Abstract
We hypothesized that direct AT2R stimulation improves albuminuria in diabetes by preventing renal inflammation and improving oxidative stress. Normoglycemic controls (NC) and streptozotocin-induced diabetes Sprague-Dawley rats (DM) were treated for 4 weeks with vehicle (V) or the AT2R agonist Compound 21 (C21). At the end of study, we evaluated BP, urinary albumin to creatinine ratio (UACR), renal interstitial fluid (RIF) levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), nitric oxide (NO), cGMP, and 8-isoprostane, and renal expression of TNF-α, IL-6, and AT2R. There were no significant differences in BP between different treatments. DM rats demonstrated increased UACR, RIF TNF-α, IL-6 and 8-isoprostane, and mRNA for TNF-α and IL-6. DM rats also had reduced RIF NO and cGMP. C21 treatment of DM rats limited the increase in UACR, normalized RIF TNF-α, IL-6 and 8-isoprostane, and in mRNA for TNF-α and IL-6, and increased RIF NO and cGMP. In NC rats, C21 treatment did not change these parameters. AT2R mRNA and protein expressions increased in DM rats compared to NC but were not influenced by C21 treatment. We conclude that direct AT2R stimulation in diabetic rats improves diabetic albuminuria through the prevention of renal inflammation and improved production of NO and cGMP.
Keywords: AT2 receptor, C21, inflammation, diabetes, kidney, oxidative stress
INTRODUCTION
Diabetes is the most common cause of end-stage renal disease (ESRD), accounting for nearly half of new cases in the United States (1). Diabetes is associated with increased renal activity of the renin-angiotensin system (RAS), leading to enhanced angiotensin (Ang) II generation (2) and stimulation of the Ang II type 1 receptor (AT1R) (3). Accordingly, suppression of the RAS by angiotensin I converting enzyme inhibitors (4–6) or AT1R blockers (7–9) exert renoprotective effects, and have become a mainstay in the treatment of diabetic nephropathy. However, despite wide utilization of RAS inhibitors, the progression of diabetic kidney disease to ESRD remains common (1).
Several pathophysiologic mechanisms may contribute to the development of diabetic kidney disease including renal inflammation (10–11), reduction in vasodilatory factors, and increased oxidative stress (12). While most of the known Ang II effects in the diabetic kidney are mediated by AT1R, the role of the Ang II type 2 receptor (AT2R) in this disease still needs to be elucidated. The AT2R counteracts most of the AT1R effects by inhibiting cell growth, promoting cell differentiation, stimulating apoptosis and the renal production of nitric oxide (NO), cyclic guanosine 3’,5’-monophosphate (cGMP), and bradykinin (13–16). Recent reports from our laboratory and others demonstrated that in non-diabetic rodents, AT2R activation with its nonpeptide agonist Compound 21 (C21) (17) reduced inflammation and fibrosis, and improved renal production of NO-cGMP (18–20).
The current study was conducted to evaluate the effects of AT2R stimulation with C21 on renal inflammation, NO-cGMP generation, oxidative stress and albuminuria in diabetic rats.
METHODS
Animal preparation
The University of Virginia Animal Care and Use Committee approved all study protocols. Experiments were conducted in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 230 to 260g. Animals were given normal-sodium diet and tap water ad libitum for the whole experiment and a minimum of one week was allowed to adjust to our animal care facility. Rats were randomly allocated into four groups: control groups receiving vehicle (C+V; n = 10) or the AT2R agonist C21 (C+C21; n = 10), and diabetes groups receiving vehicle (DM+V; n = 10) or C21 (DM+C21; n = 10). Diabetes was induced by intraperitoneal injection of 65 mg/kg of streptozotocin (STZ; Sigma-Aldrich, Saint Louis, MO, USA). Control rats (C+V and DM+V) were injected with an equal volume of vehicle (0.9% NaCl). Treatments were initiated the day after STZ injection and lasted for a period of 4 weeks. Insulin was not given to any of the animals utilized in this study. C21 (Vicore, Uppsala, Sweden) was infused at a rate of 0.3 mg/kg/day via osmotic minipump (model 2004; Alzet, Cupertino, CA, USA). The control groups of rats were implanted with a sham osmotic minipump containing 0.9% NaCl. For minipump implantation, one day after STZ or vehicle injection, rats were anesthetized with a combination of ketamine (80 mg/kg; I.P.) and xylazine (8 mg/kg; I.P.). The osmotic minipumps were surgically implanted subcutaneously in the subscapular region of all rats.
