Abstract
A growing body of evidence has indicated that dipeptidyl peptidase-4 (DPP-4) inhibitors have antihypertensive effects. Here, we aim to examine the effect of vildagliptin, a DPP-4-specific inhibitor, on blood pressure and its circadian-dipping pattern during the development of salt-dependent hypertension in Dahl salt-sensitive (DSS) rats. DSS rats were treated with a high-salt diet (8% NaCl) plus vehicle or vildagliptin (3 or 10 mg kg−1 twice daily by oral gavage) for 7 days. Blood pressure was measured by the telemetry system. High-salt diet for 7 days significantly increased the mean arterial pressure (MAP), systolic blood pressure (SBP) and were also associated with an extreme dipping pattern of blood pressure in DSS rats. Treatment with vildagliptin dose-dependently decreased plasma DPP-4 activity, increased plasma glucagon-like peptide 1 (GLP-1) levels and attenuated the development of salt-induced hypertension. Furthermore, vildagliptin significantly increased urine sodium excretion and normalized the dipping pattern of blood pressure. In contrast, intracerebroventricular infusion of vildagliptin (50, 500 or 2500 μg) did not alter MAP and heart rate in DSS rats. These data suggest that salt-dependent hypertension initially develops with an extreme blood pressure dipping pattern. The DPP-4 inhibitor, vildagliptin, may elicit beneficial antihypertensive effects, including the improvement of abnormal circadian blood pressure pattern, by enhancing urinary sodium excretion.
Keywords: blood pressure, Dahl salt-sensitive rats, dipeptidyl peptidase-4 (DPP-4) inhibitors, dipping pattern
INTRODUCTION
Blood pressure (BP) physiologically dips at night from day time levels (dipper pattern). Excessive lowering of BP at night is called an extreme dipper and the absence of this dipping pattern is referred to as non-dipper.1 In rodents, BP dips during the day time (sleeping period) compared with night time (working period).2 During the development of salt-dependent hypertension, there is changing of the dipping pattern, which is associated with a greater risk of cardiovascular complications.3-5 Recently, Kamezaki et al.6 also demonstrated the role of circannual rhythms in the prevalence of metabolic syndrome with insulin resistance. In patients with chronic kidney disease, the non-dipping BP pattern is predictive of heart failure, stroke and myocardial infarction.7 In addition, previous studies have shown that abnormal BP-dipping pattern is also associated with proteinuria.8
Dipeptidyl peptidase-4 (DPP-4) is a ubiquitous cell-membrane-associated enzyme responsible for cleaving and inactivating incretins, including glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide,9 which are released by cells in the small intestine after the ingestion of food and stimulate insulin secretion.10 Pharmacological inhibition of DPP-4 activity increases insulin secretion and improves glycemic control in diabetic animals11-13 and humans.14,15 In parallel with its antihyperglycemic actions, treatment with DPP-4 inhibitors has been reported to exert BP-lowering effects in both spontaneously hypertensive rats16,17 and hypertensive patients.18,19 However, the effects of DPP-4 inhibition on salt-sensitive hypertension and its alteration of the circadian-dipping pattern are yet to be investigated.
In the present study, we aimed to investigate whether vildagliptin, a DPP-4-specific inhibitor, has antihypertensive effects in salt-induced hypertension and improves the circadian BP-dipping pattern in Dahl salt-sensitive (DSS) rats. We have found that DSS rats initially developed salt-induced hypertension with an extreme BP dipper pattern. Furthermore, vildagliptin significantly attenuated high-salt (HS)-induced hypertension and improved the BP-dipping pattern in DSS rats through enhanced urinary sodium excretion.
MATERIALS AND METHODS
Experimental animals
Experiments were performed in 6-week-old male DSS rats (SLC, Shizuoka, Japan). Rats were maintained in a temperature-controlled (24 ± 2 °C) room with a 12-h light/dark cycle and 55 ± 5% humidity. Experimental protocols and animal care were performed according to the guidelines for the care and use of animals established by Kagawa University, Japan.
Implantation of the telemetry system
To measure BP in conscious animals, we used a telemetry technique as described previously.20 The rats were anesthetized with isoflurane and an abdominal incision was made for implantation of the radiotelemetry device. The system consists of a radiofrequency transmitter (TA11PA-C40) with a receiver panel (RPC-1) and an adaptor (R11CPA) with an ambient pressure monitor (APR-1; Data Science International, St Paul, MN, USA). The telemetry transmitter catheter was positioned into the abdominal aorta and glued (3M vetbond; 3M Animal Care Products, St Paul, MN, USA) into position. The transmitter was secured to the abdominal wall with sutures. Data were collected and analyzed using Dataquest ART version 4.3 (Data Science International).
