Abstract
This study compared second generation chimeric antigen receptors encoding signaling domains composed of CD28, ICOS and 4-1BB. Here we report that certain CARs endow T cells with the ability to undergo long-term autonomous proliferation. Transduction of primary human T-cell with lentiviral vectors encoding some of the CARs resulted in sustained proliferation for up to three months following a single stimulation through the TCR. Sustained numeric expansion was independent of cognate antigen and did not require the addition of exogenous cytokines or feeder cells after a single stimulation of the TCR and CD28. Results from gene array and functional assays linked sustained cytokine secretion and expression of T-bet, EOMES and GATA-3 to the effect. Sustained expression of the endogenous IL2 locus has not been reported in primary T cells. Sustained proliferation was dependent on CAR structure and high expression, the latter of which was necessary but not sufficient. The mechanism involves constitutive signaling through NF-kB, Akt, Erk and NFAT. The propagated CAR T cells retained a diverse TCR repertoire and cellular transformation was not observed. The CARs with a constitutive growth phenotype displayed inferior antitumor effects and engraftment in vivo. Therefore the design of CARs that have a non-constitutive growth phenotype may be a strategy to improve efficacy and engraftment of CAR T cells. The identification of CARs that confer constitutive or non-constitutive growth patterns may explain observations that CAR T cells have differential survival patterns in clinical trials.
Introduction
The creation of tumor-specific T lymphocytes by genetic modification to express chimeric antigen receptors (CAR) is gaining traction as a form of synthetic biology generating powerful antitumor effects (1-6). Because the specificity is conferred by antibody fragments, the CAR T cells are not MHC- restricted and are therefore more practical than approaches based on T cell receptors (TCR) that require MHC matching.
Clinical data from patients treated with CD19-specific CAR+ T cells indicate that robust in vivo proliferation of the infused T cells is a key requirement for immunoablation of tumors (7, 8). Therefore, efforts have been made to incorporate the signaling endodomains of co-stimulatory molecules such as CD28, ICOS, OX40, and 4-1BB into CARs. It was first reported in 1998 that the use of gene-engineered T cells expressing chimeric single-chain (scFv) receptors capable of co-delivering CD28 costimulation and TCR/CD3 zeta chain (CD3ζ) activation signals increased the function and proliferation of CAR T cells (9). A number of laboratories have confirmed that incorporation of CD28 signaling domains enhances the function of CARs in pre-clinical studies compared to CD3ζ or FcεR1. In a study in patients with B-cell malignancies, CD28:CD3ζ CARs had improved survival compared to CARs endowed only with the CD3ζ signaling domain (5).
Here we report the unexpected finding that expression of some CARs containing CD28 and CD3ζ tandem signaling domains leads to constitutive activation and proliferation of the transduced primary human T cells. The CAR T cells that we have identified have constitutive secretion of large amounts of diverse cytokines and consequently do not require the addition of exogenous cytokine or feeder cells in order to maintain proliferation. This was surprising because in numerous previous reports that described CARs endowed with CD28 domains (9-28), the proliferation of such tandem CARs has been ligand-dependent, and required restimulation of the CAR T cells to maintain proliferation. Here we report that one mechanism that can result in the phenotype of CARs with continuous T-cell proliferation is the density of CARs at the cell surface.
Materials and Methods
Construction of lentiviral vectors with differing eukaryotic promoters and CARs
Supplemental figure 1A shows schematic diagrams of the CARs used in this study. All CARs contain an scFv that recognizes either human CD19, mesothelin or c-Met.
In vivo assessment of anti-c-Met CAR T cells
Xenograft tumors in NSG mice were established by intraperitoneal injection of 0.791×106 SK-OV3 ovarian cancer cells or subcutaneous injection of 1×106 L55 human lung adenocarcinoma cells, transduced to express click-beetle-green. Tumor growth was measured by bioluminescent imaging. Peripheral blood was obtained from retro-orbital bleeding or intracardiac puncture and was stained for the presence of human CD45+ T cells. The human CD45+ population was quantified using TruCount tubes (BD Biosciences). All experiments were performed in anonymized fashion.
Construction of deletion variants of PGK (phosphoglycerate kinase1) promoter
A series of 5′ deletion mutations of the human PGK promoter was prepared by PCR using specific 5′ primers with an incorporated PmeI site, indicated below, and a common 3′ primer with an incorporated NheI site (5′-gtggctggagagaggggtgctagccgc-3′). The PCR product was digested and then inserted into the pELNS c-Met-IgG4-28z plasmid to substitute the EF-1α promoter with PGK promoter deletion mutants. PGK100, PGK200, PGK300 and PGK400 encompasses from nucleotides -38, -141, -243 and -341 of transcription start site of PGK to +84, respectively.
PGK100 5′- gcggtttaaacgtggggcggtagtgtgggccctg-3′
PGK200 5′- gcggtttaaacgcaatggcagcgcgccgaccg-3′
PGK300 5′-gcggtttaaacgcccctaagtcgggaaggttccttg-3′
PGK400 5′-gcggtttaaacgccgaccctgggtctcgcacattc-3′
Construction and characterization of chimeric antigen receptors
Lentiviral vectors from previously published work were used to express the anti-CD19 FMC63 CD8α (29), the anti-mesothelin SS1 CD8α, and the anti-mesothelin SS1 CD8α Δtail CAR constructs (30). The c-Met 5D5 IgG4 construct was used as a template to generate the SS1 IgG4 and CD19 IgG4 CAR constructs through PCR splicing and overlap extension. Restriction sites were introduced via PCR primers, which allowed for cloning into third generation self-inactivating lentiviral plasmids. The cytomegalovirus (CMV) and elongation factor-1α (EF-1α) promoter sequences were amplified via PCR from previously constructed plasmids and introduced into pre-existing CAR-containing constructs (29) using standard molecular biology techniques.
Microarray studies
Sample Collection
Human CD4+ T cells from three healthy donors were stimulated and transduced with either the c-Met IgG4 or CD19 CD8α CAR construct. Cell pellets were collected and frozen on day 0 prior to stimulation, day 6 and day 11 at rest down for all samples and day 24 for the c-Met IgG4 CAR.
Microarray Target Preparation and Hybridization
Microarray services were provided by the UPenn Microarray Facility, including quality control tests of the total RNA samples by Agilent Bioanalyzer and Nanodrop spectrophotometry. All protocols were conducted as described in the Affymetrix GeneChip Expression Analysis Technical Manual. Briefly, 100ng of total RNA was converted into first-strand cDNA using reverse transcriptase primed by poly(T) and random oligomers that incorporated the T7 promoter sequence. Second-strand cDNA synthesis was followed by in vitro transcription with T7 RNA polymerase for linear amplification of each transcript, and the resulting cRNA was converted into cDNA, fragmented, assessed by Bioanalyzer, and biotinylated by terminal transferase end-labeling. cRNA yields ranged from 36-89μg, and cDNA was added to Affymetrix hybridization cocktails, heated at 99°C for 5 min and hybridized for 16 h at 45°C to Human Gene 1.0ST GeneChips (Affymetrix Inc., Santa Clara CA). The microarrays were then washed at low (6× SSPE) and high (100mM MES, 0.1M NaCl) stringency and stained with streptavidin-phycoerythrin. A confocal scanner was used to collect fluorescence signal after excitation at 570 nm.