Body weight, blood glucose, kidney mass index, 24-h urine measurements, and systolic blood pressure monitoring
Body weight, blood glucose, 24-h urine collections, and systolic blood pressure (SBP) were obtained at baseline and at the end of study. For blood glucose determination, blood was collected from a tail vein after overnight fasting, and glucose was measured using a glucometer (Bayer HealthCare, Mishawaka, IN, USA). For urine collections, rats were placed in individual metabolic cages for a period of 24-h. The volume of collected urine was determined gravimetrically and urine samples were kept at −80°C until assayed. Urinary albumin concentration was determined by using a sensitive rat albumin enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Harbor, MI, USA). Urine creatinine was determined by a creatinine assay kit (Cayman). Urinary albumin to creatinine ratio (UACR) was used as a marker for diabetic nephropathy. UACR was calculated and presented as albumin in milligrams divided by creatinine in grams. SBP was measured in non-anesthetized rats using a tail-cuff non-invasive multi channel blood pressure system (IITC Life Sciences, Woodland Hills, CA, USA). The mean values of the recorded SBP were calculated.
In vivo renal interstitial fluid (RIF) collections and kidney mass indexes
To determine the RIF levels of tumor necrosis factor (TNF)-α, Interleukin (IL)-6, NO, cGMP, and the oxidative stress marker 8-isoprostane we utilized a microdialysis technique as previously described (13–14). In this technique, substances with a molecular mass > 40,000 Da cannot cross the dialysis membrane, which nevertheless allows the free passage of smaller molecules. At the end of the study, RIF collections were performed in each animal under sodium pentobarbital anesthesia (50 mg/kg I.P.; Sigma). A dialysis probe was placed in the renal cortex of both kidneys through a midline laparotomy. In brief, a 30-gauge needle was tunneled approximately 1–2 mm from the outer renal surface for about 0.5 cm before it exited by penetrating the capsule again. The tip of the needle was then inserted into one end of the dialysis probe, and the needle was pulled together with the dialysis tube until the dialysis fiber was situated into the renal cortex. To prevent dislodging, the dialysis probe was glued to the surface of the kidney using Vetbond (3M Animal Care Products, Saint Paul, MN, USA). Thereafter, the inflow tube of the dialysis probe was connected to a gas-tight syringe filled with saline. Perfusion was done at a rate of 3 µl/min using an infusion pump. After a 60-min period for stabilization following completion of surgical procedures, the effluent was collected from the outflow tube in non-heparinized plastic tubes over ice through five periods of 60-min each with an amount of around 180 µl in each sample. At the end of each experiment, animals were euthanized and kidneys were harvested and weighed. Total kidney mass index was calculated as kidney weights in milligrams divided by body weight in grams. Kidney tissues were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
RIF storage and assays
The RIF collections were immediately stored at −80°C until assayed. RIF TNF-α and IL-6 were measured using an EIA kit (R&D Systems, Minneapolis, MN, USA). RIF NO recovery levels were measured using a nitrate/nitrite fluorometric assay kit (Cayman) and presented as µmol/min. RIF cGMP recovery levels were measured using a cyclic GMP EIA kit (Cayman). RIF 8-isoprostane recovery levels were measured using a 8-isoprostane EIA kit (Cayman). Both RIF cGMP and RIF 8-isoprostane are presented as fmol/min.
Determination of mRNA expression
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was used to determine changes in renal expression of TNF-α, IL-6, and AT2R mRNAs. RNA (n = 5, each group) was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). Reverse transcription of the RNA was performed by the first strand cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The PCR was analyzed using SYBR Green Supermix (Bio-Rad). Primer sequences were: TNF-α forward, 5’-ACTCCCAGAAAAGCAAGCAA-3’, reverse, 5’-CGAGCAGGAATGAGAAGAGG-3’; IL-6 forward, 5’-CCGGAGAGGAGACTTCACAG-3’, reverse, 5’-ACAGTGCATCATCGCTGTTC-3’; and AT2R forward, 5’-TTCTTGGGAGCAAACAGACC-3’, reverse, 5’-CTGGAACTGTGCCCAGAAAT-3’. RT-PCR was performed using iCycler (Bio-Rad) and threshold cycle number was determined using iCycler software version 3.0 (Bio-Rad). Reactions were performed in triplicate, and threshold cycle numbers were averaged. The mRNA results for specific target genes were calculated with normalization to 18S rRNA.
