 44Sc in successive fractions of deionized water. The first 400 μL fraction, with an approximate [H+] of 1 M was used as the 44Sc stock solution for labeling of DOTA and DTPA.
Fig. 2 shows a schematic of the automated module with the numbered arrows indicating the sequence of steps used in the separation. Trace metal analysis was performed on samples from three separation runs dissolving the target in 10 mL of concentrated HCl for a final solution concentration of ~10.5 M HCl. Table 2 describes the samples that were analyzed using Agilent’s 4200 MP-AES system.
Fig. 2.
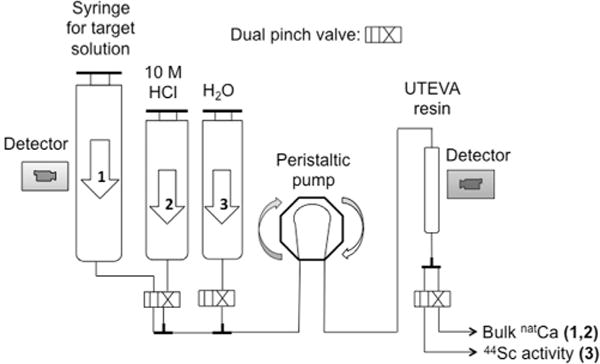
Scheme of the separation process overlaid in a schematic of the automated module.
Table 2.
Samples analyzed by MP-AES.
| Volume (μL) | Sample description | |
|---|---|---|
| i) | 50 | Target dissolution in 10 mL |
| ii) | 50 | Eluate after trapping Sc |
| iii) | 50 | Eluate from 5 mL 10 M HCl wash |
| iv) | 400 | First eluted fraction with isolated Sc |
| v) | 200 | Second eluted fraction with isolated Sc |
2.4. Determination of Kd values of Sc(III) and Ca(II) in UTEVA and HCl solution at various concentrations
Kd values for Sc(III) and Ca(II) in UTEVA were determined in batch experiments using different concentrations of HCl similar to the method described in (Filosofov et al., 2010). 44Sc dissolved in ~1 M HCl from one of the separations was used as a tracer of Sc(III). 26.2 mg of 99.99% natCa were dissolved in 6 M HCl, dried down and re-dissolved in 0.05 M HCl in order to have a concentration of 1 mg/20 μL. Aliquots were prepared in Eppendorf 1.5 mL vials with 100 mg of UTEVA resin. To all solutions 1 mL of HCl was added, then 20 μL of the 44Sc tracer solution (12.1 MBq), followed by 20 μL of the natCa solution (1.0 mg). Each of the vials was shaken at room temperature for 4 h in a shaker set at 500 rpm. 400 μL of the supernatant was taken from every vial and radioactivity A′ was measured on the Capintec. The mass of calcium m′ in this fraction was assayed using the MP-AES system. The dimensionless Kd value for 44Sc was calculated from the following equation:
A being the activity of the whole vial and A′ being the activity of a 400 μL sample of the solution after the extraction, both decay-corrected to the time when the vials were removed from the shaker.
In the case of calcium, Kd was calculated from the analogous equation:
2.5. Reactivity with DOTA and DTPA
The reactivity of a radiometal towards a chelating ligand is an indirect method for quantifying the amount of competing nonradioactive metal impurities in a solution. The reactivity of the separated 44Sc was assayed using the macrocycle 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) as well as the acyclic chelating ligand diethylene triamine pentaacetic acid (DTPA) following the method described in (Severin et al., 2012a). First, a stock solution is made by mixing 100 μL (> 74 MBq) of the 44Sc eluate with 1 mL of deionized water and 1 mL of 0.25 M sodium acetate (NaOAc). Fractions of 100 μL (~3.7MBq) from this buffered solution are distributed into two sets of 10 1.5 mL Eppendorf vials, each containing 100 μL of an aqueous solution of DOTA or DTPA with a known mass of chelator that ranges from zero to 10 μg. All reaction vials had a pH of 4.5. The DOTA solutions were incubated at 85–95 °C for 1 h and the DTPA solutions at room temperature for 30 min and then spotted onto aluminum backed silica gel ITLC plates (EMD Chemicals, Gibbstown NJ) for Thin Layer Chromatography (TLC), using 0.25 M ammonium hydroxide as the mobile phase. The activity distribution on the plates was assessed by autoradiography with a Packard Cyclone Phosphor-Plate imaging system.
