Abstract
Opioids are well known for their robust analgesic effects. Chronic activation of mu opioid receptors (MOPs) is however accompanied by various unwanted effects such as analgesic tolerance. Among other mechanisms, interactions between MOP and delta opioid receptor (DOP) are thought to play an important role in morphine-induced behavioral adaptations. Interestingly, certain conditions such as inflammation enhance the function of the DOP through a MOP-dependent mechanism. Here, we investigated the role of DOP during the development of morphine-tolerance in an animal model of chronic inflammatory pain. Using behavioral approaches we first established that repeated systemic morphine treatment induces morphine analgesic tolerance in rats coping with chronic inflammatory pain. We then observed that blockade of DOP with subcutaneous naltrindole (NTI), a selective DOP antagonist, significantly attenuates the development of morphine tolerance in a dose-dependent manner. We confirmed that this effect was DOP-mediated by showing that an acute injection of NTI had no effect on morphine-induced analgesia in naïve animals. Previous pharmacological characterizations revealed the existence of DOP1 and DOP2 subtypes. As opposed to NTI, 7-benzylidenenaltrexone (BNTX) and naltriben (NTB) were reported to be selective DOP1 and DOP2 antagonists, respectively. Interestingly, NTB but not BNTX was able to attenuate the development of morphine analgesic tolerance in inflamed rats. Altogether, our results suggest that targeting of DOP2 with antagonists provides a valuable strategy to attenuate the analgesic tolerance that develops after repeated morphine administration in the setting of chronic inflammatory pain.
Keywords: hyperalgesia, opioid, heteromer, inflammation, antagonist
INTRODUCTION
Opioids are the most commonly used drugs for severe pain management. However, a significant reduction in the analgesic properties of opioids as well as the development of physical dependence resulting from prolonged use negatively impact the clinical benefits of these drugs. Although the exact mechanisms of opioid tolerance remain unknown, a putative role for a crosstalk between delta (DOP) and mu (MOP) opioid receptors has recently emerged (for a review see (Costantino et al., 2012)).
We and others have shown that the blockade of DOPs or a lack of functional DOPs is associated with a reduction in the rewarding properties (Chefer & Shippenberg, 2009; Shippenberg et al., 2009; Billa et al., 2010; Moron et al., 2010; Le Merrer et al., 2011), and in the withdrawal symptoms of morphine (Crain & Shen, 1995; Fundytus et al., 1995; Hepburn et al., 1997; Nitsche et al., 2002). Interestingly, the selective blockade of DOPs was deemed sufficient to prevent morphine analgesic tolerance in rodents (Abdelhamid et al., 1991; Crain & Shen, 1995; Fundytus et al., 1995; Hepburn et al., 1997; Roy et al., 2005; Abul-Husn et al., 2007; McNaull et al., 2007). Similar prevention of morphine analgesic tolerance was observed in mice treated with antisense oligonucleotide to knock-down DOP or in DOP-KO mice (Kest et al., 1996; Zhu et al., 1999; Nitsche et al., 2002). While two DOP subtypes (DOP1 and DOP2) have been identified (Jiang et al., 1991; Mattia et al., 1991; Sofuoglu et al., 1991), pharmacological studies using selective antagonists have shown that blockade of DOP2, and not DOP1, is implicated in the modulation of morphine-induced behaviors (Miyamoto et al., 1993; Shippenberg et al., 2009; Billa et al., 2010). Altogether, these results suggest a mechanism by which DOP can regulate MOP functions. Interestingly, the use of bivalent opioid ligands has brought new insights on DOP-MOP interactions in opioid analgesic tolerance. Indeed, single molecules combining a MOP agonist and a DOP antagonist, MDANs (MOP-agonist-DOP-antagonist), were shown to produce less analgesic tolerance and dependence than classical opioids (Daniels et al., 2005; Lenard et al., 2007).
As opposed to most GPCRs, the DOP is mainly localized intracellularly. However, under specific conditions such as inflammation and chronic morphine treatment, the targeting of DOP to the plasma membrane is enhanced (for a review see (Gendron et al., 2014)). Interestingly, we and others have shown that MOP is essential for the functional emergence of DOP (Morinville et al., 2003; Morinville et al., 2004a; Gendron et al., 2007b), suggesting functional interactions between these receptors.
As stated above, DOPs have been shown to mediate morphine analgesic tolerance in acute pain models but surprisingly, giving the fact that inflammatory pain enhances DOP functions, there are no studies investigating the role of DOP in the development of morphine-induced analgesic tolerance in the setting of chronic pain. In the present study, we sought to investigate the role of DOP in morphine analgesic tolerance in an inflammatory pain model. Interestingly, we show that the selective blockade of DOP2, but not DOP1, prevented morphine tolerance in Complete Freund Adjuvant’s (CFA)-inflamed rats.
EXPERIMENTAL PROCEDURES
Animals
Experiments were carried out in adult male Sprague-Dawley rats weighting 225–250 g (Charles River, St-Constant, QC, Canada) maintained on a 12 h light/dark cycle (06:00–18:00 h). Laboratory chow and water were available ad libitum. Behavioral tests were conducted between 07:00 and 11:30 (light cycle). All experiments were approved by the animal care committee of the Université de Sherbrooke (Protocol #242-10B) and all procedures conformed to the directives of the Canadian Council on Animal Care and guidelines of the International Association for the Study of Pain. All animal experiments were designed to minimize the number of animals used and their suffering.
