Abstract
Purpose
Black patients with diabetes are at greater risk for underuse of antidepressants even when they have equal access to health insurance. This study aims to evaluate the impact of removing a significant financial barrier to prescription medications (drug caps) on existing black-white disparities in antidepressant treatment rates among patients with diabetes and comorbid depression.
Methods
We used an interrupted time series with comparison series (ITS) design and a 5% representative sample of all fee-for-service Medicare and Medicaid dual enrollees to evaluate the removal of drug caps on monthly antidepressant treatment rates. We evaluated the impact of drug cap removal on racial gaps in treatment by modeling the month-to-month white-black difference in use within age strata (<65, 65 or older). We compared adult dual enrollees with diabetes and comorbid depression living in states with strict drug caps (n=221) and without drug caps (n=1133) prior to the policy change.
Our primary outcome measures were the proportion of patients with any antidepressant use per month and the mean standardized monthly doses of antidepressants per month.
Findings
The removal of drug caps in strict cap states was associated with a sudden increase in the proportion treated for depression (4 percentage points; 95% Confidence Interval: 0.03, 0.05, <0.0001) and in the intensity of antidepressant use (SMD: 0.05; 95% CI: 0.03, 0.07, p<0.001). While antidepressant treatment rates increased for both whites and blacks, the white-black treatment gap increased immediately after Part D [0.04 percentage points; 95% CI: 0.01, 0.08] and grew over time [0.04 percentage points per month (0.002, 0.01); P<0.001].
Implications
Policies that remove financial barriers to medicines may increase depression treatment rates among patients with diabetes overall while exacerbating treatment disparities. Tailored outreach may be needed to address non-financial barriers to mental health services use among African Americans with diabetes.
INTRODUCTION
Diabetes affects more than 25 million Americans, costs an estimated $245 billion per year in health care costs and lost productivity, and is a major contributor to racial and ethnic disparities in morbidity and mortality.(1,2) More than half of adults with diabetes have at least one other chronic physical or mental health condition.(3) Depression is one of the most prevalent comorbidities, with an estimated 20% of adult diabetes patients suffering from major depression.(4)
Comorbid depression is independently associated with poorer outcomes among individuals with diabetes.(5–7) Identifying and treating depression has become an increasingly important component of diabetes management.(8) While antidepressant medications are highly effective tools in the co-management of depression among adults with diabetes, rates of pharmacologic treatment of depression remain suboptimal and are especially low among African Americans.(9,10)
The reasons for these differences likely include patient preferences for psychotherapy versus medications, stigma associated with depression, variation in care quality, competing demands, differences in prescribing, and out of pocket medication costs.(11–14) Previous studies suggest that African Americans may be at greater risk for cost-related non-adherence and that reducing economic barriers to prescription drugs may reduce disparities in adherence to chronic disease medications.(15,16) Natural experiments that increase economic access to prescription drugs provide a unique opportunity to evaluate the degree to which such policy changes may reduce disparities in antidepressant treatment among diabetes patients.
When Medicare Part D was implemented as an outpatient prescription benefit for Medicare enrollees in 2006, seven million people who were dually enrolled in both Medicare and Medicaid due to permanent disability or low income were automatically transitioned from Medicaid drug coverage to Part D.(17,18) For dual enrollees living in states with strict caps on the number of reimbursable prescriptions per month (drug caps), the transition to Part D, which disallowed drug caps, eliminated a significant financial barrier to drug treatment.(19) Our central research question was whether the transition to Part D increased antidepressant treatment rates among dual enrollees with diabetes and depression living in states with strict drug caps at the time of the transition relative to those living in states without drug caps. Secondarily, we examined whether there were differences in response by race, in order to assess the potential for such policies to reduce disparities in antidepressant treatment among diabetes patients.
We hypothesized that the transition would increase overall rates of treatment of depression and reduce the racial disparity in antidepressant use. The findings from this study are highly relevant as newly eligible dual enrollees continue to transition from states with restrictive drug caps to Medicare Part D on an ongoing basis following the two-year waiting period for Medicare eligibility. In addition, they have the potential to inform evaluation of Medicaid coverage expansion under the Affordable Care Act and the need to assess the impact of the ACA on disparities populations. Specifically, the findings from this study provide information about the potential for reducing out-of-pocket costs for medications as a strategy to address under-treatment among patients with diabetes and as a mechanism to reduce racial disparities in depression treatment.
RESEARCH DESIGN AND METHODS
We used an interrupted time series with comparison series (ITS) design to examine changes in the monthly rate of antidepressant treatment for 24 months before and after the transition of dual enrollees from Medicaid to Medicare Part D drug coverage. This quasi-experimental design provides strong evidence of the immediate and ongoing effects of Part D on antidepressant treatment among diabetes patients with comorbid depression.
Our study protocol was reviewed and approved by the Institutional Review Board of the Kaiser Foundation Research Institute.
Data Sources and Study Population
We used a 5% nationally representative sample of dual Medicare and Medicaid enrollees provided by the Centers for Medicare and Medicaid Services. These data contain all health insurance claims and enrollment data between January 2004 and December 2007.(20,21) Using these data, we identified a cohort of fee-for-service dual beneficiaries who were at least 18 years old in 2004 and who had at least one hospital diagnosis or two physician diagnoses (no more than 12 months apart) of diabetes (International Classification of Diseases, ICD9: 250.XX) (22) at any time during the study period (2004–2007). We excluded enrollees residing in Ohio, Arizona, and Louisiana due to data anomalies such as concurrent changes in coding and reporting methods.(23) The resulting population included 60,288 adults with diabetes.