Measurement of BP by the telemetry system
After 2-week acclimatization, radiotelemetry transmitters were implanted in the abdominal aorta of rats at 6 weeks of age. One week after surgery, baseline BP was measured by the telemetry system. At 7 weeks of age rats were divided into four groups as follows: group I (n = 4): normal salt diet (NS, 0.3% NaCl) +vehicle (0.5% carboxymethyl cellulose sodium), group II (n = 4): high-salt diet (8% NaCl)+vehicle (0.5% carboxymethyl cellulose sodium), group III (n = 4): HS+vildagliptin-low dose (3 mg kg−1 by oral gavage twice daily) and group IV (n = 5): HS+vildagliptin-high dose (10 mg kg−1 by oral gavage twice daily). Vildagliptin was provided by Novartis (Basel, Switzerland). Rats in groups II–IV were placed on the HS diet for 7 days followed by LS diet for 7 days. We measured 24-h BP (5 min of automatic sampling per rat per hour) using the telemetry system before starting the HS diet, 7 days after beginning the HS diet and 7 days after beginning the NS diet for each treatment group. In addition to weekly 24-h BP measurement, we also measured the BP daily at five time points per day using the telemetry system: in the morning before gavage, 2 h after gavage, afternoon, at night before gavage and 2 h after gavage.
Active GLP-1 level and DPP-4 activity in the plasma
At the end of the experiment, rats were killed with an excessive dose of sodium pentobarbital (200 mg kg−1, i.p.), and arterial blood was collected with EDTA. The active plasma GLP-1 level was measured by ELISA (Immuno-Biological Laboratories, Gunma, Japan; catalog number 27784). The plasma DPP-4 activity was also measured using a commercial assay (Enzo Life Sciences, Farmingdale, NY, USA; catalog number BML-AK498).
Urinary sodium excretion
Twenty-four-hour urine samples were collected at 7 weeks (before HS diet), 8 weeks (after HS diet) and 9 weeks (after NS diet) of age after a 12-h acclimatization period in their metabolic cages. Urine samples were stored at −20 °C for future use. Urinary sodium was measured by an automated analyzer (7020-Automatic Analyzer, Hitachi High-Technologies, Tokyo, Japan). Daily sodium balance was calculated as the difference between the Na+ ingested and the Na+ excreted through urine.
Intracerebroventricular (ICV) infusion of vildagliptin
In a separate group of animals, we examined the effects of ICV infusion of vildagliptin in DSS rats (n = 4). After 3-week acclimatization with NS diet, 7-week-old DSS rats were fed a HS diet for 1 week. Then, ICV injection of vildagliptin was performed using an injector needle (30 gauge stainless steel), as described previously.21,22 Briefly, under isoflurane anesthesia, a guide cannula was implanted into the left cerebroventricular region for ICV infusion. Rats were placed on a stereotaxic frame (Narishige Scientific Instruments, Tokyo, Japan) in the prone position and the guide cannula was fixed to the skull. In addition, a polyethylene catheter (PE-50) was inserted into the right femoral artery for measurement of BP and another catheter was inserted into the right femoral vein for administration of saline (2 ml h−1) to maintain body fluid. Before infusion of vildagliptin, artificial cerebrospinal fluid (a-CSF, 10 μl; Artcereb, Otsuka Pharmaceutical, Tokushima, Japan) was injected into the lateral cerebroventricular region. BP and heart rate were measured for 30 min. After that, vildagliptin (50, 500 or 2500 μg in 10 μl a-CSF) was injected intracerebroventricularly into rats at 30-min intervals. The changes in BP and heart rate with respect to the basal values were observed for 30 min following each injection.
Statistical analysis
All values are presented as the mean ± s.e.m. Statistical comparisons of the differences were performed using one-way repeated-measures analysis of variance, followed by the Newman–Keuls post hoc test. P-values <0.05 were considered statistically significant. Data and statistical analyses were performed using GraphPad Prism version 5 for Windows (Graph Pad Software, San Diego, CA, USA).
RESULTS
Effect of vildagliptin on body weight, food intake and urine volume
Changes in body weight, food intake and urine volume are shown in Table 1. Body weight gain continued during the 2-week treatment period in all groups, and none of the treatments significantly affected body weight gain. Daily food intake was also no different among the groups. An 8% NaCl diet has often been used in HS diet studies in rats.23,24 There is no clear evidence for appetite loss during a short period of treatment using this 8% NaCl diet.23,24 During the HS diet, urine volume was significantly increased in all groups compared with NS diet-fed animals. The higher dose of vildagliptin significantly increased the urine volume compared with the HS diet vehicle-treated DSS rats. After switching back to the NS diet, urine volume was significantly decreased in all the groups.