Induction of inflammation and morphine tolerance
Unilateral inflammation of the hind limb and development of hyperalgesia was induced by a single injection of 100 μL emulsified complete Freund’s adjuvant 50 μg/100 μL (CFA; Calbiochem, San Diego, CA, USA) in the plantar surface of the left hind paw of rats under brief isoflurane anesthesia. Inflammation was used to enhance cell surface availability of DOP (Cahill et al., 2003; Morinville et al., 2004b; Gendron et al., 2006; Gendron et al., 2007a; Gendron et al., 2007b). Hargreaves tests (heat hyperalgesia) were carried out 72 h after CFA injection as described below. Morphine analgesic tolerance was induced as previously showed (Beaudry et al., 2009). Morphine sulfate was injected subcutaneously every 12 hours for 72 h (5 injections of 10 mg/kg), starting 12h after CFA injection. Hargreaves tests were carried out 72 h after CFA injection (see Figure 1 for timeline representation).
Figure 1. Timeline representation of drug administration and behavioral measurements.
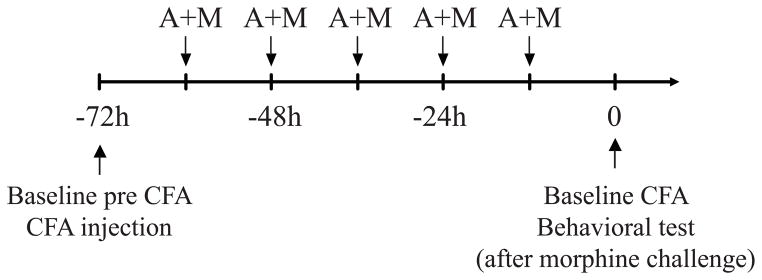
Basal latencies to paw withdrawal were measured before CFA administration (Baseline pre CFA) and CFA was injected in the left hindpaw. Twelve hours later, DOP antagonist or vehicle (A) was administered s.c. 15 min before morphine 10 mg/kg or vehicle (M) every 12h for 5 consecutive injections. Twelve hours after the last A+M treatment, thermal latencies to paw withdrawal were measured (Baseline CFA) and Hargreaves test was performed as described in the text.
Drugs
Morphine sulfate (lots BK8689 and CC0630; Sandoz, Montréal, QC, Canada and lot 43156/C; Medisca, Montréal, QC, Canada; an additional lot was obtained from the National Institute on Drug Abuse Drug Program) was diluted in sterile saline solution (0,9 % NaCl) to concentrations of 0.3, 1, 3 and 10 mg/ml and stored at room temperature protected from light. Control rats received equivalent volume of sterile saline. As demonstrated in pharmacological studies, different DOP subtypes selective antagonists are available: the DOP 1/2 antagonist (naltrindole; NTI) (Portoghese et al., 1988), the DOP2 antagonist (naltriben; NTB) (Sofuoglu et al., 1991), and the DOP1 antagonist (7-benzylidenenaltrexone; BNTX) (Portoghese et al., 1992). NTI (Tocris Bioscience, Minneapolis MN, USA) was dissolved in DMSO at 100 mM and stored in aliquots at −20 °C until use. For experiments, NTI was diluted in sterile saline to 0.003 to 0.03 mg/ml and control rats received equivalent volume of sterile saline. NTB (Tocris Bioscience, Minneapolis MN, USA) was dissolved in a sterile saline solution at 5 mg/ml and stored in aliquots at −20 °C until use. For experiments, NTB was diluted in sterile saline to 0.1 mg/ml and control rats received equivalent volume of sterile saline. BNTX (Tocris; Tocris Bioscience, Minneapolis MN, USA) was dissolved in a sterile saline solution at 50 mg/ml and stored in aliquots at −20 °C until use. For experiments, BNTX was diluted in sterile saline to 1 mg/ml and control rats received equivalent volume of sterile saline. NTI, NTB and BNTX doses have been used elsewhere (Shippenberg et al., 2009) and are known to fully block DOP agonists effects (Suzuki et al., 1997; Schultz et al., 1998; Broom et al., 2002; Maslov et al., 2010; Zeng et al., 2011; Maslov et al., 2014). All drugs and vehicles were administered subcutaneously.
Hargreaves test
Response to noxious heat stimulus was evaluated using the Hargreaves test in hyperalgesic conditions (induced by CFA intraplantar injection) to determine antihyperalgesic effects of drugs. Animals were acclimatized 30 min to the Hargreaves test environment and were placed in Plexiglas boxes positioned on a glass surface (IITC Life Science Inc., Woodland Hills, CA, USA), 24 h prior to baseline measurements. The following day, corresponding to −72 h, the heat source was positioned under the plantar surface of the hind paw after a 15 min habituation period and the latency for each hind paw withdrawal in response to radiant heat was measured three times in alternation. Subsequently, CFA was injected in the left hind paw as described above. Seventy-two hours after injection of CFA, baseline withdrawal latencies (identified as 0 min) of each hind paw were measured two times in alternation preceding subcutaneous injection of a challenging dose of morphine. Afterward, latencies to paw withdrawal were recorded every 15 min for 60 min. To prevent tissue damage, a cut-off time of 20 s was imposed. If an animal reached the cut-off, the light beam was automatically turned off and the animal was assigned the maximum score. Area under curve was calculated with Prism 6.0 on the curve obtained between 0 and 60 min after morphine challenge dose (Y baseline set for each animal according to its latency to paw withdrawal after inflammation).