In order to ensure stable population characteristics over time and complete data on utilization, we also required continuous enrollment for at least 10 months per year in fee-for-service Medicaid and Medicare during the study period (n=29,373) and excluded patients with more than 90 consecutive days in any year in an institution such as a nursing home (n=22,774).
Drug Reimbursement before Part D
We assigned strict drug cap status to four states (Texas, Oklahoma, Mississippi, Arkansas) that consistently limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during the 24-month baseline period 2004–2005. We excluded from analysis 11 states where true exposure to or enforcement of drug caps was uncertain. For example, some states required caps, but allowed for exceptions for chronic disease medications. We conducted sensitivity analysis to assess the impact of excluding these 11 states and found that rates of medication use in the excluded states mimicked those of the no drug cap group. In addition, we excluded Tennessee, which instituted a strict drug cap late in the baseline period.(24)
We identified a comparison subgroup of dual enrollees who lived in 31 states (AK, CO, CT, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY) and the District of Columbia where there were no prescription drug caps during the 24-month baseline period. The strict drug cap and no drug cap states included 10,992 adults diagnosed with diabetes. Enrollees in our sample were similar to the overall fee-for-service dual enrollee population at the time with respect to race and ethnicity distribution, but slightly more likely to be under 65 (40% vs. 36%) and female (70% vs. 62%).(25)
Comorbid Major Depression
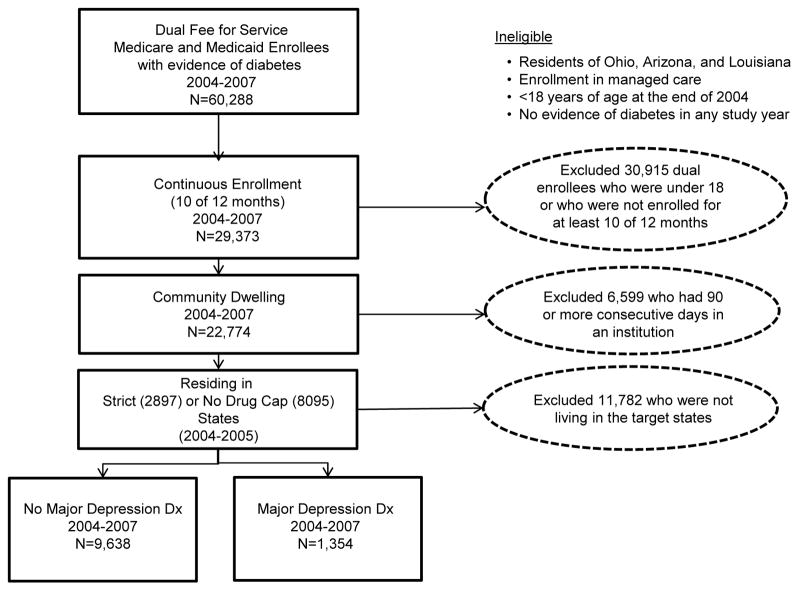
Within the cohort of 10,992 adult dual enrollees with diabetes, we identified a subset of people with evidence of major depression, defined as having at least one hospital or two physician visits with a diagnosis of major depression.(26) The resulting 1,354 dual enrollees with diabetes and comorbid major depression, representing 27,080 dual enrollees, served as the analytic cohort for this paper. (Figure 1)
Figure 1.
Description of the application of inclusion and exclusion criteria and impact on sample size.
Outcomes
Our primary outcomes were (1) the monthly proportion of patients with any use of antidepressants, including tricyclics, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, serotonin modulators, and selective norepinephrine reuptake inhibitors, and (2) the intensity of use of these medications as represented by standardized monthly doses. To assess intensity of use, we first defined a standardized monthly dose (SMD) (27,28) for each unique molecular entity of interest, which was equal to the median number of milligrams dispensed per month across person-months with any use during the entire study period. Thus, SMDs represent the population’s “typical” monthly dose for a given medication in this population. We then calculated average SMDs dispensed per patient per month across all antidepressant medications.
Policy Variables
We controlled for prior trends in the outcomes of interest using segmented time series regression, as described in previous studies.(19,27,29–31) We included a dichotomous indicator for months before and after Part D implementation, as well as a variable to estimate changes in post-Part D trends. We identified state drug cap status in 2005 (strict drug cap vs. no drug cap) using a dichotomous indicator. We then estimated the effect of Part D within strict cap and no cap states, separately.
Covariates
We used Medicare administrative files to determine racial identity, age, and gender. Given higher rates of long-term disability among younger dual enrollees, we stratified all analyses by age: aged 65 or older (elderly) versus 18–65 years of age (non-elderly). Only black and white dual enrollees are included in this analysis due to the lack of sensitivity and specificity for other racial and ethnic categories during the study period.(32, 33)
Statistical Analysis
We used interrupted time series (ITS) with comparison series to evaluate changes from baseline in the level and trend of antidepressant use.(30) Segmented regression analysis of interrupted time series data allowed us to evaluate the immediate discontinuity and longer term slope change in medication after the introduction of Part D. In these models, potential confounding was limited to factors that were both correlated with the outcome and that changed at the same time as the intervention. Therefore, factors that changed gradually over time such as age, education, income and comorbidity were not potential confounders and were not included in the regression models. (30)
We first estimated the overall impact of transitioning to Part D by cap status using separate ITS models for dual enrollees with major depression in strict drug cap and no drug cap states. To control for anticipatory effects of the policy (e.g., changes in prescribing practices) and the phase-in period of the transition, we excluded the observations between December 2005 and March 2006. Our time series models controlled for autocorrelation by testing for first-order autoregressive processes and correcting for significant correlations. We also tested for non-linearity of the models.(30)
We next examined changes in rates of antidepressant use and in intensity of use, stratifying by black and white race within non-elderly (under 65) and elderly (65+) subgroups. We then directly evaluated the impact of Part D on racial gaps in treatment by modeling the month-to-month white-black difference in use within age strata.