Western blot analysis
Preparations of kidney tissue lysate and protein quantitation were performed as previously described (21). Antibody to AT2R (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used in the Western blot. Signal detection was carried out by using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). The blots were treated using Restore Western Blot Stripping Buffer (Thermo Fisher) according to the manufacturer's recommendation and followed by reprobing with a monoclonal antibody against β-actin (Sigma). Densitometry evaluation of the bands was done using ImageMaster TotalLab software version 2.0 (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The bands densities of target proteins were normalized to the corresponding density of β-actin. The arbitrary unit of band densities was represented as the expression level.
Statistical analysis
Comparisons among different treatment groups were assessed by ANOVA followed by a Tukey test for post-hoc comparisons. Data are expressed as mean ± SE. P < 0.05 is considered statistically significant.
RESULTS
Body weight, blood glucose, total kidney mass index, urine output, and SBP data of all rats at the end of study are presented in Table 1. At baseline there were no significant differences in these parameters among groups. At four weeks, body weight was significantly reduced and the total kidney mass index was increased in diabetic rats. Fasting blood glucose of all diabetic rats at baseline was 71 ± 3 mg/dl and increased to 486 ± 19 mg/dl (P < 0.001) four weeks after STZ injection. There were no significant changes in any of these parameters in response to C21 treatment in both control and diabetic groups. There were no significant differences in SBP levels between groups of normoglycemic controls and STZ-induced diabetes rats.
TABLE 1.
