Abstract
BACKGROUND/OBJECTIVES
Cesarean section (CS) and antibiotic use during pregnancy may alter normal maternal-offspring microbiota exchange, thereby contributing to aberrant microbial colonization of the infant gut and increased susceptibility to obesity later in life. We hypothesized that (i) maternal use of antibiotics in the second or third trimester of pregnancy and (ii) CS are independently associated with higher risk of childhood obesity in the offspring.
SUBJECTS/METHODS
Of the 727 mothers enrolled in the Northern Manhattan Mothers and Children Study, we analyzed the 436 mother–child dyads followed until 7 years of age with complete data. We ascertained prenatal antibiotic use by a questionnaire administered late in the third trimester, and delivery mode by medical record. We derived age- and sex-specific body mass index (BMI) z-scores using the CDC SAS Macro, and defined obesity as BMI z ≥ 95th percentile. We used binary regression with robust variance and linear regression models adjusted for maternal age, ethnicity, pre-gravid BMI, maternal receipt of public assistance, birth weight, sex, breastfeeding in the first year and gestational antibiotics or delivery mode.
RESULTS
Compared with children not exposed to antibiotics during the second or third trimester, those exposed had 84% (33–154%) higher risk of obesity, after multivariable adjustment. Second or third trimester antibiotic exposure was also positively associated with BMI z-scores, waist circumference and % body fat (all P<0.05). Independent of prenatal antibiotic usage, CS was associated with 46% (8–98%) higher offspring risk of childhood obesity. Associations were similar for elective and non-elective CS.
CONCLUSIONS
In our cohort, CS and exposure to antibiotics in the second or third trimester were associated with higher offspring risk of childhood obesity. Future studies that address the limitations of our study are warranted to determine if prenatal antibiotic use is associated with offspring obesity. Research is also needed to determine if alterations in neonatal gut microbiota underlie the observed associations.
INTRODUCTION
The ensemble of microogranisms, or microbiota, in the human intestine have an important role in adipogenesis.1 Cesarean section (CS)2 and early-life antibiotics3 deprive normal microbial colonization of the newborn gut. Mounting evidence also links these exposures to development of obesity later in life.4–10
However, important questions remain. First, although meta-analyses5,6 have concluded that CS compared with vaginal delivery (VD) is associated with greater risk of obesity in children,6 adolescents6 and adults,5,6 none of the studies included in these meta-analyses simultaneously controlled for differences in pre-gravid maternal body mass index (BMI), birth weight, breastfeeding and gestational antibiotics—all potential confounders to the CS–obesity association. Second, studies7–10 on early-life antibiotics and childhood obesity have thus far focused on postnatal exposure to antibiotics, with one of these studies reporting that earlier (<6 months) but not later (≥6 months) administration of antibiotics was associated with higher body mass from 10 to 38 months.8 No studies have reported on whether maternal antibiotic use in the second or third trimester of pregnancy—when the fetal intestine has developed11—increases risk of offspring obesity. Given the evidence that antibiotics alter microbial communities in the mother12–14 and can enter fetal circulation via the placenta,15 it is plausible that antibiotic use in mid-to-late pregnancy may disrupt seeding of the offspring’s intestinal microbiome by way of the placenta16 before birth, and/or the mother’s vagina, feces, skin or breast milk during and after birth.17
In response to these literature gaps, we examined the hypotheses that CS and antibiotics taken in second or third trimester of pregnancy are independently associated with higher childhood obesity risk and levels of adiposity after controlling for important confounders, including maternal pre-gravid BMI, birth weight and breastfeeding in the first year of life.
MATERIALS AND METHODS
Independent variables
Data are from participants in the Columbia Center for Children’s Environmental Health Mothers and Newborns Study in Northern Manhattan and the South Bronx in New York—a previously described18 longitudinal birth cohort of mother–offspring dyads. Healthy, non-smoking, pregnant women (n = 727) were recruited from prenatal clinics at New York-Presbyterian Hospital and Harlem Hospital Center between 1998 and 2006. The cohort was restricted to women aged 18–35 years who self-identified as either African-American or Dominican and had resided in the study area for at least 1 year. Women were excluded from the study if their first prenatal visit was after 20 weeks gestation or if they self-reported diabetes, hypertension, known HIV or use of illicit drugs or cigarettes during pregnancy.