Initial Data Analysis
Affymetrix Command Console and Expression Console were used to quantify expression levels for targeted genes; default values provided by Affymetrix were applied to all analysis parameters. Border pixels were removed, and the average intensity of pixels within the 75th percentile was computed for each probe. The average of the lowest 2% of probe intensities occurring in each of 16 microarray sectors was set as background and subtracted from all features in that sector. Probe sets for positive and negative controls were examined in Expression Console, and Facility quality control parameters were confirmed to fall within normal ranges. Probes for each targeted gene were averaged and inter-array normalization performed using the robust multichip average (RMA) algorithm.
Analysis of terminal telomeric restriction fragment lengths
Telomeric restriction fragment length analysis was performed essentially as described (31). Briefly, 2μg of genomic DNA was digested with RsaI + HinfI and resolved on a 0.5% agarose gel, which was then dried and probed with a 32P- labeled (CCCTAA)4 oligonucleotide. After washing, the samples were visualized with a Phosphor imager.
Accession numbers
Micro array data will be deposited in the GEO repository upon acceptance of this manuscript for publication.
Statistical analysis
Raw data obtained from microarray core was normalized with RMA. Analysis was performed using a 3- way mixed model ANOVA with factors being sample date, treatment group and donor ID. An interaction term between sample and collection date was added. In conjunction with the multiple pair-wise comparisons the p-value and fold-change were determined. For all p-values we calculated the FDR corrected p-value using the method of Benjamini and Hochberg as implemented by Partek Genomic Suite (Partek). For transcription factor and cytokine dot plots the normalized absolute log2 gene expression intensities were plotted. Cluster analysis was performed using Euclidean distance of median normalized log2 gene expression intensities with average linkage. All growth curves, MFI and engraftment plots were plotted using Prism (GraphPad Software). All error bars are representative of standard deviation. A two tailed Mann-Whitney test was performed for the in vivo engraftment studies.
Additional methods are described in the Supplementary Materials.
Results
Construction and characterization of chimeric antigen receptors
A plethora of CARs have been generated that express CD28 and CD3ζ downstream of antibody fragments that mediate surrogate antigen recognition (12-18, 20-28). Given that these transgenes were constructed differently and by different investigators at different institutes, it remains unknown how these CARs would perform using a common expression system and a standardized culture system that has been optimized for clinical use. Therefore, a set of 12 CARs targeting c-Met, mesothelin and CD19 was expressed in primary human T cells (Supplementary Fig S1A and C). The CARs encoded IgG4 or CD8α hinge domains, CD28, ICOS or CD8α transmembrane domains and the signaling domains were composed of CD28, 4-1BB, ICOS and CD3ζ. A CAR with a truncated signaling domain, and CART19, a CD19 4-1BB:CD3ζ CAR used in a previous clinical trial (7) served as controls. All CARs were expressed constitutively using an EF-1α promoter, and in a typical experiment 50% of the cells initially expressed the CAR and had similar levels of expression on the surface by day 6 after transduction (Supplementary Fig S1B). The c-Met CAR T cells had specific and potent cytotoxicity (Supplementary Fig S2), and previous studies have shown that the CARs specific for CD19 and mesothelin have similarly potent effector functions (29, 30).
Chimeric antigen receptors with CD28 and CD3ζ can induce constitutive T-cell proliferation
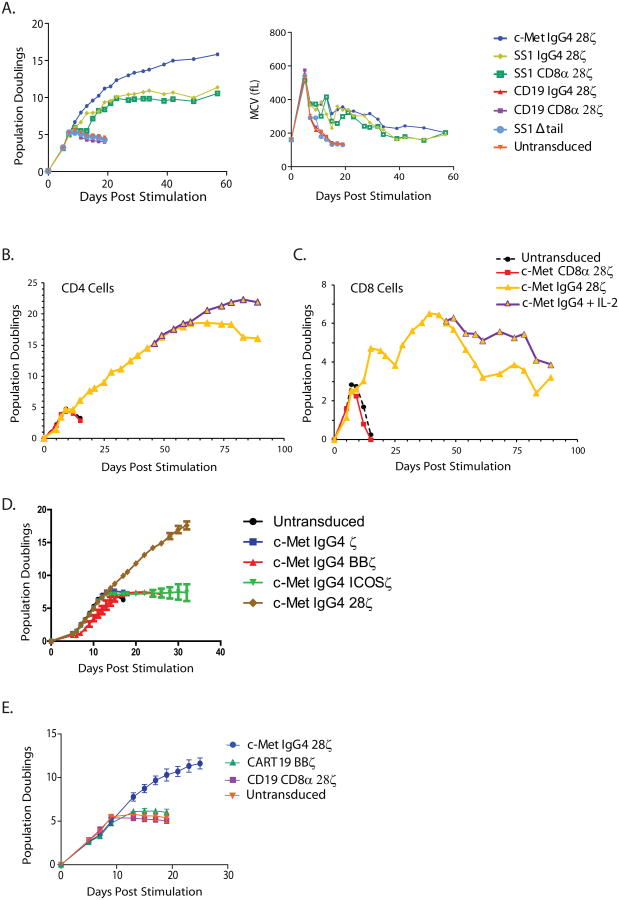
Previous studies suggested that antitumor effects after CAR T-cell infusions require sustained expansion of CAR T cells in vivo after adoptive transfer (8). To determine the proliferative capacity of the CAR T cells, CD4+ T cells were activated with anti-CD3 and anti-CD28 beads, transduced with the lentiviral vector encoding the CAR and then propagated without further stimulation in the absence of exogenous cytokines or feeder cells. Unexpectedly, we observed constitutive proliferation of some of the CAR T-cell populations (Fig 1A, left). Exponential growth was observed for 60 to 90 days in CAR T cells transduced with the c-Met IgG4 construct encoding the CD28 and CD3ζ signaling domains (Fig 1 A and B). Similarly, the T cells expressing the anti-mesothelin SS1:IgG4 and SS1:CD8α CARs that signaled through chimeric CD28 and CD3ζ domains also had sustained proliferation that was independent of supplementation with exogenous growth factors. We also observed long-term proliferation of CD8+ T cells that was independent of antigen stimulation and did not require the addition of exogenous cytokines or feeder cells (Fig 1C). To minimize experimental variables, we used bulk CD4+ T cells for most of the experiments in this study.
Figure 1. Induction of constitutive, ligand-independent CAR T-cell proliferation.
A) In vitro proliferation of human CD4+ T cells following 5 days of αCD3/CD28-coated magnetic bead stimulation and lentiviral transduction with the indicated CAR constructs (left panel). No cytokines were added to culture media at any point during expansion. The CAR T cells with constitutive proliferation also maintain a larger mean cell volume (right panel). Results are representative of n>10 normal human donors. B and C) CD4+ and CD8+ T cells were stimulated as in (A), with or without exogenous IL2. In vitro proliferation of human CD4+ (D) T cells following lentiviral transduction with the indicated CAR constructs. No cytokines were added to culture media at any point during expansion for CD4+ T cells. Results are representative of n>4 normal human donors. E) CD4+ T cells from 3 healthy donors were isolated, stimulated and transduced with lentivirus encoding the c-Met IgG4, CD19 CD8-α, and CART19 CAR constructs or mock transduced, and cultured with addition of fresh media and no exogenous cytokines. Error bars denote standard deviation. The design of the various CAR constructs is shown in Supplemental Figure S1.