All statistical analyses were conducted using the SAS system (SAS, v.9.0, Durham, NC).(34)
RESULTS
Baseline Characteristics by Depression Status
Table 1 examines the characteristics of dual enrollees with diabetes at baseline (2005) overall and compares demographic characteristics between patients with and without evidence of diabetes and comorbid major depression during the study period. Dual enrollees with a major depression diagnosis were more likely to be white, under the age of 65, and female compared to those without concurrent depression. In addition, those with diagnoses of comorbid major depression were more likely to be living in states without drug caps prior to Part D.
Table 1.
Baseline Characteristics of Dual Medicare and Medicare Enrollees with Diabetes in 2005 by State Drug Cap Status
| All | No Depression | Depressiona | p-value | ||||
|---|---|---|---|---|---|---|---|
| n=10992 | %/sd | N=9638 | 88% | N=1354 | 12% | ||
| Race | |||||||
| White | 6555 | 60% | 5607 | 58% | 948 | 70% | <0.0001 |
| Asian | 474 | 4% | 461 | 5% | 13 | 1% | |
| Black | 2340 | 21% | 2141 | 22% | 199 | 15% | |
| Hispanic | 1189 | 11% | 1030 | 11% | 159 | 12% | |
| Native American | 191 | 2% | 170 | 2% | 21 | 1% | |
| Other | 226 | 2% | 212 | 2% | 14 | 1% | |
| Missing | 17 | <1% | 17 | <1% | 0 | 0% | |
| Age group (%) | |||||||
| 65 and older | 6617 | 60.198 | 6145 | 64% | 472 | 35% | <0.0001 |
| less than 65 | 4375 | 39.802 | 3493 | 36% | 882 | 65% | |
| Gender (%) | |||||||
| Female | 7663 | 69.714 | 6683 | 69% | 980 | 72% | 0.0227 |
| Male | 3329 | 30.286 | 2955 | 31% | 374 | 28% | |
| Drug Cap Statusb | |||||||
| None | 8095 | 74% | 6962 | 72% | 1133 | 84% | <0.0001 |
| Strict Drug Caps | 2897 | 26% | 2676 | 28% | 221 | 16% | |
: At least one hospital or two physician claims (within 12 months of each other) with ICD 9=296.2, 296.20, 296.21, 296.22, 296,23, 296.24, 296.25, 296.26, 296.3, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 296.82, 298.0, 300.4, 300.40, 301.12, 309, 309.0, 309.1, 309.28, or 311.
: State Medicaid program limited monthly drug coverage to five or fewer total prescriptions or to three or fewer brand name drugs during the 24-month baseline period, 2004–2005. Included in the no drug cap category are DC and 31 states, including AK, CO, CT, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY. The 4 strict cap states were: AR, MS, OK, TX. The following 11 states had less restrictive caps: AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC. Three states were excluded from the study due to data anomalies: AZ, LA, OH. We also excluded one state due to the introduction of a drug cap during the baseline period: TN.
Impact of Part D on Antidepressant Use in States with and without Drug Caps
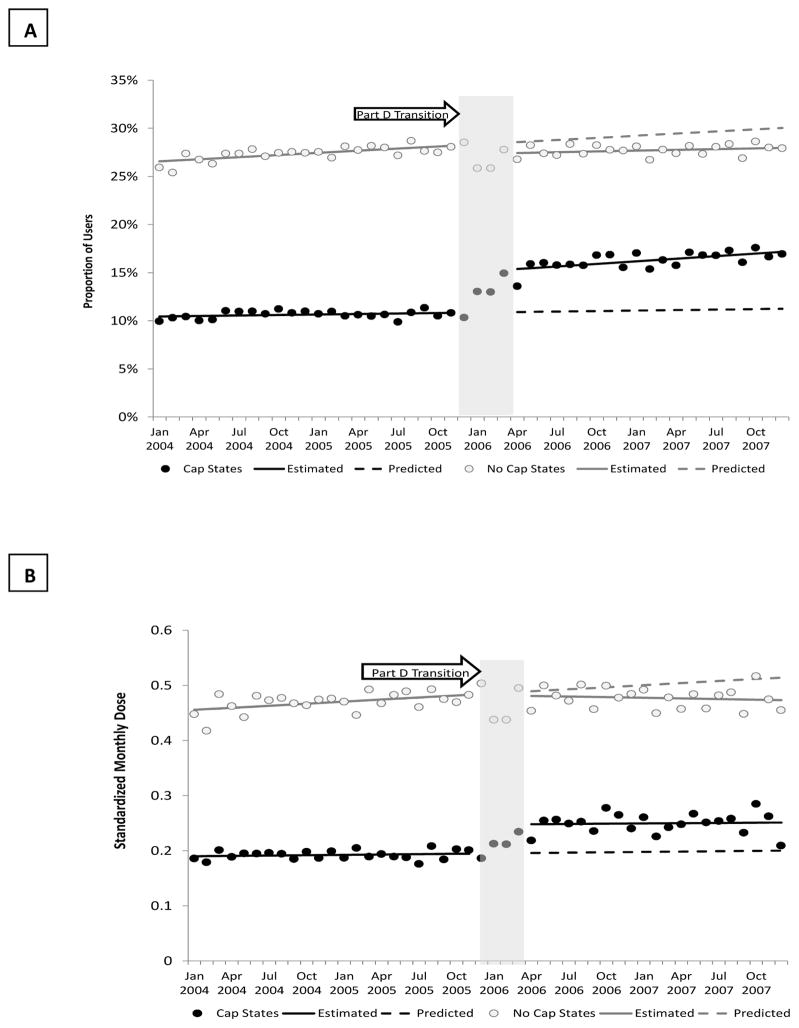
At baseline (Table 2), the proportion using antidepressants in states without drug caps was more than twice that for an equivalent population of dual enrollees living in strict cap states.[11% (cap) vs. 28% (no cap), November 2005] The transition to Part D was associated with a statistically significant increase in the proportion of antidepressant users in drug cap states (4 percentage points; 95% Confidence Interval: 0.03, 0.05, <0.0001) and an increasing trend in this proportion post policy (0.1 percentage points per month (0.0002, 0.001), p=0.01).(Figure 2) The estimated relative increase over the value predicted by baseline trend in the proportion of patients using any antidepressants at 12 months post Part D implementation (December 2007) was 45%. There was a corresponding small decrease in the proportion of people with any use in no drug cap states (−1 percentage point; 95% CI: −0.01, −0.003; p=0.007) and a slight declining trend post policy (−0.05 percentage points per month; 95% CI: −0.001, −0.0001, p=−0.026) in these states. The corresponding relative decrease in use at 12 months post Part D was 3%.