Measures
Prenatal antibiotic use
A questionnaire, administered to each woman in her home by a bilingual interviewer during the third trimester of pregnancy, elicited information on the timing (first, second and/or third trimester) and duration (in days) of antibiotic use during pregnancy. Prenatal antibiotic use was determined if a mother marked ‘yes’ to antibiotic use during pregnancy, and indicated a duration (days) greater than zero during the trimester of interest. It did not include intrapartum antibiotics.
Mode of delivery
Information on mode of delivery (VD, elective CS, non-elective CS) was abstracted from the mothers’ and infants’ medical records by research staff following delivery. As the effect sizes for elective and non-elective CS were similar (see Results), we combined these two types of CS into one exposure group.
Body mass and composition
At age 7 years, weight (to the nearest 0.1 kg) and body composition were measured using a Tanita scale (model BC-418; Tanita Corporation of America, Arlington Heights, IL, USA) while the child wore light clothes and no shoes. The Tanita scale calculated percentage of body fat (% body fat), fat mass and lean mass using bioimpedance formulas validated in children as young as 7 years. Height (to the nearest 0.1 cm) was obtained using a Detecto Cardinal 750 digital scale/stadiometer (Cardinal Scale Manufacturing Company, Webb City, MO, USA) until January 2010, and thereafter with the Holtain-Harpenden Wall Mounted Stadiometer Counter 602VR (Holtain Limited, Crymych, UK). Waist circumference was measured halfway between the iliac crest and the lowest rib to the nearest 0.5 cm using non-stretchable tape.
Potential confounders
The questionnaire administered during pregnancy collected information on demographics, history of passive smoke exposure, educational and income levels, receipt of public assistance during pregnancy, maternal height and pre-gravid weight, and number of previous live births. Infant sex, birth weight, and whether the mother was diagnosed with diabetes or hypertension during pregnancy were determined from medical records, and breastfeeding was determined by questionnaire during follow-up.
Statistical analysis
Of the 727 mother–child dyads, we excluded those missing information for delivery mode (23), maternal receipt of public assistance (5), maternal pregravid BMI (14), birth weight (50) and gestational age (2). We also excluded those (22) born prematurely (that is, <37 weeks) to rule out premature birth as a confounding factor. Of the 611 remaining, we successfully followed-up and measured BMI in 436 children (71.4%) at 7 years of age. Children’s BMI z-scores and percentiles were calculated using the SAS Macro provided by the Centers for Disease Control and Prevention.19 Children were classified as obese if their BMI percentile was greater than or equal to the 95% percentile.
Binomial regression models with robust variance20 were used to determine if relative risks (RRs) of obesity were higher for children whose mothers had CS (compared with VD), or for those whose mothers used antibiotics (compared with not having used antibiotics) in second or third trimester. General linear regression models were used to examine mode of delivery and gestational antibiotics in relation to continuous BMI z-score units, % body fat and waist circumference at 7 years of age. A separate linear regression analysis was conducted to examine whether first trimester antibiotics were associated with BMI z-score units. To determine whether gestational antibiotics and CS had compounding impacts, we further compared multivariable adjusted means for BMI z-score units across four groups: VD+no second or third trimester antibiotics (referent), VD+second or third trimester antibiotics, CS+no second or third trimester antibiotics and CS+second or third trimester antibiotics.