Table 1. Body weight, food intake and urine output in DSS rats.
|
Baseline (NS diet)
|
7 Days HS-diet feeding
|
7 Days NS-diet feeding
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Bwt (g) |
Food intake
(g per day) |
Urine volume
(ml per day) |
Bwt (g) |
Food intake
(g per day) |
Urine volume
(ml per day) |
Bwt(g) |
Food intake
(g per day) |
Urine volume
(ml per day) |
| DSS/NS+vehicle | 254 ± 8 | 24.3 ± 2.2 | 8.4 ± 0.2 | 288 ± 4 | 22.0 ± 0.5 | 8.2 ± 1.0 | 326 ± 6 | 23.0 ±0.5 | 10.3 ± 0.6 |
| DSS/HS+vehicle | 257 ± 4 | 21.7 ± 0.3 | 7.2 ± 0.7 | 287 ± 5 | 21.5 ± 1.0 | 57.3 ± 2.8***, ∥∥∥ | 324 ± 2 | 21.7 ± 0.4 | 12.9 ± 1.6### |
| DSS/HS+vildagliptin-low dose | 262 ± 4 | 19.1 ± 1.2 | 8.7 ± 0.6 | 291 ±4 | 22.0 ± 0.6 | 62.7 ± 2.5∥∥∥ | 332 ± 2 | 21.0 ± 0.4 | 10.5 ± 0.6### |
| DSS/HS+vildagliptin-high dose | 255 ± 5 | 20.0 ± 0.4 | 8.8 ± 1.5 | 282 ± 4 | 20.7 ± 0.9 | 65.6 ± 0.9†, ∥∥∥ | 315 ± 4 | 20.0 ± 0.4 | 12.93 ± 1.6### |
Abbreviations: Bwt, body weight; DSS, Dahl salt-sensitive; HS, high-salt diet (8% NaCI); NS, normal salt diet (0.3% NaCI).
P<0.005 NS+vehicle versus HS+vehicle
P<0.05 HS+vehicle versus vildagliptin-high dose
P<0.005 versus baseline in each group
P<0.005 versus 7 days after HS diet in each group.
Effect of vildagliptin on BP
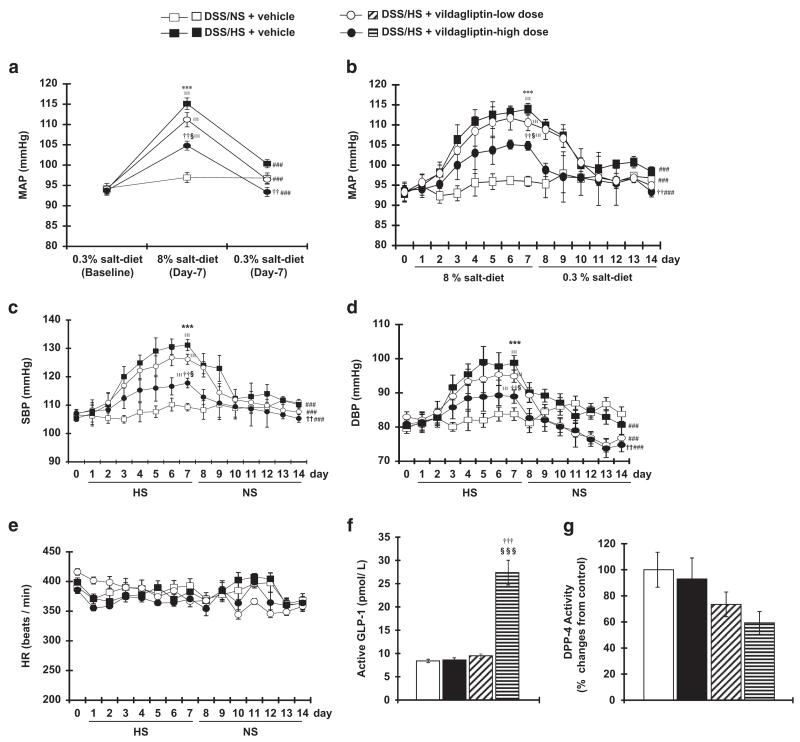
Changes in the mean arterial pressure (MAP) are shown in Figures 1a and b. NS diet for 2 weeks did not alter MAP in DSS rats, whereas rats on the HS diet exhibited increased MAP after 7 days. Treatment with vildagliptin dose-dependently suppressed the progression of HS-induced hypertension in DSS rats. Interestingly, after 7 days of the HS diet switching to the NS diet for 7 days reduced the MAP. A further reduction in MAP was observed with dietary salt reduction plus vildagliptin treatment in DSS rats.
Figure 1.
