Abstract
The striatum is the major input nucleus of the basal ganglia involved in reward processing, goal-directed behaviors, habit learning, and motor control. The striatum projects to the basal ganglia output nuclei via the “direct” and “indirect” pathways, which can be distinguished by their projection fields and their opposing effects on behavior. In adult animals, the functional opposition is modulated by the differential actions of D1 and D2 dopamine receptors (D1R, D2R), the expression of which is largely separated between these pathways. To determine whether a similar degree of separation exists earlier in development, we used dual-label immunohistochemistry to map dorsal-striatal D1R and D2R expression at the promoter level in postnatal day 0 (PD0) Drd1a-tdTomato/Drd2-GFP BAC transgenic mice, and at the receptor level by co-staining for native D1R and D2R in wild-type PD0 animals. To assess for potential molecular interactions between the D1R and the D2R we also employed a recently developed proximity-ligation assay (PLA). Limited co-expression and co-localization of the D1R and D2R proteins was found in clusters of neurons endemic to the “patch” compartment as identified by co-staining with tyrosine hydroxylase, but not outside these clusters. Moreover, in contrast to our recent findings where we failed to detect a D1R-D2R PLA signal in the adult striatum, in PD0 striatum we did identify a clear PLA signal for this pair of receptors. This co-localization at close proximity points to a possible role for D1R/D2R-mediated crosstalk in early striatal ontogeny.
Keywords: Striatum, Patch, Matrix, Neonate, Dopamine Receptors, Proximity-Ligation Assay
Introduction
In the classical model of basal ganglia circuitry, the striatal output projections are organized into two distinct pathways, the direct “striatonigral” pathway – which monosynaptically projects to the substantia nigra pars reticulata (SNr) – and the indirect “striatopallidal” pathway – which projects to the external globus pallidus (GPe) and then through intermediate neurons relays to the SNr (Gerfen and Surmeier, 2011; Kreitzer and Malenka, 2008). These two pathways are thought to exert opposing effects on motor activity, cognition, and motivational processes in the adult animal (Durieux et al., 2009; Ferguson et al., 2011; Hikida et al., 2010; Kravitz et al., 2010; Kravitz et al., 2012; Lobo et al., 2010). The direct and indirect pathways can also be distinguished at the molecular level by their differential expression of dopamine receptors and neuropeptides. Whereas dopamine Drd1a receptors (D1R) and the neurokinin receptor-ligand substance P are predominantly expressed in medium spiny neurons (MSNs) of the direct pathway, Drd2 receptors (D2R) and the opioid enkephalin are strongly enriched in MSNs of the indirect pathway (Aubert et al., 2000; Gerfen et al., 1990; Le Moine and Bloch, 1995).
The degree of overlap between D1R- and D2R-expressing MSN populations has been a matter of long debate (Bertran-Gonzalez et al., 2010). In situ hybridization methods combined with retrograde labeling have shown an almost complete separation between D1R/substance P-expressing MSNs labeled from the SNr versus D2R/enkephalin-expressing MSNs labeled from the GPe (Aubert et al., 2000; Gerfen et al., 1990; Le Moine and Bloch, 1995). In contrast, use of single-cell PCR methods to more sensitively measure receptor mRNA levels have indicated a larger degree of D1R-D2R co-expression, with around 20% of enkephalin/substance P mRNA-positive MSNs co-expressing both receptor transcripts (Surmeier et al., 1996). Studies using immunohistochemistry (IHC) to assess expression at the receptor level in adult animals have also found differing degrees of co-labeling in the same MSNs of the dorsal striatum, ranging from low (~7%) (Perreault et al., 2010) to moderate (15–20%) (Deng et al., 2006), with at least one study reporting an almost complete co-expression of both receptors (Aizman et al., 2000). Two factors complicate the interpretation of these IHC results: the specificity of the antibodies used and the fact that dopamine receptors are mainly expressed on neuropil, the cellular origin of which is difficult to determine (Caille et al., 1995). In line with anatomical binding studies using radiolabeled D1R and D2R antagonists, antibodies specific to these receptors should show extremely low staining in the cortex relative to strong staining throughout the striatum (Schambra et al., 1994). After generating and validating such antibodies, Hersch et al. (1995) used electron microscopy to show that although some striatal MSNs may co-express both receptors, these receptors nevertheless do not co-localize at the subcellular level (Hersch et al., 1995). Consistent with this study, we recently found that the D1R and D2R do not directly interact or co-localize in the adult ventral striatum of the mouse as assessed by proximity-ligation assays (PLA) and immunohistochemistry (IHC), even in the small fraction of neurons that co-express both receptors (Frederick et al., 2014).
With the generation of Drd1a-GFP (D1-GFP), Drd2-GFP (D2-GFP), and Drd1a-tdTomato (D1-Tom) BAC transgenic mice, the question of co-expression could be addressed indirectly by assessing the expression of fluorescent marker proteins driven by particular dopamine receptor promoters. More specifically, these mice express green fluorescent (GFP) and a red fluorescent protein derivative (tdTomato) under large regulatory elements of the D1R or D2R genes, which faithfully recapitulate the endogenous pattern of expression (Gong et al., 2003). Paralleling previous studies on endogenous expression, co-expression between D1R- and D2R-promoter driven fluorescent proteins in dorsal-striatal MSNs has been shown to be less than 5% in adult animals (Ade et al., 2011; Bertran-Gonzalez et al., 2008; Gangarossa et al., 2013; Matamales et al., 2009; Shuen et al., 2008; Thibault et al., 2013).
Despite the plethora of studies investigating the question of dopamine receptor overlap in the adult, relatively little research has been done to address this issue in younger animals. In rats, it has been well-documented that during mid- to late-gestation the striatum develops distinct patch and matrix compartments bearing neurons with specific developmental, output projection, and receptor profiles (Fishell and van der Kooy, 1987; Gerfen, 1985). Classically, the patch compartment of the neonatal striatum has been delineated from the surrounding matrix by high levels of D1R and mu-opioid receptor expression, as well as specific innervation by dopaminergic fibers revealed by staining for tyrosine hydroxylase (TH) (Fishell and van der Kooy, 1987; Gerfen et al., 1987; Gerfen and Young, 1988; Kent et al., 1981). However, studies mapping the ontogeny of dopamine receptor gene expression have found that in addition to prominent expression within the matrix, the D2R also shows enriched expression in clusters throughout the dorsal striatum up until 7–10 days post-parturition (Schambra et al., 1994). Unfortunately, these early efforts did not employ double-label or serial section analysis to determine whether both receptors are expressed in the same or distinct neuron clusters. It is therefore unclear whether at this particular stage of striatal development the D1R and the D2R coexist within the patch compartment. Furthermore, if D1R and D2R expression does indeed overlap in patches, it remains to be determined to what extent the D1R and D2R are co-expressed and co-localized within the same patch neurons where they could potentially interact through direct heteromerization and/or signaling crosstalk (George et al., 2014; Perreault et al., 2010; Rashid et al., 2007) and thereby play a role in in the development and function of neurons within the patch compartment.