Body weight, blood glucose, total kidney mass index, urinary volume, and systolic blood pressure (SBP) of control + vehicle, control + compound 21 (C21), diabetes + vehicle, and diabetes + C21 rats at the end of study.
| Control |
Diabetes |
|||
|---|---|---|---|---|
| Vehicle | C21 | Vehicle | C21 | |
| Body Weight (g) | 411±14 | 389±10 | 291±10*‡ | 288±10*‡ |
| Blood Glucose (mg/dl) | 83±5 | 78±2 | 473±27*‡ | 499±26*‡ |
| Total Kidney Mass Index (mg/g) | 3.18±0.05 | 3.28±0.06 | 5.51±0.13*‡ | 5.27±0.10*‡ |
| Urinary Volume (ml) | 31±4 | 40±4 | 217±12*‡ | 215±7*‡ |
| SBP (mmHg) | 115±3 | 120±2 | 119±2 | 120±3 |
Values are mean ± SEM.
P < 0.05 vs. control+vehicle;
P < 0.05 vs. control+C21.
Renal expressions of TNF-α and IL-6 mRNA
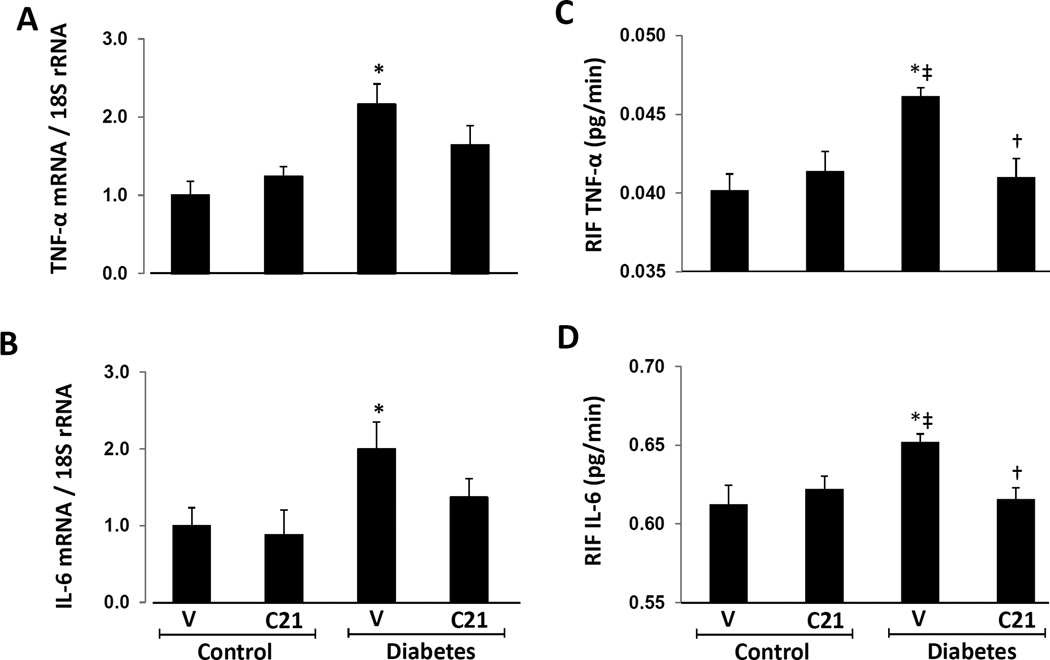
Renal TNF-α (Figure 1A) and IL-6 (Figure 1B) mRNA significantly increased in vehicle-treated DM rats compared to normoglycemic rats. The comparison between both groups of DM rats showed no statistical difference in the renal expression of TNF-α and IL-6 mRNA, although a trend towards reduced expression of these inflammatory markers was observed in C21-treated DM rats. There were no significant differences in renal expression of TNF-α and IL-6 mRNA between the vehicle- and C21 treated normoglycemic groups of rats.
Figure 1.
Renal mRNA expression and RIF levels of transforming growth factor-alpha (TNF--α) and interleukine-6 (IL-6) in kidneys of normoglycemic controls and STZ-induced diabetes rats treated with vehicle (V) or compound 21 (C21). A, mRNA levels of TNF-α. B, mRNA levels of IL-6. C, RIF levels of TNF-α. D, RIF levels of IL-6. Data are mean ± SEM. *P < 0.05 vs. control+V; ‡P < 0.05 vs. control+C21; †P < 0.05 vs. DM+V.
RIF TNF-α and IL-6 in normal and diabetic rats
RIF TNF-α (Figure 1C) and RIF IL-6 (Figure 1D) were significantly increased in DM rats receiving vehicle (P < 0.01) compared to normoglycemic control animals treated with vehicle or C21. Compared to vehicle treated DM rats, RIF TNF-α and RIF IL-6 were significantly reduced in DM rats receiving C21 (P < 0.01). There were no differences in RIF TNF-α and RIF IL-6 between both groups of normoglycemic control rats receiving vehicle or C21.