3. Results and discussion
3.1. Cyclotron targetry and irradiations
The metallic calcium target is able to withstand a current of 25 μA without any noticeable effect in its integrity. The 25 μm molybdenum foil degrader drops the 16 MeV proton beam from the PETtrace cyclotron down to 15.56 ± 0.04 MeV according to a SRIM simulation (Ziegler et al., 2013). The exit energy after passing through 2.2 mm of calcium is 3.4 ± 0.4 MeV also according to a SRIM simulation. This constitutes a thick target since 4.5 MeV is the threshold energy for the 44Ca(p,n)44gSc reaction.
3.2. Target yields, separation yields and radionuclidic contaminants
Table 3 lists the experimental activity saturation yields at end of bombardment (EoB) of the scandium isotopes produced after our irradiation setting of 15.56 MeV protons, at 25 μA for one hour. These experimental yields are in agreement with the saturation yields calculated from the excitation functions published in (Levkovskij, 1991) after a correction of 20% (Takacs et al., 2002) and the yields reported in (Severin et al., 2012a).
Table 3.
Natural abundances of calcium isotopes and their radioactive scandium reaction products after proton bombardment at 15.56 MeV.
| Target | % abundance | Nuclear reaction | t1/2 | Measured saturation yield (MBq/μA) | Predicted yield using corrected data in (Levkovskij, 1991) (MBq/μA) | % of predicted yield | % of yield measured in (Severin et al., 2012a) at 16 MeV |
|---|---|---|---|---|---|---|---|
| 40Ca | 96.941 | 40Ca(p,n)40Sca | 182 ms | – | |||
| 42Ca | 0.647 | 42Ca(p,n)42mSca | 61.7 s | – | |||
| 42Ca(p,n)42gScb | 681 ms | – | |||||
| 43Ca | 0.135 | 43Ca(p,n)43Scb | 3.89 h | 4.870.8 | 7.06 | 68% | 90% |
| 43Ca(p,2n)42Sca | 681 ms | – | |||||
| 44Ca | 2.086 | 44Ca(p,n)44gSc | 3.93 h | 179 ± 22 | 257 | 70% | 84% |
| 44Ca(p,n)44mSc | 58.6 h | 15.2 ± 2.0 | 20.0 | 76% | 88% | ||
| 44Ca(p,2n)43Scb | 3.89 h | ||||||
| 46Ca | 0.004 | 46Ca(p,n)46Sc | 83.8 h | Unobserved | |||
| 48Ca | 0.187 | 48Ca(p,n)48Sc | 43.7 h | 15.6 ± 2.4 | – | – | 75% |
| 48Ca(p,2n)47Sc | 80.4 h | 14.5 ± 2.5 | – | – | 129% |
These reactions are practically negligible because of the too short half-life of the produced Sc-isotope.
The 43Sc produced from 44Ca(p,2n), with an energy threshold of 14.5 MeV, is not distinguished from 43Ca(p,n) in this study.
From our irradiation setting it is reasonable to expect about 0.79 ± 0.08 GBq of 44Sc in the target. The percentage impurities at EoB and 9 h, the timeframe of maximum uptake for many radiolabeled peptides (Hernandez et al., 2014), are presented in Table 4.
Table 4.