We assessed confounding by variables previously found to be associated with either exposure (delivery mode or prenatal antibiotics), associated with the outcome (BMI), and that were not considered to be in the causal pathway between the exposure and the outcome.21 Our core multivariable model included adjustment for child’s sex and birth weight (tertiles), and mother’s age (tertiles), ethnicity (African-American/Dominican), pre-gravid BMI (tertiles), receipt of public assistance during pregnancy (yes/no; as a proxy for socioeconomic status), and prenatal antibiotic use or delivery mode (depending on the main predictor variable). In a second model (model 2), we further controlled for breastfeeding (yes/no) in the first year to determine whether this might mitigate the observed associations. In a sensitivity analysis, we excluded 25 children born to mothers who were diagnosed with gestational diabetes or preeclampsia. We also considered models that were additionally adjusted for family income, maternal educational achievement at pregnancy, parity and mother’s report of a smoker living in the home during pregnancy.
Incomplete follow-up
We conducted sensitivity analyses using inverse probability weights for successful follow-up to assess potential bias because of incomplete follow-up on effect estimates.22–24 In estimating the weights, we included all variables in our final model that estimated BMI z-score, and household income, mother’s satisfaction with living conditions, mother’s years of school completed at time of pregnancy, mother’s neighborhood linguistic isolation and neighborhood socioeconomic status (poverty rate and median household income) measured using 2000 US Census block group data aggregated to the 1-km radial neighborhood buffers around the home.25
All study procedures used at enrollment and at 7 years were approved by the Columbia University Institutional Review Board. Informed consent was obtained from all participating women, and assent was provided by the children at age 7 years.
RESULTS
Table 1 shows the mean (s.d.) for continuous or n (%) for categorical representation of baseline characteristics for the overall analytic sample, and for the sample according to second or third trimester antibiotic exposure and delivery mode. Of the 436 children included, 70 (16%) had mothers who used antibiotics in the second or trimester, and 99 (22%) were born by CS (46% of which were elective CS). Although not statistically significant, children with mothers who used antibiotics in the second or third trimester were more likely to be female (P = 0.17), have Dominican ethnicity (P = 0.10) and have mothers with greater than high-school education (P = 0.15). These children did not differ appreciably (all P ≥ 0.25) by delivery mode, gestational age, birth weight, having been breastfed in first year, or by mother’s age, parity, receipt of public assistance during pregnancy, or pre-gravid BMI. Children born by CS were less likely to be male (P = 0.04) and Dominican (P = 0.03) than their VD counterparts. CS children were also marginally more likely to have older mothers (P = 0.07) without a previous birth (P = 0.15). These groups did not appear to differ on gestational age, birth weight, having been breastfed, or mother’s use of second or third trimester antibiotics, pre-gravid BMI, educational achievement, or receipt of public assistance in pregnancy (all P ≥ 0.25).
Table 1.
Characteristics of mothers and children by prenatal antibiotics and mode of delivery: the Mothers and Newborns Study in Northern Manhattan and South Bronx
| 2nd or 3rd trimester antibiotics |
Delivery mode |
||||
|---|---|---|---|---|---|
| Variables | Total sample, n = 436 (%) | No, n = 366 (%) | Yes, n = 70 (%) | Vaginal, n = 337 (%) | C-section, n = 99 (%) |
| Children | |||||
| Boys | 47.5 | 48.9 | 40.0 | 50.1 | 38.4 |
| Dominican | 39.7 | 38.0 | 48.6 | 42.4 | 30.3 |
| 2nd or 3rd trimester antibiotics | 16.1 | — | — | 15.7 | 17.2 |
| C-section | 22.7 | 22.4 | 24.3 | — | — |
| Gestational age, weeks, mean (s.d.) | 39.5 (1.2) | 39.5 (1.2) | 39.3 (1.1) | 39.5 (1.2) | 39.4 (1.1) |
| Birth weight, g, mean (s.d.) | 3418.5 (473.4) | 3429.9 (481.3) | 3358.6 (428.0) | 3411.9 (471.3) | 3441.0 (482.4) |
| Ever breastfed | 74.7 | 74.7 | 74.3 | 74.6 | 74.7 |
| Mothers | |||||
| Age of mother at birth (years) | |||||
| Mean (s.d.) | 25.5 (4.9) | 25.5 (4.9) | 25.2 (4.9) | 25.2 (4.8) | 26.3 (5.1) |
| Previous births | |||||
| 0 | 24.8 | 24.7 | 25.7 | 23.2 | 30.3 |
| 40 | 75.2 | 75.3 | 74.3 | 76.8 | 69.7 |
| Education level of mother at birth | |||||
| <High school | 37.6 | 39.1 | 30.0 | 38.3 | 35.4 |
| ≥ High school | 62.4 | 60.0 | 70.0 | 61.7 | 64.7 |
| Public assistance in pregnancy | 57.3 | 57.1 | 59.0 | 57.3 | 57.6 |
| Pre-gravid BMI, kg m2, mean (s.d.) | 25.8 (6.0) | 25.8 (6.0) | 26.0 (6.0) | 25.6 (5.9) | 26.4 (6.2) |
Abbreviation: BMI, body mass index.