To address the question of D1R and D2R co-localization during the early neonatal stage, we used dual-label IHC to map striatal D1R and D2R expression at the promoter level in postnatal day 0 (PD0) Drd1a-tdTomato/Drd2-GFP BAC transgenic mice, and at the receptor level by co-staining for native D1R and D2R in wild-type PD0 animals. To assess for potential molecular interactions between D1Rs and D2Rs in the same neurons we also employed the recently developed PLA (Trifilieff et al., 2011).
Materials and Methods
Animals and Husbandry
With the exception of promoter activity mapping, all other protocols in this study used wild-type C57BL/6 mice harvested on the day of birth (postnatal day 0, PD0); adult animals (>PD90) and pups genetically null for the D2R (D2R-KO) were included for PLA validation. For promoter mapping, we used heterozygous double-transgenic first generation PD0 pups from crossed D1Rp-tdTomato (Tg(Drd1a-tdTomato)6Calak/J, (Ade et al., 2011), RRID:IMSR_JAX:016204) and D2Rp-GFP (Tg(Drd2-EGFP)S118Gsat/Mmnc, (Gong et al., 2003), RRID:IMSR_MMRRC:000230) mice. Each experiment was performed on at least 3 brains and repeated at least three independent times to ensure reproducibility. Transgenic mice were genotyped by PCR as described in Shuen et al. (2008) (D1Rp-tdTomato) and the MMRRC protocol (D2Rp-GFP). All mice were housed under a 12h light/dark cycle in a temperature-controlled environment with food and water available ad lib. All animal protocols used in the present study were approved by the Institutional Animal Care and Use Committee at Columbia University.
Antibodies
All details regarding the antibodies used in the present studies are listed in Table 1.
| Antibody | Immunogen |
|
|
| Anti-D1 Receptor | 97 amino acid C-terminal of the human D1 receptor |
|
|
| Anti-D2 Receptor | Amino acids 246-305 (third intracellular loop) of the mouse D2 receptor |
|
|
| Anti-A2A Receptor | SQPLPGER sequence in the third intracellular loop of the human A2A receptor |
|
|
| Anti-Tyrosine Hydroxylase (TH) |
Synthetic peptide corresponding to human TH amino acids 30-100 (N-terminal) |
|
|
| Anti-Choline Acetyltransferase (ChAT) |
Extracted human placental enzyme |
|
|
Antibody Characterization
The D1 and D2 receptor antibodies were characterized by fluorescence immunohistochemistry of coronal brain sections from PD0 animals. Staining for the D1R revealed the expected patchy expression of this receptor in the striatum with little detectable signal in the surrounding cortex (see Figure 1), a region of the brain known to exhibit lower levels of D1R expression (Schambra et al., 1994). Consistent with the known expression of D2 receptors within the striatal matrix, our D2 receptor antibody enabled specific detection of this protein ubiquitously throughout the striatum with almost no staining evident in the cortex as expected from the literature (Schambra et al., 1994). For further validation of this specific antibody in knock-out mice, see Trifilieff et al. (2011) and Trifilieff et al. (2013).
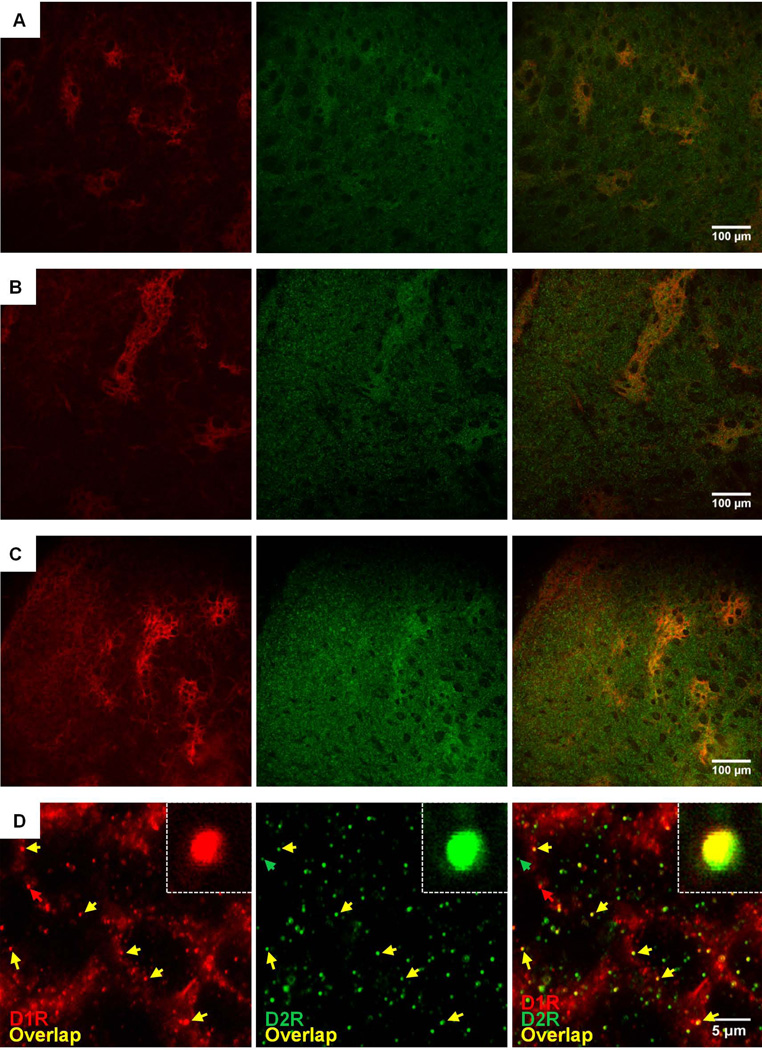
Figure 1. Immunohistochemical mapping of endogenous D1 and D2 receptors reveals co-expression and co-localization in the same striatal clusters of neonatal (PD0) mice.
Photomicrographs showing immunohistochemical double-labeling for endogenous D1R (red, left), D2R (green, middle), and D1R/D2R overlay (right) in the same coronal slice from a wild-type PD0 mouse. (A, B, C) Representative images showing D1R and D2R double-staining in rostral (A), mid (B), and caudal (C) sections of the dorsal striatum. Note the co-appearance of D1R- and D2R-specific staining in the same clusters across the medio-lateral as well as the rostral-caudal axis of the PD0 striatum, with weaker D2R expression also evident outside these regions. (D) Representative, high-magnification photomicrograph of a mid-striatal patch reveals a modest level of D1-D2 receptor co-localization (yellow arrows) amidst singular staining for either the D1R (red arrows) or the D2R (green arrows). Inset shows a magnified example of co-localized D1R-D2R in a single puncta.