Effect of high-salt (HS) diet and vildagliptin on blood pressure (BP) profile and heart rate, glucagon-like peptide 1 (GLP-1) and dipeptidyl peptidase-4 (DPP-4) in Dahl salt-sensitive (DSS) rats. Twenty-four-hour mean arterial pressure (MAP) (a) and hourly MAP at five time points per day (b) as measured by the telemetry system. Normal salt (NS) diet for 2 weeks does not alter the MAP, whereas HS diet significantly increases MAP after 7 days in DSS rats. Vildagliptin dose-dependently suppressed salt-dependent hypertension. After switching to the NS diet, MAP is reduced. Further, BP reduction is observed by NS+vildagliptin treatment. Systolic blood pressure (SBP, c), diastolic blood pressure (DBP, d) and heart rate (HR, e) in DSS rats fed a HS-diet for 7 days followed by NS diet for 7 days. The SBP and DBP of DSS rats fed a NS diet for 2 weeks do not change, whereas HS diet significantly increased the SBP and DBP after 7 days. Vildagliptin dose-dependently suppressed the BP elevation induced by HS diet. After switching to NS diet, SBP and DBP significantly decreased, and further BP reduction was observed in dietary salt reduction with vildagliptin treatment. However, HR was not significantly different between the treatment groups. Effects of vildagliptin on active plasma GLP-1 (f) and DPP-4 activity (g). The concentration of active GLP-1 in plasma is increased by high doses of vildagliptin in DSS rats. Plasma DPP-4 activity is reduced by vildagliptin in a dose-dependent manner. ***P<0.005 NS+vehicle vs. HS+vehicle; ††P<0.01, †††P<0.005 HS+vehicle vs. HS+vildagliptin-high dose; §P<0.05, §§§P<0.005 HS+vildagliptin-low dose vs. HS+vildagliptin-high dose; ∥∥∥P<0.005 vs. baseline in respective group; ###P<0.005 vs. 7 days after HS diet in respective group.
Changes in systolic blood pressure (SBP) and diastolic blood pressure are shown in Figures 1c and d, respectively. Treatment with vildagliptin dose-dependently suppressed the progression of HS-induced elevation of SBP and diastolic blood pressure in DSS rats. However, none of the treatments significantly affected heart rate (HR) in DSS rats (Figure 1e).
Effect of vildagliptin on plasma GLP-1 and DPP-4
We measured the active GLP-1 level and DPP-4 activity in plasma. The active GLP-1 level in plasma was significantly increased in vildagliptin-treated DSS rats (Figure 1f). In contrast, plasma DPP-4 activity was dose-dependently decreased by vildagliptin (Figure 1g).
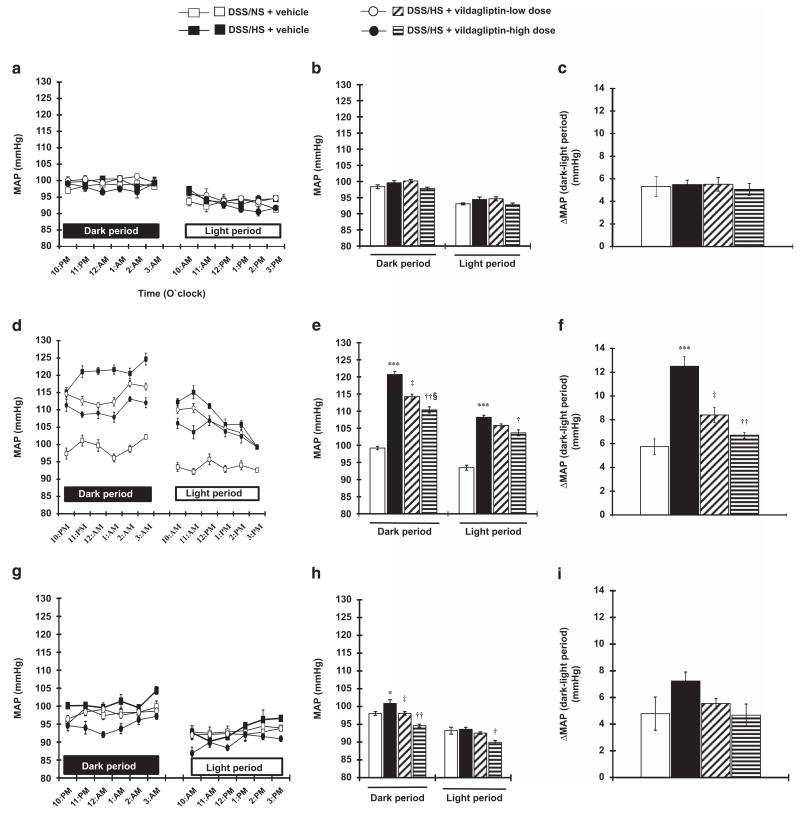
Effect of vildagliptin on circadian BP-dipping pattern
The baseline MAP during the NS diet treatment was higher during the dark period compared with the light period (98.4 ± 0.6, 99.6 ± 0.6, 100.1 ± 0.5 and 97.8 ± 0.4 mm Hg vs. 93.1 ± 0.3, 94.4 ± 0.8, 94.6 ± 0.6 and 92.8 ± 0.5 mm Hg; P<0.001 for each group; Figures 2a–c), indicating that the animals showed a dipper-type circadian BP pattern at baseline.2 After 7 days of HS diet, the MAP in DSS rats was higher during the dark period compared with the light period (120.7 ± 0.8 vs. 108.2 ± 0.6 mm Hg; P<0.001). Importantly, the difference in MAP values between dark time and light time was significantly greater after treatment with HS diet for 7 days (12.5 ± 0.8 vs. 5.7 ± 0.7 mm Hg; P<0.005). These data suggest that HS feeding for 7 days in DSS rats induced an extreme dipper type of BP pattern. Vildagliptin treatment dose-dependently attenuated the extreme BP dipper-type (dark period MAP was 114.2 ± 0.7 and 110.3 ± 