Abstract
The immune-modulating effects of radiation therapy have gained considerable interest recently and there have been multiple reports of synergy between radiation and immunotherapy. However, additional pre-clinical studies are needed to demonstrate the antigen-specific nature of radiation-induced immune responses and elucidate potential mechanisms of synergy with immunotherapy. Here we demonstrate the ability of stereotactic radiotherapy to induce endogenous antigen-specific immune responses when combined with anti-PD-1 checkpoint blockade immunotherapy. Using the small animal radiation research platform (SARRP), image-guided stereotactic radiotherapy delivered to B16-OVA melanoma or 4T1-HA breast carcinoma tumors resulted in the development of antigen-specific T and B cell-mediated immune responses. These immune-stimulating effects of radiotherapy were significantly increased when combined with either anti-PD-1 therapy or regulatory T cell (Treg) depletion, resulting in improved local tumor control. Phenotypic analyses of antigen-specific CD8 T cells revealed that radiotherapy increased the percentage of antigen-experienced T cells and effector memory T cells. Mechanistically we found that radiotherapy up-regulates tumor-associated antigen-MHC complexes, enhances antigen cross-presentation in the draining lymph node, and increased T-cell infiltration into tumors. These findings demonstrate the ability of radiotherapy to prime an endogenous antigen-specific immune response and provide additional mechanistic rationale for combining radiation with PD-1 blockade in the clinic.
Keywords: Radiation, Radiotherapy, T Cell, CD8, immunotherapy, PD-1, cross-presentation, APC, lymphocyte
Introduction
Ionizing radiation is a locally directed therapy that induces lethal chromosomal aberrations and activates the DNA damage response pathways, including ATM and p53, resulting in cell-cycle arrest and apoptosis or mitotic catastrophe [1, 2]. However, radiotherapy also activates other signal transduction pathways and transcription factors including protein kinase C (PKC) and mitogen-activated protein kinases (MAPK) [3, 4], as well as nuclear factor-KappaB (NFκB) [5], which is a master regulator of immune responses. Activation of these signaling pathways and transcription factors can result in significant changes to the phenotype of cancer cells prior to cell death. Supporting this is a growing body of literature demonstrating how radiotherapy can change the immunophenotype of cancer cells and alter how the immune system interacts with cancer cells [6-12]. For example, in a study of 23 human carcinoma cell lines treated in vitro with radiation, 91% of the cell lines up-regulated one or more of the surface molecules including Fas, intercellular adhesion molecule-1 (ICAM-1), mucin-1, carcinoembryonic antigen (CEA), and/or major histocompatibility (MHC) class I [7]. Furthermore, the irradiated CEA/A2 colon tumor cells were more susceptible to killing by CEA-specific CD8 cytotoxic T lymphocytes (CTL) as compared with non-irradiated tumor cells [7]. Similar direct effects of radiation on the immunophenotype of tumor cells and responding immune cells have been corroborated by several groups [8-12].
There is evidence supporting the hypothesis that the immune system itself may play a critical role in the therapeutic efficacy of radiotherapy [13-17]. Early data showed that the radiation dose required to control a fibrosarcoma tumor in 50% of mice (TCD50) was significantly increased in immunocompromised mice as compared to control mice [13]. Conversely, when the immune system was activated with bacterial pathogens the radiation dose required to control the tumor was significantly reduced [13]. More recent data show that CD8 T cells play a key role in the antitumor effect of standard radiotherapy applied to B16 melanoma tumors. Specifically, depleting CD8 T cells reduced the antitumor effect of radiotherapy and decreased survival of mice with melanoma tumors [14, 15]. These findings run counter to the conventional paradigm that radiotherapy induces tumor cell kill primarily through DNA damage alone and instead suggest that the immune system may play an underappreciated role in the therapeutic effects of radiotherapy.
Immunotherapy has recently gained mainstream recognition as a viable anti-cancer therapy [18, 19]. Much of the excitement about immunotherapy revolves around checkpoint blockade using antibodies blocking the negative regulatory molecules cytotoxic T-lymphocyte antigen-4 (CTLA-4) and/or programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PDL-1) [20, 21]. These blocking antibodies have shown activity in multiple different tumor types, and when combined have shown synergistic effects in metastatic melanoma [22-24]. Given that immunotherapy is now a likely fourth pillar in the armamentarium against cancer, additional efforts are required to understand how immunotherapy can be best incorporated with surgery, chemotherapy, and radiotherapy (XRT) [25]. Along these lines, radiotherapy may be uniquely suited to synergize with immunotherapy because it can be delivered precisely to the tumor and may enhance expression of targets for the immune system [8, 26-28]. Moreover, there are several clinical case reports providing evidence of synergy between combined radiotherapy and immune checkpoint blockade [29, 30].
A number of preclinical studies have combined XRT and immunotherapy with intriguing results, including effects outside of the radiation field - termed the abscopal effect. Initial pioneering work by Demaria, Formenti, and others combined radiotherapy with Flt3-L and documented an abscopal effect in contralateral shielded tumors that was immune-mediated [31, 32]. A subsequent study combined radiotherapy with anti-CTLA-4 antibody in TSA breast carcinoma and MC38 colorectal carcinoma and reported abscopal effects which correlated with the frequency of IFNγ+ CD8 T cells [33]. Our group previously used the Small Animal Radiation Research Platform (SARRP) [34] to combine XRT with a cell-based vaccine in an autochthonous model of prostate cancer, and showed an additive treatment effect [35]. Additionally, we were the first to use the SARRP to deliver stereotactic radiotherapy combined with anti-PD-1 antibody in a glioma model and reported long-term survival of mice receiving combination therapy [36]. Recently a report combining radiotherapy with anti-PD-L1, an antibody against the ligand of PD-1, demonstrated enhanced efficacy through a cytotoxic T cell-dependent mechanism with a synergistic reduction in tumor-infiltrating myeloid-derived suppressor cells (MDSC) [37]. Furthermore a recent study combining radiation with blockade of PD-L1 demonstrated improved local control, survival, and protection against tumor-rechallenge in colorectal and breast cancer mouse models [38]. While these pre-clinical studies have shown additive or synergistic effects on tumor control, the question of whether XRT-induced priming augments an endogenous antigen-specific immune response, specifically in combination with antibody-mediated blockade of the PD-1 receptor, requires further investigation.