Data Analysis
Calculations were done with Excel (2010), graphs with SigmaPlot11.0, and statistical analysis with Prism GraphPad 6. Data are expressed as the mean ± SEM. P-values are presented in figure legends.
RESULTS
Effect of acute naltrindole treatment on morphine analgesic efficacy
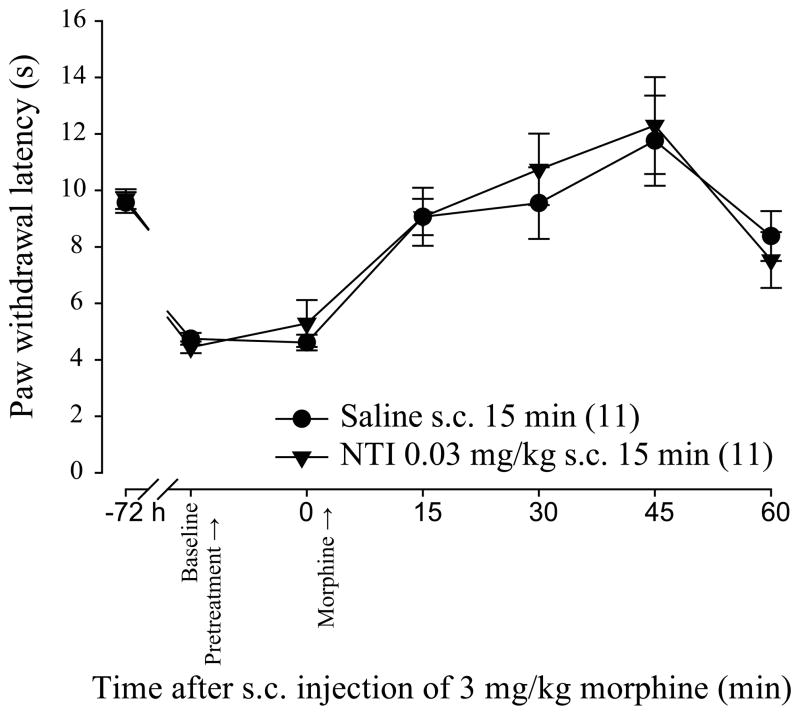
Naltrindole (NTI) is a selective DOP antagonist that does not interfere with morphine analgesic properties in naïve animals (Abdelhamid et al., 1991; Abul-Husn et al., 2007). However, its selectivity has not been tested in models known to upregulate DOP such as CFA-induced inflammation (Cahill et al., 2003; Gendron et al., 2006; Gendron et al., 2007a). In this first set of experiments we verified the effect of an acute injection of NTI on morphine analgesic efficacy in a model of chronic inflammatory pain. As it can be seen in Figure 2, CFA-induced inflammation triggered heat hyperalgesia. In inflamed rats receiving saline for 15 min before morphine, 3 mg/kg morphine induced a robust time-dependent alleviation of CFA-induced thermal hyperalgesia with maximal effect at 45 min (11.8 ± 1.6 sec compared to 4,7 ± 0.2 sec for 0 min and 45 min respectively). When s.c. NTI 0.03 mg/kg was given 15 min before morphine, morphine antihyperalgesic effect was similar that in Saline s.c. 15 min group rats (p=0.9594 using Two-way ANOVA). Moreover, NTI pretreatment did not modify CFA-induced hyperalgesia (5.3 ± 0.8 sec compared to 4.6 ± 0.3 sec at 0 for NTI 0.03 mg/kg s.c. 15 min and Saline s.c. 15 min respectively). These results indicate that NTI 0.03 mg/kg does not interfere with morphine antihyperalgesic effect in CFA-inflamed rats.
Figure 2. Effect of acute NTI injection on morphine antihyperalgesic effect in inflamed rats.
Sprague–Dawley rats were injected with CFA in the plantar surface of the hind paw. Seventy-two hours after CFA injection, the latency to paw withdrawal (in sec) was tested before (Baseline) and after (0) a pretreatment with NTI or saline (s.c.; 15 min) using the Hargreaves test. Morphine 3 mg/kg (Morphine) was administered in both groups to compare morphine’s antihyperalgesic effect every 15 min (from 15 to 60 min). Number given in the legend inset represents the number of animals per group. Acute NTI did not modify morphine antihyperalgesic effect.