0.9 mm Hg and light period MAP was 105.8 ± 0.5 and 103.6 ± 0.9 mm Hg for 3 and 10 mg kg−1 doses of vildagliptin, respectively; P<0.001; Figures 2d–f). After switching to NS diet for the following 1 week, the animals reverted to a normal BP dipper type and vildagliptin treatment did not show any extra effect (dark period MAP was 98.0 ± 0.5, 100.8 ± 1.1, 98.0 ± 0.5 and 94.6 ± 0.4 mm Hg and light period MAP was 93.2 ± 0.9, 93.6 ± 0.5, 92.5 ± 0.3 and 89.9 ± 0.5 mm Hg; P<0.001 for each group; Figures 2g–i). Similar results were observed when the dipping pattern of BP was evaluated using SBP data (Supplementary Figures S1–S3). Detailed results are shown in the Supplementary Results section.
Figure 2.
Circadian rhythms of the mean arterial pressure (MAP) measured hourly by the telemetry system in Dahl salt-sensitive (DSS) rats. At baseline MAP in DSS rats with NS diet (a), the MAP during the dark and light periods (b) and differences in MAP between the dark and light periods (c). Baseline MAP during normal salt (NS) diet treatment is higher in the dark period compared with the light period, indicating that the animal has circadian dipper pattern of blood pressure (BP). (d–f) Hourly MAP in DSS rats with high-salt (HS) diet. After 1 week of HS diet treatment, the MAP is increased (d). Averaged value of dark and light periods (e) and differences between the dark and light periods (f) are shown. DSS rats with HS diet feeding for 7 days show greater increases in MAP in the dark period compared with the light period, suggesting that DSS rats have an extreme dipper pattern of BP. Vildagliptin treatment dose-dependently attenuated these changes in BP. (g–i) Hourly MAP in DSS rats after switching to NS diet. After 1 week treatment of NS diet, the MAP is decreased (g). The mean values of the dark and light periods (h) and differences between the dark and light periods (i) are shown. After switching from HS diet to NS diet, the animals demonstrated a normal BP dipper pattern. ***P<0.005 NS+vehicle vs. HS+vehicle; †P<0.05, ††P<0.01 HS+vehicle vs. HS+vildagliptin-high dose; ‡<0.05 HS+vehicle vs. HS+vildagliptin-low dose; §P<0.05 HS+vildagliptin-low dose vs. HS+vildagliptin-high dose.
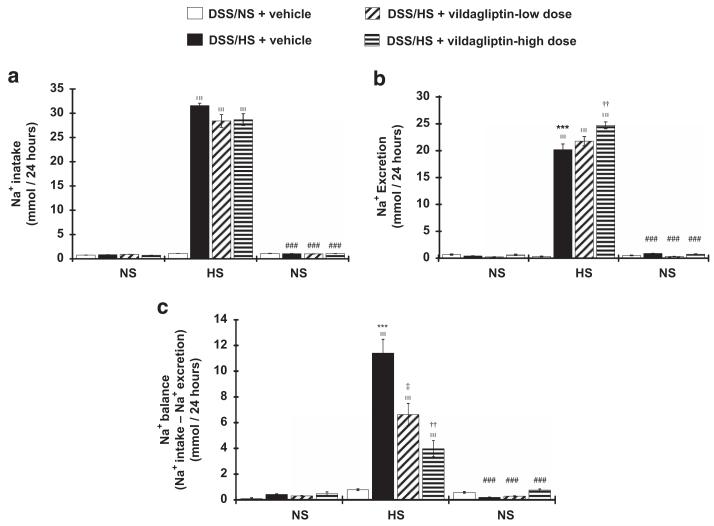
Effect of vildagliptin on urinary sodium excretion
HS diet markedly increased sodium intake in all animals (0.83 ± 0.05, 0.93 ± 0.02, 0.72 ± 0.03 mmol per day during NS diet feeding at baseline vs. 31.57 ± 0.5, 28.40 ± 1.3, 28.68 ± 1.2 mmol per day after 7-day-HS diet feeding; P<0.005 for each group; Figure 3a). DSS rats on a HS diet showed higher urinary sodium excretion compared with NS-fed rats (20.17 ± 1.06 vs. 0.32 ± 0.06 mmol per day; P<0.005). Vildagliptin dose-dependently increased urinary sodium excretion (21.77 ± 0.85 and 24.71 ± 0.62 mmol per day for 3 and 10 mg kg−1 doses of vildagliptin respectively; P<0.005; Figure 3b). During HS feeding, the sodium balance was increased compared with NS-feeding rats (11.41 ± 1.06 vs. 0.78 ± 0.06 mmol per day; P<0.005). Vildagliptin treatment dose-dependently decreased the sodium balance (6.63 ± 0.86 and 3.98 ± 0.62 mmol per day for 3 and 10 mg kg−1 doses of vildagliptin, respectively; Figure 3c). Linear regression analyses showed that the difference in BP between the active and inactive periods (dipping of BP) was significantly correlated with urinary sodium excretion in DSS hypertensive rats treated with a high dose of vildagliptin (r2 = 0.76, P = 0.05).