Specificity of the A2A antibody was established as part of the proximity-ligation assay validation by Trifilieff et al. (2011). The antibodies used against TH and ChAT are standard in the field for detecting these proteins, and their specificity has been validated elsewhere (Betts et al., 2014; Bhagwandin et al., 2006)
Tissue Preparation for Immunohistochemistry and Proximity-Ligation Assays
For histological analysis of PD0 pups, litters were collected on the day of birth after which the pups were decapitated and brains removed into 0.1 M Na2HPO4/NaH2PO4 (PBS, 150 mM NaCl; pH 7.4) for rinsing. Brains were then fixed in ice-cold 4% paraformaldehyde in PBS for 48 h, rinsed in PBS, and subsequently immersed in melted (<55°C) 4% agarose (in PBS) to aid sectioning. Agarose was allowed to solidify at 4°C after which coronal sections were cut at 40 µm using a vibratome (Leica, Wetslar, Germany) and stored short-term in PBS at 4°C until processing. Adult animals were anesthetized with a ketamine/xylazine (100 mg/kg, 20 mg/kg) mixture and transcardially perfused with 4% paraformaldehyde in PB (0.1 M Na2HPO4/NaH2PO4; pH 7.4) before brains were extracted and post-fixed in the same solution overnight at 4°C. Sections were sliced at 30 µm using a vibratome and stored at −20°C in a cryoprotective solution (30% glycerol, 30% ethylene glycol in 0.1 M Tris-buffer, pH 7.4) until processing.
Fluorescence Immunohistochemistry (IHC)
Floating sections were rinsed 4x with ice-cold TBS (150 mM NaCl in 0.1 M Tris, pH 7.4), and blocked/permeabilized for >1h in TBS-T (Triton-x, 0.5%) containing 10% fetal bovine serum (v/v) and 0.5% bovine serum albumin (w/v); this solution was used for all subsequent antibody incubations. For endogenous dopamine receptor staining, sections were incubated overnight at 4°C with rat-generated anti-D1R antibody (1:250, RRID:AB_1840787) and/or a rabbit-generated anti-D2R antibody (1:400). Staining for tyrosine hydroxylase (TH) was accomplished with use of a goat anti-TH antibody (1:200, RRID:AB_10710873) while choline acetyltransferase (ChAT) was detected with a goat anti-ChAT antibody at a dilution of 1:100 (RRID:AB_2079751). Sections were washed repeatedly in TBS-T (0.2%) and then incubated with fluorophore-labeled secondary antibodies (1:500) directed against rat (Alexa 488, Life Technologies, Grand Island, NY), rabbit (Alexa 568), and/or goat IgGs (Alexa 405). For GFP and tdTomato staining, we respectively used a chicken anti-GFP antibody (1:250, Abcam, Cambridge, MA; cat# ab13970, RRID:AB_300798) and a rabbit anti-dsRED antibody (1:250, Clontech, Mountain View, CA; cat# 632496, RRID:AB_10013483) before washes and subsequent incubation with an anti-chicken Alexa 488 (1:500) and anti-rabbit Alexa 568 (1:500). Following secondary antibody incubation, all slices were washed again in TBS-T (0.2%), rinsed in 50 mM Tris (pH 7.4) to remove salts, and then mounted and coverslipped with Vectashield with or without DAPI (Vector Labs, Burlingame, CA) on Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA).
Proximity-Ligation Assay (PLA)
Dual-antigen recognition PLA experiments were conducted as described in Trifilieff et al. (2011). Briefly, PLA was performed using the DuoLink in situ kit (Olink Bioscience, Uppsala, Sweden) according to the manufacturer’s instructions with the following modifications: incubation with PLA probes and the ligation step were extended to 2h and to 45 min, respectively; the amplification step consisted of a 2h incubation at 37°C with a 1/60 concentration of polymerase. Anti-D1R (rat) and anti-D2R (rabbit) primary antibodies were used at a concentration of 1:200. The anti-A2AR primary antibody (mouse) was used at a dilution of 1:1000. Subsequent secondary labeling of the D2R and A2AR for this assay was accomplished with the use of kit-provided anti-rabbit and anti-mouse PLA probes, respectively, while the D1R was detected using an anti-rat PLA probe generated according to the manufacturer’s instructions using the DuoLink Probemaker kit (Olink Bioscience) and goat anti-rat IgGs (Santa Cruz Biotechnology, Dallas, TX). For this assay, slices were coverslipped with Fluorsave (Millipore, Billerica, MA).
Microscopy and Initial Image Processing
Fluorescent sections were imaged with a Nikon A1 laser-scanning confocal microscope (Nikon Instruments Inc., Melville, NY), using a 40x N.A. = 1.0 oil objective (with or without digital zoom) for high-magnification images. Bandpass filters were as follows: 405, 425–475 nm; 488, 500–550 nm; 568, 570–620 nm. Z-stacking was used only with PLA-processed sections (5 µm with a 0.5 µm interval) as the majority of signal resides within the first 5–10 µm of each slice. Acquired images were then processed using ImageJ software (NIH, Bethesda, MD, RRID:nif-0000-30467). Sections stained for endogenous D1R and D2R were reverse-pseudocolored (green to red and vice versa) in ImageJ to parallel native fluorophore color in D1-Tom/D2-GFP mice. For PLA analysis, each z-stack was collapsed into a single image based on the maximum intensity signal across sections using standard ImageJ algorithms. Signal bleed-through between channels was negligible.