In Table 2, we present RRs and 95% confidence intervals (CIs) for obesity at age 7 years. Children exposed to antibiotics in second or third trimester had 84% (33–154%) higher risk of obesity, after full covariate adjustment. Independent of gestational antibiotics, and all other covariates, children born by CS had 46% (8–98%) higher risk of obesity compared with those born vaginally. The association was not significantly different for elective (RR = 1.53; 95% CI = 1.04, 2.25) vs non-elective CS (RR = 1.41; 95% CI = 0.97, 2.05) compared with VDs. Furthermore, both exposures (second or third trimester antibiotics and CS) remained significantly associated with higher risk of childhood obesity in analyses that excluded children born to mothers diagnosed with gestational diabetes and preeclampsia.
Table 2.
Relative risks (and 95% confidence intervals) for childhood obesitya by prenatal exposure to second or third trimester antibiotics and mode of delivery: the Mothers and Newborns Study in Northern Manhattan and South Bronx
|
Cases/ n |
Model 1 | Model 2 |
Model 2+exclusion of
children whose mothers had gestational diabetes or preeclampsia |
|
|---|---|---|---|---|
| 2nd or 3rd trimester antibiotics | ||||
| No | 85/366 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Yes | 25/70 | 1.83 (1.36, 2.47) | 1.84 (1.33, 2.54) | 1.77 (1.25, 2.51) |
| Mode of delivery | ||||
| Vaginal | 77/337 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Cesarean | 33/99 | 1.43 (1.06, 1.92) | 1.46 (1.08, 1.98) | 1.41 (1.01, 1.96) |
All models exclude those missing information on pre-gravid BMI, gestational age and birth weight, and only include those with gestational age <37 weeks. Model 1: adjusted for sex, ethnicity (Dominican/African-American), offspring birth weight (tertiles), maternal age (tertiles), maternal pre-gravid BMI (tertiles), receipt of public assistance during pregnancy (yes/no) and prenatal antibiotic use or delivery mode. Model 2: additionally adjusted for having breastfed in first year (yes/no). Log-binomial models were used to generate relative risk and 95% confidence interval. N =411 after exclusion of mothers diagnosed with gestational diabetes and preeclampsia during pregnancy
Obesity defined as BMI percentile ≥95th percentile.
In Table 3, we present findings from multivariable linear regression analyses using continuous BMI z-score units, waist circumference and % body fat measured at 7 years of age that are consistent with the RRs for these exposures in relation to obesity. Adding family income, mother’s educational achievement at pregnancy, parity or mother’s report of a smoker in the home did not alter our β-coefficient for BMI z-score by >10%, and to maintain a parsimonious model these variables were not included in the final analyses.26
Table 3.