The cultures with the non-continuous CAR T-cell populations had an initial period of exponential proliferation at the same rate, and after day 10, a decreasing rate of growth followed by death of the culture within 20 days (Fig 1A and B). Notably, in the absence of exogenous IL2, the CD19 CARs expressing the 4-1BB domain returned to a resting state with similar kinetics as that of the CD19:28ζ CAR T cells (Fig 1E). This expected pattern of initial growth followed by a return to a resting state by CD19 CARs and the mock transduced cells has been reported by our laboratory and others (15, 21, 24, 29, 32). For simplicity and clarity the CAR constructs that induce constitutive proliferation are henceforth referred to as “continuous CARs”, while the CARs that exhibit inducible proliferation similar to that described in previous reports are referred to as “non-continuous CARs”.
The mean cell volumes were monitored at frequent intervals as a measure of metabolic status and cell cycle (Fig 1A, right). T-cell cultures transduced with the various CAR constructs increased from a resting (G0) cell volume of ∼160 fl to nearly 600 fl by day 6 of culture, consistent with the induction of DNA synthesis and the exponential increase in cell numbers. However, the non-continuous CAR T cells and non-transduced T cells rapidly returned to a resting cell volume, while the continuous CAR T cells (c-Met IgG4, SS1 IgG4 and SS1 CD8α) failed to return to a resting cell volume, consistent with continued cellular proliferation. On day 20 of culture, the mean cell volume in cultures of continuous CARs and non-continuous CARs was ∼400 fl and 180 fl, respectively. Notably, the long-term proliferation of the CAR T cells was independent of cognate antigen, because the surrogate ligands c-Met and mesothelin are not expressed at detectable levels on the surface of activated human CD4+ T cells (Supplementary Fig S3), consistent with previous reports (33). Q-PCR analysis did not detect transcripts for mesothelin or c-Met in resting CD4 T cells (Supplementary Table S1). However, activated T cells, either mock transduced or transduced with a continuous CAR and cultured under conditions that lead to long-term growth expressed very low but detectable transcripts specific for c-Met, while mesothelin transcripts remained undetectable. Given that both c-Met and mesothelin-specific CARs displayed the continuous growth phenotype, the low level of c-Met expression in activated T cells is unlikely to be necessary for the sustained growth of T cells. In addition, the absence of fratricide in the cultures is consistent with ligand-independent continuous growth. Finally the results described above were replicated in T cells obtained from at least 10 different healthy donors.
Signaling CD28 and CD3ζ domains is required for constitutive CAR T-cell proliferation
To determine the contribution of signaling to the observed phenotype, we constructed a series of CARs that were identical except that the endodomain was replaced with ICOS, 4-1BB, CD28 and zeta only (Supplementary Fig S1). When T cells were transduced with lentiviral vectors encoding these CARs, only the c-Met IgG4 28ζ CAR T cells exhibited continuous proliferation (Fig 1D). Given that the scFv was held constant in these experiments, signaling from the CD28 transmembrane and cytosolic domain is required for the phenotype.
Constitutive expression of IL2 and a diverse array of cytokines and chemokines
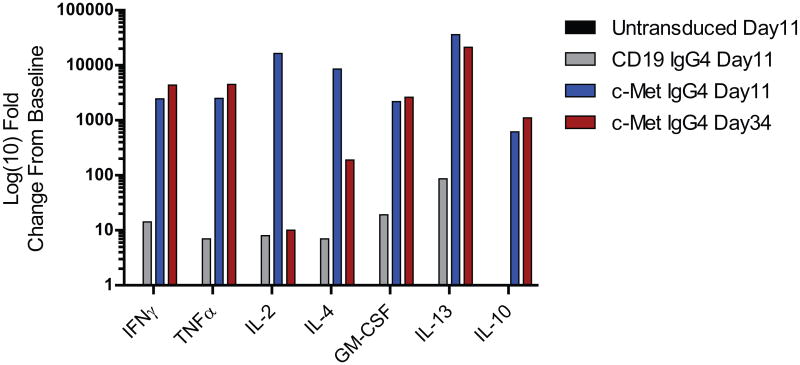
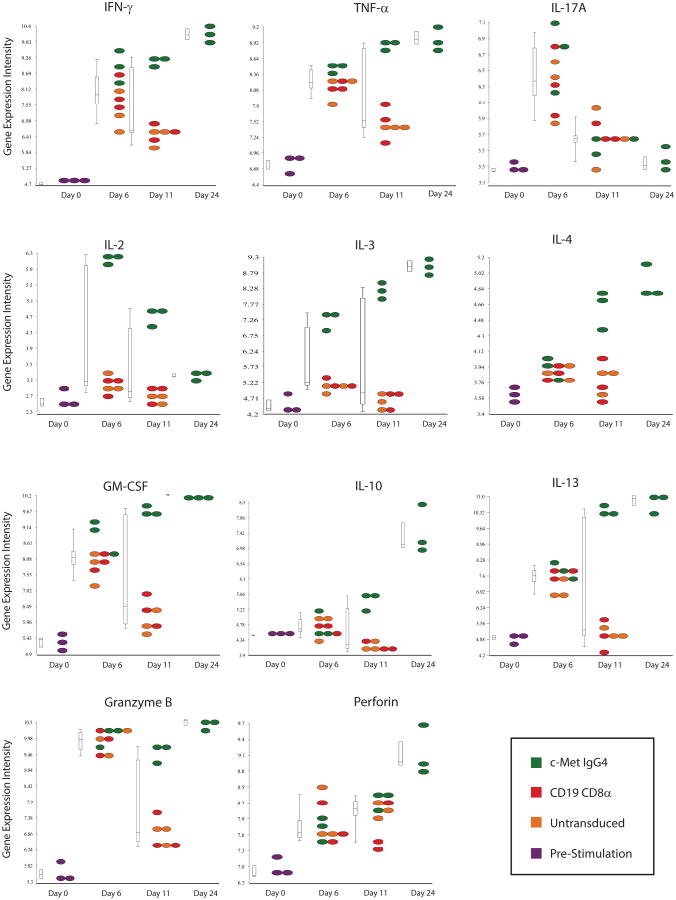
To our knowledge, the observation that CARs can mediate long-term constitutive proliferation of primary T cells has not been reported previously. To begin to understand the mechanism leading to constitutive proliferation, we first determined the levels of various cytokines and other immune-related factors in the supernatants from the cultures that might be sustaining their unusual longevity. Analysis at the protein level revealed that the culture supernatants from continuous CARs contained high levels of cytokines characteristic of both Th1 and Th2 CD4+ T cells (Fig 2). In contrast, the supernatants of non-continuous CAR T cells had low levels of cytokines that decreased with time of culture. The differences were large in magnitude, as the cytokine concentrations in the supernatants of continuous CARs were 100 to >1000-fold higher than those in the non-continuous CAR cultures. The cytokines likely contributed to the proliferation because transfer of day 56-conditioned medium from continuous CAR T cell cultures induced activation of unstimulated naïve CD4+ T cells (Supplementary Fig S4). These results were confirmed at the transcriptional level, with prominent expression of transcripts for IFNγ, TNFα, IL2, IL4, IL13, IL3 and GM-CSF in cells isolated from the constitutively proliferating CAR T cells compared to those from non-continuous CAR T cells (Fig 3). Consistent with this finding, we observed that continuous CAR T cells outgrew normal T cells in cultures that were initially composed of mixtures of CAR T cells and T cells that did not express CARs (Fig 4A). In addition to the sustained transcription and secretion of cytokines and chemokines, continuous CAR CD4+ T cells had elevated levels of granzyme B and perforin (Fig 3), consistent with the potent cytotoxic effector function that was observed (Supplementary Fig S2) and reported(30). The growth was not driven by fetal growth factors because the continuous CAR phenotype occurred in culture medium supplemented with human serum as well as with fetal bovine serum (Supplementary Fig S5).