Figure 3.
Effects of vildagliptin on 24-h urinary sodium excretion. Sodium intake by food (a), urinary sodium excretion after salt loading (b) and sodium balance (c). Sodium intake does not vary between the groups, high-salt (HS)-diet feeding increased sodium intake in all animals with no significant differences between the groups. Vildagliptin dose-dependently increased urinary sodium excretion and decreased the sodium balance. ***P<0.005 normal salt (NS)+vehicle vs. HS+vehicle; ††P<0.01 HS+vehicle vs. HS+vildagliptin-high dose; ‡P<0.05 HS+vehicle vs. HS+vildagliptin-low dose; ∥∥∥P<0.005 vs. baseline in each group; ###P<0.005 vs. 7 days after HS diet in each group.
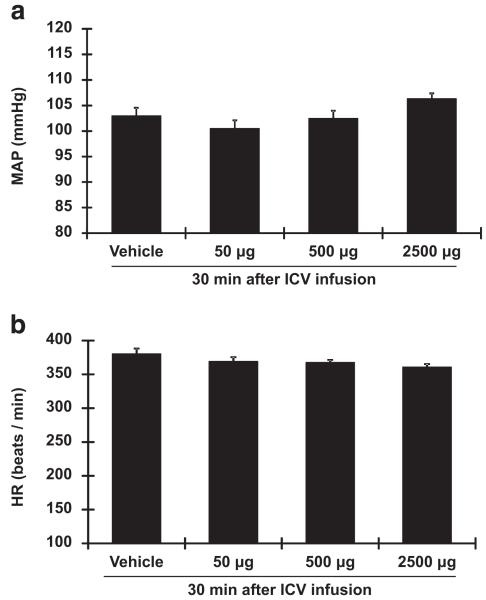
Effect of ICV infusion of vildagliptin on BP and HR
Infusion of a-CSF solution had no effect on baseline parameters (Figures 4a and b) in DSS rats fed a HS diet. Similarly, ICV infusion of vildagliptin (50, 500 or 2500 μg in 10 μl a-CSF solution) did not change either BP or HR in these animals (Figures 4a and b).
Figure 4.
Intracerebroventricular (ICV) administration of vildagliptin in high-salt (HS)-fed Dahl salt-sensitive (DSS) rats. Seven days after HS diet treatment, ICV infusion of vildagliptin (50, 500 and 2500 μg per 10 μl solution) or vehicle (10 μl solution) were performed. Changes in blood pressure (BP; a) and heart rate (HR) (b) were measured for 30 min following the infusion. ICV infusion of vildagliptin does not change either BP or HR.
DISCUSSION
A growing body of evidence has indicated that orally active DPP-4 inhibitors have antihypertensive actions both in animals16,17 and humans.18,19 The present study examines the effects of vildagliptin, a potent and specific DPP-4 inhibitor, on the development of salt-dependent hypertension in DSS rats. The results have demonstrated for the first time that vildagliptin treatment dose-dependently attenuates the development of HS-induced hypertension and improves the circadian-dipping pattern in DSS rats.