Image Processing and Counting of Positive Signal (“Dots”) Generated by PLA
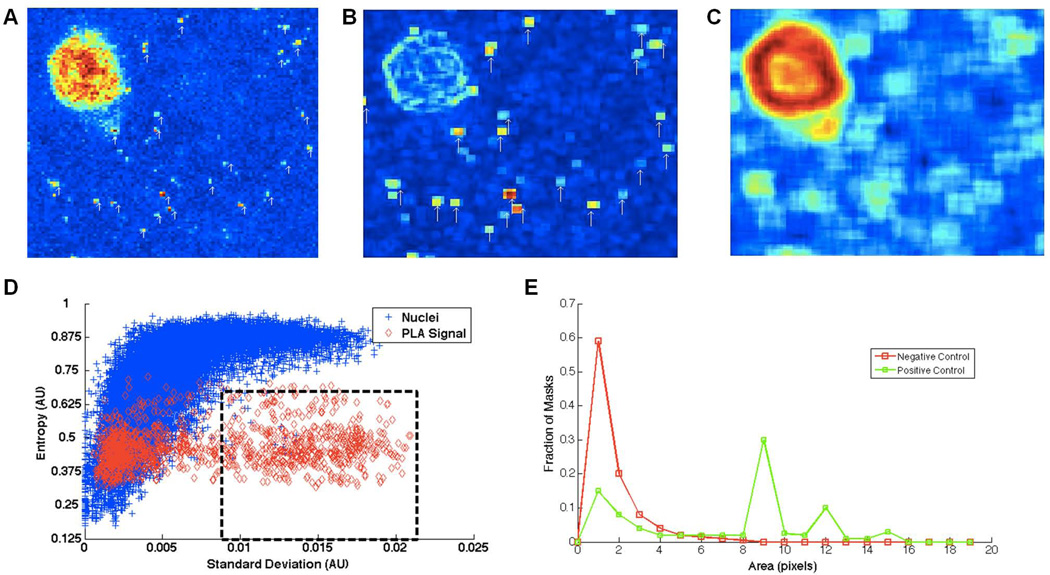
The PLA procedure yields three types of signal: 1) fluorescent “dots” indicative of proteins in close physical proximity (Trifilieff et al., 2011); 2) fluorescent cell nuclei (an artifact caused by probe infiltration); 3) background. To separate genuine PLA dots from cell nuclei and background, we used Matlab (The MathWorks, Natick, MA, RRID:nlx_153890) to develop a computational method for sorting that requires minimal user intervention (Figure 4). First, we noted that a pure intensity threshold was insufficient to separate extended areas of increased fluorescence inside and around cell nuclei from concentrated areas of PLA fluorescence (i.e., positive dots) (Figure 4A). Nuclear fluorescence occurred even within negative control conditions (e.g., D1R-D2R PLA in D2-KO tissue) and it differed from genuine PLA plots by not exhibiting sharp signal peaks. We therefore combined standard deviation and entropy filtering methods to determine optimal thresholds for segregating nuclear fluorescence and background from genuine PLA–generated dots. First, the original raw confocal images were standard deviation-filtered, a process which replaced every value in the image with the standard deviation of 8 neighboring pixels, effectively elevating local intensity differences far above background while suppressing intensity differences spread out over a larger area (Figure 4B). Because nuclear boundaries occasionally lead to locally high standard deviation values over background, we also employed an entropy filter to locate the edges of these nuclei (Figure 4C). In parallel, a naïve user manually placed 40 masks, between 1 and 20 µm in area, around isolated regions of high fluorescence in a positive control image (i.e., adult D2R-A2AR PLA, see Results), including randomly selected nuclei. Every pixel within a putative positive signal region and within a nuclear region was then analyzed. The entropy and standard deviation values within these regions showed a clear clustering/separation into either nuclei or PLA-positive dots (Figure 4D). Because the user masks were user-selected, they inevitably contained a large number of background (non-nuclear/non-dot-specific) pixels, which are seen as signal (red/blue) overlap in the bottom left corner of the plot in Figure 4D. As panel D illustrates, results from our analysis provided the rationale for thresholding standard deviation filtered images at 0.009 and entropy-filtered images at 0.65 in order to distinguish PLA signal from nuclei and background noise. The thresholded images were then transformed into individual masks that captured the putative PLA signal. We found that some of these masks seemed to reflect background noise, as they were present even in negative controls. To ascertain the source of this additional noise, we plotted histograms of mask areas for both negative and positive control images (Figure 4E). As evidenced by the prominent peak (9–11 pixels) in the positive control condition, we concluded that the smallest detectable PLA signals were at least 8 pixels in area and that any smaller signal could be attributed to high standard deviation/low entropy noise. Therefore, any masks smaller than 8 pixels were excluded from further analysis. These methods were then systematically applied to all images to yield total PLA dot counts for every condition.
Figure 4. Software-guided image analysis method for selecting PLA-generated dots.
(A) Heat map of the fluorescence intensity values in an unfiltered image. Before any transformation of the image is performed, both the nuclei and the PLA signal exhibit high intensity fluorescence. White arrows point to putative PLA-generated dots. (B) To elevate isolated areas of high fluorescence intensity, a standard deviation filter is employed. White arrows highlight areas that have high standard deviation and low entropy. (C) To locate edges of nuclei the image is entropy filtered. Nuclei are then filled inward to specify their location. (D) The entropy and standard deviation of all pixels within user-selected dots (red) and user-selected nuclei (blue) illustrates that nuclei and PLA-generated dots can be distinguished on the basis of differential clustering in this parameter space. (E) Using the thresholds from “D” (standard deviation = 0.009, entropy = 0.65) to gate (perforated box) only PLA dots for further analysis, masks are produced for each individual image from each experimental condition. In our analyses, masks smaller than 8 pixels were counted as noise, while those over 8 pixels were counted as positive PLA-generated signal. See Materials and Methods for more details regarding this method.
Statistical Analysis
For D1R-D2R PLA, multiple images for statistical comparison were acquired across the striatum in WT as well as D2R-knockout (KO) PD0 animals. Data were analyzed in Graphpad Prism (RRID:rid_000081) and are presented as mean ± SEM. Dot counts in PD0 WT vs. D2-KO striatum were compared by way of a one-tailed unpaired student’s t-test using Welch’s correction for unequal variance. For more comprehensive analysis of D1R-D2R PLA in the striatum of adult animals, see Trifilieff et al. (2011) and Frederick et al. (2014).
Results
Immunohistochemical mapping of endogenous D1 and D2 receptors reveals co-expression and co-localization within the patch compartment of neonatal (PD0) mice
To address whether D1R and D2R are co-expressed during neonatal development, we mapped the expression of these receptors across the whole rostral-caudal axis of the dorsal striatum in coronal brain slices from PD0 mice by double-label immunohistochemistry (IHC) using antibodies that have previously been validated using knock-out mice (Trifilieff et al., 2013; Trifilieff et al., 2011).
We found that relative to the expected weak staining in the cortex (see Antibody Characterization in the methods section), D1 and D2 receptors co-stained prominently in the same clusters across the medio-lateral as well as rostral-caudal axis of the PD0 striatum (Figure 1A, B, C). We further characterized the nature of these clusters by co-staining for tyrosine hydroxylase (TH), a known marker of the striatal patch compartment in neonatal animals (Graybiel, 1984). We found a complete overlap between the D1R and TH signal clusters throughout the striatum (Figure 3A, B, C), in line with published literature showing enrichment of D1Rs in striatal patches during early postnatal development (Schambra et al., 1994). Outside the D1R/D2R-intense patches - presumably in the "matrix" compartment - we detected lower levels of the D2R, while D1R expression was almost absent (Figure 1A, B, C). Single-label experiments found no evidence of signal crossover between the two fluorescence channels (data not shown).