Radionuclidic purity of the separated scandium at EoB and 8 h after EoB (n=8).
| Scandium isotope | % of total activity at EoB | % of total activity 9 h after EoB |
|---|---|---|
| 44gSc | 95.7 ± 0.3 | 90.6a |
| 44mSc | 0.6 ± 0.1 | 2.3 |
| 43Sc | 2.6 ± 0.3 | 2.3 |
| 47Sc | 0.40 ± 0.05 | 1.7 |
| 48Sc | 0.8 ± 0.1 | 3.0 |
Bateman’s equilibrium equation between 44mSc and 44Sc was included in the computation of this value.
The radioactivity of the product is > 95% 44Sc at EoB and the radionuclidic purity will remain above 90% up to 9 h after EoB. This level of purity is tolerable for preclinical PET studies and perhaps even human studies if dosimetry studies demonstrate tolerable doses from the longer lived impurities 44mSc, 47Sc and 48Sc, particularly if they potentially accumulate in bone tissue after detaching from an unstable chelate complex. If the radioactive scandium is completely excreted from the body, then we believe that 44Sc from irradiated natural calcium targets is suitable for clinical applications. Natural calcium targets are in metallic form with a thermal conductivity of 200 W/m K, which is comparable to aluminum, 235 W/m K. Calcium is inexpensive and readily available so thick targets for higher yields are economically feasible and target recycling is unnecessary. However, if a higher radionuclidic purity is needed (> 99%, with the coproduced 44mSc representing the remaining 1%), then isotopically enriched 44Ca targets are mandatory. To our knowledge, only two groups have reported the use of these target (Krajewski et al., 2013; Muller et al., 2013), both of them employing encapsulated enriched calcium carbonate, 44CaCO3.
3.3. Radiochemical separation
The activity measured with the Capintec dose calibrator was < 10% higher than the 44Sc activity measured with the HPGe detector. Hence, this reading was used for the determination of separation yields.
Loading the target at a H+ concentration of ~10.5 M, 80 ± 4% (n = 7) of the original activity (decay corrected) was recovered in 400 μL of deionized water. The 44Sc from this separation setting offered the highest separation yield with a high reactivity with DOTA, as will be shown in Section 3.5, and is highlighted in bold in Table 5. Table 5 contains these and other results, together with the parameters involved in the separation. All activities are decay corrected to EoB.
As it was pointed out in Section 2.3, ~50 mg of resin was selected for the optimization experiments since we observed a significant drop in the trapping efficiency, from 83% to 52%, when we reduced the amount of resin by half (~25 mg, Table 5, row a) when loading the target solution in a HCl concentration of ~ 9.1 M. Combining the results in Tables 5 and 8 we can see that there is a trade off between the separation efficiency and the reactivity of the radioactive scandium. Loading the target solution onto the resin at a concentration of 10.5 M HCl results in the optimum with both high separation yield and reactivity. The final product from this separation method was further characterized by analyzing it with MP-AES for trace metal contaminants and by titration with the acyclic ligand DTPA. The results from the MP-AES analysis indicated that the most significant metal impurities are: calcium (2.1 mM), iron (93 μM), zinc (72 μM), nickel (29 μM), aluminum (6.4 μM) and manganese (2.0 μM). These results are summarized in Table 6. The concentrations highlighted in bold correspond to the 400 μL fraction of 44Sc employed in the radiolabeling experiments.
Table 8.
Comparison of the separation methods used for the separation of radioactive Sc from calcium-based targets.
| Method | Target | Proton energy (MeV) | Activity yield (MBq/μAh) | Separation yield (%) | Volume of 44Sc eluate (mL) | Matrix of separated 44Sc | Calcium conc. in separated 44Sc (mM) | Specific Activity with DOTA (GBq/μmol) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Filtration | natCa (99.99%) | 16 | 38 ± 3 | 40 ± 18 | 0.15 | 0.1 M HCl | 2.3 | 54 ± 14 | (Severin et al., 2012a) |
| Hydroxamate resin | natCa (99.99%) | 16 | 38 ± 3 | 63 ± 15 | 1.0 | 0.1 M HCl | Not reported | 50 | (Severin et al., 2012b) |
| Chelex 100 | 44CaCO3 (94.53%) | 9.0 | 8.9 | 70 | 0.50 | 1 M HCl | < 0.025 | 22 | (Krajewski et al., 2013) |
| DGA+DOWEX-50 | 44CaCO3 (97.00%) | 17.6 ± 1.8 | 11.7 | 85 | 0.20–0.40 | 1 M NH4OAc | Not reported | 5.2 | (Muller et al., 2013) |
| UTEVA | natCa (99.99%) | 15.56 ± 0.04 | 32 ± 3 | 80 ± 4 | 0.40 | ~1 M HCl | 2.1 ± 0.4 | 18.1 ± 6.7 | This work |
Table 6.