Difference in adjusted means (and 95% CI) for BMI z-scores, waist circumference, and % body fat in childhood (7 years) by prenatal antibiotics and delivery mode: the Mothers and Newborns Study in Northern Manhattan and South Bronx
| BMI z-score |
Waist
circumference (cm) |
% Body fat | |
|---|---|---|---|
| 2nd or 3rd trimester antibiotics | |||
| No | Reference | Reference | Reference |
| Yes | 0.47 (0.19, 0.74) | 3.13 (0.68, 5.59) | 1.86 (0.33, 3.39) |
| Delivery mode | |||
| Vaginal | Reference | Reference | Reference |
| Cesarean | 0.22 (−0.02, 0.46) | 1.35 (−0.82, 3.50) | 1.04 (−0.33, 2.41) |
Abbreviation: BMI, body mass index. All models exclude those missing information on pre-gravid BMI, gestational age and birth weight, and only include those with gestational age <37 weeks. Models adjusted for sex, ethnicity (Dominican/African-American), offspring birth weight (tertiles), maternal age (tertiles), maternal pre-gravid BMI (tertiles), maternal receipt of public assistance during pregnancy (yes/no), having breastfed in first year (yes/no) and either prenatal antibiotic use (yes/no) or mode of delivery. n=396 (67 with and 329 without 2nd or 3rd trimester antibiotics; 90 with and 306 without cesarean section) for % body fat analyses and 391 (65 with and 326 without 2nd or 3rd trimester antibiotics; 91 with and 300 without cesarean section) for waist circumference analyses (CS).
In linear regression analyses (not shown in tables) adjusted for the same covariates above, we found no evidence that first trimester antibiotics were associated with childhood BMI (difference in mean BMI z-score units between first trimester antibiotics and no antibiotics = 0.05; 95% CI = − 0.41, 0.52). In assessing the independent and joint effect of delivery mode and prenatal antibiotics (Figure 1), we found that associations for delivery mode and second or third trimester antibiotic exposure were similar when stratified by each other. Thus, there was little evidence of interaction on the additive scale. Weighting the data by the inverse probability of follow-up and complete data collection at 7 years did not appreciably change the size of the estimated effect of second or third trimester antibiotics or C-section on BMI z-score. The covariate-adjusted weighted β-coefficient for exposure to antibiotics in the second or third trimester vs no exposure in the second or third trimester was 0.42 BMI z-score units (95% CI = 0.15, 0.68), and the covariate-adjusted weighted β-coefficient for the C-section delivered children was 0.23 BMI z-score units (95% CI = 0.01, 0.46).
Figure 1.
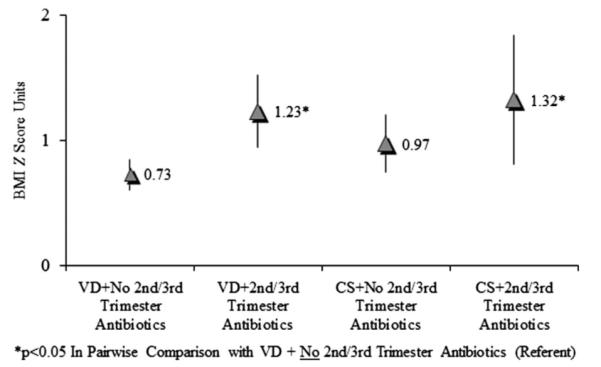
Multivariable adjusted BMI z-score means (and confidence intervals) for secomd or third trimester antibiotic use according to vaginal delivery (VD) and cesarean section (CS).
DISCUSSION
In the present cohort study of 436 mother–child dyads followed-up for 7 years from birth of the child, we found that children born to mothers who self-reported taking antibiotics in the second or third trimester of pregnancy had 84% higher risk of obesity at age 7 years compared with children whose mothers did not report antibiotic exposure during this time. Moreover, independent of prenatal antibiotics, and other confounders, children born via CS had 46% higher obesity risk than VD children.