Figure 2. CAR T cells with continuous T-cell proliferation have constitutive cytokine secretion.
Serial measurements of cytokine production by various CAR constructs following αCD3/CD28 stimulation and expansion. At each noted time point c-Met IgG4, CD19 IgG4 CAR transduced, and untransduced CD4+ T cells were collected from culture, washed and re-plated at 1×106/mL. Cells were kept in culture for 24 hrs at which time supernatant from each culture was collected. Supernatants were analyzed via luminex assay and values plotted as log(10) fold-change from the pre-stimulated cells (baseline). Baseline values (pg/ml) for each analyte were: IFNγ: 5 pg/mL; TNFα: 2 pg/mL; IL2: 1 pg/mL; GM-CSF: 15.25 pg/mL; IL13: 1 pg/mL; IL10: 1 pg/mL. The design of the CAR constructs is shown in Supplemental Figure S1.
Figure 3. CARs with a constitutive growth phenotype display a unique gene signature.
Cytokines, perforin and granzyme expression. Microarray analysis comparing cytokine expression of c-Met IgG4 (green), CD19 CD8-α (red), CART19 (blue) CARs and untransduced (orange) T cells at baseline and on days 6, 22 and 24 of culture; only the c-Met IgG4 culture was analyzed on day 24 because the other cultures were terminated due to cell death. No exogenous cytokines were added to the culture media. Normalized absolute log2 gene expression intensities are plotted for IFNγ, TNFα, IL17A, IL2, IL3, IL4, GM-CSF, IL10, IL13, Granzyme B and Perforin, The design of the CAR constructs is shown in Supplemental Figure S1.
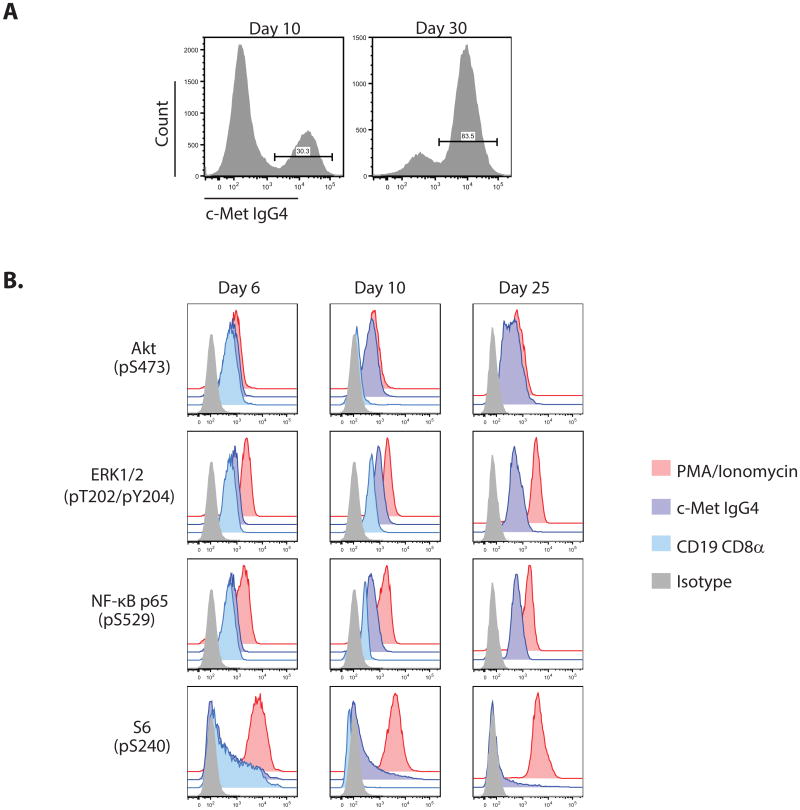
Figure 4. Constitutive activation of AKT, NF-kB and MAPK signaling pathways is associated with the CAR T-cell proliferative phenotype.
A) Representative FACS histograms displaying enrichment of c-Met IgG4 CAR+ T cells during culture from day 10 to day 30 of culture. B) PhosFlow plots of CD4+ T cells stimulated and transduced with the c-Met IgG4 constitutive or CD19 CD8α non-constitutive CARs as previously described. On days 6, 10 and 25 cells were fixed, permeabilized and stained using PE anti-Erk1/2 (pT202/pY204), PE anti-Akt (pS473), PE anti-NF-kB p65 (pS529) and PE anti-S6 (pS235/pS236); the CD19 CD8α CAR culture did not continue to proliferate to day 25, and therefore is only analyzed on days 6 and 10. Positive controls were samples from each condition stimulated for 10 min using PMA/Ionomycin prior to fixation, while negative controls cells were fully stimulated T cells stained using PE-conjugated IgG2b κ isotype control. The design of the CAR constructs is shown in Supplemental Figure S1.
Molecular signature of constitutive CAR T-cell proliferation
We performed gene array analysis to investigate the mechanism leading to long-term CAR T-cell proliferation. The molecular signature of key transcription factors and genes involved in T-cell polarization, growth and survival is shown in Supplementary Fig S6. The master transcription factors T-bet (TBX21), Eomes, and GATA-3 were induced and maintained at high levels in the continuous CAR CD4+ T cells. In contrast, FoxP3 and RORC were expressed at comparable levels in continuous CAR T cells, untransduced activated T cells, and in CAR T cells with the non-continuous proliferative phenotype. As early as day 11, Bcl-xL was highly expressed in continuous CAR T cells compared to the non- continuous CAR and other control T-cell populations (p < 0.001), suggesting that resistance to apoptosis as well as enhanced proliferation contributes to the long-term proliferation of CAR T cells. The day 11 microarray samples were derived from cells that were >90% CAR-positive. Consistent with their substantial proliferative capacity, continuous CAR T cells maintained low level expression of KLRG1, a gene often expressed in terminally differentiated and senescent CD4+ T cells (34).