Here we report that combining radiotherapy with anti-PD-1 antibody results in the development of endogenous antigen-specific antitumor immune responses in models of melanoma and breast cancer in conjunction with enhanced tumor control. Importantly, we identify that the immune responses induced by combined radiotherapy and checkpoint blockade are not limited to T cells and include development of specific B cell-mediated antitumor antibodies. Mechanistically we found that XRT is capable of increasing immune-cell tumor infiltrates and direct presentation of tumor antigens, although in vivo the increase in antigen recognition mediated by XRT likely requires cross-presentation.
Materials and Methods
Mouse strains and cell lines
C57BL/6, BALB/cJ, and MHC Class I knockout (KO) female 6-8 week old mice were purchased from The Jackson Laboratory (Bar Harbor, ME). OT1-Rag KO mice were bred in-house. Animal experiments were performed in specific pathogen-free facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. MC38-OVA cells were a kind gift from Dr. Mark Smyth (Melbourne, Australia) on April 2013. B16-OVA melanoma cells were obtained from the lab of Dr. Hyam Levitsky and cultured in RPMI complete media plus G418 selection. 4T1-HA breast carcinoma cells were obtained from Dr. Katharine Whartenby. B3Z T-cell Hybridoma was a kind gift from Dr. Nilabh Shastri (Berkeley, CA) on September 2013. All tumor cell lines were tested prior to in vivo use and found to be free of mycoplasma. In addition model antigen expression for OVA and HA was confirmed by flow cytometry and western blotting.
Flow cytometry, intracellular cytokine staining and cytokine analysis
Single-cell suspensions were prepared from spleens, inguinal lymph nodes, and tumors. Cells were stained with fluorescent-labeled antibodies (BioLegend, San Diego, CA; BD-Bioscience Pharmingen, San Diego, CA; or eBiosciences, San Diego, CA) and analyzed by either FACSCalibur or LSR II flow cytometer (BD, San Diego, CA). The following clones were used: CD4 (RM4-5), CD8 (5H1), CD11c (HL3), Cd11b (M170), CD25 (PC61.5), SIINFEKL/H-2Kb (25-D1.16), CD44 (IM7), CD62L (MEL-14), IFNγ (XMG1.2), TNF-α (MP6-XT22), FAS (2495), CD40 (3/23) and Foxp3 (FJK-16s). For Pentamer staining H-2Kb (SIINFEKL) or H-2Kd (IYSTVASSL) Pentamers were used followed by Pro5 R-PE Fluorotag (ProImmune, Oxford UK). For intracellular cytokine staining, cells were activated with OVA peptide-pulsed splenocytes or with PMA (100ng/ml) plus ionomycin (500ng/ml) for 4 hours in the presence of GolgiPlug™ (32), processed with a Cytofix/Cytoperm™ kit (32), and stained as indicated. Gates and quadrants were set based on isotype control staining.
B3Z Assay
B3Z cells were cultured in complete media and split 1:20 every 2 days. Semi-confluent B16-OVA cells were trypsinized and washed in complete media and irradiated using a Fixed Cs source Gamma Irradiator with 0Gy, 10Gy or 20Gy. B16-OVA cells were washed and then immediately seeded into 24-well plates at 1×10^5 cells/well and cultured in complete media+G418 for 36 hours. Wells were washed twice in serum-free media and 5×10^5 B3Z cells and/or whole splenocytes were then added to the wells and co-cultured for 16 hours. For control experiments OVA peptide-pulsed splenocytes alone were added to wells 4 hours prior to B3Z cells. Supernatant was aspirated and IL2 concentrations were measured by IL2 ELISA (eBioscience) according to manufacturer’s specifications.
Adoptive Transfer Experiments and CFSE Labeling
T cells from spleens and lymph nodes (LN) of OT1-Rag KO mice were washed twice in PBS and labeled in 5nM CFSE (25×10^6 cells/ml) for 8 min at 37deg C on a shaker followed by quenching with ice-cold media. Cells were washed twice in PBS and 3×10^6 cells were injected per mouse via retro-orbital injection. 3 days later mice were sacrificed and the spleen and lymph node cell suspensions were analyzed via flow cytometry.
Serum Antibody Analysis
Mouse serum was isolated by allowing whole blood collected via cardiac puncture to coagulate at XRT for 45 min. Samples were centrifuged at 10000 RPM for 10 min and serum supernatant was aspirated and analyzed via Anti-Ovalbumin IgG1 Mouse EIA Kit (Cayman Chemical, Ann Arbor, MI).
Tumor growth experiments and TIL preparation
B16-OVA melanoma and 4T1-HA breast carcinoma models were performed as previously described with some modifications (33, 34). Briefly, on day 0 mice were injected with 1.25 – 5.0×10^5 B16 cells intradermally (i.d.) in the back or 2.0 – 5.0×10^6 4T1-HA cells subcutaneously (s.c) in the right flank. Tumor diameter was measured every 2-3 days with an electronic caliper and reported as volume using the formula (m1*m2^2)/2. Mice were treated with 200ug anti-PD-1 antibody via IP injection every 3 days for a total of 3 injections per mouse. To isolate tumor-infiltrating lymphocytes (TIL), solid tumors were excised after 12-14 days, single-cell suspensions prepared by mechanical dissociation, followed by density gradient centrifugation on an 80%/40% Percoll (GE Healthcare, Piscataway, NJ) gradient.
SARRP Irradiation
For in vivo experiments, mice with established palpable tumors were treated using the Small Animal Radiation Research Platform (SARRP) previously described by Wong and colleagues [34]. The anesthesized mice underwent computed tomographic (CT) imaging on the SARRP for image-guided localization of the tumor and placement of isocenter prior to irradiation. The dose rate was 1.9 Gy/min.
Statistical Analysis
Statistical analysis was performed using Prism 5 (GraphPad, La Jolla, CA). Unpaired two-tailed t-tests were conducted and considered statistically significant at p-values≤0.05 (*), 0.01 (**) and 0.001 (***).