In the present study, vildagliptin substantially inhibited plasma DPP-4 activity in DSS rats. In addition, the antihypertensive action of vildagliptin was accompanied by (1) a marked increase in plasma active GLP-1 level and (2) enhanced urinary sodium excretion. These findings are consistent with those of previous studies in hypertensive animal models,25,26 which demonstrated that GLP-1 and its analogs significantly increase urinary sodium excretion and decrease BP. Multiple factors contribute to the pathogenesis of salt-dependent hypertension, among which the kidney is thought to have a fundamental role.27 This is supported by the development of hypertension in DSS rats being associated with increased urinary sodium reabsorption.28 The present study showed that treatment with vildagliptin significantly increased urinary volume and sodium excretion in HS-diet DSS rats, suggesting that vildagliptin enhances diuresis and natriuresis. Previous studies showed that GLP-1-infused rats exhibited a 13-fold increase in urinary sodium excretion.29 In the present study, we showed that HS-fed DSS rats retained sodium, as reflected by the sodium balance. In contrast, sodium excretion was significantly increased by vildagliptin. These results indicate that the ability of vildagliptin to promote urinary salt and water excretion contribute to its antihypertensive effects. The occurrence of HS-induced increases in MAP during dark time (working period) suggests that the BP elevation may be related to a nocturnal increase in intravascular volume that is then reversed during the day through enhanced renal excretion of sodium and water, as proposed by the previous study.30 In agreement with the present findings, a previous study showed that GLP-1 increases natriuresis and diuresis because of the inhibition of tubular sodium reabsorption.29 The DPP-4 inhibitor, sitagliptin, also exhibited enhanced cumulative urinary flow and sodium excretion in young spontaneously hypertensive rats.17
We evaluated, for the first time, the dipping pattern by analyzing differences between dark time (working period) and light time (sleeping period) MAP as well as SBP during the development of salt-induced hypertension in DSS rats. We observed that the MAP and SBP were significantly higher in dark time compared with light time. These data are consistent with those of a previous report that MAP was higher at dark time (working period) than light time (sleeping period) in DSS rats with HS diet.2 Although the definitions of dipper and non-dipper types of BP are well established in humans, there is no clear definition for the circadian pattern of BP in other species. In the present study, we found that in normotensive NS-treated DSS rats, BP was 5.7 mm Hg lower during the light (inactive period) than during the dark (active period; P<0.001). These data are consistent with those obtained by Sueta et al.31 who showed that normotensive Wistar-Kyoto rats and spontaneously hypertensive rats have normal dipper patterns of BP, similar to those observed in the present study.
During the NS diet, DSS rats showed a normal BP-dipping pattern, whereas HS-diet feeding changed this to an extreme BP dipper pattern. Vildagliptin dose-dependently reversed the extreme dipping pattern to a normal BP-dipping pattern. BP follows a circadian rhythm, with lower values during night time than day time in humans.32 The absence of a nocturnal BP decrease (dipping) is associated with target organ damage.33 Kario et al.34,35 reported that the relative risk for stroke was greater in patients with an extreme dipper pattern than in patients with a non-dipper BP pattern. Collectively, the present data suggest that HS diet causes hypertension with an extreme dipper pattern of BP in DSS rats at the early phase of the development of hypertension, which may contribute to the pathogenesis of cardiovascular injury in these animals.
Previous studies have demonstrated the potential role of the sympathetic nervous system in the development of salt-dependent hypertension.36 To test whether the antihypertensive effect of vildagliptin is mediated by the central nervous system, we performed acute ICV administration of vildagliptin in HS-fed DSS rats. However, ICV administration of vildagliptin did not alter BP or HR, suggesting that the antihypertensive effect of vildagliptin is not mediated by the central nervous system. Alternatively, the observation period (30 min) may not have been a sufficient duration to evaluate the effectiveness of vildagliptin. Previous studies have shown that GLP-1 and GLP-1 receptor agonists increase BP and HR in Sprague–Dawley rats.37,38 However, a limitation of this study is that there is no direct evidence that the antihypertensive effect of vildagliptin is actually mediated by an increase in urinary sodium excretion. To address this issue directly, future studies should be performed in tubular-specific DPP-4 knockout animals. Notably, GLP-1 is able to penetrate the blood–brain barrier,39 but DPP-4 inhibitors may not easily cross the blood–brain barrier.40,41 Further studies are required to examine the effect of vildagliptin on brain GLP-1 levels during the development of salt-dependent hypertension.
In conclusion, the present study demonstrates that vildagliptin attenuates the development of salt-induced hypertension and extreme BP-dipping pattern in DSS rats fed a HS diet. Vildagliptin exerts its antihypertensive effects through enhanced urinary sodium excretion.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI; 26460343 to Akira Nishiyama). Kazi Rafiq is a recipient of a JSPS Postdoctoral Fellowship for Foreign Researchers (P12420).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLOSURE
Vildagliptin was provided by Novartis A.G. (Basel, Switzerland).