Although previous studies have found that antibiotics administered early in life are associated with increased risk of obesity in childhood,7,8 to the best of our knowledge, ours is the first study to report that antibiotic exposure in the second or third trimester of pregnancy may be associated with higher risk of childhood obesity, and greater central adiposity and percent body fat. To determine whether these associations were explained by neonatal exposure to an antibiotic-altered maternal microbiota at birth, we stratified the percent body fat analyses by delivery mode. In these analyses, we found a similar magnitude of association, indicating that altered maternal-offspring exchange of vaginal and/or fecal microbiota at birth does not explain the prenatal antibiotics-adiposity link. Rather, it may be due to immunologic and/or metabolic programming occurring before birth.
Until recently, it was believed that the fetus and the intrauterine environments were sterile, and, thus, that the intestinal microbiome development began at birth after the newborn’s first microbial inoculum in the vaginal canal or, if delivered CS, contact with skin microbiota.2,27,28 However, studies have found evidence of microbes in amniotic fluid,29 umbilical cord blood,30 meconium31 and placental32,33 and fetal34 membranes. These findings, and a recent paper reporting the existence of a distinct human placental microbiome,16 call into question the notion of in utero sterility. Moreover, they allow for the hypothesis that maternal-fetal exchange of commensal microbiota may occur before birth, through exchange of placental bacteria, thereby seeding the intestinal microbiome of the fetus. In this light, antibiotics taken in pregnancy, which can cross the placenta and enter the fetal circulation,15 might disrupt normal colonization of the developing intestinal microbiome in utero similar to their effect after birth.3
A role for antibiotics in prenatal bacterial ecology has been indicated in other immunological diseases known to be associated with the intestinal microbiome. The Copenhagen Prospective Study on Asthma in Childhood reported an increased risk of asthma exacerbation in children (hazard ratio = 1.98; 95% CI = 1.08, 3.63) if their mothers had used antibiotics in the third trimester.35 This evidence is supported by other observational studies,36–38 and mechanistic research39 that demonstrates the involvement of gastric T-cell populations. A systematic review40 also found that early-life, including prenatal, exposure to antibiotics was linked to greater risk of eczema in childhood.
Antibiotics taken during pregnancy have also been associated with low birth weight and methylation of imprinted genes.41 In our study, we observed an inverse association between prenatal antibiotics and birth weight, which was not statistically significant (P = 0.25), but adjustment for birth weight did not alter associations between antibiotics and measures of childhood adiposity. Needed still is investigation on whether methylation of imprinting regions of adipogenesis promoting genes may be on the causal pathway from prenatal antibiotics to childhood obesity.
Independent of prenatal antibiotics, we found that CS was associated with 46% higher risk for obesity in offspring at 7 years. Our results are largely consistent with recent meta-analyses of studies on this topic. One meta-analysis concluded that CS was associated with 33%, 24% and 50% greater odds of overweight/obesity in children, adolescents and adults, respectively.6 Another meta-analysis of adult studies found that CS compared with VD was associated with 22% greater odds of obesity.5 Others have suggested that confounding by maternal pre-gravid body mass may explain at least some of the observed association.42 Yet in our study, the association persisted after control for pre-gravid BMI, and other potentially confounding factors, including birth weight, use of prenatal antibiotics and having breastfed in the first year of life. It has also been suggested that childhood outcomes differ by whether the CS was elective or emergent,43 but consistent with a meta-analysis5 of four studies with data on CS type we did not observe such evidence in our cohort. Thus, our findings provide new evidence in support of the hypothesis that CS independently contributes to offspring development of adiposity.
Mounting evidence suggests the CS–obesity association might be attributed to surgically delivered newborns bypassing the bacterial inoculum of the vaginal canal at birth. A growing body of literature has reported differences in the structure of microbial communities between children delivered by CS and those born vaginally.2,44–47 Dominguez-Bello et al. demonstrated that the microbiota (across several body habitats, including the skin, oral, nasopharynx and feces) of vaginally delivered neonates resembled the vaginal microflora of their own mother, whereas the microbiota of neonates born by CS resembled that of the mother’s skin.2 Other studies have found that stools of CS delivered children have lower counts of bifidobacteria and higher counts of Clostridium difficile than VD children.44–46 And a longitudinal study found that babies delivered by CS had lower overall bacterial diversity up to the age of 2 years, and delayed colonization of the gut by bacteriodetes, compared with their VD counterparts.48 Exactly how these differences in richness and diversity of overall and specific bacterial communities relate to accumulation of adipose tissue later in life remains to be elucidated.