Results
Radiotherapy enhances presentation of tumor-associated antigens
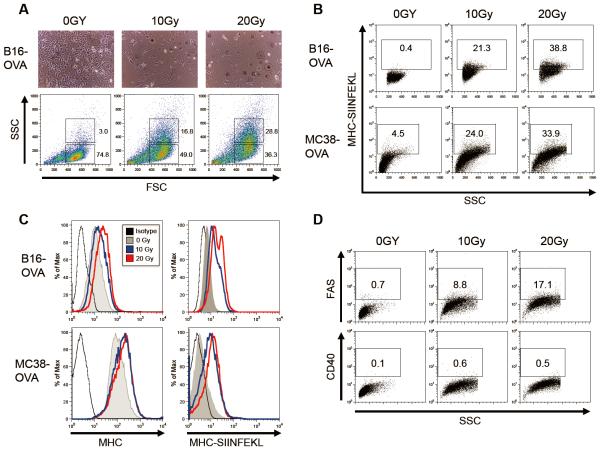
Tumor cell lines expressing model antigens such as ovalbumin (OVA) or hemagglutinin (HA) are useful models with which to evaluate the effects of radiotherapy (XRT) on the development of antigen-specific immune responses. To better understand how radiation therapy affects antigen presentation, we utilized B16 melanoma and MC38 colorectal carcinoma cell lines which were engineered to express OVA. The gross morphologic effects of XRT on these cell lines were as expected; light microscopy of cell cultures at 48 hours post XRT showed increased cell size, an increased nuclear to cytoplasmic ratio, and prominent nucleoli (Figure 1A, upper panels). These effects were mirrored in the increased FSC and SSC of the viable cell population as assayed by flow cytometry (Figure 1A, lower panels) To test whether XRT could result in increased direct (i.e. tumor-cell intrinsic) antigen-presentation, we used a commercially available antibody that binds specifically to the immunodominant OVA peptide SIINFEKL complexed within the MHC Class I molecule Kb [39-41]. Using this reagent, we observed a significant increase in antigen presentation on irradiated cells (Figure 1B). Although B16-OVA had a lower baseline expression of MHC Class I as compared to MC38-OVA, both cell lines up-regulated MHC and OVA antigen presentation upon irradiation in a dose-dependent manner (Figure 1C). As a positive control for the pro-immunogenic effects of XRT, we confirmed that XRT up-regulated expression of FAS, as previously described [7] (Figure 1D). As a negative control, we evaluated CD40 expression and found no-significant up-regulation (Figure 1D), suggesting that XRT does not lead to a general, non-specific expression of cell surface antigens on these tumor cell lines.
Figure 1. Radiotherapy enhances MHC presentation of tumor-associated antigens in B16-OVA melanoma and MC38-OVA colorectal carcinoma.
(A, upper panel) Light microscopy images of irradiated B16-OVA melanoma cells cultured for 48 hrs. (A, upper panel) Flow cytometry forward scatter and side scatter plots of unstained, irradiated B16-OVA cultures after 48 hrs. (B) Flow plots and percentages of irradiated B16-OVA and MC38-OVA cell cultures at 48 hrs stained with anti-mouse SIINFEKL/H-2Kb antibody [eBio25-D1.16]. (C) Representative histograms of irradiated cells stained with isotype, H-2Kb, or SIINFEKL/H-2Kb anti-mouse antibodies. (D) Flow plots of irradiated cells stained with anti-mouse FAS or CD40 antibodies. Experiments repeated three times with similar results.
Radiation increases T-cell recognition of tumor cells in vitro
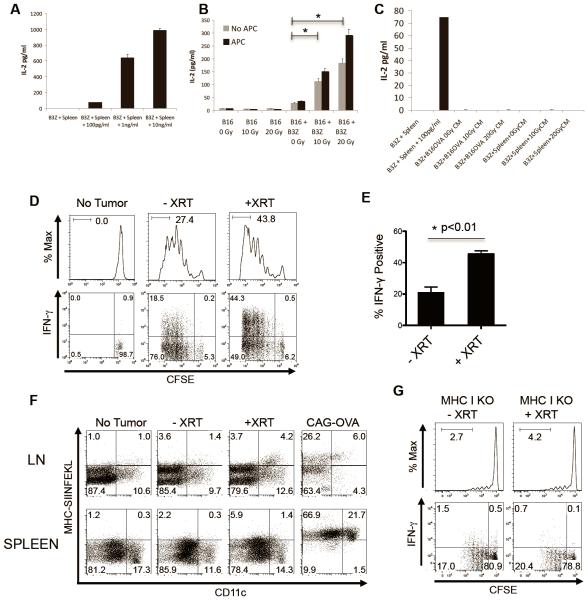
To study whether increased MHC-OVA expression secondary to XRT leads to specific T-cell recognition in vitro, we utilized the B3Z hybridoma [42, 43]. These immortalized T cells have a TCR specific for the OVA peptide SIINFEKL, and secrete IL2 in response to antigen recognition in a dose-dependent manner [42]. We first verified B3Z function by co-culturing these cells with peptide-pulsed, syngeneic splenocytes as antigen presenting cells (APC), and confirmed their dose-dependent IL2 secretion as assayed by ELISA (Figure 2A). Surprisingly, XRT of B16-OVA cells in the absence of APC resulted in increased IL2 secretion by co-cultured B3Z T cells, showing that XRT enhanced direct antigen presentation from tumor cells (Figure 2B). In addition to this direct presentation, it is also possible that XRT-induced cell death could result in the release of antigen, which could then be taken up by APCs and cross-presented. To investigate this possibility we irradiated B16-OVA cells, harvested the cell-free supernatant from those cultures 24 hours post-radiation, and pulsed APCs with the cell-free conditioned media. As show in Figure 2C, the addition of conditioned media from irradiated B16-OVA to APCs did not result in detectable IL2 secretion (i.e. activation) from B3Z. Along with the data from Figure 1, these data suggest that increased direct antigen presentation could be one of the effects of XRT on tumor cells.
Figure 2. Radiotherapy enhances cell-mediated tumor antigen presentation and results in increased proliferation and activation of antigen-specific T cells in the draining LN.