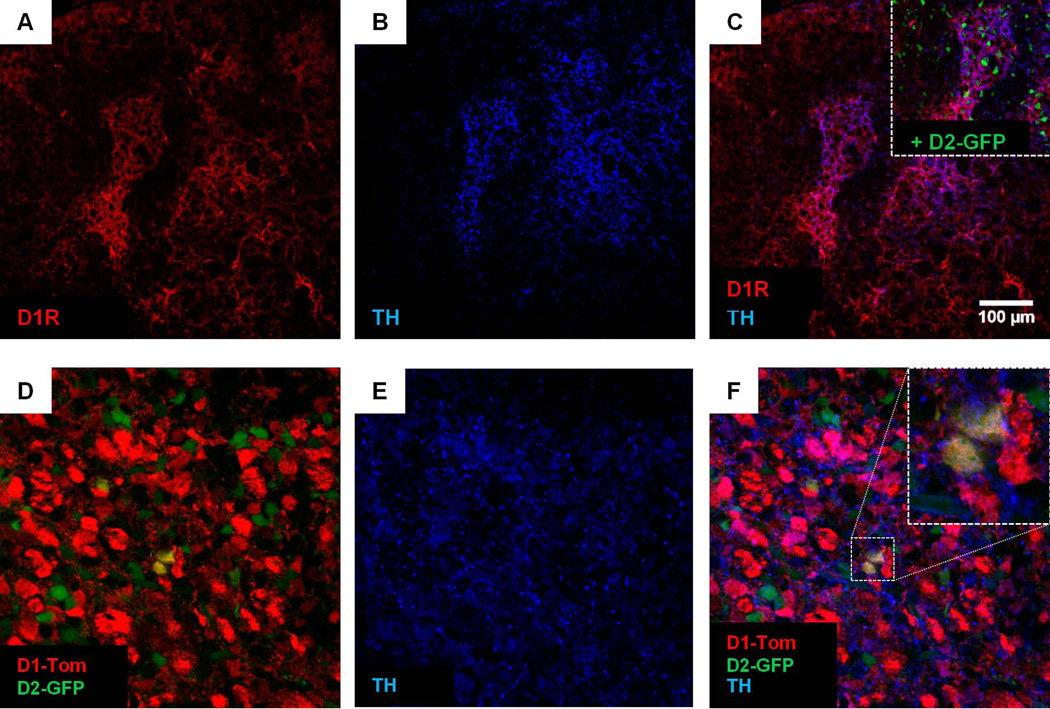
Figure 3. Dopamine receptor co-expression occurs within the patch compartment of the neonatal striatum.
(A, B, C) A representative photomicrograph showing double staining for native D1R (A) and tyrosine hydroxylase (TH,B), a marker for the patch compartment in neonatal striatum. The almost complete overlap between D1R and TH signal is shown in (C), suggesting that clusters of D1R expression are endemic to the patch compartment. For cross-methodological comparison, the inset in (C) illustrates the higher fluorescence intensity of D2-GFP expressing cells within TH-positive clusters than outside these regions, which agrees with our finding of higher native D2R expression within D1R-positive patches (Figure 1). (D, E, F) A representative photomicrograph showing that cells with high D1-Tom and/or D2-GFP levels (D) are clustered within TH-positive regions (E,F), suggesting that these clusters are patch-specific. Inset in (F) illustrates an example of dual-expressing D1-Tom/D2-GFP cells within the patch compartment.
To determine whether D1R and D2R that are expressed in the same patches in the PD0 striatum co-localize at the molecular level, we visualized D1R and D2R expression in these patches using high-magnification confocal microscopy. A representative patch is shown in Figure 1D. Amidst singular staining for either receptor alone (D1R, red arrows; D2R, green arrows), D1R and D2R showed evidence of co-localization within the boundary of this patch (yellow arrows).
Mapping of reporter gene expression in D1-Tom/D2-GFP mice reveals modest promoter co-activity within patch neurons of the neonatal (PD0) striatum
Given the high degree of endogenous D1R and D2R co-staining in patches across the PD0 striatum, we investigated whether both receptors are co-expressed by the same cells, or in different interdigitated cells as is generally found in adult animals (Matamales et al., 2009). Dopamine receptors, however, are highly enriched in neuropil relative to the perikarya thereby causing difficulties in pinpointing their cellular origin. We circumvented this problem by mapping the striatal expression of fluorescent reporter genes Tom (td-Tomato) and GFP driven respectively by the D1 and D2 receptor promoters in the brains of PD0 mice heterozygous for both transgenes (i.e., using D1-Tom/D2-GFP mice); both Tom and GFP concentrate within the cell body making individual neurons easy to distinguish. In correspondence with more intense D1R and D2R expression within the same clusters of the PD0 striatum (Figure 1), signal intensification of Tom and GFP by IHC revealed a clustered co-appearance of cells exhibiting stronger signal intensity of both markers surrounded by cells with weaker expression outside these clusters (Figure 2A, B, C). This was particularly evident across the mid-caudal axis of the striatum. Further characterization revealed that the cell clusters exhibiting higher Tom and GFP signal intensity overlapped with prominent TH staining throughout the striatum (Figure 3D, E, F; also see inset Figure 3C). These findings suggest that D1R and D2R promoter activity is stronger in the patch compartment, complementing our previous finding of stronger receptor staining within these regions relative to surrounding neurons. Co-expression of both reporter genes in the same neurons was limited to patches (Figure 2D; Figure 3F). Cell count analysis of 10 representative clusters imaged from multiple striatal regions of 3 independently stained mouse brains revealed Tom/GFP overlap (i.e., MSNs with co-active D1R and D2R promoters) to be in the range of 5.6 ± 3.28% (average number of labeled cells per cluster: D1-Tom: 27.6 ± 12.15; D2-GFP: 26.9 ± 7.85; Tom/GFP: 3 ± 1.49). Importantly, additional characterization revealed that co-expression of both fluorophores was not observed in cholinergic interneurons positive for choline acetyltransferase (ChAT), as these cells only weakly expressed D2-GFP and not D1-Tom (Figure 2D, right panel).
Figure 2. Mapping of reporter gene expression in D1-Tom/D2-GFP mice reveals modest promoter co-activity in the neonatal (PD0) striatum.
(A, B, C) Photomicrographs showing fluorescence immunohistochemistry-based intensification of D1Rp-driven tdTomato (D1-Tom, left), D2Rp-driven green fluorescent protein (D2-GFP, middle), and Tom/GFP overlay (right) signals in rostral (A), mid (B), and caudal (C) coronal striatal slices obtained from a PD0 mouse heterozygous for both transgenes. Note the stronger expression of both fluorophores in overlapping neural clusters across the medio-lateral axis and in particular the mid-to-caudal levels of the PD0 dorsal striatum. (D) A magnified portion of a representative MSN cluster of the PD0 dorsal striatum shows evidence of modest D1 and D2 receptor promoter co-activity within some neurons (yellow arrows; images organized exactly as in “A”), suggesting that these cells endogenously co-express both receptor proteins. Additional characterization of this patch revealed that cells co-expressing Tom and GFP are not cholinergic in nature (D, right), as the choline acetyltransferase (ChAT)-positive cell was only weakly positive for D2-GFP (D, middle, green arrow) and not D1-Tom (D, left).