Results from MP-AES analysis on the samples described in Table 2.
| Sample | i) | ii) | iii) | iv) | v) | |
|---|---|---|---|---|---|---|
| Caa | (ppm) | 32770 ± 932 | Overrange | 1110 ± 127 | 82.2 ± 16.5 | 57.3 ± 7.4 |
| (mM) | 818 ± 23 | Overrange | 27.7 ± 3.2 | 2.1 ± 0.4 | 1.4 ± 0.2 | |
| Mass in total volume (mg) | 309.6 ± 5.2 | Overrange | 5.55 ± 0.64 | 0.027 ± 0.001 | 0.012 ± 0.002 | |
| Separation factor | 1.2 × 104 | |||||
| Fe | (ppm) | 0.6 ± 0.3 | 0.32 ± 0.09 | 0.16 ± 0.14 | 5.2 ± 3.2 | 1.2 ± 0.8 |
| (μM) | 10 ± 6 | 5.6 ± 1.5 | 2.8 ± 2.5 | 93 ± 58 | 22 ± 15 | |
| Mass in total volume (μg) | 5.6 ± 3.2 | 5.1 ± 3.6 | 0.90 ± 0.54 | 1.8 ± 0.8 | 0.27 ± 0.21 | |
| Separation factor | 3 | |||||
| Zn | (ppm) | 0.7 ± 0.4 | 1.6 ± 0.8b | 2.7 ± 1.1 | 4.7 ± 1.3 | 5.8 ± 2.4 |
| (μM) | 10 ± 6 | 25 ± 12 | 52 ± 16 | 72 ± 20 | 88 ± 36 | |
| Mass in total volume (μg) | 6.6 ± 3.9 | 15.5 ± 7.8 | 14 ± 5 | 1.7 ± 0.7 | 1.2 ± 0.4 | |
| Separation factor | 4 | |||||
| Ni | (ppm) | 2.7 ± 1.5 | 2.8 ± 1.9 b | 2.7 ± 1.2 | 1.7 ± 1.3 | 0.90 ± 0.59 |
| (μM) | 46 ± 26 | 48 ± 33 | 47 ± 21 | 29 ± 22 | 15 ± 10 | |
| Mass in total volume (μg) | 26 ± 18 | 26 ± 18 | 10 ± 6 | 0.58 ± 0.39 | 0.18 ± 0.17 | |
| Separation factor | 45 | |||||
| Al | (ppm) | 9.6 ± 0.1 | 7.6 ± 0.1 | 1.2 ± 0.7 | 0.17 ± 0.02 | 0.18 ± 0.09 |
| (μM) | 354 ± 1 | 281 ± 2 | 44 ± 26 | 6.4 ± 0.7 | 6.7 ± 3.4 | |
| Mass in total volume (μg) | 91.3 ± 0.4 | 71.7 ± 0.4 | 8.4 ± 1.2 | 0.059 ± 0.017 | 0.046 ± 0.017 | |
| Separation factor | 1559 | |||||
| Mn | (ppm) | 0.77 ± 0.30 | 0.90 ± 0.79b | 0.12 ± 0.90 | 0.11 ± 0.07 | 0.07 ± 0.06 |
| (μM) | 14 ± 5 | 16 ± 14 | 2.2 ± 1. 5 | 2.0 ± 1.3 | 1.3 ± 1.0 | |
| Mass in total volume (μg) | 7.3 ± 2.7 | 8.4 ± 7.2 | 0.59 ± 0.43 | 0.041 ± 0.031 | 0.016 ± 0.012 | |
| Separation factor | 178 |
The calcium content in sample i) was calculated from the measured mass of calcium dismounted from the target system.