(A) IL2 ELISA concentrations in 16 hr supernatant of B3Z cells co-cultured with splenocytes (Spleen) with or without indicated concentrations of OVA peptides. (B) IL2 concentrations in supernatant of untreated (0Gy) or irradiated (10Gy or 20Gy) B16-OVA melanoma cells alone or co-cultured with B3Z cells or whole splenocytes as antigen-presenting cells (APC), experiment repeated three times with similar results. (C) IL2 concentrations in supernatant of B3Z cells with or without splenocytes (Spleen) cultured with indicated concentration of OVA peptides, or with cell-free conditioned media (CM) from untreated or irradiated B16-OVA cells. (D) Flow histograms and dot plots of ex vivo CFSE-labeled OT1 T cells harvested from DLNs, representative of three independent experiments (n=4 per group). (E) Change in IFNγ positivity in OT1 T cells from D, students t-test p<0.01. (F) Dot plots of ex vivo CD11b+CD11c+ dendritic cells isolated from non-tumor-bearing or B16-OVA tumor-bearing mice, or CAG-OVA mice constitutively expressing OVA driven via the beta-actin promoter, representative of three independent experiments. (G) Flow histograms and dot plots of ex vivo CFSE-labeled OT1 T cells harvested from untreated or irradiated MHC Class I KO mice bearing B16-OVA tumors, repeated twice with similar results.
Radiation increases T-cell recognition of tumor antigen in vivo by cross presentation
To further explore the effects of XRT on tumor antigen presentation, we used an in vivo model, in which congenically marked, CFSE-labeled OVA-specific CD8 T cells (OT-1) were adoptively transferred into animals bearing established B16-OVA tumors in their flanks. Using the SARRP [34], animals received 12Gy x 1 of image-guided stereotactic radiotherapy to their flank-tumors 1 day prior to adoptive transfer of CFSE-labeled OT-1, and their draining LNs were harvested on day 4. As shown in Figure 2D, radiotherapy resulted in significantly increased antigen-specific proliferation as measured by CFSE dilution. We tested whether this recognition was pro-inflammatory by performing intracellular staining for effector cytokines, and found increased expression of IFNγ and TNFα (Figures 2D, 2E, and Data not shown). Similar levels of specific T-cell activation were noted with a fractionated XRT scheme of 7Gy x 3 (Supplemental Figure 1). We next tested whether CD11c+ dendritic cells (DC) in the tumor draining lymph nodes (DLN) were presenting antigen by staining them with the SIINFEKL/Kb-specific antibody used in Figure 1. For these studies we used mice that express OVA ubiquitously (CAG-OVA) as a positive control. As shown in Figure 2F, CD11c+ DCs in the tumor DLNs indeed expressed the specific MHC/OVA complex, suggesting that in addition to direct presentation, in vivo XRT might also augment tumor antigen recognition via cross-presentation. To evaluate the relative importance of direct versus cross-presentation in XRT-mediated up-regulation of tumor antigen recognition, we repeated these studies using MHC Class I KO mice, in which only direct presentation would be possible. In these animals, we observed a reduction in the proliferation of adoptively transferred OT-1 T cells (Figure 2G). Furthermore, the absence of MHC Class I on host cells also abrogated the ability of radiation to enhance activation (i.e cytokine secretion) of tumor antigen-specific T cells (Figure 2G). Taken together these results demonstrate that locally directed radiotherapy can increase the activation and proliferation of an antigen-specific antitumor T-cell population in the DLN and that this effect likely involves cross presentation via MHC Class I-expressing professional APCs. It should be noted that although we did not directly measure the radiation dose to the DLNs in these experiments, in previous studies we used IHC for γH2AX to demonstrate that the SARRP is capable of precisely irradiating a target structure while sparing nearby normal tissues [35].
Priming of endogenous antigen-specific T and B cells by radiation therapy
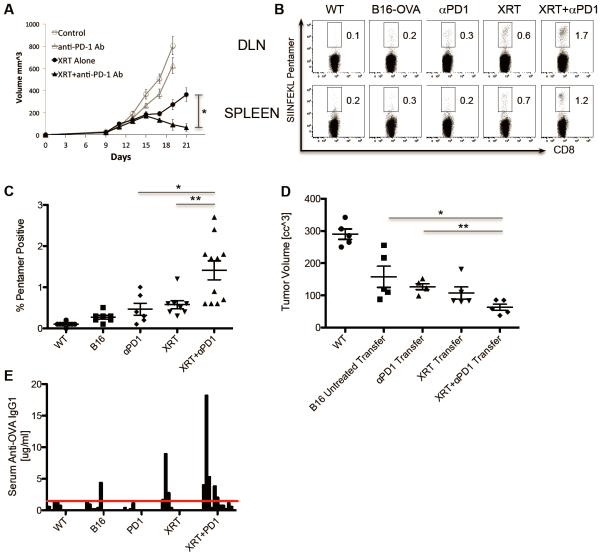
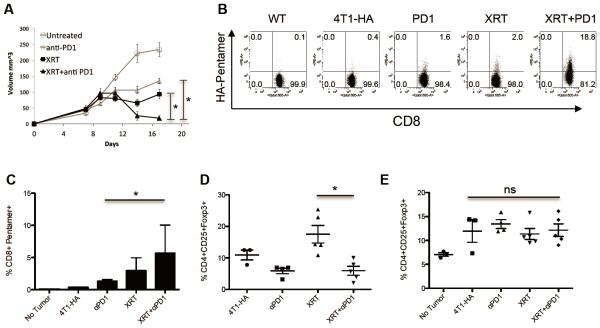
To test whether XRT could increase specific recognition of tumors by endogenous (as opposed to adoptively transferred) T cells, we performed antigen-specific pentamer staining in an in vivo treatment model. We first established such a model by treating implanted B16-OVA tumors with either XRT, anti-PD-1, or the combination. For these studies we chose to block the immune checkpoint PD-1, because of clinical activity, as well as its generally tolerable side effect profile. As previously demonstrated by our group [36] and others [33, 37], the combination of directed XRT + immune checkpoint blockade resulted in increased inhibition of tumor outgrowth (Figure 3A). Using SIINFEKL-MHC pentamer staining, we next tested whether the combined treatment increased antigen-recognition and drove the expansion of tumor antigen-specific T cells. As shown in Figure 3B, radiotherapy alone resulted in a moderate increase in OVA-specific T cells in the DLNs and spleens. However, when single fraction XRT was combined with PD-1 blockade we observed significant increases in OVA-specific T-cells (Figure 3B & 3C). Of interest, the increased prevalence of pentamer-positive T cells in the spleens supports the notion that local irradiation can result in the development of a systemic immune response outside of the radiation field. To assay the functionality of the endogenous immune cells induced by either single or combined treatment, we assayed the ability of these cells to prevent tumor outgrowth after adoptive transfer into naïve mice. To perform those studies, we adoptively transferred splenocytes from untreated or treated mice into WT naïve mice, and then challenged those animals with primary B16-OVA tumor cells and followed tumor outgrowth. As shown in Figure 3D, endogenous immune cells primed by XRT + anti-PD-1 were able to significantly delay tumor cell outgrowth in naïve hosts, supporting their functionality. Although previous groups have described the ability of XRT to induce or expand antibodies [44], the relative specificity of those antibodies for tumor cells is less clear. To address that issue, we assayed the ability of XRT + PD-1 blockade to induce OVA-specific antibodies in mice bearing OVA-expressing tumors. As shown in Figure 3E, although the ability of XRT +/− PD-1 blockade to induce IgG antibodies to OVA was quite variable, we observed an increased frequency and magnitude of IgG1 OVA-specific antibodies in the sera from animals in the combined treatment group. Taken together these data suggest that combined XRT + checkpoint blockade can induce tumor antigen-specific responses among endogenous immune cells.