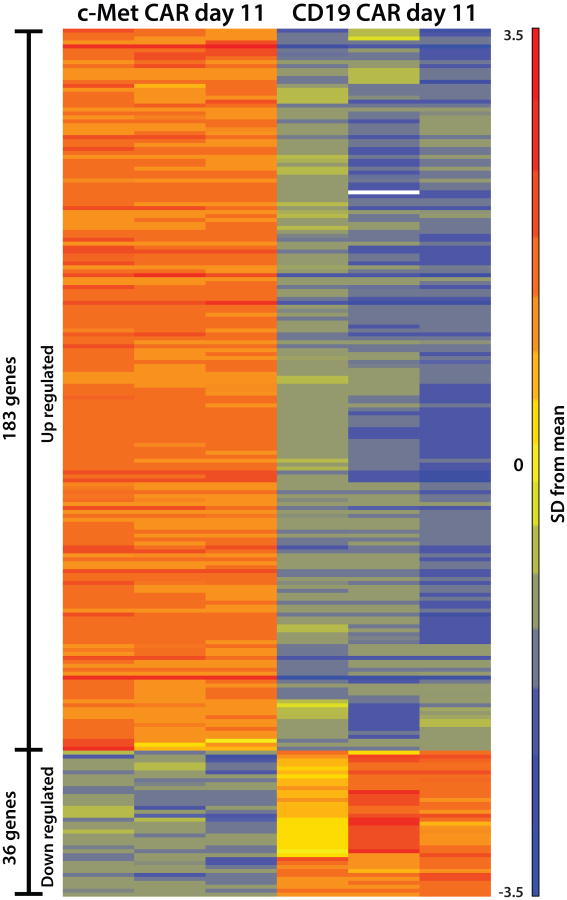
Hierarchical clustering analysis of the microarray data set indicates that the CAR T cells with constitutive T-cell proliferation have a unique molecular signature (Supplementary Fig S7). It is notable that by day 11, cMet IgG4 28ζ CAR T cells with the long-term growth phenotype closely cluster in the dendrogram. In contrast, naïve T cells were most closely related to untransduced T cells and non-continuous CARs on day 11 of culture (Supplementary Fig S7). Similarly, fully activated day 6 T cells from all groups cluster together, while T cells expressing the continuous CAR constructs diverge by day 11 to display a unique RNA signature differs from that in untransduced or non-continuous CAR T cells on day 6 (Supplementary Fig S8). The differentially expressed genes in the continuous CAR (c-Met IgG4) and non-continuous CAR (CD19 CD8α) T cells were plotted as a heat map to depict the relationship of the two populations (Fig 5). When analyzed using a stringent 5-fold cutoff on day 11 of culture, 183 genes were upregulated and 36 genes were down regulated in continuous CARs compared to the non-continuous CAR T cells. A list of the differentially expressed genes is presented in Supplemental Tables 2 and 3. Most notably the continuous CAR T cells are enriched for genes related to control of the cell cycle and a diverse group of cytokines.
Figure 5. Heat map showing relative intensities of the differentially expressed genes in CD4+ T cells expressing continuous CARs or non-continuous CARs.
The differentially expressed genes with a 5-fold cutoff in CD4+ cells from 3 healthy donors are shown for c-Met IgG4 CAR and CD19 CD8α CAR on day 11. The expression level of each gene is represented by the number of standard deviations above (red) or below (blue) the average value for that gene across all samples. The list of the differentially expressed genes is shown in Supplemental Tables S2 and S3. The design of the CAR constructs is shown in Supplemental Figure S1.
Constitutive signal transduction by continuous CARs
To further investigate the mechanisms of the continuous CAR-dependent and ligand-independent T-cell growth, we interrogated the canonical signal transduction pathways that are implicated in T-cell activation and growth (Fig 4B). T cells expressing non-continuous or continuous CARs had similar levels of phosphorylation on Akt, ERK1/2, NF-κB p65 (RelA) and S6 on day 6 of culture. In contrast, only the continuous CAR T cells had sustained activation of Akt pS473, ERK1/2 pT202 and pY204, and RelA pS529 at days 10 and 25 of culture. However, the expression of continuous CARs in cells had only a minor effect on S6 pS240 phosphorylation, indicating that the expression of CARs do not lead to universal activation of T-cell signaling pathways. Constitutive signal transduction together with sustained cytokine secretion indicate that both cell intrinsic and extrinsic effects of CARs can lead to the long-term expansion of primary human T cells.
In the experiments described above, primary human T cells were subjected to a single round of activation with anti-CD3 and anti-CD28 beads, and then followed in culture without the addition of exogenous cytokines. This method of culture was chosen because it has been used in clinical trials, and the initial activation is necessary to mediate high-efficiency transduction of CARs. To determine if the initial activation of T cells by anti-CD3 and anti-CD28 signaling is required for the subsequent constitutive signaling by CARs, we expressed various CARs in a Jurkat T-cell line that stably expresses GFP under the control of the NFAT promoter (Supplementary Fig S9). The cells were analyzed 3 days after transduction; only the continuous CARs as classified by the growth phenotype in primary T cells, led to constitutive NFAT activation in Jurkat cells. This effect was cell intrinsic as only the Jurkat cells that expressed CARs on the surface had GFP expression. In contrast, expression of non-continuous CARs (SS1 CAR with a truncated cytosolic domain and the CD19 CARs) did not lead to constitutive NFAT activation in Jurkat cells.
Level of surface expression contributes to continuous CAR T-cell phenotype
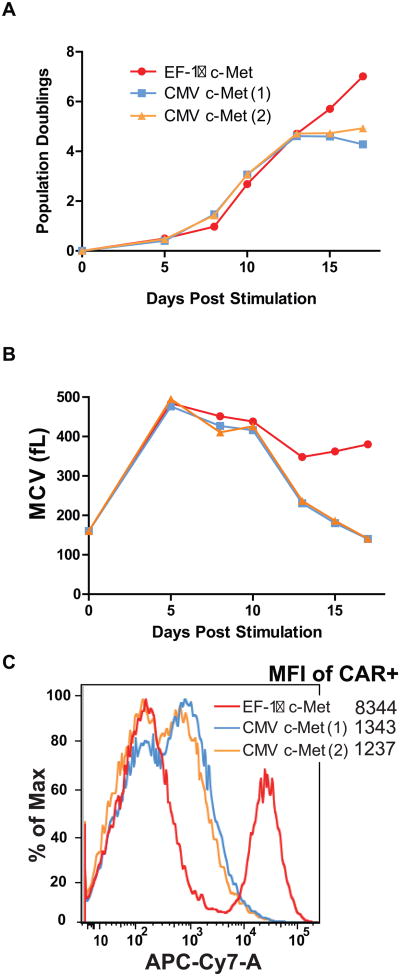
In previous studies we showed that CARs expressed under the control of different eukaryotic promoters in primary T cells had widely varying levels of surface expression (29). To determine if the level of surface expression contributes to the continuous CAR phenotype, the initial experiments were conducted using the EF-1α or CMV promoters to express the CARs, resulting in a higher or lower expression (Fig 6C). By day 9 of culture, there was a 5-fold reduction in surface expression of the CAR driven by the CMV promoter. As in previous experiments, the c-Met CAR displayed a continuous phenotype when under the control of EF-1α. In contrast, the same CAR reverted to a non-continuous CAR phenotype when expressed under the control of the CMV promoter (Fig 6A and B).
Figure 6. A,B and C. Transgene expression levels are sufficient to convey the constitutive CAR growth phenotype.
In vitro proliferation of human CD4+ T cells following 5 days of anti-CD3 plus anti-CD28 stimulation and lentiviral transduction with c-MET-expressing CARs under the control of the indicated promoter. CMV(1) and CMV (2) represent replications of lentiviral vector production in the same human donor. A) Population doublings were determined for both CMV and EF-1α driven c-MET CAR cells. After ∼12 days in culture, CMV-c-MET CAR cells were unable to sustain proliferation and died, while EF-1α c-MET CAR T cells continue to proliferate. B) Mean cell volume (MCV) was also determined. The CMV-c-MET CAR T cells decreased in cell size after 10 days, indicative of the cells resting down. C) Comparison of the level of expression between CARs expressed with the CMV and EF-1α promoters is shown at day 6 post-transduction. The mean fluorescence intensity is indicated. The design of the CAR constructs is shown in Supplemental Figure S1.