Table 2.
Estimated Effects of the Removal of Drug Caps Under Medication Part D on Prevalence and Intensity of Antidepressant Use among Dual Enrollees with Diabetes and Co-Morbid Major Depression by State Drug Cap Status*
| All | Observed At Baseline (Nov 2005) | Baseline Trend | Part D | Trend Change | Absolute Effect 12 Months Post (Dec 2007)† | Relative Effect 12 Months Post (Dec 2007)† | |||
|---|---|---|---|---|---|---|---|---|---|
| Cap States | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | p-value | ||||
| Any Use | 11% | 0.0002 (−0.0002,0.0005) | 0.344 | 0.04 (0.03,0.05) | <0.0001 | 0.001 (0.0002, 0.001) | 0.010 | 0.05 | 45% |
| SMD‡ | 0.20 | 0.0002 (−0.001,0.001) | 0.628 | 0.05 (0.03,0.07) | <0.001 | −0.0001 (−0.001,0.001) | 0.916 | 0.04 | 25% |
| No Cap States | |||||||||
| Any Use | 28% | 0.001 (0.0004,0.001) | <0.001 | −0.01 (−0.01, −0.003) | 0.007 | −0.0005 (−0.001, −0.0001) | −0.026 | −0.02 | −3% |
| SMD‡ | 0.48 | 0.001 (0.0005,0.002) | 0.001 | −0.002 (−0.02,0.01) | 0.828 | −0.002 (−0.003, −0.0005) | 0.005 | −0.03 | −4% |
: The 32 no-cap states were: AK, CO, CT, DC, DE, FL, HI, IA, ID, IN, MA, MD, MI, MN, MO, MT, ND, NE, NH, NJ, NM, NV, OR, RI, SD, UT, VA, VT, WA, WI, WV, WY. The 4 strict cap states were: AR, MS, OK, TX. The following 11 states had less restrictive caps: AL, CA, GA, IL, KS, KY, ME, NC, NY, PA, SC. Three states were excluded from the study due to data anomalies: AZ, LA, OH. We also excluded one state due to the introduction of a drug cap during the baseline period: TN.
: Attributable differences (absolute and relative) as compared to expected values 1 year after Part D implementation were estimated for December 2007.
: Mean standardized monthly doses (SMDs) calculated across all individuals in cohort, not only those with dispensed claims.
Figure 2. Changes in the Prevalence of Antidepressant Use Before and After Part D by Drug Cap Status*.
*Monthly Prevalence (A) and Intensity of Antidepressant Use (B) Before and After the Transition from Medicaid to Part D among Dual Enrollees with Diabetes and Co-Morbid Major Depression in Strict Drug Cap Compared to No Drug Cap States; Solid black circle=observations in drug cap states; solid black line=estimated utilization in drug cap states from time series models; Black dashed line=predicted utilization in drug cap states based on baseline trends; Open grey circle=observations in non-drug cap states; solid grey line=estimated utilization in non-drug cap states from time series models; Black dashed line=predicted utilization in non-drug cap states based on baseline trends
Dual enrollees in strict drug cap states also experienced a one-time increase in the standardized monthly dose of antidepressant use (SMD: 0.05; 95% CI: 0.03, 0.07, p<0.001), but no change was observed among dual enrollees in no drug cap states (SMD: −0.002; 95% CI: −0.02, 0.01, p=0.828), although we observed a slightly declining trend in the intensity of use in those states post policy (−0.002 standard monthly doses per month; 95% CI: −0.003, −0.0005, p=0.005). At 12 months post Part D implementation, mean SMD per month within strict cap states increased 25% relative to what would have been predicted according to baseline trends. Within no drug cap states, there was an estimated 4% decrease in the mean SMD per month relative to baseline.