Figure 3. Stereotactic radiotherapy combined with anti-PD1 immunotherapy significantly improves tumor control and enhances development of antigen-specific T cell- and B cell-mediated antitumor immune responses.
(A) Tumor volumes of mice (n=8 per group) inoculated with 3x10^5 B16-OVA tumor cells and irradiated (12 Gy x1) on day 12 and/or treated with IP injections of 200ug anti-PD1 Abs starting 1 day prior to irradiation and every 3 days for a total of three injections, experiment repeated three times with similar results. (B) Percentages of CD8+SIINFEKL Pentamer+ T cells isolated on day 10 after irradiation from WT (non-tumor-bearing) or B16-OVA tumor-bearing mice treated as indicated, representative of three independent experiments. (C) Scatter plot quantifying significant increase in SIINFEKL Pentamer+ T cells (* p< 0.05, ** p<0.01) in mice treated with XRT + anti-PD1 Abs. (D) Tumor volumes measured at day 18 in WT mice that received 12×10^6 IV adoptively transferred splenocytes from mice treated as indicated (harvested on day 14) followed 1 day later by tumor challenge with 2×10^5 B16-OVA cells. (E) OVA Ab ELISA measuring concentration of anti-OVA IgG1 present in sera of mice (n=8-11 mice per group) treated as indicated harvested on day 14 post-XRT.
Combining XRT with PD-1 blockade increases endogenous T-cell infiltration of established B16 tumors
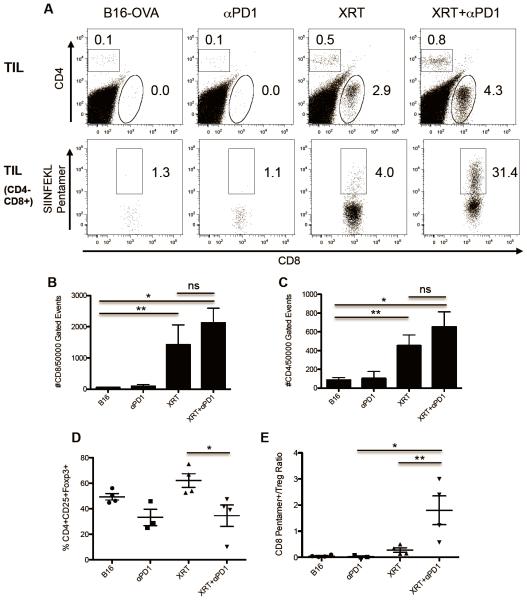
To assay the effects of increased antigen-recognition mediated by XRT + PD-1 blockade on the endogenous T-cell population, we tested whether the combined treatment increased tumor infiltration with either bulk or specific (OVA-pentamer positive) T cells. As shown in Figure 4A, untreated B16-OVA tumors or tumors treated with anti-PD-1 alone had scant immune-cell infiltrates and a notable absence of CD4 or CD8 lymphocytes. Remarkably, XRT alone drove a significant intratumoral infiltrate, composed of both endogenous CD4 and CD8 T cells (Figure 4A, upper panel). Adding anti-PD-1 to radiotherapy further enhanced the percentage of CD8 T cells within B16-OVA tumors, with a significant proportion of these cells being specific for OVA as determined by pentamer staining (Figure 4A, lower panel) In order to further quantify these changes, we calculated the absolute numbers of CD4 and CD8 T cells per 50,000 gated events. As shown in Figures 4B and 4C, XRT alone significantly increased CD4 and CD8 T-cell infiltration, but this was not further increased by the addition of PD-1 blockade. As the immunologic effects of effector cells within the tumor parenchyma likely represent a balance between regulatory (Treg) and effector T-cell function, we also quantified Treg infiltration, as well as the ratio of CD8 / Treg for each of the treatment groups. These data (Figures 4D and 4E) show that XRT alone significantly increases the percentage of CD4 TILs that are FoxP3+, but that this effect is significantly abrogated by the addition of PD-1 blockade to XRT. Indeed, the combination of XRT + PD-1 blockade results in an increase in the ratio of antigen-specific (OVA pentamer positive) / Treg ratio; this parameter in the tumor microenvironment appears to correlate with the treatment effects shown in Figure 4A. To further explore the role of Tregs in this model, we performed depletion studies using the anti-CD25 antibody PC-61. As shown in Supplemental Figure 2, PC-61 mediated Treg depletion added to XRT in tumor control, confirming the notion that Tregs likely attenuate the capacity of XRT to augment antitumor immune responses.
Figure 4. Single-dose stereotactic radiotherapy induces tumor-infiltrating lymphocytes and synergizes with anti-PD1 checkpoint blockade.