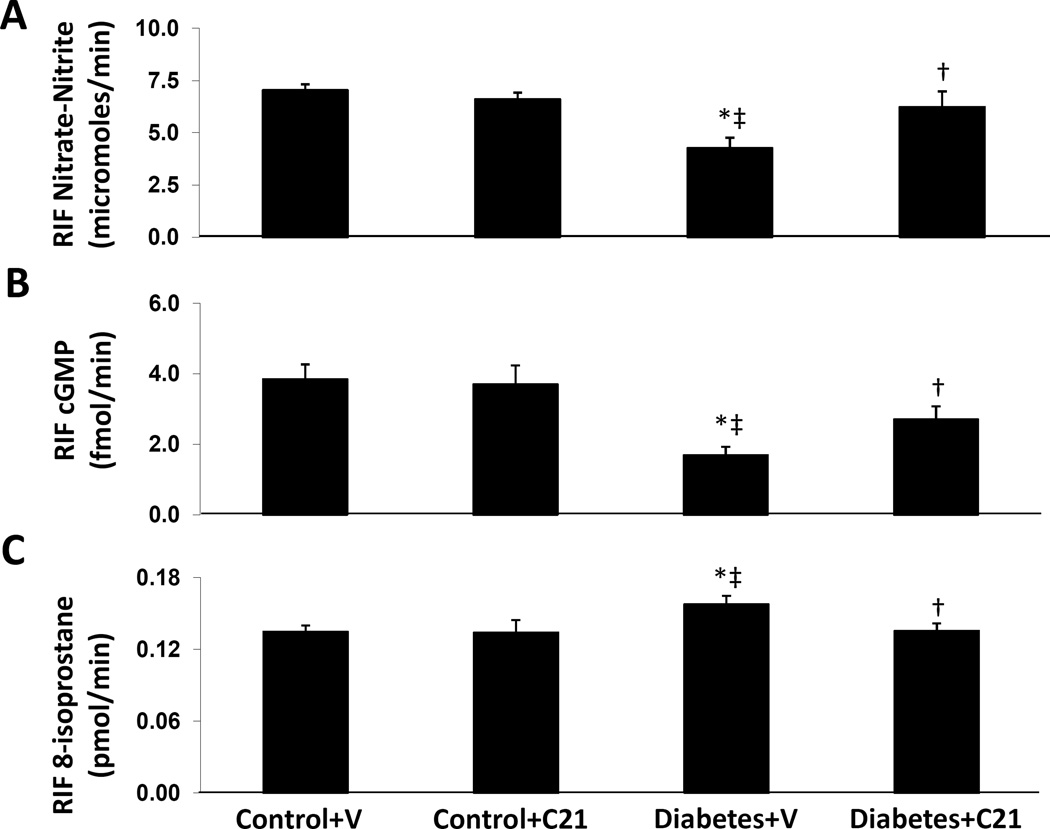
RIF NO, cGMP, and 8-isoprostane in normal and DM rats
RIF NO (Figure 2A) and RIF cGMP (Figure 2B) levels were significantly reduced in vehicle treated DM rats compared to normoglycemic controls receiving vehicle (−39% and −56% respectively, P < 0.01) or C21 (−36% and −54%, P < 0.01). C21 treatment significantly increased RIF NO and RIF cGMP levels in diabetic rats by 46% and 60%, respectively (P < 0.04). Conversely, compared to normoglycemic controls receiving vehicle, RIF 8-isoprostane levels (Figure 2C) increased by 17% (P < 0.02) in vehicle treated DM rats. Compared with this group of animals, C21 treatment significantly reduced RIF 8-isoprostane levels by 14% in diabetic rats (P < 0.03). However, C21 treatment did not cause significant changes in levels of RIF NO, RIF cGMP, and RIF 8-isoprostane in the control group.
Figure 2.
Renal interstitial fluid (RIF) levels of nitric oxide (NO, A) , cGMP (B), and 8-isoprostane (C) in kidneys of normoglycemic control rats treated with vehicle (V) or compound 21 (C21), and STZ-induced diabetes rats treated with V and C21. Data are mean ± SEM. *P < 0.05 vs. control+V; ‡P < 0.05 vs. control+C21; †P < 0.05 vs. DM+V.
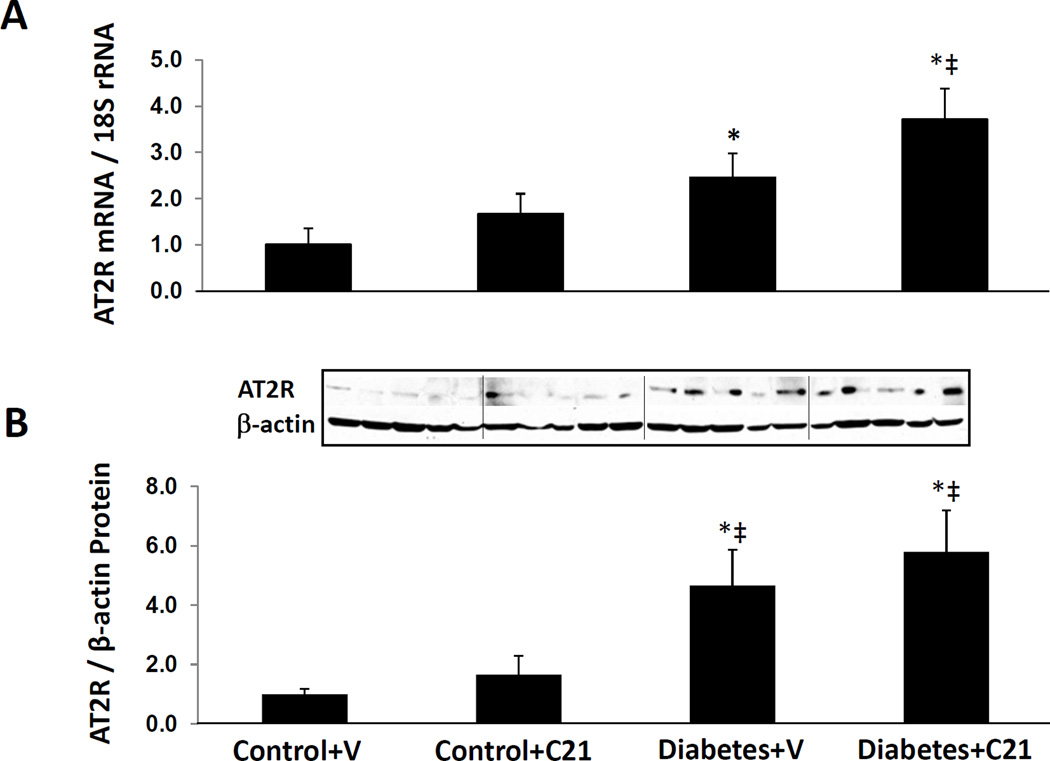
AT2R expression in normal and diabetic rat kidneys
Figure 3 shows that compared to vehicle treated normoglycemic control rats, renal AT2R mRNA and protein expressions were significantly increased in both groups of diabetic rats (P < 0.04). Compared to C21 treated normoglycemic controls, renal AT2R mRNA increased significantly in C21 treated DM rats (P < 0.05) but the comparison with the vehicle treated DM rats was not significant, despite a trend to increased AT2R mRNA and protein expressions in C21 treated DM rats.