Racial Differences in Antidepressant Use Pre and Post Policy in Cap States
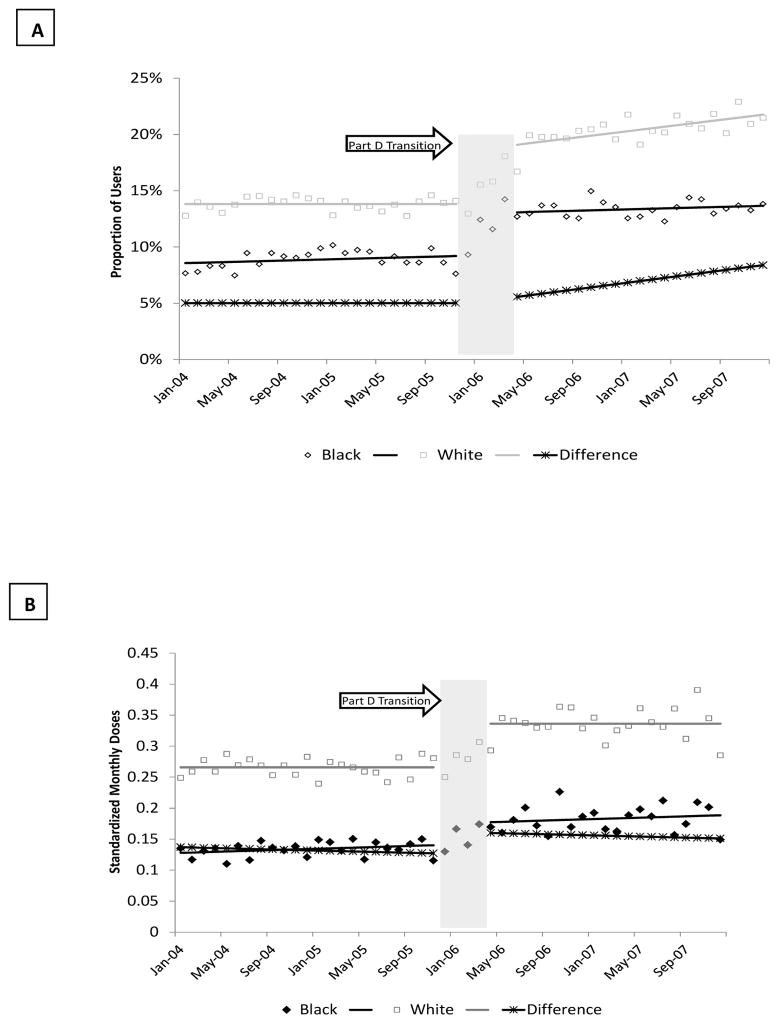
Prior to the policy change, the observed proportion of depressed patients using antidepressants in strict cap states was half as much for blacks compared to whites.(Figure 3A) The Part D transition was associated with an increase in the proportion treated among whites [non-elderly: 0.08 percentage points; 95% CI: 0.06, 0.11; elderly: 0.03 percentage points; 95% CI: 0.02, 0.04] and blacks [non-elderly: 0.04 percentage points; 95% CI: 0.002,0.01; elderly: 0.04 percentage points; 95% CI: 0.03,0.04] in strict drug cap states, that was not observed among dual enrollees living in states without drug caps.(Table 3, Columns 2–4) The greater increase among white non-elderly dual enrollees in strict cap states resulted in a statistically significant increase in the gap between white and black patients [0.04 percentage points; 95% CI: 0.01, 0.08] This gap grew gradually wider during the post-Part D period [0.04 percentage points per month (0.002, 0.01); P<0.001].
Figure 3. Changes in the Prevalence and Intensity of Antidepressant Use among Dual Enrollees Pre and Post Part D in States with Drug Cap by Race*.
*Monthly Prevalence (A) and Intensity of Antidepressant Use (B) Before and After the Transition from Medicaid to Part D among Black and White Dual Enrollees with Diabetes and Co-Morbid Major Depression in Strict Drug Cap States Only; Solid black diamond=observed utilization of black patients; solid black line=estimated utilization for black patients from time series model; Open grey square=observed utilization of white patients; solid grey line=estimated utilization for white patients from time series model; dotted black line=estimated difference in utilization between whites and blacks from time series models
Table 3.
Effects of Part D on Antidepressant Use among Dual Enrollees with Diabetes and Co-Morbid Major Depression by Drug Cap Status, Age and Race
| Proportion with any Use | Standardized Monthly Dose | |||||
|---|---|---|---|---|---|---|
| CAP STATES Non-Elderly | Level Change in the % with Any Use (%) | % with Any Use Pre Part D (Nov 2005) | % with Any Use 12 Mos Post Part D (Jan 2007) | Level Change in SMDs per Month | Mean SMDs Pre Part D | Mean SMD 12 Mos Post Part D |
| Whites | 0.08 (0.06,0.11)a | 21.5% | 31.8% | 0.12 (0.09,0.15)a | 0.49 | 0.62 |
| Blacks | 0.04 (0.002,0.01)a | 13.9% | 17.7% | 0.05 (0.01,0.09)b | 0.24 | 0.29 |
| Difference | 0.04 (0.01,0.08)b | 0.10 (0.03,0.16)a | ||||
| Elderly | ||||||
| Whites | 0.03 (0.02,0.04)a | 10.4% | 15.0% | 0.03 (0.0002,0.06)b | 0.16 | 0.20 |
| Blacks | 0.04 (0.03,0.04)a | 6.7% | 10.9% | 0.03 (0.02,0.03)a | 0.08 | 0.12 |
| Difference | −0.01 (−0.02,0.003) | 0.004 (−0.03, 0.04) | ||||
| NO CAP STATES Non-Elderly | ||||||
| Whites | −0.01 (−0.02, −0.004) | 43.6% | 42.8% | 0.004 (−0.03,0.03) | 0.87 | 0.87 |
| Blacks | 0.01 (−0.0004,0.03) | 23.1% | 23.2% | 0.02 (−0.01,0.05) | 0.42 | 0.42 |
| Difference | −0.02 (−0.03, −0.01)a | −0.01 (−0.04, 0.01) | ||||
| Elderly | ||||||
| Whites | −0.01 (−0.02,0.001) | 25.0% | 24.7% | −0.001 (−0.002,0.00001) | 0.34 | 0.34 |
| Blacks | −0.01 (−0.02, −0.01) | 12.6% | 11.8% | −0.02 (−0.04, −0.01)a | 0.15 | 0.14 |
| Difference | 0.01 (−0.003, 0.02) | 0.01 (−0.01, 0.04) | ||||
p<0.01
p<0.05
In contrast, following Part D, there was a slight reduction in the treatment gap between white and black non-elderly dual enrollees in no drug cap states [−0.02 percentage points; 95% CI: −0.03, −0.01] due to a decrease in the proportion treated among whites [−0.01; 95% CI: −0.02, −0.004]. The treatment gap continued to decline over time among elderly dual enrollees in no cap states [−0.01 percentage points per month; 95% CI: −0.001, −0.00004; p<0.05].