To explore the contribution of CAR expression to continuous phenotype, we constructed a panel of promoters driving surface expression that varied by 10- to 20-fold (Supplementary Fig S10 and Fig S1D) using a series of PGK truncation mutants. The growth characteristics of c–Met IgG4 28ζ CAR T cells were compared to the same CAR expressed with the EF-1a promoter (Suppl Fig S10). In both CD4+ and CD8+ T cells the c–Met IgG4 28ζ CAR was continuous when driven by EF-1α and non-continuous when driven by the PGK100 truncation mutant (Suppl Fig S10A and B). However, bright surface expression is necessary but not sufficient for the continuous CAR phenotype because as was shown in figure 1, when the CD19 CARs are expressed at similar levels using the EF-1α promoter they display the non-continuous CAR phenotype. This result suggests that structural characteristics of the particular scFv, in addition to constitutive signaling through CD28 contribute to the continuous CAR phenotype.
Continuous CARs induce T-cell differentiation and proliferation without transformation
Polychromatic flow cytometry was used to characterize CAR T cells with constitutive proliferation. The expression of T-cell molecules associated with activation and differentiation was examined in cultures of cells expressing or not expressing the CAR (Supplementary Fig S11). Additionally, untransduced T cells were followed over time after a single round of stimulation with anti-CD3 and anti-CD28 beads (Supplementary Fig S12). The results show that a progressive enrichment for CAR T cells, so that by day 23 of culture, essentially all cells expressed the CAR. This was associated with bright expression of CD25 at all times on the CAR T cells, whereas CD25 became undetectable by day 14 in the non- transduced companion control culture (Supplementary Fig S12). Similarly, CD70 was expressed at progressively higher frequencies in CAR T-cell culture, a feature not observed in the control culture. In contrast, CD27, the ligand for CD70, was expressed in the control cultures, while CD27 progressively decreased in the CAR T-cell cultures. CD28, CD62L and CCR7 expression was maintained in the control cultures while many of the continuous CAR T cells became dim or negative for these molecules. In contrast, PD-1 was transiently expressed in the control cultures at day 6, while the CAR T cells had a prominent subpopulation of cells that retained expression of PD-1. Finally, Crtam, a molecule associated with cell polarity regulation (35), was induced in the continuous CAR T cell cultures and expression of Crtam was notably restricted to the T cells expressing CARs at the surface.
The potential for the CAR T cells to transform was assessed by long-term cultures in vitro and by transfer of CAR T cells to immunodeficient mice. The long-term cultured CAR T cells do not have constitutive expression of telomerase, as assessed by hTERT expression (Supplementary Fig S6B), and telomere length decreases with time in cultures of continuous CAR T cells (Supplementary Fig S13). In contrast, transformed human T cells have been reported to have constitutive telomerase activity (36). To date, in more than 20 experiments, transformation has not been observed in T cells transduced with continuous CARs. The continuous cytokine-independent polyclonal T-cell proliferation mediated by the CD28:CD3ζ CARs was independent of the specificity of the endogenous TCR, and was not the result of clonal outgrowth because the T-cell populations remained diverse during culture (Supplementary Fig S14).
As a potentially more sensitive assay to detect cellular transformation, NSG (NOD-SCID-γc−/−) mice were used, as previous studies have shown that adoptively transferred transformed and malignant T cells can form tumors in immunodeficient mice (37). Groups of mice were infused with fully activated T cells or with continuous CAR T cells and proliferation assessed by quantification of T cells in the mice and effector function assessed by the induction of xenogeneic graft versus host disease in the mice. By day 60, xeno-reactivity (grade 1-3 xGVHD) was observed in 5/10 mice in the untransduced group compared to 3/10 in the c-Met IgG4 CAR group. Tumor formation was not observed at necropsy, and the levels of T-cell engraftment were similar (p= 0.39) in mice engrafted with continuous CAR T cells or untransduced primary T cells that were stimulated with anti-CD3 and anti-CD28 (Supplementary Fig S15).
Comparison of antitumor effects mediated by continuous and non-continuous CAR T cells
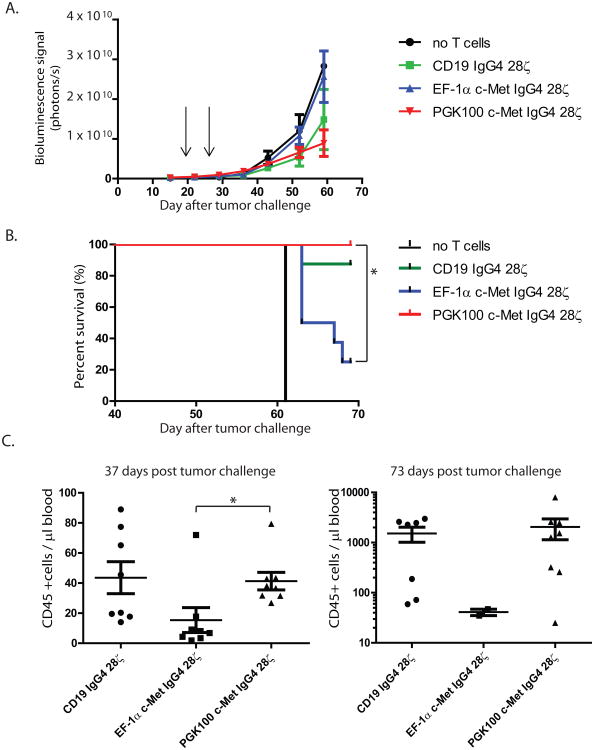
To extend the above phenotypic, functional and transcriptional studies, a series of experiments were conducted in NSG mice with advanced vascularized tumor xenografts. The human ovarian cancer cell line SK-OV3 was selected as a representative c-Met-expressing tumor. We compared the antitumor efficacy of c-Met IgG4 28ζ CAR T cells expressed under continuous or non-continuous conditions using the promoter system shown in Supplementary figures S10. Mock transduced and CD19 IgG4 28ζ CAR T cells served as specificity controls. NSG mice bearing day 16 intraperitoneal tumors were injected intravenously with the T-cell preparations and serial bioluminescence imaging was used as a measure of tumor growth. Surprisingly, the non-continuous c-Met CAR cells with the PGK100 promoter had improved antitumor efficacy compared to the EF-1α group as measured by bioluminescence and survival (Fig 7A and B). Consistent with the improved antitumor effects, the engraftment and persistence of the non-continuous PGK100 CAR T cells was better than that of the continuous EF-1α CAR T cells (Fig 7C). Analysis of tumors from mice with flank tumors showed that there are many more T cells infiltrating the tumors in mice with the CARs using the weaker promoter (Supplementary Figure S16). In addition, the numbers of circulating CAR T cells were significantly higher when mice were treated with CARs driven by weaker rather than by stronger promoters. Together these results suggest that efficacy of CAR T cells in vivo is a function of the density of CAR expression and that this can have a substantial impact on antitumor efficacy and persistence of CAR T cells both systemically and at the tumor site. Mice treated with the irrelevant CD19 CAR had improved survival compared to mice given no T-cell injection, consistent with an allogeneic effect. However mice treated with the continuous c-Met CAR T cells using the EF-1α promoter were inferior in all experimental endpoints: bioluminescence, survival and in vivo CAR T-cell persistence.
Figure 7. A-C. In vivo efficacy of c-Met IgG4 28ζ CAR T cells.