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
References
- 1.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, Robertson D, Biaggioni I. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;53:363–369. doi: 10.1161/HYPERTENSIONAHA.108.124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DR, Morgan DA, Peuler JD, Thoren P. 24-hour blood pressure recordings in Dahl rats on high- and low-salt diets. Am J Physiol. 1989;257:R1225–R1231. doi: 10.1152/ajpregu.1989.257.5.R1225. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M, Goto N, Kimura G. Hypothesis on renal mechanism of non-dipper pattern of circadian blood pressure rhythm. Med Hypotheses. 2006;67:802–806. doi: 10.1016/j.mehy.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 4.White WB. Ambulatory blood pressure monitoring: dippers compared with non-dippers. Blood Press Monit. 2000;5:S17–S23. [PubMed] [Google Scholar]
- 5.White WB. Importance of blood pressure control over a 24-hour period. J Manag Care Pharm. 2007;13:34–39. doi: 10.18553/jmcp.2007.13.s8-b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamezaki F, Sonoda S, Nakata S, Muraoka Y, Okazaki M, Tamura M, Abe H, Tekeuchi M, Otsuji Y. Association of seasonal variation in the prevalence of metabolic syndrome with insulin resistance. Hypertens Res. 2013;36:398–402. doi: 10.1038/hr.2012.197. [DOI] [PubMed] [Google Scholar]
- 7.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, Systolic Hypertension in Europe Trial Investigators Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, Kamiya Y, Yoshida A, Kimura G. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 11.Burkey BF, Li X, Bolognese L, Balkan B, Mone M, Russell M, Hughes TE, Wang PR. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther. 2005;315:688–695. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- 12.Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009;40:536–544. doi: 10.1016/j.arcmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Nakagami H, Pang Z, Shimosato T, Moritani T, Kurinami H, Koriyama H, Tenma A, Shimamura M, Morishita R. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertens Res. 2014;37:629–635. doi: 10.1038/hr.2014.53. [DOI] [PubMed] [Google Scholar]
- 14.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 15.Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–257. doi: 10.1111/dom.12020. [DOI] [PubMed] [Google Scholar]
- 16.Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol. 2012;60:467–473. doi: 10.1097/FJC.0b013e31826be204. [DOI] [PubMed] [Google Scholar]
- 17.Pacheco BP, Crajoinas RO, Couto GK, Davel AP, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 18.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223:133–135. doi: 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 20.Johansson ME, Andersson IJ, Alexanderson C, Skott O, Holmang A, Bergstrom G. Hyperinsulinemic rats are normotensive but sensitized to angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1240–R1247. doi: 10.1152/ajpregu.00493.2007. [DOI] [PubMed] [Google Scholar]
- 21.Fujisawa Y, Nagai Y, Lei B, Nakano D, Fukui T, Hitomi H, Mori H, Masaki T, Nishiyama A. Roles of central renin-angiotensin system and afferent renal nerve in the control of systemic hemodynamics in rats. Hypertens Res. 2011;34:1228–1232. doi: 10.1038/hr.2011.115. [DOI] [PubMed] [Google Scholar]
- 22.Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension. 2003;41:266–273. doi: 10.1161/01.hyp.0000049621.85474.cf. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 24.Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, Nishiyama A. Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–368. doi: 10.1038/ajh.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–49. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int. 1988;33:1119–1129. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 28.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33:419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 29.Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol. 2002;434:163–167. doi: 10.1016/s0014-2999(01)01542-4. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun DA, Zhu S, Wyss JM, Oparil S. Diurnal blood pressure variation and dietary salt in spontaneously hypertensive rats. Hypertension. 1994;24:1–7. doi: 10.1161/01.hyp.24.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Sueta D, Kataoka K, Koibuchi N, Toyama K, Uekawa K, Katayama T, Mingjie M, Nakagawa T, Waki H, Maeda M, Yasuda O, Matsui K, Ogawa H, Kim-Mitsuyama S. Novel mechanism for disrupted circadian blood pressure rhythm in a rat model of metabolic syndrome–the critical role of angiotensin II. J Am Heart Assoc. 2013;2:e000035. doi: 10.1161/JAHA.113.000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda M, Wakamatsu-Yamanaka T, Mizuno M, Miura T, Tomonari T, Kato Y, Ichikawa T, Miyagi S, Shirasawa Y, Ito A, Yoshida A, Kimura G. Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am J Physiol Renal Physiol. 2011;301:F953–F957. doi: 10.1152/ajprenal.00167.2011. [DOI] [PubMed] [Google Scholar]
- 33.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 34.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 35.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 36.Nagasu H, Satoh M, Fujimoto S, Tomita N, Sasaki T, Kashihara N. Azelnidipine attenuates glomerular damage in Dahl salt-sensitive rats by suppressing sympathetic nerve activity. Hypertens Res. 2012;35:348–355. doi: 10.1038/hr.2011.184. [DOI] [PubMed] [Google Scholar]
- 37.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 40.Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 41.Shannon RP. DPP-4 inhibition and neuroprotection: do mechanisms matter? Diabetes. 2013;62:1029–1031. doi: 10.2337/db12-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.