Figure 3.
Renal mRNA and protein expressions of angiotensin II type 2 receptor (AT2R) in kidneys of normoglycemic control rats treated with vehicle (V) or compound 21 (C21), and STZ-induced diabetes rats treated with V or C21. A, mRNA levels of AT2R. B, Western blot analyzes of AT2R. Top, Representative blots. Bottom, Quantitative results normalized to β-actin. Data are mean ± SEM. *P < 0.05 vs. control+V; ‡P < 0.05 vs. control+C21; †P < 0.05 vs. DM+V.
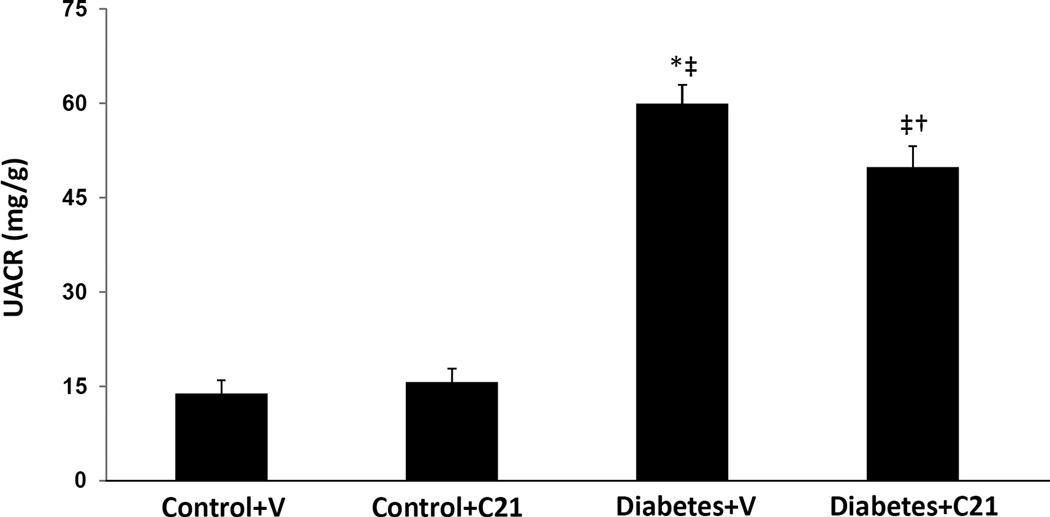
UACR in normal and diabetic rats
Compared to normoglycemic control rats receiving vehicle or C21, UACR was significantly increased in DM rats receiving vehicle (13.9 ± 2.1 and 15.7 ± 2.1 respectively vs. 60.0 ± 3.0; P < 0.001) (Figure 4). C21 treatment reduced UACR in DM rats by about 17% to 49.9 ± 3.3 mg/g (P < 0.04) but did not affect UACR in normoglycemic controls.
Figure 4.
Urinary albumin to creatinine ratio (UACR) of normoglycemic control rats treated with vehicle (V) or compound 21 (C21), and STZ-induced diabetes rats treated with V or C21. Data are mean ± SEM. *P < 0.001 vs. control+V; ‡P < 0.001 vs. control+C21; †P < 0.04 vs. DM+V.