Proximity-ligation assays confirm D1 and D2 receptor co-localization in the neonatal (PD0) striatum
One limitation of using IHC to determine protein co-localization is that given the diffraction limit of light of ~250 nm, this method does not provide adequate spatial resolution for detecting true molecular proximity. We therefore addressed the possibility of D1R-D2R interaction more directly by way of a proximity-ligation assay (PLA). PLA is a novel technique that relies on close physical proximity of antigens in order to generate a positive signal at the point of co-localization, in this case a fluorescent “dot” (Trifilieff et al., 2011). Furthermore, previous data suggest that for a signal to be generated, the proteins must be in close proximity, maximally ~20–30 nm (Soderberg et al., 2006; Trifilieff et al., 2011). We performed PLA on PD0 brain slices using the same antibodies as were used for endogenous D1R and D2R IHC staining, and counted the resulting PLA-generated dots using the method described in Materials and Methods and Figure 4. Results from this assay revealed a significant number of dots (per µm2 of entire image field) in clusters within the dorsal striatum corresponding to co-localized D1R-D2Rs (Figure 5A, E, I, M, N), which were absent outside these clusters (Figure 5B, F, J, M). As expected, no signal was detected in the striatum of D2R-KO mice (Figure 5D, H, L, M) while very low signal was seen in the cortex of wild-type mice (Figure 5C, G, K, M), the cortex known to express low levels of D1R and D2R. In contrast, we observed a high number of dots in our PLA positive control, where PLA was performed on adult striatal tissue with antibodies against D2R and the adenosine 2A receptor (A2AR) (Figure 5M, O), proteins that have previously been shown to interact as assessed by this method (Trifilieff et al., 2011). Quantification of the D1R-D2R PLA signal in PD0 animals (Figure 5M) revealed 0.2828 ± 0.09471 dots/µm2 in clusters within the dorsal striatum, 0.0275 ± 0.01181 dots/µm2 in the striatum outside the clusters, 0.05 dots/µm2 in the cortex, and 0.0250 ± 0.0050 dots in the negative D2R-KO control; the adult D2R-A2AR positive control yielded 8.554 dots/µm2. D1R-D2R PLA counts from WT PD0 striatum were 10-fold higher than in the D2R-KO control (t(5) = 2.719, p = 0.0209).
Figure 5. Proximity-ligation assays confirm D1 and D2 receptor co-localization in the neonatal (PD0) striatum.
Results of proximity-ligation assays (PLA) using the same D1 and D2 receptor antibodies as were used in IHC analysis. A-D show the original raw images for each of the four regions/conditions, E-H show the respective fluorescence intensity standard deviation heat maps (see Methods) for each condition, and I-L show binary PLA dot-sorted images ultimately used to count PLA dots for each condition. Nuclear staining is an artifact of the PLA procedure and does not reflect positive signal. PLA analysis confirmed the co-localization of D1R and D2R ("dots", see yellow arrows) within clusters presumed to reflect striatal “patches” (A,E,I; for clarity, N shows a higher-magnification image of the raw dot-positive region) but not outside these clusters (presumed “matrix”) (B,F,J) in wild-type (WT) PD0 mice. Little to no positive signal was observed in the cortex (C,G,K), a region known to express low levels of D1R and D2R, or when D1R-D2R PLA was performed on mice lacking the D2R (D2R-KO) (D,H,L). In contrast, a large number of dots were evident in the striatum (O; high magnification image of the raw dot-positive region) of our positive control when PLA was performed on adult tissue using antibodies against D2R and the adenosine 2A receptor (A2AR), which are known to associate in adult animals. (M) Quantification of PLA dot counts (per µm2 of entire image field) across conditions revealed a significant, 10-fold higher number of D1R-D2R PLA dots in WT neonatal striatum than in D2R-KOs (#, t(5) = 2.719, p = 0.0209). Gtype, genotype. Bottom-left descriptor for images A-D, N, O, Gtype & Age: PLA condition.
Discussion
The cellular architecture of the perinatal striatum is divided between islands of striosomal (patch) neurons within a broader matrix compartment, distinguished by distinct neural birthdates, molecular signatures, and output targets. In the rat the patch compartment consists of neurons born between embryonic day (ED) 12–15, approximately half of which send a unique direct-pathway projection to the SN pars compacta that is already evident by the end of gestation (Fishell and van der Kooy, 1987); the projection fields of the remaining patch neurons have not been investigated but have been presumed to be striatopallidal in nature (Gerfen and Young, 1988). In contrast, ubiquitously distributed matrix neurons arise between ED17-20 and gradually connect with both the SNr and/or the globus pallidus within the first post-natal week (Fishell and van der Kooy, 1987; Fishell and van der Kooy, 1991; Gerfen, 1984; Gerfen, 1985; Gerfen et al., 1987; Gerfen and Young, 1988). In terms of molecular phenotype, previous studies investigating the molecular constituents of the patch compartment in the neonate have generally focused on characterizing specifically the SNc-projecting neurons, showing that these MSNs express both D1R and substance P (Bolam et al., 1988; Caille et al., 1995), proteins mainly expressed by direct-pathway MSNs in adults. In addition, the neonatal patch compartment can be delineated from the matrix by high expression of the mu-opioid receptor, higher levels of acetylcholinesterase (AChE), and specific innervation by TH-positive dopamine fibers originating from the SNc (Fishell and van der Kooy, 1987; Graybiel, 1984; Tokuno et al., 1996). Within the next few weeks the distribution of dopamine receptors and dopaminergic afferents becomes homogenous across the striatum (Graybiel, 1984; Schambra et al., 1994), and patch neurons in juvenile and adult animals become identifiable mainly by their selective expression of mu-opioid receptors (Szele et al., 1991); within these patches, the levels of proenkephalin and D2R are lower relative to the matrix (Besson et al., 1988; Korf and Loopuijt, 1988; Koshimizu et al., 2008). Importantly, in neonatal animals, at least one study has reported a “clustered” appearance of neurons radiolabeled for D2R within the putative patch compartment identified by AChE staining (Lowenstein et al., 1989), suggesting that at this developmental time point D2R-expressing neurons may co-exist in the same patch clusters as direct-pathway neurons containing the D1R. To test this directly, we used dual-label immunohistochemistry to show that native D1Rs exhibit overlapping patterns of expression with TH clusters across the neonatal striatum, and are thus endemic to the patch compartment of this region. Moreover, these D1R-expressing patches also show high levels of the D2R, indicating that the neonatal patch compartment is comprised of MSNs that can express either (or both) receptor subtype.
Taking advantage of transgenic mice that express fluorescent reporters driven by the D1R (Tom) and D2R (GFP) promoters (Gong et al., 2003), we also mapped the fluorophore expression patterns of cells throughout the neonatal striatum and found a clustered appearance of neurons enriched for either marker, surrounded by cells with weaker expression. As with native D1Rs, these clusters largely overlapped with TH-positive regions, suggesting that they were patch-specific. Presumably, the higher level of fluophore expression in these cells may reflect higher promoter activity driving the generation of native D1Rs and D2Rs, which coincides with our finding of higher receptor expression within TH-positive clusters. Cell count analysis of clusters high in Tom and/or GFP signal intensity revealed that approximately 5–6% of these cells co-expressed both fluorophores in the dorsal striatum, up to 2-fold higher than has been reported in adults (Ade et al., 2011; Bertran-Gonzalez et al., 2008; Gangarossa et al., 2013; Matamales et al., 2009; Shuen et al., 2008; Thibault et al., 2013). These dual-expressing cells were most likely MSNs as such signal overlap was not found to occur in cholinergic cells, which only weakly expressed GFP and not Tom.