CD4+ and CD8+ T cells transduced to express CD19 IgG4 28ζ or c-Met IgG4 28ζ CAR under the influence of either EF-1α promoter or PGK100 promoter were infused (two administrations, 16 × 106 cells in total) into mice (for no T cells n=2; for the rest n=8 per group) bearing intraperitoneal SKOV3 tumors pre-established for 16 days. (A) Bioluminescence signal was acquired every week as a surrogate for tumor growth. p < 0.01 EF-1α vs PGK100 group. (B) Kaplan-Meier analysis. * indicates p < 0.05, EF-1α vs PGK100, log-rank (Mantel-Cox) test was used for statistical analysis. (C) The absolute number of human CD45+ T cells was determined in the blood on days 37 (left panel) and 73 (right panel), respectively. Only 2 mice survived in the EF-1α c-Met IgG4 28ζ CAR group on d73. * indicates p < 0.05. Two-tailed student T-test was used for statistical analysis. The design of the CAR constructs is shown in Supplemental Figure S1.
Discussion
To our knowledge this is the first description of “continuous CARs”, i.e. primary T cells that exhibit prolonged exponential expansion in culture that is independent of ligand and of addition of exogenous cytokines or feeder cells. The constitutive secretion for several months of large amounts of cytokines by non-transformed T cells was unexpected. The continuous CAR T cells progressively differentiate during culture towards terminal effector cells and transformation has not been observed. The mechanism leading to the growth phenotype includes signal transduction involving canonical TCR and CD28 signal transduction pathways that is independent of cognate antigen. Another mechanism identified is the level of scFv surface expression, as CARs that expressed brightly at the cell surface had sustained proliferation, while CARs that expressed at lower levels did not exhibit sustained proliferation and cytokine secretion. Furthermore, the scFv appears to have important effects on determining the growth phenotype. We have not investigated the role of the hinge domain in these studies.
These results are notable for several reasons. The nature of the scFv has a role in the phenotype, as we have observed continuous CAR phenotype with scFvs that are specific for c-Met and mesothelin but not in the case of FMC63 that is specific for CD19. An implication of this finding is that one cannot assume that the behavior of a signaling domain coupled to a given scFv will be the same when expressed with a distinct scFv. The method of CAR expression also contributes to the growth phenotype. To date we have not observed constitutive growth of T cells when the CARs are expressed by electroporation of mRNA or plasmids encoding Sleeping Beauty transposons (38-40). When expressed using lentiviral vectors, we have only observed continuous growth in vectors that employ the EF-1α promoter but not when driven by CMV or truncated PGK promoters. In previous studies comparing several promoters in lentiviral vectors, we found that this promoter resulted in more stable and higher level expression in primary CD4 and CD8 T cells (29). The particular design of the hinge and extracellular domain does not appear to have a major contribution to the continuous growth phenotype as we have observed this phenomenon with CARs that encode either the longer IgG4 hinge or the shorter CD8α scaffold. High level expression of the CAR appears to be necessary for the continuous growth phenotype. However high level expression is not sufficient to induce constitutive growth, as this phenomenon is only observed when the CAR encodes the CD28 transmembrane and cytosolic domain.
As far as we are aware, this is the first report of constitutive expression of the endogenous IL2 gene in primary non-transformed T cells. Previous studies have shown that constitutive expression of IL2 and CD25 occurs under conditions that lead to transformation of T cells, most prominently in HTLV-1 infection (41). It is likely that sustained signaling of the CD28 cytosolic domain encoded by the CAR is responsible for the constitutive secretion of IL2 and numerous other cytokines. It is interesting that both HTLV-1-mediated expression of IL2 by tax and IL2 secretion driven by the endogenous CD28 pathway have been reported to be resistant to cyclosporine (42, 43), an immunosuppressant that inhibits the calcineurin phosphatase. Consistent with the above, we have not observed constitutive proliferation of CAR T cells encoding ICOS, a signaling molecule that is closely related to CD28 (44).
Our collective results suggest that overexpression of the CD28 transmembrane and cytosolic domains in the context of some CARs can lead to constitutive signaling. Thus, it is likely that the regulation of endogenous CD28 gene expression is a critical determinant of T-cell homeostasis, consistent with studies showing that overexpression of CD28 ligands leads to T-cell hyperplasia in mice (45).
It is not well understood why human T cells progressively downregulate CD28 expression with age and cell division (46). The constitutive CAR T cells maintained CAR expression at bright levels and had far more rapid downregulation of the endogenous CD28 molecule than non-continuous CARs or non- transduced T cells. A dileucine motif in CD28 contributes to limiting expression of CARs on mouse T cells, and mutating this sequence leads to increased expression of the CAR (47). The constitutive CAR T cells that we have tested employed the wild type dileucine motif in the CD28 endodomain.
One of the limitations of our results is that we do not yet have a complete mechanistic understanding of the properties of CAR design that result in non-continuous CAR T-cell growth that is ligand-dependent or continuous CARs that are ligand-independent. Our data indicate that given a permissive scFv, a 5- to 10-fold change in the level of expression can lead to the continuous CAR phenotype. This may explain why other laboratories have not detected this phenomenon using other expression systems. In addition we have not examined the role of the hinge region in these studies. Hudecek and colleagues have recently compared the influence of a CH2-CH3 hinge (229 amino acids (AA)), CH3 hinge (119 AA), and short hinge (12AA) on the effector function of T cells expressing ROR1-specific CARs and concluded that T cells expressing ‘short hinge’ CARs had superior antitumor activity when ROR1 is targeted (48).
The role, if any, of CAR T cells with continuous proliferation in potential clinical applications remains to be determined. We recently reported safety and clinical benefit with CD19 CARs that use the 4-1BB signaling domain (7, 8). T cells expressing this CAR have enhanced ligand-independent proliferation (29) but do not have the long-term continuous growth phenotype that we describe in this report. CARs containing CD28 signaling domains have now been tested with safety in several clinical trials (5, 49-52). However it is important to note that those trials expressed the CARs after manufacturing with a different cell culture system and with a retroviral vector rather than the lentiviral vector that we have used in the present work. Whether continuous CARs such as those that we report here would be useful and safe can only be established in future clinical trials. Overall our present data suggest that strategies to identify CARs with a non-continuous growth phenotype should be used to optimize antitumor efficacy and CAR persistence.
Supplementary Material
Key points.
Chimeric antigen receptors (CARs) have promise for a variety of cancers
An unexpected observation is that some CAR modified T cells that encode a CD28 domain have antigen independent proliferation and cytokine secretion, leading to inferior antitumor effects
Acknowledgments
The authors thank John Tobias and the Microarray Core, Brian Keith for advice, Spencer Small for experimental help, and the Human Immunology Core for healthy donor lymphocytes. This work was supported by funding from NIH grants: R01CA120409, R01CA105216, 2PN2EY016586, 2P30CA016520, P01CA066726, 3T32GM007170-36S1, the Abramson Family Cancer Institute, and Novartis. Data and materials are available from the authors under a material transfer agreement.
Footnotes
Disclosure of Conflicts of Interest: M.J.F, Y.Z, J.S., M.K., M.C.M and C.H.J. have intellectual property rights, including patents, to some of the cell culture technologies and CARs described in the manuscript. A.P, M.C.M and C.H.J have sponsored research grants from Novartis. L.J.N.C received honoraria from Speakers Bureau from Miltenyi, and has ownership interests, including patents, with Targazyme, and is a consultant with Ferring Pharmaceuticals, Janssen Pharmaceuticals and Cellectis. All other authors declare no competing financial interests. Conflicts of interest are managed in accordance with University of Pennsylvania policy and oversight.