The increase in Zn, Ni and Mn content in the eluate after loading the Sc may come from the UTEVA resin or the tubing in the separation module.
The stability constants (log KML) between metals and ligands, such as DOTA and DTPA, provide a convenient gauge of the ligand’s relative affinity for a specific metal (Wadas et al., 2010). The main contaminants in the final product are calcium, iron, zinc and nickel, the three latter ones being important competitors for DOTA chelation based on the magnitude of their thermodynamic stability constants when bound to this chelator: 29.4, 20.8 and 20.0, respectively (Anderegg et al., 2005; Martell et al., 2004), which are comparable to that of Sc(III), 22.5 (Huclier-Markai et al., 2011). The main impurity calcium, on the other hand, is not a strong competitor for DOTA chelation, since its log KML value is 17.2 (Anderegg et al., 2005). A typical production run of 25 μA h generates 0.637 GBq of 44Sc in 400 μL, which corresponds to an activity concentration of 1593 GBq/L Dividing this by the sum of the concentrations, in μmol/L, of the main metallic impurities that behave chemically similar to Sc3+ ions in aqueous solution (Fe3+, Zn2+, Ni2+) results in an effective specific activity of 8.2 GBq/μmol, which can be seen in Section 3.5, has the same order of magnitude of the reactivity between scandium and DOTA, 18.1 ± 6.7 GBq/μmol.
3.4. Determination of Kd values of Sc(III) and Ca(II) in UTEVA and HCl solution at various concentrations
Fig. 3 shows the distribution coefficients (Kd) between resin and acid of scandium and calcium for HCl concentrations ranging from 0 to 12.1 mol/L Clearly, this plot confirms our hypothesis that the UTEVA resin and TBP have similar extraction properties with respect to calcium and scandium in hydrochloric acid medium.
Fig. 3.
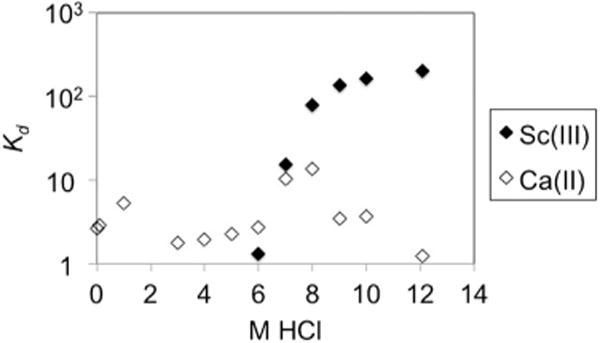
Distribution coefficients of Sc(III) and Ca(II) in UTEVA resin at different HCl concentrations.
Furthermore, the separation was possible in a “trap-and-release” fashion employing ~50 mg of UTEVA packed in a 5 mm diameter column with the solution flowing at 1.1 mL/min thanks to the > 10-fold difference in the distribution coefficients between calcium and scandium at a HCl concentration > 9 M.
3.5. Reactivity with DOTA and DTPA
Thin layer chromatography of the DOTA and DTPA titrations showed retention factors of 0.7 and 1.0, respectively, consistent with the production of the scandium complex since these peaks were not present in the control vial with no chelator. These peaks increased in activity concentration as a greater mass of chelating ligand was added to the reaction vial. Plotting the percentage of chelated 44Sc against mass of chelator, a sigmoid curve is obtained from which the reactivity or effective specific activity is calculated by dividing the activity in each vial over the amount of mass with which 50% of the radioactive scandium is chelated, and then multiplying this value times two. When the solution is loaded onto the UTEVA column at a concentration of ~10.5 M, the reactivity was 18.1 ± 6.7 GBq/μmol, which was enough for radiolabeling the DOTA conjugated cyclic arginine-glycine-aspartate (RGD) dimer E[c(RGDyK)]2 (Hernandez et al., 2014) as well as the DTPA derivative cyclohexyldiethylenetriaminepentaacetic acid ligand (CHX-A″-DTPA) conjugated to an antibody fragment at a nmol scale with > 90% yield. The rest of the results are presented in Table 7.