DISCUSSION
The present investigation was designed to evaluate the effects of AT2R stimulation on renal inflammation, production of NO-cGMP and 8-isoprostane, and albuminuria in the development of diabetic nephropathy. Our results demonstrated in diabetic rats that direct stimulation of AT2R by the nonpeptide agonist C21 reduced renal inflammatory markers TNF-α and IL-6 and the oxidative stress marker 8-isoprostane; and increased renal NO-cGMP production. These changes were accompanied by reduction of albuminuria. All these effects were independent of changes in blood pressure.
Renal RAS activity is increased in diabetes (2–3). Both clinically and experimentally, it is well demonstrated that renal injury in diabetes can be slowed by the blockade of RAS with angiotensin converting enzyme inhibitors or angiotensin receptor antagonists (4–9). Although these pharmacological tools have been widely used for several years, diabetes is still the leading cause of ESRD (1). Thus the finding of new therapies that could lead to further improvement of diabetic nephropathy is of great interest. Recently, the renal and cardiovascular benefits of AT2R stimulation by C21 were reported in several different disease models (18–20). Overall, these studies have consistently demonstrated that direct stimulation of AT2R reduces inflammation and fibrosis in the kidneys and heart. The selectivity of C21 to AT2R was well demonstrated in these in vivo studies through the effective blockade of its actions by the AT2R antagonist PD123319 (18–20). In addition, studies in vitro also demonstrated a high selectivity of this compound to AT2R (17, 22). However, the benefits of AT2R stimulation in diabetic kidney disease still need to be elucidated.
In diabetes, increased formation of Ang II enhances oxidative stress and the release of renal inflammatory cytokines (23–24) contributing to the development and progression of diabetic nephropathy (10). These changes can be improved by RAS inhibition (25–29). Our current results demonstrated a reduction in renal inflammation and improvement of oxidative stress and albuminuria in diabetic rats treated with the AT2R agonist C21. Interestingly, in C21 treated diabetic animals, there were significant reductions in renal TNF-α and IL-6 expressions at the protein but not at the mRNA levels, although a trend towards reduced expression of the mRNA levels of these inflammatory markers was noted. This observation could be due to the limited number of animals used in this study. In addition, the finding that blood pressure was not affected by treatment with the AT2R agonist C21 is consistent with most previous studies exploring the effects of this compound on the cardiovascular system (18–20).
AT2R stimulation did not affect albuminuria in normal control animals but attenuated it in the diabetic rats. This beneficial response was noted after a short 4 weeks period of drug administration that was initiated during the onset and development of diabetes. This short duration study was not associated with significant renal histopathological changes (data not shown). Future long duration experiments should be considered to evaluate the effects of C21 treatment on established diabetic kidney disease. It is highly likely that the anti-inflammatory and anti-oxidant effects of AT2R stimulation contributed to amelioration of albuminuria in diabetic animals. In addition, increased formation of NO-cGMP due AT2R stimulation was previously demonstrated to induce vasodilation in human resistance vessels (30–31) and to enhance renal blood flow in rats (32). These hemodynamic changes may have played a role in attenuating renal injury in the diabetic rats. Although the current study was not designed to explore signaling mechanisms associated with AT2R activation, a previous study using human primary dermal fibroblasts demonstrated that C21 reduced TNF-α and IL-6 production by inhibition of the pro-inflammatory transcription factor nuclear factor-kappa B (33). Our study results are in agreement with these findings.
Our current data also demonstrated that AT2R expression was increased in diabetic kidney, which is consistent with previous reports (34–35). However, other studies failed to show similar findings (36–37). It is possible that these discrepancies are related to variations in duration of diabetes, differential expression among diverse renal cells, and the use of different animal models (35–36). We also demonstrated that AT2R stimulation did not cause significant changes in renal AT2R expression in both non-diabetic and diabetic rats. In contrast to these findings, we previously reported in non-diabetic hypertensive rats that AT2R stimulation further enhanced AT2R expression (20), which may suggest that AT2R stimulation may have a positive feedback on its gene activity in non-diabetic state. Despite the increased renal AT2R expression observed in our diabetic animals, its function is reduced as suggested by the observed reductions in NO and cGMP production. A possible explanation could be related to decrease in its stimulating agonists. This possibility is supported by the observed improvements in AT2R function with the administration of the C21. The exact mechanisms underlying this reduction in AT2R activity in diabetes need to be elucidated.