Effect of chronic naltrindole treatment on morphine analgesic efficacy in inflamed rats
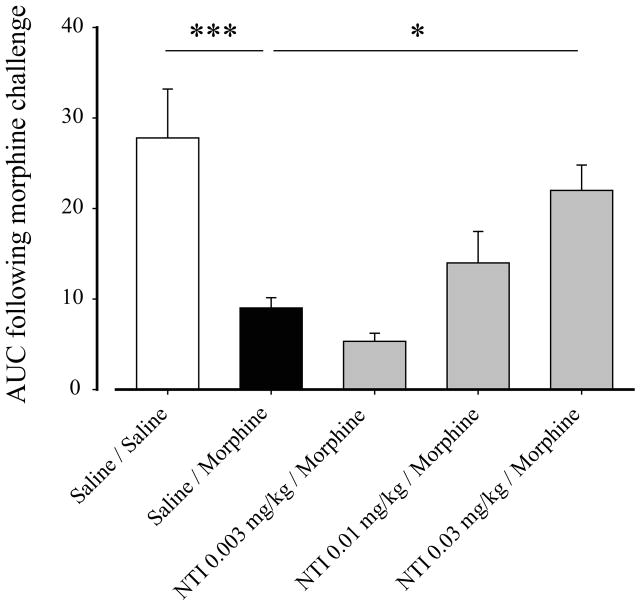
In the next set of experiments, we sought to determine the effect of chronic NTI on morphine analgesic tolerance in CFA-inflamed rats. We first examined the dose of NTI needed to prevent morphine analgesic tolerance in inflamed rats. As it can be seen in Figure 3, morphine has a robust analgesic effect in control inflamed rats, reaching an AUC of 27.3 ± 5.4 (Fig. 3; Saline/Saline). Following chronic morphine pretreatment, the analgesic effect of morphine was significantly reduced compared to saline-treated rats (Fig. 3; AUC of 9.0 ± 1.1 compared to 27.3 ± 5.4 for Saline/Morphine and Saline/Saline respectively; p<0.001 using One-way ANOVA with Bonferroni’s multiple comparisons test). Interestingly, when NTI was administered 15 min before every morphine administration, we observed a dose-dependent prevention of morphine analgesic tolerance. This effect was significant with a dose of 0.03 mg/kg NTI (Fig. 3; AUC of 22.0 ± 2.8 compared to 9.0 ± 1.1 for NTI 0.03mg/kg/Morphine and Saline/Morphine respectively; p<0.05 using One-way ANOVA with Bonferroni’s multiple comparisons test). Moreover, the analgesic effect of morphine in NTI 0.03 mg/kg pretreated rats was not different from analgesia measured in Saline/Saline rats. These results indicate that NTI pretreatment completely prevented morphine analgesic tolerance (Fig. 3; AUC of 22.0 ± 2.8 compared to 27.3 ± 5.4 for NTI 0.03mg/kg/Morphine and Saline/Saline respectively; p>0.05 using One-way ANOVA with Bonferroni’s multiple comparisons test).
Figure 3. Determination of NTI dose necessary to prevent morphine analgesic tolerance in inflamed rats.
CFA inflamed rats were pretreated as illustrated in Fig. 1 with NTI (0.003 to 0.3 mg/kg) as a DOP antagonist. Twelve hours after the last pretreatment injection (saline followed 15 min later by saline (□), saline followed 15 min later by morphine (■) or three different doses of NTI (■)), the analgesic effect of a challenging dose of morphine (3 mg/kg) was measured using the Hargreaves test. Results are expressed as area under curve obtained between 0 and 60 min after morphine challenge dose (Y baseline set for each animal according to its latency to paw withdrawal after inflammation). (N = 9–15 rats), *, p<0.05 and ***, p<0.001 when groups were compared to Saline/Morphine group. NTI at 0.03 mg/kg is sufficient to prevent morphine analgesic tolerance.
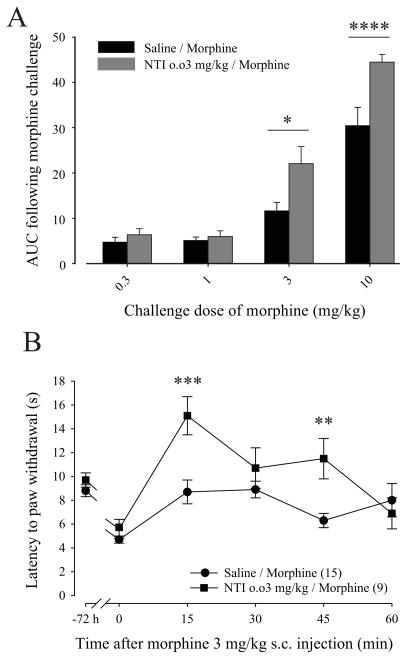
We next compared the analgesic effect of different doses of the morphine challenge in inflamed rats pretreated with a combination of saline/morphine or NTI 0.03 mg/kg/morphine. As it is shown in Figure 4A, NTI 0.03 mg/kg prevented morphine analgesic tolerance which resulted in a significant increase in the analgesic effect of morphine when the dose of the challenge was 3 mg/kg (Fig. 4A; AUC of 22.1 ± 3.8 compared to 11.6 ± 1.9 for NTI 0.03 mg/kg/Morphine and Saline/Morphine respectively; p<0.05 using Two-way ANOVA with Bonferroni’s multiple comparisons test) or 10 mg/kg (Fig. 4A; AUC of 44.5 ± 1.7 compared to 30.4 ± 4.0 for NTI 0.03 mg/kg/Morphine and Saline/Morphine respectively; p<0.0001 using Two-way ANOVA with Bonferroni’s multiple comparisons test). In Figure 4B, results are expressed as latency to paw withdrawal in function of time after morphine injection. Chronic pretreatment with NTI did not affect CFA-induced thermal hyperalgesia (Fig. 4B; 5.5 ± 0.7 sec compared to 9.9 ± 0.6 sec for 0 min and −72h respectively; p<0.05 using Two-way ANOVA with Bonferroni’s multiple comparisons test). Moreover, NTI and saline pretreated groups developed similar