Table 7.
Results of the DOTA-Sc and DTPA-Sc reactivity experiments from the separation runs explained in Table 5.
| Reactivity of 1st fraction with DOTA (GBq/μmol) | Reactivity of 1st fraction with DTPA (GBq/μmol) | |
|---|---|---|
| a) | 15.2 | – |
| b) | 18.5 ± 3.5 | – |
| c) | 18.1 ± 6.7 | 49 ± 37 |
| d) | 3.8 ± 0.7 | – |
The Sc-DOTA reactivity is about 3 and 5 times less than reported in (Severin et al., 2012a, 2012b), 54 and 100 GBq/μmol, respectively. Nevertheless, it has the same order of magnitude as the one inferred from the pioneer work in (Pruszynski et al., 2010), 7.0 GBq/μmol, in which the peptide DOTATOC was successfully labeled to 44Sc from a 44Ti generator with > 98% yield. The Sc-DTPA reactivity is more than two times higher than with DOTA, which renders this ligand or its derivatives as potential labels of targeted agents, such as the FDA-approved 90Y-Ibritumomab tiuxetan, tiuxetan being the DTPA derivative 1B4M-DTPA (Camera et al., 1994). We believe that an explanation for the greater reactivity of our separated 44Sc towards DTPA compared to DOTA is due to the much lower thermodynamic stability constant of Ca-DTPA compared to Ca-DOTA, 10.7 and 17.2, respectively (Anderegg et al., 2005). Hence, the remaining bulk calcium in our isolated product, with ~2.1 mM concentration, offers less competition for DTPA occupation by the 44Sc isotopes.
Huclier-Markai, et al. (Huclier-Markai et al., 2011) measured the in vitro stability of Sc3+ complexes with DOTA, DTPA, NOTA, TETA and EDTA in the presence of hydroxyapatite and rat serum and discovered that the most stable one was Sc-DOTA, followed by Sc-DTPA. However, the same kind of stability has not been determined for scandium complexed with modified versions of DTPA. For instance CHX-A″-DTPA was proven to have a greater in vivo stability than DTPA when labeled with 90Y (Camera et al., 1994).
3.6. Comparison of our separation method to previous publications
Comparing the separation chemistry of this work with the ones in (Severin et al., 2012a, 2012b) we found many improvements. First, the UTEVA resin is commercially available unlike the hydroxamate resin, which could potentially introduce deviations in the resin’s chemical properties. Second, the dissolved target is directly loaded onto the column without having to adjust the pH, which is a process difficult to automate. Third, the 44Sc radioactivity is eluted at high concentration in 400 μL of deionized water ready for labeling, instead of eluting in 1 mL of 0.1 M HCl. And fourth, 80 ± 4% of the produced radioactive scandium was separated, compared to the overall separation efficiencies of 53% and 63%, reported in (Severin et al., 2012a, 2012b), respectively.