(A) Dot plots of CD4 vs CD8 (upper panel) and SIINFEKL Pentamer+ vs CD8 (lower panel) tumor-infiltrating lymphocytes (TIL) isolated on day 14 from tumor-bearing mice treated as indicated, representative of three independent experiments. (B and C) Quantification of significant increase in absolute number of CD8 and CD4 TILs in mice treated with radiotherapy and anti-PD1 Abs. (D) Changes in percentages of CD4+CD25+Foxp3+ T-regulatory cells (Treg) in TILs from treated groups. (E) Significant increase in the ratio of CD8+Pentamer+ Effector T cells to CD4+CD25+Foxp3+ Tregs in TILs of mice treated with radiotherapy plus anti-PD1 Abs (* p <0.01, ** p<0.05).
Combining XRT with PD-1 blockade increases endogenous T-cell infiltration of established 4T1 tumors
To investigate whether the ability of combined XRT + PD-1 blockade to enhance an endogenous antigen-specific immune response was confined to the poorly immunogenic B16 cell line, we used an engineered version of the 4T1 breast carcinoma line which expresses hemagglutinin (HA) as a model antigen. Similar to B16-OVA, established 4T1-HA tumors treated with anti-PD-1 or XRT alone showed a significant growth delay (Figure 5A). However, when radiotherapy was combined with anti-PD-1 we observed significant regression of tumors and tumor control (Figure 5A). We analyzed the development of antigen-specific CD8 T-cell responses using HA peptide-MHC pentamers and found that either anti-PD-1 or radiation alone resulted in increased HA-specific CD8 T cells (Figures 5B & 5C). However, when XRT was combined with anti-PD-1, there was a statistically significant increase in HA-specific CD8 T cells compared to that treated with anti-PD-1 alone. Interestingly, and as was the case for B16-OVA, we found that XRT increased the proportion of intratumoral CD4 T cells with a Treg phenotype, and this increase was abrogated by the combined treatment (Figure 5D). This XRT-mediated increase in Treg percentages appeared to be a localized phenomenon; we did not observe any increase in T-regulatory cells in the draining lymph nodes (Figure 5E) or in the spleens (data not shown) in either the 4T1-HA or B16-OVA models.
Figure 5. Stereotactic radiotherapy combined with anti-PD1 immunotherapy significantly improves 4T1-HA tumor control and enhances development of antigen-specific T cells.
(A) Tumor volumes in mice (n=6 per group) inoculated with 1x10^6 4T1-HA cells irradiated on day 9 and/or treated with IP injections of 200ug anti-PD1 Abs starting 1 day prior to irradiation and every 3 days for a total of three injections, representative of two independent experiments. (B) Percentages of CD8+ HA Pentamer+ (H2-Kd IYSTVASSL) cells harvested at day 14 from the DLNs of WT or 4T1-HA tumor-bearing mice treated as indicated, representative of two independent experiments. (C) Quantification of increase in CD8+Pentamer+ cells with radiotherapy and anti-PD1 immunotherapy. (D) Changes in CD4+CD25+Foxp3+ T-regulatory cells in the TILs of 4T1-HA tumor-bearing mice treated as indicated. (E) No significant changes of CD4+CD25+Foxp3+ Tregs in the DLNs, representative of two independent experiments.
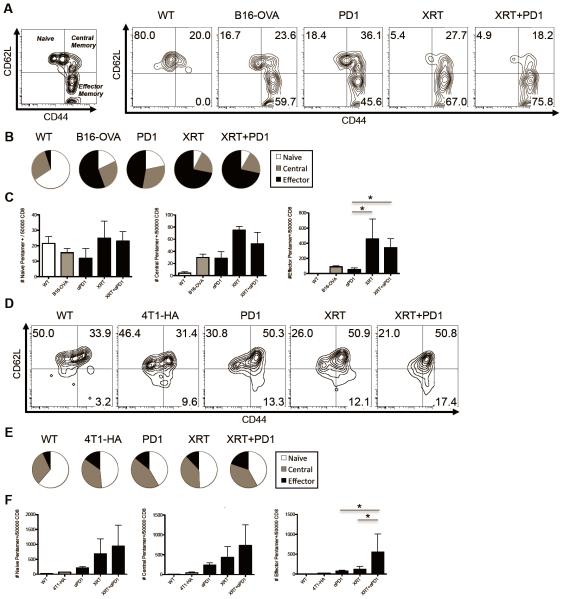
XRT + increases tumor-antigen specific central memory cells in the draining lymph node
Our ability to identify tumor antigen-specific endogenous CD8 T cells in two different models afforded us with the opportunity to quantify the effects of either single or combined treatment on the phenotype of induced / expanded CD8 T cells. For these studies, we defined naïve cells as CD62L+ and CD44− (Figure 6A). In a similar manner, the effector memory phenotype was defined as CD44+CD62L−, and central memory cells as CD44+CD62L+ [45]. As in previous experiments, we identified and gated on tumor antigen-specific CD8 T cells in the DLNs using pentamer staining (See Supplemental Figure 3 for gating scheme). In the poorly immunogenic B16-OVA model, we found that XRT decreased the relative percentage of naïve tumor-specific CD8 T cells and increased the proportion of tumor-specific CD8 T cells with an effector memory phenotype (Figure 6A & 6B), but that PD-1 blockade did not significantly add to this skewing. We also observed significant increases in the absolute numbers of tumor-specific effector memory CD8 T cells induced by XRT alone or XRT combined with anti-PD-1 immunotherapy (Figure 6C). The phenotype of tumor antigen-specific CD8 T cells was also altered by XRT in the 4T1-HA model (Figures 6D-F), but here XRT alone was less effective in increasing the absolute number of effector memory CD8 T cells compared to that of combine therapy (Figure 6F), possibly reflecting differences in the tumor microenvironment and immunogenicity of 4T1 compared to B16 cells. Taken together these two datasets support the concept that XRT combined with PD-1 blockade increases tumor-specific CD8 T cells with a memory phenotype.
Figure 6. Radiotherapy decreases the percentage of naïve antigen-specific T cells in the DLNs and increases antigen-specific effector memory T cells.