Changes in the intensity of use by race are presented in Figure 3B and Table 3, Columns 5–7. Part D was associated with an increase in the intensity of use for both white [non-elderly: 0.12 SMDs; 95% CI: 0.09, 0.15; elderly: 0.03 SMDs; 95% CI: 0.0002, 0.06] and black [non-elderly: 0.05 SMDs; 95% CI: 0.01, 0.09; elderly: 0.03 SMDs; 95% CI: 0.02, 0.03] dual enrollees in drug cap states, regardless of age. The magnitude of change was greatest among non-elderly whites [0.12 SMDs; 95% CI: 0.09, 0.15], resulting in a statistically significant increase in the gap between racial groups of 0.10 standard monthly doses at the time of the policy [95% CI: 0.03, 0.16]. No statistically significant changes in level or trend in the intensity of use of antidepressants were observed among dual enrollees in non-drug cap states with the exception of a decrease among black elderly patients at the time of the policy [−0.02 SMDs; 95%CI: −0.04. −0.01].
DISCUSSION
Among dual enrollees with diabetes and comorbid major depression living in states with drug caps, we observed a modest increase in the proportion using antidepressants and in the intensity of antidepressant use after the transition to Part D. The absence of a corresponding increase in use in non-drug cap states is consistent with our hypothesis that the removal of drug caps under Part D would be associated with an increase in access to antidepressant therapy. However, we also observed an unexpected increase in the racial gap in antidepressant use among dual enrollees with diabetes and major depression in strict drug cap states immediately following Part D implementation that was driven by a greater response to the policy among non-elderly whites.
Two previous studies of Part D effects reported no changes in medication use associated with Part D among elderly dual enrollees.(35,36) Differences between our work and previous studies of changes in utilization may be explained by our focus on those living in states with strict drug caps. In addition, we observed a slight decrease in use within non-drug cap states; combining all dual enrollees without regard to coverage changes would lead to a conclusion of no change at the population level.
To our knowledge, our study is the first to show an increase in the racial gap in antidepressant treatment associated with Medicare Part D implementation. In a related study, we reported that Part D was equally effective in increasing the use of lipid lowering medications among black and white dual enrollees with diabetes.(37) The reasons for differential response to coverage expansion are likely multifactorial. One possible explanation for this difference is that use among African Americans may be driven by non-financial factors to a greater extent than it is among whites..(38) For example,, several studies have reported cultural differences in preferences for mental health treatment and illness-related beliefs that may contribute to reduced price sensitivity among African Americans.(3,11) In addition, differences in knowledge of coverage changes, may also contribute to the observed differential policy response.(39) For example, Haviland and colleagues reported that African Americans, Hispanics and Asian Pacific Islanders were all more likely to report lack of knowledge about Part D plan coverage.(40) More studies are needed to identify the potential mechanisms for differential response in order to identify mediating factors that are potentially modifiable through health system intervention (e.g., knowledge gaps).
This quasi-experimental study has some limitations that deserve consideration. During the study period, there were other changes in Medicaid and Medicare coverage and utilization management that may have contributed to the observed changes. By our design, we focused on a population of dual enrollees who experienced a substantial change in exposure to drug caps for simplicity of analysis and because caps are known to have especially strong effects.(9,27,29) We limited analysis to enrollees who had evidence of major depression during the study period. Since antidepressants may be used for other indications, this allowed us to focus on a more homogenous population. However, our findings may apply to the treatment of milder forms of depression as well that are not as reliably identified in claims data. In addition, with the exception of black and white dual enrollees, race and ethnicity data in Medicaid are generally unreliable for the period of this study. As a result, we did not include subgroups other than blacks and whites in our comparison of race differences in use, nor could we accurately exclude dual enrollees of Hispanic origin from our comparisons. Therefore, there may have been additional variation in response in these subgroups that we failed to capture. Allowing beneficiaries to have a diagnosis at any time during the study period means that some patients included in the denominator at any given time may have been ineligible for treatment. However, this error should be distributed equally across subgroups of patients and change gradually over time, and is therefore unlikely to have introduced bias in our findings. Finally, our observations of a study population living in selected states with substantial financial barriers in Medicaid drug coverage do not necessarily reflect the experience of the entire dual enrollee population.