inflammation-induced thermal hyperalgesia (5.6 ± 0.8 sec compared to 4.7 ± 0.3 sec for NTI 0.03 mg/kg/Morphine and Saline/Morphine respectively) but significantly increased the analgesic effect of morphine at 15 min (Fig. 4B; 15.1 ± 1.6 sec compared to 8.7 ± 1.0 sec for NTI 0.03 mg/kg/Morphine and Saline/Morphine respectively; p<0.05 using Two-way ANOVA with Bonferroni’s multiple comparisons test) and 45 min (Fig. 4B; 11.5 ± 1.7 sec compared to 6.3 ± 0.6 sec for NTI 0.03 mg/kg/Morphine and Saline/Morphine respectively; p<0.01 using Two-way ANOVA with Bonferroni’s multiple comparisons test). Taken together, these results show that NTI pretreatment is sufficient to prevent morphine analgesic tolerance in inflamed rats.
Figure 4. Effect of NTI pretreatment on morphine analgesic tolerance in inflamed rats.
(A) CFA inflamed rats were pretreated as illustrated in Fig. 1 with NTI 0.3 mg/kg as a DOP antagonist. Twelve hours after the last treatment, the analgesic effect of a challenging dose of morphine (0.3 to 10 mg/kg) was measured using the Hargreaves test. Results are expressed as area under curve obtained between 0 and 60 min after morphine challenge dose (Y baseline set for each animal according to its latency to paw withdrawal after inflammation). (N = 8–13 rats), *, p<0.05 and ****, p<0.0001 when groups were compared together within a similar morphine challenge dose. (B) CFA inflamed rats were pretreated as illustrated in Fig. 1 with NTI 0.3 mg/kg as a DOP antagonist. Twelve hours after the last treatment, the analgesic effect of a challenging dose of morphine (3 mg/kg) was measured using the Hargreaves test. The latency to paw withdrawal (in sec) was tested every 15 min (from 0 to 60 min) after morphine injection. Number given in the legend inset represents the number of animals per group. **, p<0.01 and ***, p<0.001 when groups were compared together. NTI pretreatment prevented morphine analgesic tolerance in inflamed rats.
Effect of chronic 7-benzylidenenaltrexone and naltriben treatment on morphine analgesic tolerance in inflamed rats
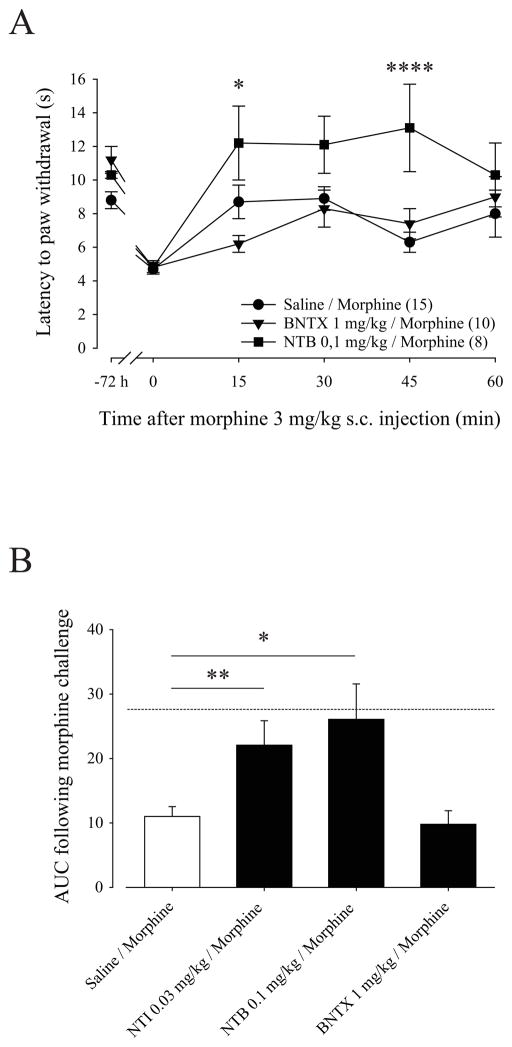
In order to assess the role of DOP1 and DOP2 in morphine analgesic tolerance in inflamed rats, we used BNTX or NTB selective antagonists for DOP1 and DOP2, respectively. As shown in Figure 5A, pretreatment with BNTX or NTB did not affect CFA-induced thermal hyperalgesia (Fig. 4B BNTX 1 mg/kg/Morphine; 4.4 ± 0.1 sec compared to 11.6 ± 1.0 sec for 0 min and −72h respectively; p<0.01 using Two-way ANOVA with Bonferroni’s multiple comparisons test) (Fig. 4B NTB 0.1 mg/kg/Morphine; 4.8 ± 0.3 sec compared to 10.2 ± 0.1 sec for 0 min and -72h respectively; p<0.05 using Two-way ANOVA with Bonferroni’s multiple comparisons test). Moreover, BNTX, NTB and saline pretreated groups developed similar inflammation-induced thermal hyperalgesia (4.7 ± 0.3 sec compared to 4.8 ± 0.4 sec and 4.8 ± 0.3 sec for Saline/Morphine, BNTX 1 mg/kg/Morphine, and NTB 0.1 mg/kg/Morphine respectively). Interestingly, inflamed rats pretreated with 0.1 mg/kg NTB had a significantly increased morphine analgesic effect at 15 min (12.2.5 ± 2.2 compared to 8.7 ± 1.0 for NTB 0.1 mg/kg/Morphine and Saline/Morphine respectively; p<0.05 using Two-way ANOVA with Bonferroni’s multiple comparisons test) and 45 min compared to tolerant rats (11.5 ± 2.0 compared to 6.3 ± 0.6 for NTB 0.1 mg/kg/Morphine and Saline/Morphine respectively; p<0.0001 using Two-way ANOVA with Bonferroni’s multiple comparisons test). Surprisingly, BNTX-pretreated rats showed similar paw withdrawal latencies than saline-pretreated rats after morphine challenge indicating that morphine analgesic tolerance still develop after DOP blockade with