The strengths of our study included the prospective design, comprehensive set of covariates, and the direct measurement of weight and body composition measured in childhood by trained research staff. Our cohort study was also restricted to non-smoking mothers, thus eliminating an important potential source of confounding. Another strength of our study was the use of medical records to ascertain mode of delivery, gestational diabetes and preeclampsia.
Our study has some key limitations. Prenatal use of antibiotics data—including timing and duration—were based on maternal interview. Although misclassification of our exposure was inevitable, we do not believe our results were influenced by maternal recall bias, because antibiotic use was self-reported (in the third trimester of pregnancy) before any adverse pregnancy outcomes would have occurred. Another limitation was the lack of information on antibiotic type, dose and route of administration during pregnancy. We also did not have information about specific medical indications for prescription of antibiotics or data on the severity of the given infections. Thus, it is possible that the maternal infection for which the antibiotics were administered was responsible for the association between prenatal antibiotic use and offspring obesity. There was also substantial drop out in our study, which could potentially bias the results. We addressed potential loss-to-follow-up bias through additional analyses that incorporated inverse probability weights for successful follow-up; the findings from these analyses were not appreciably different from non-weighted models. Nevertheless, inverse probability weighting is only as valid as the predictive model for loss to follow-up. Thus, we cannot rule out residual bias from unmeasured factors that influence drop out, and that themselves were associated with exposure and child anthropometric outcomes. Finally, because we did not have data on infant antibiotic use in our cohort, we cannot exclude the possibility that prenatal antibiotic exposure is acting as a proxy for the child having been treated with antibiotics during infancy, and that infant antibiotic usage, not prenatal antibiotic exposure, is causally associated with childhood BMI. However, to the best of our knowledge, there is no literature to support the supposition that maternal prenatal antibiotics use is strongly associated with the child being exposed to antibiotics during infancy.
Because of the considerable limitations for, and the novelty of, our findings on prenatal antibiotics and childhood obesity, we urge replication in other prospective cohort studies before any conclusions or clinical implications are drawn. Ideally, these studies should be prospective in nature, and extract information on prenatal antibiotic use from medical records, including the indication for the prescription, the types of prenatal antibiotics (for example, broad- vs narrow-spectrum) and their mode of administration.
In summary, children born by CS were at higher risk of obesity in childhood, as were children born to mothers who self-reported taking antibiotics in the second or third trimester of pregnancy. These exposures were independent of each and other potential confounders, including maternal pre-gravid BMI, birth weight and breastfeeding. If other observational studies can replicate our findings on gestational antibiotics and childhood obesity, then a logical next step to determine whether this association is causal might be to follow-up children whose mothers participated in randomized controlled trials of antibiotics during pregnancy. Prospective observational studies from conception through childhood, with serial measurement of microbial communities, body weight and body composition, and a comprehensive set of potential confounding factors are needed to further evaluate relationships between CS and childhood obesity. A better understanding of how mode of delivery and early-life antibiotics affect our microbiome may pave new avenues toward the prevention of obesity and related diseases.
ACKNOWLEDGEMENTS
This publication was made possible by US Environmental Protection Agency (US EPA) grants RD8260901 and R827027, and National Institute for Environmental Health Sciences (NIEHS) grants 5P01ES09600, 5R01ES08977, R01ES010165 and R01ES015282. This study was also supported by the Irving General Clinical Research Center (grant RR00645), the Educational Foundation of America, the John and Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund. Finally, NTM and EMW received fellowships from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK 5T32DK091227-03).
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of intrest.