(A) Flow plots of putative memory markers on CD4−CD8+SIINFEKL Pentamer+ endogenous T cells (See Supp Figure for gating scheme) isolated from DLNs of WT or B16-OVA tumor-bearing mice on day 14 treated as indicated, representative of three independent experiments. (B) Pie charts showing relative percentages of memory populations in treated groups. (C) Quantification of absolute number of naïve, putative central and effector memory T-cells in DLNs of WT of B16-OVA treatment groups. (D,E,F) Same respective memory charts as above except isolating CD4−CD8+IYSTVASSL Pentamer+ T cells from 4T1-HA tumor-bearing mice, representative of two independent experiments.
Discussion
Tumor cell lines expressing model antigens are useful for studying induction of endogenous antigen-specific immune responses because of the numerous epitope-specific reagents available to detect responses to these model antigens. Here we utilized these reagents to test the ability of radiotherapy alone or in combination with immune checkpoint blockade to induce immune responses against OVA expressed by B16 melanoma and HA expressed by 4T1 breast carcinoma. It has been shown by multiple groups that radiotherapy increases the expression of MHC Class I in a dose-dependent manner in multiple different tumor types [7, 12]. This is a critically important finding because many tumor types, including melanoma, may down-regulate MHC expression in order to protect from CD8 T-cell mediated cytotoxicity. By up-regulating MHC, radiotherapy may prevent tumor cells from remaining undetected by CD8 T cells. Indeed we found that radiotherapy increased the expression of specific tumor model antigenic epitopes presented in MHC on the cell surface (Figure 1). The H2kb-SIINFEKL antibody stains for the direct targets of CD8 OT1 T cells or endogenously generated CD8 T cells specific for the SIINFEKL epitope. Thus, up-regulating the targets for CD8 T cells is one mechanism by which radiotherapy directly increases the susceptibility of tumor cells to CD8 T cell-mediated cytotoxicity. One potential limitation of model antigen systems like these is that the relative immunogenicity of exogenously introduced antigens like OVA or HA could render them more susceptible to immunologic intervention. For B16, this is unlikely; implanted B16 and B16-OVA have similar growth curves in vivo (data not shown), but it does remain a minor concern for 4T1-HA. The interpretation of these studies should also be tempered by observation that both B16 [46], and 4T1 [47] express PD-L1 in vivo, whereas expression of PD-L1 in human tumors is variable.
In addition to up regulation of MHC Class I, multiple other potential mechanisms exist by which radiotherapy can increase tumor-cell susceptibility. Up-regulation of FAS [7] could increase tumor-cell susceptibility to undergoing FAS-L-mediated apoptosis (Figure 1D). Interestingly, FAS-L-mediated induction of apoptosis could be triggered by tumor-specific immune cells as well as bystander immune cells which are not necessarily specific for tumor antigens. Taken together this would support the hypothesis generated by previous studies that the ability of radiotherapy to control certain tumors depends on the general immune status of the host [13], and more specifically on CD8 T cells [14, 15].
The B3Z T-cell hybridoma is an important cell line for probing the expression of OVA antigen presented by tumor cells or other engineered cell lines. The B3Z T-cell hybridoma is selected to secrete IL2 in a dose-dependent manner upon TCR ligation with MHC loaded with SIINFEKL epitope, and thus is similar in some respects to an immortalized OT-1 cell line. Here we adapted this cell line to quantify the level of OVA antigen presentation in tumor cells after irradiation. We confirmed that radiotherapy increases direct tumor-mediated cell-cell antigen presentation as measured by IL2 ELISA (Figure 2). This raised the question whether radiotherapy could potentially convert tumor cells into antigen presenting cells capable of priming immune responses. Using an in vitro assay co-culturing irradiated B16-OVA melanoma cells with naïve CFSE-labeled OT-1 T-cells we found no evidence of T-cell priming or proliferation (Data not shown). Furthermore using MHC Class I knockout mice in which only the injected B16-OVA tumor cells were capable of presenting antigen to adoptively transferred OT-1 T-cells, we confirmed that the ability of radiotherapy to enhance T-cell proliferation in the draining lymph node requires MHC Class I-expressing antigen presenting cells. This raises the intriguing question of whether stereotactic radiotherapy, which does not generally include the draining lymph nodes, may be superior to larger field or conventional radiotherapy in terms of inducing antitumor immune responses. One limitation of these results is that we did not specifically quantify the radiation dose to the DLNs, although in previous studies we were able to target the prostate gland without irradiating either the bladder or DLNs [35]. Studies in which the DLNs are specifically targeted or spared are ongoing in order to more precisely address this issue.
One potential downside of increased direct tumor cell-mediated antigen presentation is increased T-cell anergy or conversion of naïve to T-regulatory cells. Indeed we did observe that radiotherapy alone increased the relative percentage of T-regulatory cells in the tumor microenvironment but not in the draining lymph node (Figure 4 and Figure 5). Increased T-regulatory cells would be deleterious to antitumor immune responses, and thus we investigated strategies to block T-regulatory cells using PC61-depleting antibody. We observed increases in MHC pentamer-specific T-cell populations and B16-OVA tumor control when radiotherapy was combined with T-regulatory cell depletion (Supplementary Figure 2). It remains to be determined whether this increase in T-regulatory cells is a general phenomenon of radiotherapy for multiple tumor types. However, strategies combining radiotherapy with T-regulatory cell-depleting antibodies such as anti-GITR or low dose cyclophosphamide may be worthy of further clinical investigation.
The presence of immune-cell infiltrate has been correlated with improved patient outcomes in multiple different solid tumors including, melanoma, colorectal, breast, and prostate carcinoma [48-51]. In fact, the type and location of immune cells in human colorectal cancer was previously reported to be a better predictor of survival than traditional stage groupings [48]. Similarly, the absence of immune-cell infiltrate has been associated with worse patient outcomes, possibly as a sign of tumor immunosuppression or physical barriers to immune-cell migration and tumor infiltration. Thus strategies that enhance immune-cell engagement and infiltration into tumors could potentially result in significant clinical benefit. Here we demonstrated that intact, untreated B16 melanoma has a relatively scant immune-cell infiltrate consistent with the poorly immunogenic nature of this tumor line (Figure 4A). Radiotherapy of B16 melanoma significantly increased both CD4 and CD8 T-cell infiltrates (Figure 4). Interestingly, simply driving recruitment of T cells into a tumor is likely not sufficient to activate an antitumor immune response given the immunosuppressive tumor microenvironment. Indeed analysis of the CD4 T-cell population showed that the majority of these cells are CD25+FoxP3+ T-regulatory cells (Figure 4D and 5D). However, the combination of radiation and anti-PD-1 immunotherapy altered the radio of CD4 to CD8 T-cells and resulted in decreased percentages of CD4 T-regulatory cells and absolute increases in CD8 T-cell populations, likely as a result of diminished inhibitory signaling from the PD-1 pathway. These findings provide a clear rationale for combining radiotherapy with checkpoint blockade immunotherapy and further work is certainly warranted to determine whether these findings hold true in human tumors.