CONCLUSION
Depression is frequently undertreated among patients with diabetes and African Americans may be at higher risk for under-treatment of this high cost and potentially disabling comorbidity.(9,10) Inadequate medication coverage poses a significant barrier to treatment for many patients, particularly low income Medicaid beneficiaries.(12–14) In this natural experiment, we demonstrated the potential for expanded drug coverage to improve rates of antidepressant use. However, coverage expansions may also have the unintended consequence of widening racial disparities in antidepressant treatment. The ongoing transition of dual enrollees from state Medicaid drug coverage to Medicare Part D programs following a two-year waiting period may perpetuate these disproportionate impacts in certain states.(18) At a minimum, federal and state health systems leaders should be aware of the potential for unintended effects of coverage expansions for disparities reduction. Interventions at the point of care may be needed to counteract these effects through increased education and outreach to African Americans with diabetes about the importance of depression treatment as a component of diabetes care.
Acknowledgments
2) Funders: This study was supported by grants from the National Institute on Aging [5R01AG032249] and the Agency for Health Care Research and Quality [1R01HS018577]. Additional support was provided by the National Cancer Institute, HMO Cancer Research Network [U24 CA171524], the Patient-Centered Outcomes Research Institute [CE-1304-7250], the Learnings in Diabetes Prevention from an Integrated Delivery System [U58 DP002721], the Medication Adherence and Social Disparities in Diabetes [R01DK080726] study, the Health Delivery Systems Center for Diabetes Translational Research funded by the National Institute for Diabetes, Digestive and Kidney Diseases [P30DK092924], the Agency for Health Care Research and Quality [K01 HS018072], and the National Institute of Mental Health [K01 MH092338].
Footnotes
1.) Contributors: The authors would like to thank Dr. Christine Bishop at the Heller School for Social Policy and Management at Brandeis University for facilitating access to data from the Centers for Medicare and Medicaid Services. In addition, we would like to acknowledge Dr. Andrea Altschuler at Kaiser Permanente Division of Research, PhD, Ms. Angelina Lee and Dr. Meredith Chace, PhD, at JEN Associates, Inc., and Ms. Rosa Hippler, MA, at Kaiser Permanente Division of Research for assistance with project management, data collection, data verification and editing. We are indebted to Drs. Joseph P. Newhouse at the Department of Health Care Policy at Harvard Medical School and Larissa Nekhlyudov at the Department of Population Medicine at Harvard Medical School and Harvard Pilgrim Health Care Institute for lending their advice and comments on an earlier version of this manuscript. Written permission has been obtained from each of these individuals.
3) Prior Presentations: An abstract of these preliminary findings was presented at the Academy Health Annual Research Meeting in Orlando, Florida in June 2012.
The authors report no conflicts of interest that could inappropriately influence this work. The study finders had not role in the design, collection, analysis, interpretation of the data, writing of the manuscript or in the decision to submit the manuscript for publication.
The authors report no conflicts of interest.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002 Nov 14;347(20):1585–92. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 3.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: Estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:120203. doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: Impact of depressive symptoms on adherence, function and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ, Rutter C, Simon G, Lin EHB, Ludman E, Cienchanowski P, Kinder L, Young B, Von Korff M. The association of comorbid depression with mortality in patients with type 2 diabetes. Diab Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 7.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diab Care. 2002;25:464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013 Jan;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer AM, Schillinger D, Parker MM, Katon W, Adler N, Adams AS, Moffet HH, Karter AJ. Health Literacy and Antidepressant Medication Adherence Among Adults with Diabetes: The Diabetes Study of Northern California (DISTANCE) J Gen Intern Med. 2013 Mar 20; doi: 10.1007/s11606-013-2402-8. Epub 2013 March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn 2010
- 11.Jimenez DE, Bartels SJ, Cardenas V, Dhaliwal SS, Alegría M. Cultural beliefs and mental health treatment preferences of ethnically diverse older adult consumers in primary care. Am J Geriatr Psychiatry. 2012 Jun;20(6):533–42. doi: 10.1097/JGP.0b013e318227f876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akincigil A, Olfson M, Siegel M, Zurlo KA, Walkup JT, Crystal S. Racial and ethnic disparities in depression care in community-dwelling elderly in the United States. Am J Public Health. 2012 Feb;102(2):319–28. doi: 10.2105/AJPH.2011.300349. Epub 2011 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sclar DA, Robison LM, Schmidt JM, Bowen KA, Castillo LV, Oganov AM. Diagnosis of depression and use of antidepressant pharmacotherapy among adults in the United States: does a disparity persist by ethnicity/race? Clin Drug Investig. 2012 Feb 1;32(2):139–44. doi: 10.2165/11598950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Skaer TL, Sclar DA, Robison LM, Galin RS. Trends in the rate of depressive illness and use of antidepressant pharmacotherapy by ethnicity/race: an assessment of office-based visits in the United States, 1992–1997. Clin Ther. 2000 Dec;22(12):1575–89. doi: 10.1016/s0149-2918(00)83055-6. [DOI] [PubMed] [Google Scholar]
- 15.Madden JM, Graves AJ, Zhang F, Adams AS, Briesacher BA, Ross-Degnan D, Gurwitz JH, Pierre-Jacques M, Safran DG, Adler GS, Soumerai SB. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008 Apr 23;299(16):1922–8. doi: 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams AS, Uratsu C, Dyer W, Magid D, O’Connor P, Beck A, Butler M, Ho PM, Schmittdiel JA. Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users. JAMA Intern Med. 2013 Jan 14;173(1):54–61. doi: 10.1001/2013.jamainternmed.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Henry J. Kaiser Family Foundation. [Accessed September 15, 2009];Dual Eligibles: Medicaid’s Role for Low-Income Medicare Beneficiaries. 2006 Available at: www.kff.org/medicaid/upload/4091_06.pdf.