BNTX. Comparing the AUC for the pretreatment with each DOP antagonist we did not observe an effect on morphine analgesic efficacy following BNTX pretreatment compared to tolerant rats (Fig. 5B; AUC of 10.5 ± 2.5 compared to 11.0 ± 1.5 for BNTX 1 mg/kg/Morphine and Saline/Morphine respectively). In contrast, NTI and NTB pretreatment induced a significant increase in morphine analgesic efficacy (AUC of 22.1 ± 3.8 and 26.0 ± 5.6 for NTI 0.03 mg/kg/Morphine and NTB 0.1 mg/kg/Morphine respectively) and this effect was similar to the morphine analgesic effect obtained in naïve inflamed-rats (Fig. 5B; dashed line corresponding to AUC of 27.8 obtained in Saline/Saline group as illustrated in Fig. 3). Taken together, our results show that DOP inhibition with NTI (a non-selective DOP antagonist) or NTB (a selective DOP2 antagonist) but not BNTX (a selective DOP1 antagonist), is sufficient to prevent morphine analgesic tolerance in inflamed rats.
Figure 5. Effect of BNTX and NTB pretreatment on morphine analgesic tolerance in inflamed rats.
(A) CFA inflamed rats were pretreated as illustrated in Fig. 1 with BNTX 1 mg/kg or NTB 0.1 mg/kg as DOP antagonists. Twelve hours after the last treatment, the analgesic effect of a challenging dose of morphine (3 mg/kg) was measured using the Hargreaves test. The latency to paw withdrawal (in sec) was tested every 15 min (from 0 to 60 min) after morphine injection. Number given in the legend inset represents the number of animals per group. *, p<0.05 and ****, p<0.0001 when groups were compared to Saline/Morphine group. (B) Results obtained with the three DOP selective antagonists are expressed as area under curve obtained between 0 and 60 min after morphine challenge dose (Y baseline set for each animal according to its latency to paw withdrawal after inflammation). Dashed lined represent AUC obtained for control Saline/Saline group. (N = 8–13 rats). *, p<0.05 and **, p<0.01 when groups were compared to Saline/Morphine group. Chronic injections of NTI and NTB, but not BNTX, prevented morphine analgesic tolerance in inflamed-rats and the morphine analgesic efficacy in NTI- or NTB-pretreated rats reached the efficacy obtained in control rats.
DISCUSSION
Opioid tolerance has been shown to be an adaptive cellular process that involves modulation of MOP function, but growing evidence suggest that DOP is also implicated (Zhang et al., 2006). Indeed, DOP has been shown to prevent morphine analgesic tolerance in acute pain models but surprisingly, there are no studies investigating the role of DOP in the development of morphine-induced analgesic tolerance in the setting of chronic pain. Several studies have demonstrated that DOP function is increased in the presence of inflammatory pain (Hylden et al., 1991; Hurley & Hammond, 2000; Cahill et al., 2003; Gendron et al., 2006; Gendron et al., 2007a), suggesting a role for DOP in morphine tolerance in the presence of chronic pain. In the present study, we investigated the role of DOP in morphine analgesic tolerance in an inflammatory pain model known to upregulate DOP (Cahill et al., 2003). CFA-inflamed rats were treated repeatedly with systemic morphine to induce analgesic tolerance and this morphine regimen induced a robust decrease in morphine analgesic efficacy. In morphine tolerant and inflamed rats, we compared the ability of NTI (non-selective DOP antagonist), NTB (selective DOP2 antagonist) or BNTX (selective DOP1 antagonist) to prevent morphine analgesic tolerance. Interestingly, our results show that NTI and NTB, but not BNTX, prevented morphine analgesic tolerance. These results indicate that the role of DOP in morphine analgesic tolerance is mainly mediated by DOP2.