We believe that our separation method based on the UTEVA resin can also be applied with 44CaCO3 targets in order to get a highly concentrated radioactive product and also to improve the recycling efficiency of the expensive target material. This would be recovered by collecting and then processing the eluate with the bulk calcium after trapping the 44Sc just like (Krajewski et al., 2013) did in his separation method based on Chelex 100, in which they achieved a 60% 44Ca recycling efficiency. They claim to have achieved a separation yield of > 70%, which is slightly lower than our 80 ± 4% separation yield. However, they elute in three 0.5 mL fractions of 1 M HCl, which means that the activity is at a lower concentration and also in a more acidic medium that will be more difficult to buffer for radiolabeling DOTA or DTPA, compared to the product obtained with our method: 400 μL of deionized water. Of course, our eluate will also be acidic (~1M) due to the residual H+ ions that remain in the UTEVA resin after loading and washing with 10 M HCl. Nevertheless, it is clear that the smaller volume of our product, 400 μL, is much easier to buffer than 1.5 mL of 1 M HCl. Muller et al. 2013 achieved a slightly higher separation efficiency of ~85% after using 50–70 mg of DGA resin and then concentrating the activity using a cation exchange resin DOWEX-50. Their isolated 44Sc is in 200–400 μL of 1 M ammonium acetate. Table 8 compares our work with the results from these publications.
4. Conclusions
In conclusion, we have described a rapid and facile method to recover 44Sc from proton-irradiated metallic calcium targets with excellent yield and high chemical and radiochemical purities. Additionally, the usage of a single column and minimal washing/elution steps provides a convenient framework for the implementation of automatic separation modules. Even though the focus of this work was to study the isolation of 44Sc from natural calcium targets, our procedure is readily applicable to the isolation of 44Sc from isotopically enriched 44CaCO3 targets or even liquid targets employing Ca(NO3)2 solutions. This separation system coupled to improved cyclotron production approaches might be the long waited answer to make 44Sc production feasible for translation into the clinic.
HIGHLIGHTS.
Cyclotron-produced 44Sc is loaded into 50 mg of UTEVA resin after dissolution of 300 mg of natCa target in concentrated HCl.
80 ± 4% of the activity at end of bombardment (EoB) is eluted using 400 μL of deionized water in < 20 min after EoB.
The separation factor of the bulk calcium is 1.2 × 104.
44Sc has reactivities or effective specific activities with DOTA and DTPA of 18.1 ± 6.7 and 49 ± 37 GBq/μmol, respectively.
Metal impurities in the isolated 44Sc are: Ca (2.1 mM), Fe (93 μM), Zn (72 μM), Ni (29 μM), Al (6.4 μM) and Mn (2.0 μM).
Acknowledgments
We gratefully acknowledge the financial support of the US Department of Energy (DOE-SC0008384), the US National Science Foundation (DGE-1256259) and the US National Institutes of Health (5T32GM08349). H.F. Valdovinos gratefully acknowledges the support of the Advanced Opportunity Fellowship offered by the Science and Medicine Graduate Research Scholars (SciMed GRS) Program at UW-Madison.
Footnotes
All nuclear physics data in this report come from UC Berkeley’s Table of the Isotopes and were accessed via http://ie.lbl.gov/education/isotopes.htm.
References
- Anderegg G, Arnaud-Neu F, Delgado R, Felcman J, Popov K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (iupac technical report) Pure Appl Chem. 2005;77:1445–1495. [Google Scholar]
- Braun T, Ghersini G. Extraction Chromatography. Elsevier, Scientific Pub. Co; Amsterdam, New York: 1975. [Google Scholar]
- Camera L, Kinuya S, Garmestani K, Wu C, Brechbiel MW, Pai LH, McMurry TJ, Gansow OA, Pastan I, Paik CH. Evaluation of the serum stability and in vivo biodistribution of CHX-DTPA and other ligands for yttrium labeling of monoclonal antibodies. J Nucl Med. 1994;35:882–889. [PubMed] [Google Scholar]