- 18.Elliott RA, Majumdar SR, Gillick MR, et al. Benefits and consequences of the new Medicare drug benefit for the poor and the disabled. N Engl J Med. 2005;353:2739–2741. doi: 10.1056/NEJMp058242. [DOI] [PubMed] [Google Scholar]
- 19.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of Limiting Medicaid Drug-Reimbursement Benefits on the Use of Psychotropic Agents and Acute Mental Health Services by Patients with Schizophrenia. N Engl J Med. 1994;331:650–5. doi: 10.1056/NEJM199409083311006. [DOI] [PubMed] [Google Scholar]
- 20.Adams AS, Madden JM, Zhang F, Soumerai SB, Gilden D, Griggs J, Trinacty CM, Bishop C, Ross-Degnan D. Changes in Use of Lipid Lowering Medications among Black and White Dual Enrollees with Diabetes Transitioning from Medicaid to Medicare Part D Drug Coverage. Med Care. doi: 10.1097/MLR.0000000000000159. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chace MJ, Zhang F, Fullerton CA, Huskamp HA, Gilden D, Soumerai SB. Intended and unintended consequences of the gabapentin off-label marketing lawsuit among patients with bipolar disorder. J Clin Psychiatry. 2012 Nov;73(11):1388–94. doi: 10.4088/JCP.12m07794. Epub 2012 Oct 16. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services CfDCaP, and the Centers for Medicare and Medicaid Services; U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; Centers for Medicare and Medicaid Services. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 2005–10 http://hdl.handle.net/1902.29/CD-0177.
- 23.Centers for Medicare and Medicaid Services. [Accessed June 17, 2013];MSIS State Data Characteristics/Anomalies Report. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/downloads/anomalies1.pdf.
- 24.APS National pharmaceutical Council. [Accessed October 28, 2008];Pharmaceutical Benefits Under State Medical Assistance Programs. 2007 Available at: www.npcnow.org/Public/Research___Publications/Publications/pub_rel_research/pub_medicaid/Pharmaceutical_Benefits_Under_State_Medical_Assistance_Programs_2007.aspx.
- 25.MedPAC. [Accessed 24 March 2014];Report to the Congress: New Approaches in Medicare. 2004 Jun; Available from www.medpac.gov/publications/congressional.../June04_ch3.pdf.
- 26.Adams AS, Zhang F, LeCates RL, Graves AJ, Ross-Degnan D, Gilden D, McLaughlin TJ, Lu C, Trinacty CM, Soumerai SB. Prior Authorization for Antidepressants in Medicaid: Effects among Disabled Dual Enrollees. Archives of Internal Medicine. 2009;169(8):750–6. doi: 10.1001/archinternmed.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soumerai SB, Avorn J, Ross-Degnan D, et al. Payment restrictions for prescription drugs under Medicaid: effects on therapy, cost, and equity. N Engl J Med. 1987;317:550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- 28.Adams AS, Mah C, Soumerai SB, Zhang F, Barton MB, Ross-Degnan D. Barriers to self-monitoring of blood glucose among adults with diabetes in an HMO: a cross sectional study. BMC Health Serv Res. 2003;3(1):6. doi: 10.1186/1472-6963-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soumerai SB, Ross-Degnan D, Avorn J, et al. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 30.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009 Feb;62(2):143–8. doi: 10.1016/j.jclinepi.2008.08.007. Epub 2008 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arday SL, Arday DR, Monroe S, et al. HCFA’s Racial and ethnic data: Current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality, US Department of Health and Human Services. Final Report. Jan, 2008. Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries. Prepared by: RTI International [CMS Contract Number: 500-00-0024, Task No. 21], AHRQ Publication Number 08-0029-EF. [Google Scholar]
- 34.SAS Institute Inc. SAS OnlineDoc®, Version 9. SAS Institute Inc; Cary, NC: 2002–2006. [Google Scholar]
- 35.Basu A, Yin W, Alexander GC. Impact of Medicare Part D on Medicare-Medicaid dual-eligible beneficiaries’ prescription utilization and expenditures. Health Serv Res. 2010;45:133–51. doi: 10.1111/j.1475-6773.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrank WH, Patrick AR, Pedan A, et al. The effect of transitioning to Medicare Part D drug coverage in seniors dually eligible for Medicare and Medicaid. J Am Geriatr Soc. 2008;56:2304–10. doi: 10.1111/j.1532-5415.2008.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams AS, Madden JM, Zhang F, Soumerai SB, Gilden D, Griggs J, Trinacty CM, Bishop C, Ross-Degnan D. Changes in Use of Lipid Lowering Medications among Black and White Dual Enrollees with Diabetes Transitioning from Medicaid to Medicare Part D Drug Coverage. Med Care. 2014;52:695–703. doi: 10.1097/MLR.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padgett DK, Patrick C, Burns BJ, Schlesinger HJ. Ethnicity and the use of outpatient mental health services in a national insured population. Am J Public Health. 1994;84:222–226.38. doi: 10.2105/ajph.84.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diab Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 40.Haviland AM, Elliott MN, Weech-Macdonado R, Hambarsoomian K, Orr N, Hays RD. Racial/ethnic disparities in Medicare Part D experiences. Med Care. 2012;11:S40–S47. doi: 10.1097/MLR.0b013e3182610aa5. [DOI] [PMC free article] [PubMed] [Google Scholar]