Author Contributions: In order of appearance: M.J.F. designed research, conducted research, analyzed data, and edited the manuscript; J.L designed research, conducted research, analyzed data, and edited the manuscript; M.C.B designed research, conducted research, analyzed data, and edited the manuscript; C.C. conducted research; S.M. conducted research; J.S. designed research and conducted research; O.U.K. conducted research; S.G. conducted research and edited the manuscript; S.M. conducted research; A.D.P. conducted research and edited manuscript; S.A. designed research and provided essential protocols and reagents; L.J.N.C. designed research and provided essential protocols and reagents; J.P. conducted research; F.B.J. designed research and conducted research; C.M.P. designed research, conducted research and edited the manuscript; YZ designed research, conducted research and edited the manuscript; M.K. conducted research; M.C.M. conceptualized idea, supervised research, and edited manuscript; C.H.J. conceptualized idea, supervised research, and wrote manuscript.
References
- 1.Jena B, Dotti G, Cooper L. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonini C, Brenner MK, Heslop HE, Morgan RA. Genetic modification of T cells. Biol Blood Marrow Transplant. 2011;17:S15–20. doi: 10.1016/j.bbmt.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ertl HC, Zaia J, Rosenberg SA, June CH, Dotti G, Kahn J, et al. Considerations for the Clinical Application of Chimeric Antigen Receptor (CAR) T Cells: Observations from a Recombinant DNA Advisory Committee (RAC) Symposium June 15, 2010. Cancer Res. 2011;71:3175–81. doi: 10.1158/0008-5472.CAN-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen MC, Cooper LJ, et al. CARS on Track in the Clinic: Report of a Meeting Organized by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Sub-Committee on Cell and Gene Therapy. Washington D.C., May 18, 2010. Mol Ther. 2011;19:432–8. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–5. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finney HM, Lawson ADG, Bebbington CR, Weir ANC. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–7. [PubMed] [Google Scholar]
- 10.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–9. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 11.Feldhaus AL, Evans L, Sutherland RA, Jones LA. A CD2/CD28 chimeric receptor triggers the CD28 signaling pathway in CTLL.2 cells. Gene Ther. 1997;4:833–8. doi: 10.1038/sj.gt.3300456. [DOI] [PubMed] [Google Scholar]
- 12.Geiger TL, Nguyen P, Leitenberg D, Flavell RA. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood. 2001;98:2364–71. doi: 10.1182/blood.v98.8.2364. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa F, Shibaguchi H, Xu ZW, Kuroki M. Targeting of T cells to CEA-expressing tumor cells by chimeric immune receptors with a highly specific single-chain anti-CEA activity. Anticancer Research. 2002:4285–9. [PubMed] [Google Scholar]
- 14.Haynes NM, Trapani JA, Teng MWL, Jackson JT, Cerruti L, Jane SM, et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 2002:5780–6. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- 15.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCR zeta/CD28 receptor. Nat Biotechnol. 2002;20:70–5. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 16.Finney HM, Akbar AN, Lawson ADG. Activation of resting human primary T cells with chimeric receptors: Costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 17.Gyobu H, Tsuji T, Suzuki Y, Ohkuri T, Chamoto K, Kuroki M, et al. Generation and targeting of human tumor-specific Tc1 and Th1 cells transduced with a lentivirus containing a chimeric immunoglobulin T-cell receptor. Cancer Res. 2004;64:1490–5. doi: 10.1158/0008-5472.can-03-2780. [DOI] [PubMed] [Google Scholar]
- 18.Moeller M, Haynes NM, Trapani JA, Teng MW, Jackson JT, Tanner JE, et al. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. Cancer Gene Ther. 2004;11:371–9. doi: 10.1038/sj.cgt.7700710. [DOI] [PubMed] [Google Scholar]
- 19.Teng MW, Kershaw MH, Moeller M, Smyth MJ, Darcy PK. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. HumGene Ther. 2004;15:699–708. doi: 10.1089/1043034041361235. [DOI] [PubMed] [Google Scholar]
- 20.Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D, Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–93. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- 21.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. ProcNatlAcadSciUSA. 2005;102:19051–6. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RLH. T cell retargeting with MHC class I-restricted antibodies: The CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174:7853–8. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- 24.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 25.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 26.Shibaguchi H, Luo NX, Kuroki M, Zhao J, Huang J, Hachimine K, et al. A fully human chimeric immune receptor for retargeting T-cells to CEA-expressing tumor cells. Anticancer Res. 2006;26:4067–72. [PubMed] [Google Scholar]
- 27.Teng MWL, Kershaw MH, Jackson JT, Smyth MJ, Darcy PK. Adoptive transfer of chimeric Fc(epsilon)RI gene-modified human T cells for cancer immunotherapy. Human Gene Therapy. 2006;17:1134–43. doi: 10.1089/hum.2006.17.1134. [DOI] [PubMed] [Google Scholar]
- 28.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 29.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukens JN, Van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimer's and Dementia. 2009;5:463–9. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can ge integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001:6123–31. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 33.Skibinski G, Skibinska A, James K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology. 2001;102:506–14. doi: 10.1046/j.1365-2567.2001.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 35.Yeh JH, Sidhu SS, Chan AC. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell. 2008;132:846–59. doi: 10.1016/j.cell.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–77. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newrzela S, Cornils K, Heinrich T, Schlager J, Yi JH, Lysenko O, et al. Retroviral insertional mutagenesis can contribute to immortalization of mature T lymphocytes. Mol Med. 2011;17:1223–32. doi: 10.2119/molmed.2010.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan A, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9062–72. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X, Wilber AC, Bao L, Tuong D, Tolar J, Orchard PJ, et al. Stable gene transfer and expression in human primary T-cells by the Sleeping Beauty transposon system. Blood. 2006;107:483–91. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T- cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuire KL, Curtiss VE, Larson EL, Haseltine WA. Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J Virol. 1993;67:1590–9. doi: 10.1128/jvi.67.3.1590-1599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Good L, Maggirwar SB, Harhaj EW, Sun SC. Constitutive dephosphorylation and activation of a member of the nuclear factor of activated T cells, NF-AT1, in Tax-expressing and type I human T-cell leukemia virus-infected human T cells. JBiolChem. 1997;272:1425–8. doi: 10.1074/jbc.272.3.1425. [DOI] [PubMed] [Google Scholar]
- 43.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. MolCell Biol. 1987;7:4472–81. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–80. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Fournier S, Allison JP, Sharpe AH, Hodes RJ. The role of B7 costimulation in CD4/CD8 T cell homeostasis. J Immunol. 2000;164:3543–53. doi: 10.4049/jimmunol.164.7.3543. [DOI] [PubMed] [Google Scholar]
- 46.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - A reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012 doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen P, Moisini I, Geiger T. Identification of a murine CD28 dileucine motif that suppresses single-chain chimeric T-cell receptor expression and function. Blood. 2003;102:4320. doi: 10.1182/blood-2003-04-1255. [DOI] [PubMed] [Google Scholar]
- 48.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2014 Sep 11; doi: 10.1158/2326-6066.CIR-14-0127. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kochenderfer J, Wilson W, Janik J, Dudley M, Stetler-Stevenson M, Feldman S, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically-engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.