Here we chose to study radiation combined with anti-PD-1 checkpoint blockade primarily because a drug blocking the PD-1 pathway (Pembrolizumab, Merck) has recently been approved by the FDA and additional agents blocking the PD-1 pathway are in Phase III clinical trial development. Given that these agents are generally well tolerated with favorable adverse event profiles, strategies incorporating anti-PD-1 immunotherapy with current treatment modalities including chemotherapy, targeted therapies, and/or radiation therapy are of critical importance. To this end, there are data to suggest that radiation therapy may be uniquely suited to combine with checkpoint blockade immunotherapy and specifically PD-1 blockade. Recently there have been reports that radiation alone can result in increased expression of PD-1 ligands on the surface of tumor cells [37, 38], including melanoma and breast cancer model cell lines. Up-regulation of PD-1 ligands might serve to dampen effector immune responses and potentially counteract the positive immunogenic effects of radiation such as increased MHC expression, antigen presentation, and immune-cell infiltration. Thus concurrent blockade of the PD-1 pathway may synergize with radiation by specifically blocking a counterproductive effect of radiation on immune responses.
Taken together, these data have several clinical implications. First, these findings clearly support the co-administration of XRT with checkpoint blockade immunotherapy to improve local tumor control. The trend towards increased T-regulatory cells in the tumor after radiotherapy alone suggests that strategies combining T-regulatory cell depletion with radiotherapy may have additional clinical benefit. Furthermore, the increase in antigen-specific T cells and antitumor antibodies with concurrent XRT and anti-PD-1 immunotherapy suggest that the combination may also potentially aid in systemic or distant tumor control via the abscopal effect. Finally, the requirement of host antigen presenting cells and cross presentation for radiotherapy to enhance immune responses raises the intriguing hypothesis that stereotactic radiotherapy could be superior to conventional field radiotherapy by sparing irradiation of draining LN regions. These data provide a preclinical rationale for evaluating these questions in prospective clinical trials.
Supplementary Material
Acknowledgements
This work was supported in part by the American Society for Radiation Oncology (ASTRO) Resident/Fellow in Radiation Oncology Research Seed Grant # RA2013-1
Footnotes
Conflict of Interest Statement: The authors declare competing financial interests. Charles G. Drake has consulted for Amplimmune, Bristol Myers Squibb (BMS), Merck, and Roche-Genentech, all of whom have either anti-PD-1 or anti-PD-L1 reagents in various stages of clinical development. In addition, Dr. Drake has received sponsored research funding from BMS. The first author Dr. Andrew Sharabi has no conflicts of interest to declare.
References
- 1.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–9. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 2.Hall EJ, Giaccia A. 7th Lippincott Williams & Wilkins; Philadelphia: 2011. Radiobiology for the radiologist. [Google Scholar]
- 3.Hallahan DE, Virudachalam S, Sherman ML, Huberman E, Kufe DW, Weichselbaum RR. Tumor necrosis factor gene expression is mediated by protein kinase C following activation by ionizing radiation. Cancer Res. 1991;51:4565–9. [PubMed] [Google Scholar]
- 4.Hara T, Namba H, Yang TT, Nagayama Y, Fukata S, Kuma K, et al. Ionizing radiation activates c-Jun NH2-terminal kinase (JNK/SAPK) via a PKC-dependent pathway in human thyroid cells. Biochem Biophys Res Commun. 1998;244:41–4. doi: 10.1006/bbrc.1998.8210. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95:13012–7. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126. doi: 10.1155/2013/658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 8.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res. 2010;173:418–25. doi: 10.1667/RR1860.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morel A, Fernandez N, de La Coste A, Haddada H, Viguier M, Polla BS, et al. g-Ray irradiation induces B7.1 costimulatory molecule neoexpression in various murine tumor cells.pdf. Cancer Immunol Immunother. 1998;46:277–82. doi: 10.1007/s002620050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clin Immunol. 2013;148:44–55. doi: 10.1016/j.clim.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma.pdf. J Natl Cancer Inst. 1979;63:1229–35. [PubMed] [Google Scholar]
- 14.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 16.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 18.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 19.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–24. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardoll D, Drake CG. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–9. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–63. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–32. [PubMed] [Google Scholar]
- 33.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–9. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada S, Harris TJ, Tryggestad E, Yoshimura K, Zeng J, Yen HR, et al. Combined treatment effects of radiation and immunotherapy: studies in an autochthonous prostate cancer model. Int J Radiat Oncol Biol Phys. 2013;87:769–76. doi: 10.1016/j.ijrobp.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 39.Ackerman AL, Kyritsis C, Tampé R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–13. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 40.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 41.Messaoudi I, LeMaoult J, Nikolic-Zugic J. The mode of ligand recognition by two peptide:MHC class I-specific monoclonal antibodies. J Immunol. 1999;163:3286–94. [PubMed] [Google Scholar]
- 42.Sotomayor Cross presentation of tumor antigens by bone marrow derived antigen presenting cells is the dominant mechanism in the induction of Tcell tolerance.pdf. Blood. 2011 doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 43.Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992;89(13):6020–4. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeWeese TL, Laiho M, Drake CG. Current cancer research. xiv. Springer; New York: 2011. Molecular determinants of radiation response; p. 276. Vol. Chapter 12: Radiation-Induced Immune Modulation. [Google Scholar]
- 45.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 46.Pilon-Thomas S, Mackay A, Vohra N, Mulé JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–9. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 48.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 49.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 50.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 51.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.