- Filosofov D, Loktionova N, Rosch F. A Ti-44/Sc-44 radionuclide generator for potential application of Sc-44-based PET-radiopharmaceuticals. Radiochim Acta. 2010;98:149–156. [Google Scholar]
- Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, Cai W. Sc-44: an attractive isotope for peptide-based pet imaging. Mol Pharm. 2014;11:2954–2961. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehr C, Oehlke E, Benard F, Lee CJ, Hou X, Badesso B, Ferguson S, Miao Q, Yang H, Buckley K, Hanemaayer V, Zeisler S, Ruth T, Celler A, Schaffer P. (44g)Sc production using a water target on a 13 MeV cyclotron. Nucl Med Biol. 2014;41:401–406. doi: 10.1016/j.nucmedbio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Huclier-Markai S, Sabatie A, Ribet S, Kubicek V, Paris M, Vidaud C, Hermann P, Cutler C. Chemical and biological evaluation of scandium(III)-polyamino-polycarboxylate complexes as potential PET agents and radiopharmaceuticals. Radiochim Acta. 2011;99:653–662. [Google Scholar]
- Koumarianou E, Loktionova N, Fellner M, Roesch F, Thews O, Pawlak D, Archimandritis S, Mikolajczak R. Sc-44-DOTA-BN[2–14]NH2 in comparison to Ga-68-DOTA-BN[2–14]NH2 in pre-clinical investigation. Is Sc-44 a potential radionuclide for PET? Appl Radiat Isot. 2012;70:2669–2676. doi: 10.1016/j.apradiso.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Koumarianou E, Pawlak D, Korsak A, Mikolajczak R. Comparison of receptor affinity of natSc-DOTA-TATE versus natGa-DOTA-TATE. Nucl Med Rev Cent East Eur. 2011;14:85–89. doi: 10.5603/nmr.2011.00021. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Cydzik I, Abbas K, Bulgheroni A, Simonelli F, Holzwarth U, Bilewicz A. Cyclotron production of Sc-44 for clinical application. Radiochim Acta. 2013;101:333–338. [Google Scholar]
- Levkovskij V. Activation cross-sections by protons and alphas. Moscow: 1991. Activation cross section nuclides of average masses (A=40–100) by protons and alpha-particles with average energies (E=10–50 MeV) Accessed via EXFOR: entries A0510002, A0510005 and A0510004. [Google Scholar]
- Martell A, Smith R, Motekaitis R. NIST Critically Selected Stability Constants of Metal Complexes Database. (2004) [Google Scholar]
- Muller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Turler A, Schibli R. Promises of cyclotron-produced Sc-44 as a diagnostic match for trivalent beta(−)-emitters: in vitro and in vivo study of a Sc-44-DOTA-Folate conjugate. J Nucl Med. 2013;54:2168–2174. doi: 10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- Pruszynski M, Loktionova N, Filosofov D, Rosch F. Post-elution processing of Ti-44/Sc-44 generator-derived Sc-44 for clinical application. Appl Radiat Isot. 2010;68:1636–1641. doi: 10.1016/j.apradiso.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Pruszynski M, Majkowska-Pilip A, Loktionova N, Eppard E, Roesch F. Radiolabeling of DOTATOC with the long-lived positron emitter Sc-44. Appl Radiat Isot. 2012;70:974–979. doi: 10.1016/j.apradiso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Severin G, Engle J, Valdovinos H, Barnhart T, Nickles R. Cyclotron produced Sc-44g from natural calcium. Appl Radiat Isot. 2012a;70:1526–1530. doi: 10.1016/j.apradiso.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin GW, Gagnon K, Engle JW, Valdovinos HF, Barnhart TE, Lewis JS. 44gSc from metal calcium targets for PET. AIP Conf Proc. 2012b;1509:125–128. [Google Scholar]
- Siikanen J, Peterson M, Tran TA, Roos P, Ohlsson T, Sandell A. A peristaltic pump driven 89Zr separation module. AIP Conf Proc. 2012;1509:206–210. [Google Scholar]
- Takacs S, Tarkanyi F, Sonck M, Hermanne A. Investigation of the Mo-nat (p,x)Tc-96 mg nuclear reaction to monitor proton beams: new measurements and consequences on the earlier reported data. Nucl Instrum Methods Phys Res Sect B: Beam Interact Mater Atoms. 2002;198:183–196. [Google Scholar]
- Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radio-metals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JF, Ziegler MD, Biersack JP. SRIM – the stopping and range of ions in matter 2013 [Google Scholar]