In this study we examined the effect of DOP inhibition during the onset of morphine analgesic tolerance in an inflammatory pain model. To achieve this goal, DOP selective antagonists were administered twice a day, before each morphine injection. As it was previously reported that the genetic ablation of DOP slightly increases the CFA-induced heat hyperalgesia compared to wildtype animals (Gaveriaux-Ruff et al., 2008), one could argue that chronic DOP blockade may also affect the level of hyperalgesia induced by CFA. However, in the present study the CFA-induced heat hyperalgesia was developed similarly among all groups, independently of the pretreatment. These discrepancies could be explained by the time frame in which our experiments were done. In the study conducted by Gaveriaux-Ruff and coworkers, CFA-induced heat hyperalgesia was different between DOP−/− and wildtype mice only after 5 days whereas our study was conducted only up to 3 days following CFA injection. Therefore, we cannot rule out the possibility that repeated use of DOP antagonists would not affect heat hyperalgesia over a longer period of time.
In naïve animals, acute DOP inhibition has been shown to increase morphine analgesic efficacy (Gomes et al., 2004; Abul-Husn et al., 2007; He et al., 2011). However, our results showed no difference in morphine analgesic efficacy between acute saline- and acute NTI-treated rats. This discrepancy could be due to the fact that our experiments were conducted in inflamed rats. Indeed, we have shown that CFA-induced DOP upregulation is dependent on MOP (Morinville et al., 2004b; Gendron et al., 2007b) suggesting that opioid receptor interactions may be involved in a complex regulatory mechanism during inflammation. Interestingly, prolonged morphine treatment is also implicated in DOP regulation (Cahill et al., 2001; Morinville et al., 2003; Morinville et al., 2004a) as well as in formation of MOP/DOP dimer (Gupta et al., 2010). Actually, no data are available regarding MOP/DOP heteromerization under inflammatory conditions, but one can speculate that inflammation induces changes in MOP/DOP interactions. Therefore, the effect of acute DOP blockade on morphine analgesic efficacy in inflamed rats would be different than in naïve rats, as seen in the present study. Altogether, these data suggest that inflammation induces changes in opioid receptor function that impacts the effect of acute DOP inhibition on MOP analgesic effect.
On the other hand, our results show that repeated DOP inhibition prevented morphine analgesic tolerance during the onset of inflammatory pain, as it has been reported in acute pain models (Abdelhamid et al., 1991; Zhu et al., 1999; Abul-Husn et al., 2007; He et al., 2011). The effect of repeated DOP blockade on MOP function is likely due to events that take place over time and not a direct effect on MOP, since morphine analgesic efficacy was not affected by acute DOP antagonist pretreatment. Interestingly, we show that NTI and NTB, but not BNTX, prevented morphine analgesic tolerance in inflamed-rats, indicating that this effect is mediated by DOP2. Similarly, morphine sensitization has been shown to be prevented by NTI and NTB but not BNTX (Shippenberg et al., 2009). Pharmacological evidence revealed that the DOP2 subtype might correspond to a MOP/DOP complex (Porreca et al., 1992; Xu et al., 1993) whereas DOP1 would be a DOP/KOP complex (Portoghese & Lunzer, 2003; Bhushan et al., 2004; Xie et al., 2005). It has been proposed that chronic morphine treatment may increase MOP/DOP heteromer formation with morphine acting as a pharmaco-chaperone bringing the dimer to the cell surface (Costantino et al., 2012) and that the presence of the MOP/DOP heteromer would favor morphine tolerance. Interestingly, a strategy using a TM1MOP mimicking peptide to selectively disrupt the MOP-DOP heteromer prevented morphine analgesic tolerance (He et al., 2011). Taken together, these results together with ours suggest that NTI and NTB block DOP which then disrupts the MOP/DOP dimer, leading to prevention of morphine analgesic tolerance.
To our knowledge, we are the first to compare the effect of DOP1 and DOP2 blockade on morphine analgesic tolerance in a chronic inflammatory pain model. Altogether, results shown in this study provide more support to the idea that the selective blockade of DOP2 in combination with MOP agonists is a promising approach to treat chronic pain conditions without unwanted side effects such as analgesic tolerance.
Acknowledgments
This work was supported by National Institute of Health grant DA036826 and DA027460 to JAM and by Canadian Institutes of Health Research grant MOP273137 to LG. LG is the recipient of a FRQ-S Junior 2 salary support. HB was the recipient of a postdoctoral fellowship from the FRQ-S.