Given the high expression of both native D1R and D2R as well as Tom and GFP within TH-positive striatal regions, our findings indicate that the patch compartment of the neonatal striatum contains three subpopulations of MSNs: those expressing either the D1R, the D2R, or both. More specifically, our findings raise the interesting possibility that patch neurons of the neonatal striatum, classically defined by the presence of the D1R and projections to direct-pathway targets in adults, are mixed with a subpopulation of cells that also exhibit the molecular characteristics typical of indirect-pathway neurons (i.e., D2R expression); however, the projection fields and thus pathway-specificity of these D2R-expressing patch neurons remain to be determined (see Gerfen and Young (1988) for further discussion). Nevertheless, the patch neurons that co-express D1R and D2R are presumably simultaneously regulated by both receptors during the neonatal period, which may have implications for their further development. For instance, it is known that up to 30% of striosomal neurons undergo apoptosis within the first postnatal week (Fishell and van der Kooy, 1991), a selection process that may depend on threshold trophic support from target regions, the reception of which may in turn be dependent in part on the differential actions of the D1R and D2R.
Examination of native D1R- and D2R-positive clusters with high-magnification confocal microscopy revealed a modest incidence of signal overlap between receptor puncta, suggesting that these receptors co-localize at the molecular level. To investigate this possibility more directly, we used a proximity-ligation assay (PLA) that can detect protein interaction. Consistent with the confocal analysis the PLA confirmed that at PD0 some of the D1R and D2R exist in close molecular proximity, presumably within individual neurons. Despite the high spatial resolution of this assay, however, it is also possible that a portion of the PLA signal could have theoretically been derived from receptors expressed in axons and/or dendrites of two different neurons that are opposed to one another. Functionally, it has previously been suggested that upon agonist co-stimulation, co-expressed and co-localized D1R and D2R can activate signaling pathways not typically governed by either receptor alone (e.g., signaling via Gαq and calcium-dependent kinases), with the potential to affect cellular plasticity (Chun et al., 2013; Rashid et al., 2007). The formation of a D1/D2 receptor heteromer has been proposed to mediate this signaling pathway (Boyd and Mailman, 2012; George et al., 2014; Rashid et al., 2007). By contrast, Lee et al., (2014) and Frederick et al. (2014) have argued against a role for Gαq and phospholipase C in D1R or D1R/D2R function (Lee et al., 2014a; Lee et al., 2014b). Future studies should address whether the close proximity of native dopamine receptors in the neonatal striatum confers receptor interactions at the level of downstream effectors, and what consequences this may have for signaling and cellular function.
Recently, we used PLA in the adult ventral striatum to assess for D1R-D2R interactions and found the signal to be essentially the same as the background signal measured in slices from D2R KO animals; no interaction was evident even in the small number of cells that co-expressed both receptors (Frederick et al, 2014). In contrast, our current data show evidence of D1R-D2R association in the neonatal dorsal striatum, with a signal ~10-fold above the background detected in the D2R-KO. Although the ventral and dorsal regions of the striatum are not directly comparable, the PLA studies in the adult animals focused on the ventral striatum because this region shows the highest degree of D1-D2 receptor co-expression (Bertran-Gonzalez et al., 2008; Perreault et al., 2012). Taken together with our current results at PD0, these data suggest the possibility that a mechanism may arise at some point during development to segregate these receptors in the plasma membrane of neurons co-expressing the two receptors. In this regard, however, Frederick et al. (2014) and the current manuscript are in conflict with Perreault et al. (2012) who reported the opposite findings: high levels of D1-D2 receptor heteromerization in both juvenile and adult rats, but with the juvenile rats showing fewer heteromers relative to adults. We cannot readily explain these differences, except that methodological differences between these reports may have yielded different results.
Normal expression of striatal-mediated behaviors in adult animals is in part dependent on a proper balance of direct-indirect pathway output from the striatal complex (Cazorla et al., 2014; Ferguson et al., 2011; Kravitz et al., 2012; Lobo et al., 2010), and it is conceivable that the establishment of this balance may be subject to modulation during striatal ontogeny by the developmental expression patterns of dopamine receptors. Since the expression of dopamine receptors can be altered by genetic (Lawford et al., 2005; Le Foll et al., 2009) and environmental factors such as stress (Pani et al., 2000) as well as in utero exposure to drugs of abuse (Chang et al., 2007; Henry et al., 1995; Thompson et al., 2009), it is possible that the degree of D1R and D2R co-expression in the developing animal may be regulated and differ under various conditions. Given our evidence for D1R-D2R interaction in the PD0 striatum, it is conceivable that D1R-D2R signaling from crosstalk downstream of both receptors could play a role in the neonatal striatum in vivo. Our findings pave the way for future research on how early D1 and D2 receptor co-localization in the striatal patch compartment may regulate function and development. Moreover, it has been postulated that the degree of D1R and D2R co-expression and potentially crosstalk is altered under pathological conditions such as in schizophrenia (Grymek et al., 2009) and depression (Pei et al., 2010). Studying the function of D1R-D2R pairs during neonatal development may therefore have implications for understanding the development of psychiatric disorders.
Supplementary Material
Acknowledgments
Acknowledgment of Support: Biezonski, DK was supported on a T-32 institutional grant in child and adolescent psychiatry (NIMH 016434-33) and the Paul Janssen Fellowship in Translational Neuroscience at Columbia University. Trifilieff, P was supported by the INRA, a Research Scientist Award from the Research Foundation for Mental Hygiene, and a NARSAD Young Investigator Award from the Brain and Behavior Foundation. Meszaros, J was supported by an institutional NIH grant 5T32NS064928-04. Javitch, J was supported by NIH grants DA022413 and MH54137. Kellendonk, C was supported by NIH grant MH093672.
Other Acknowledgments
We would like to thank Bhavani Ramesh and Mariya Shegda for animal colony maintenance and animal genotyping. Images were collected in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, supported by NIH grant #P30 CA013696 (National Cancer Institute).
Footnotes
RRID: AB_11213750, AB_1840787, AB_10710873, AB_2079751, AB_300798, AB_10013483, nif-0000-30467, nlx_153890, rid_000081, IMSR_JAX:016204, IMSR_MMRRC:000230
Conflict of Interest Statement
The authors declare no conflicts of interest in relation to the present manuscript.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: DB, CK, JJ, PT. Acquisition of data: DB, PT. Analysis and interpretation of data: DB, JM, CK, PT, JJ. Drafting of the manuscript: DB, CK. Critical revision of the manuscript for important intellectual content: DB, CK, PT, JJ. Statistical analysis: DB, JM. Obtained funding: CK, DB, JJ. Administrative, technical, and material support: CK. Study supervision: CK.