CONCLUSIONS
We conclude that direct stimulation of AT2R improves albuminuria in diabetic nephropathy through the reduction of renal inflammation, stimulation of the renal NO and cGMP production, and improvement of oxidative stress. These effects are independent of blood pressure changes. Our findings suggest a protective role for AT2R, and that its stimulation is a potential therapeutic tool to prevent kidney injury in diabetes.
Acknowledgments
Sources of Funding: This study was supported by National Institutes of Health grants DK078757 and HL091535 to H.M. Siragy.
REFERENCES
- 1.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 2.Awad AS, Webb RL, Carey RM, Siragy HM. Increased renal production of angiotensin II and thromboxane B2 in conscious diabetic rats. Am J Hypertens. 2005;18:544–548. doi: 10.1016/j.amjhyper.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6:319–330. doi: 10.1038/nrneph.2010.58. [DOI] [PubMed] [Google Scholar]
- 4.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson S, Rennke HG, Garcia DL, Brenner BM. Short and long term effects of antihypertensive therapy in the diabetic rat. Kidney Int. 1989;36:526–536. doi: 10.1038/ki.1989.227. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 7.Teles F, Machado FG, Ventura BH, Malheiros DM, Fujihara CK, Silva LF, Zatz R. Regression of glomerular injury by losartan in experimental diabetic nephropathy. Kidney Int. 2009;75:72–79. doi: 10.1038/ki.2008.528. [DOI] [PubMed] [Google Scholar]
- 8.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 11.Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30:49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 13.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3’, 5’-monophosphate and AT1 receptor-mediated prostaglandin (PG) E2 production in conscious rats. J Clin Invest. 1996;97:1979–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1090–R1095. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey JJ, Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol Renal Physiol. 1999;276:F39–F45. doi: 10.1152/ajprenal.1999.276.1.F39. [DOI] [PubMed] [Google Scholar]
- 17.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 18.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlöf B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 19.Gelosa P, Pignieri A, Fändriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 20.Matavelli LC, Huang J, Siragy HM. Angiotensin II type 2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–5565. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdonk K, Durik M, Abd-Alla N, Batenburg WW, van den Bogaerdt AJ, van Veghel R, Roks AJ, Danser AH, van Esch JH. Compound 21 induces vasorelaxation via an endothelium- and angiotensin II type 2 receptor-independent mechanism. Hypertension. 2012;60:722–729. doi: 10.1161/HYPERTENSIONAHA.112.196022. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Runge MS, Braiser AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κB transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 24.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 25.Awad AS, Webb RL, Carey RM, Siragy HM. Renal nitric oxide production is decreased in diabetic rats and improved by AT1 receptor blockade. J Hypertens. 2004;22:1571–1577. doi: 10.1097/01.hjh.0000133718.86451.6a. [DOI] [PubMed] [Google Scholar]
- 26.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matavelli LC, Huang J, Siragy HM. Combined aliskiren and amlodipine reduce albuminuria via reduction in renal inflammation in diabetic rats. J Cardiovasc Pharmacol. 2012;59:281–287. doi: 10.1097/FJC.0b013e31823fc3f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matavelli LC, Huang J, Siragy HM. Reduction of aldosterone production improves renal fibrosis in diabetic rats. J Cardiovasc Pharmacol. 2013;61:17–22. doi: 10.1097/FJC.0b013e318274d2ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 31.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- 32.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59:409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 33.Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappa B. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 34.Hakam AC, Hussain T. Renal AT2 receptors are upregulated and mediate the candesartan induced natriuresis/diuresis in obese zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 35.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in STZ-induced diabetes rats. Am J Physiol Renal Physiol. 2006;290:F503–F508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- 36.Wehbi GJ, Zimpelmann J, Carey RM, Levine DZ, Burns KD. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol. 2001;280:F254–F265. doi: 10.1152/ajprenal.2001.280.2.F254. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet F, Candido R, Carey RM, Casley D, Russo LM, Osicka TM, Cooper ME, Cao Z. Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens. 2002;20:1615–1624. doi: 10.1097/00004872-200208000-00025. [DOI] [PubMed] [Google Scholar]