ABBREVIATIONS
- DOP
delta opioid receptor
- MOP
mu opioid receptor
- KO
knock-out
- DOP1
delta opioid receptor subtype 1
- DOP2
delta opioid receptor subtype 2
- MDAN
MOP-agonist-DOP-antagonist
- GPCR
G protein coupled receptor
- CFA
complete Freund’s Adjuvant
- NTI
naltrindole
- NTB
naltriben
- BNTX
7-benzylidenenaltrexone
- DMSO
dimethyl sulfoxyde
- AUC
area under curve
- KOP
kappa opioid receptor
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry H, Proteau-Gagne A, Li S, Dory Y, Chavkin C, Gendron L. Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience. 2009;161:381–391. doi: 10.1016/j.neuroscience.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. Eur J Neurosci. 2010;32:625–631. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A. 1995;92:10540–10544. doi: 10.1073/pnas.92.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[psi] Eur J Pharmacol. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Mittal N, Beaudry H, Walwyn W. Recent advances on the delta opioid receptor: From trafficking to function. Br J Pharmacol. 2014 doi: 10.1111/bph.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, Wang Q, Lu YJ, Bao L, Zhang X. Facilitation of mu-Opioid Receptor Activity by Preventing delta-Opioid Receptor-Mediated Codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Hepburn MJ, Little PJ, Gingras J, Kuhn CM. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J Pharmacol Exp Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F. Differential antagonism of opioid delta antinociception by [D-Ala2,Leu5,Cys6]enkephalin and naltrindole 5′-isothiocyanate: evidence for delta receptor subtypes. J Pharmacol Exp Ther. 1991;257:1069–1075. [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta Opioid Receptor Gene Impairs Place Conditioning But Preserves Morphine Reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Barzakh EI, Krylatov AV, Chernysheva GA, Krieg T, Solenkova NV, Lishmanov AY, Cybulnikov SY, Zhang Y. Opioid peptide deltorphin II simulates the cardioprotective effect of ischemic preconditioning: role of delta(2)-opioid receptors, protein kinase C, and K(ATP) channels. Bulletin of experimental biology and medicine. 2010;149:591–593. doi: 10.1007/s10517-010-1000-6. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Oeltgen PR, Lishmanov YB, Brown SA, Barzakh EI, Krylatov AV, Pei JM. Activation of peripheral delta opioid receptors increases cardiac tolerance to arrhythmogenic effect of ischemia/reperfusion. Acad Emerg Med. 2014;21:31–39. doi: 10.1111/acem.12286. [DOI] [PubMed] [Google Scholar]
- Mattia A, Vanderah T, Mosberg HI, Porreca F. Lack of antinociceptive cross-tolerance between [D-Pen2, D-Pen5]enkephalin and [D-Ala2]deltorphin II in mice: evidence for delta receptor subtypes. J Pharmacol Exp Ther. 1991;258:583–587. [PubMed] [Google Scholar]
- McNaull B, Trang T, Sutak M, Jhamandas K. Inhibition of tolerance to spinal morphine antinociception by low doses of opioid receptor antagonists. Eur J Pharmacol. 2007;560:132–141. doi: 10.1016/j.ejphar.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Portoghese PS, Takemori AE. Involvement of delta 2 opioid receptors in the development of morphine dependence in mice. J Pharmacol Exp Ther. 1993;264:1141–1145. [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O’Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology. 2010;35:955–966. doi: 10.1038/npp.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI. Modulation of mu-mediated antinociception in the mouse involves opioid delta-2 receptors. J Pharmacol Exp Ther. 1992;263:147–152. [PubMed] [Google Scholar]
- Portoghese PS, Lunzer MM. Identity of the putative delta1-opioid receptor as a delta-kappa heteromer in the mouse spinal cord. Eur J Pharmacol. 2003;467:233–234. doi: 10.1016/s0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Nagase H, Takemori AE. A highly selective delta 1-opioid receptor antagonist: 7-benzylidenenaltrexone. Eur J Pharmacol. 1992;218:195–196. doi: 10.1016/0014-2999(92)90167-3. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Roy S, Guo X, Kelschenbach J, Liu Y, Loh HH. In vivo activation of a mutant mu-opioid receptor by naltrexone produces a potent analgesic effect but no tolerance: role of mu-receptor activation and delta-receptor blockade in morphine tolerance. J Neurosci. 2005;25:3229–3233. doi: 10.1523/JNEUROSCI.0332-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Je-J, Hsu AK, Nagase H, Gross GJ. TAN-67, a delta 1-opioid receptor agonist, reduces infarct size via activation of Gi/o proteins and KATP channels. Am J Physiol. 1998;274:H909–H914. doi: 10.1152/ajpheart.1998.274.3.H909. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Portoghese PS, Takemori AE. Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: evidence for delta opioid receptor subtypes. J Pharmacol Exp Ther. 1991;257:676–680. [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H. Involvement of dopamine-dependent and -independent mechanisms in the rewarding effects mediated by delta opioid receptor subtypes in mice. Brain Res. 1997;744:327–334. doi: 10.1016/S0006-8993(96)01119-5. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68:1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, de Costa BR, Rice KC, Rothman RB. Differential binding of opioid peptides and other drugs to two subtypes of opioid delta ncx binding sites in mouse brain: further evidence for delta receptor heterogeneity. Peptides. 1993;14:893–907. doi: 10.1016/0196-9781(93)90064-n. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhao X, Yang Y, Kuai J, Gao C, Yu D, Zhao H, Chai W, Yao L. Opioid delta(1) and delta(2) receptor agonist attenuate myocardial injury via mPTP in rats with acute hemorrhagic shock. J Surg Res. 2011;169:267–276. doi: 10.1016/j.jss.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]