Literature Cited
- Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons. Frontiers in systems neuroscience. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nature neuroscience. 2000;3(3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. The Journal of comparative neurology. 2000;418(1):22–32. [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Frontiers in neuroanatomy. 2010;4 doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson MJ, Graybiel AM, Nastuk MA. [3H]SCH 23390 binding to D1 dopamine receptors in the basal ganglia of the cat and primate: delineation of striosomal compartments and pallidal and nigral subdivisions. Neuroscience. 1988;26(1):101–119. doi: 10.1016/0306-4522(88)90130-3. [DOI] [PubMed] [Google Scholar]
- Betts JF, Schweimer JV, Burnham KE, Burnet PW, Sharp T, Harrison PJ. D-amino acid oxidase is expressed in the ventral tegmental area and modulates cortical dopamine. Frontiers in synaptic neuroscience. 2014;6:11. doi: 10.3389/fnsyn.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwandin A, Fuxe K, Manger PR. Choline acetyltransferase immunoreactive cortical interneurons do not occur in all rodents: a study of the phylogenetic occurrence of this neural characteristic. Journal of chemical neuroanatomy. 2006;32(2–4):208–216. doi: 10.1016/j.jchemneu.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM. Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: a combined Golgi and immunocytochemical study in cat and ferret. Neuroscience. 1988;24(3):853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Mailman RB. Dopamine receptor signaling and current and future antipsychotic drugs. Handbook of experimental pharmacology. 2012;(212):53–86. doi: 10.1007/978-3-642-25761-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Le Moine C, Begueret J, Bloch B. Ontogeny of the D1 dopamine receptor in the rat striatonigral system: an immunohistochemical study. The European journal of neuroscience. 1995;7(4):714–722. doi: 10.1111/j.1460-9568.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, Ahmari SE, Moore H, Kellendonk C. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81(1):153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Molecular pharmacology. 2013;84(2):190–200. doi: 10.1124/mol.113.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. Journal of chemical neuroanatomy. 2006;32(2–4):101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d'Exaerde A. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nature neuroscience. 2009;12(4):393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature neuroscience. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, van der Kooy D. Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci. 1987;7(7):1969–1978. doi: 10.1523/JNEUROSCI.07-07-01969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, van der Kooy D. Pattern formation in the striatum: neurons with early projections to the substantia nigra survive the cell death period. The Journal of comparative neurology. 1991;312(1):33–42. doi: 10.1002/cne.903120104. [DOI] [PubMed] [Google Scholar]
- Frederick A, Yano H, Trifilieff P, Vishwasrao H, Biezonski D, Mészáros J, Sibley D, Kellendonk C, Sonntag K, Graham D, Colbran R, Stanwood G, Javitch J. Evidence against dopamine D1/D2 receptor heteromers. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.166. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, Mailly P, De Bundel D, de Kerchove d'Exaerde A, Herve D, Girault JA, Valjent E, Krieger P. Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Frontiers in neural circuits. 2013;7:124. doi: 10.3389/fncir.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Kern A, Smith RG, Franco R. Dopamine receptor heteromeric complexes and their emerging functions. Prog Brain Res. 2014;211:183–200. doi: 10.1016/B978-0-444-63425-2.00008-8. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaicICompartmental organization of projections from the striatum to the substantia nigra in the rat. The Journal of comparative neurology. 1985;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7(12):3935–3944. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain research. 1988;460(1):161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Correspondence between the dopamine islands and striosomes of the mammalian striatum. Neuroscience. 1984;13(4):1157–1187. doi: 10.1016/0306-4522(84)90293-8. [DOI] [PubMed] [Google Scholar]
- Grymek K, Lukasiewicz S, Faron-Goreckaa A, Tworzydlo M, Polit A, Dziedzicka-Wasylewska M. Role of silent polymorphisms within the dopamine D1 receptor associated with schizophrenia on D1-D2 receptor hetero-dimerization. Pharmacological reports : PR. 2009;61(6):1024–1033. doi: 10.1016/s1734-1140(09)70164-1. [DOI] [PubMed] [Google Scholar]
- Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, Demotes-Mainard J. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain research. 1995;685(1–2):179–186. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(7 Pt 2):5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kent JL, Pert CB, Herkenham M. Ontogeny of opiate receptors in rat forebrain: visualization by in vitro autoradiography. Brain research. 1981;254(4):487–504. doi: 10.1016/0165-3806(81)90018-3. [DOI] [PubMed] [Google Scholar]
- Korf J, Loopuijt LD. Synaptic and non-synaptic striatal dopamine D2 receptors: possible implications in normal and pathological behaviour. Acta morphologica Neerlando-Scandinavica. 1988;26(2–3):177–190. [PubMed] [Google Scholar]
- Koshimizu Y, Wu SX, Unzai T, Hioki H, Sonomura T, Nakamura KC, Fujiyama F, Kaneko T. Paucity of enkephalin production in neostriatal striosomal neurons: analysis with preproenkephalin-green fluorescent protein transgenic mice. The European journal of neuroscience. 2008;28(10):2053–2064. doi: 10.1111/j.1460-9568.2008.06502.x. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Swagell CD, Barnes M, Burton SC, Ward WK, Heslop KR, Shadforth S, van Daal A, Morris CP. The C/C genotype of the C957T polymorphism of the dopamine D2 receptor is associated with schizophrenia. Schizophrenia research. 2005;73(1):31–37. doi: 10.1016/j.schres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioural pharmacology. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. The Journal of comparative neurology. 1995;355(3):418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, Mailman RB. SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase. C. Neuropharmacology. 2014a;86:145–154. doi: 10.1016/j.neuropharm.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yang Y, Mailman RB. Dopamine D1 receptor signaling: does GalphaQ-phospholipase C actually play a role? The Journal of pharmacology and experimental therapeutics. 2014b;351(1):9–17. doi: 10.1124/jpet.114.214411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Slesinger PA, Singer HS, Walker LC, Casanova MF, Raskin LS, Price DL, Coyle JT. Compartment-specific changes in the density of choline and dopamine uptake sites and muscarinic and dopaminergic receptors during the development of the baboon striatum: a quantitative receptor autoradiographic study. The Journal of comparative neurology. 1989;288(3):428–446. doi: 10.1002/cne.902880306. [DOI] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Herve D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PloS one. 2009;4(3):e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Molecular psychiatry. 2000;5(1):14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nature medicine. 2010;16(12):1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. The Journal of biological chemistry. 2010;285(47):36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, O'Dowd BF, George SR. Reduced striatal dopamine D1-D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience. 2012;225:130–139. doi: 10.1016/j.neuroscience.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104(2):654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT., Jr Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62(1):65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(11):2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3(12):995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(20):6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szele FG, Artymyshyn R, Molinoff PB, Chesselet MF. Heterogeneous distribution of dopamine D2 receptor mRNA in the rat striatum: a quantitative analysis with in situ hybridization histochemistry. The Anatomical record. 1991;231(4):548–558. doi: 10.1002/ar.1092310416. [DOI] [PubMed] [Google Scholar]
- Thibault D, Loustalot F, Fortin GM, Bourque MJ, Trudeau LE. Evaluation of D1 and D2 dopamine receptor segregation in the developing striatum using BAC transgenic mice. PloS one. 2013;8(7):e67219. doi: 10.1371/journal.pone.0067219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nature reviews Neuroscience. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Takada M, Kaneko T, Shigemoto R, Mizuno N. Patchy distribution of substance P receptor immunoreactivity in the' developing rat striatum. Brain research Developmental brain research. 1996;95(1):107–117. doi: 10.1016/0165-3806(96)00080-6. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Molecular psychiatry. 2013;18(9):1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slattman M, Gullberg M, Javitch JA. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques. 2011;51(2):111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.