DISCLAIMER Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
REFERENCES
- 1.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes. 2013;37:900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS One. 2014;9:e87896. doi: 10.1371/journal.pone.0087896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes. 2013;37:893–899. doi: 10.1038/ijo.2012.195. [DOI] [PubMed] [Google Scholar]
- 7.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 8.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy R, Stewart AW, Braithwaite I, Beasley R, Hancox RJ, Mitchell EA, et al. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obesity. 2013;38:1115–1119. doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 10.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes. 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 11.Malas MA, Aslankoc R, Ungor B, Sulak O, Candir O. The development of large intestine during the fetal period. Early Hum Dev. 2004;78:1–13. doi: 10.1016/j.earlhumdev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Neu J. Perinatal and neonatal manipulation of the intestinal microbiome: a note of caution. Nutr Rev. 2007;656(Pt 1):282–285. doi: 10.1301/nr.2007.jun.282-285. [DOI] [PubMed] [Google Scholar]
- 13.Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect. 2013;20:629–635. doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]
- 14.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Database Syst Rev. 2002;4:CD002250. doi: 10.1002/14651858.CD002250. [DOI] [PubMed] [Google Scholar]
- 15.Pacifici GM. Placental transfer of antibiotics administered to the mother: a review. Int J Clin Pharmacol Ther. 2006;44:57–63. doi: 10.5414/cpp44057. [DOI] [PubMed] [Google Scholar]
- 16.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Trans Med. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J PediatrGastroenterol Nutr. 2010;51:77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 18.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevention CDCa. A SAS Program for the CDC Growth Charts. Center for Disease Control and Prevention C.D.C.; Atlanta, GA: [Accessed 1 Oct 2012]. 2011. (Centers for Disease Control and Prevention) Available http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 20.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 21.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd edn Jones and Bartlett Publishers; Sudbury, Massachusetts: 2007. [Google Scholar]
- 22.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(Suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 25.Lovasi GS, Quinn JW, Rauh VA, Perera FP, Andrews HF, Garfinkel R, et al. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am J Public Health. 2011;101:63–70. doi: 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grayson DA. Confounding confounding. Am J Epidemiol. 1987;126:546–553. doi: 10.1093/oxfordjournals.aje.a114687. [DOI] [PubMed] [Google Scholar]
- 27.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinatal Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez E, Fernandez L, Marin ML, Martin R, Odriozola JM, Nueno-Palop C, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51:270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Satokari R, Gronroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009;48:8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- 33.Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. 2010;78:1789–1796. doi: 10.1128/IAI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 35.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–838. e833. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 36.Benn CS, Thorsen P, Jensen JS, Kjaer BB, Bisgaard H, Andersen M, et al. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol. 2002;110:72–77. doi: 10.1067/mai.2002.125833. [DOI] [PubMed] [Google Scholar]
- 37.Rusconi F, Galassi C, Forastiere F, Bellasio M, De Sario M, Ciccone G, et al. Maternal complications and procedures in pregnancy and at birth and wheezing pheno-types in children. Am J Resp Critic Care Med. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]
- 38.McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Resp Critic Care Med. 2002;166:827–832. doi: 10.1164/rccm.200202-158OC. [DOI] [PubMed] [Google Scholar]
- 39.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. Br J Dermatol. 2013;169:983–991. doi: 10.1111/bjd.12476. [DOI] [PubMed] [Google Scholar]
- 41.Vidal AC, Murphy SK, Murtha AP, Schildkraut JM, Soubry A, Huang Z, et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes. 2013;37:907–913. doi: 10.1038/ijo.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flemming K, Woolcott CG, Allen AC, Veugelers PJ, Kuhle S. The association between caesarean section and childhood obesity revisited: a cohort study. Arch Dis Childhood. 2013;98:526–532. doi: 10.1136/archdischild-2012-303459. [DOI] [PubMed] [Google Scholar]
- 43.Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes. 2012;36:1320–1324. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 44.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 45.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53:1388–1389. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huurre A, Kalliomaki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008;93:236–240. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 47.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut micro-biota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]


