Abstract
Background
The purpose of this study was to determine whether patients with heart failure and a preserved ejection fraction (HFpEF) have an increase in passive myocardial stiffness and the extent to which discovered changes are dependent on changes in extracellular matrix fibrillar collagen and/or cardiomyocyte titin.
Methods and Results
Seventy patients undergoing coronary artery bypass grafting underwent an echocardiogram, plasma biomarker determination, and intra-operative left ventricular (LV) epicardial anterior wall biopsy. Patients were divided into 3 groups: referent control (n=17, no hypertension or diabetes), hypertension (HTN) without(-) HFpEF (n=31), and HTN with(+) HFpEF (n=22). One or more of the following studies were performed on the biopsies: passive stiffness measurements to determine total, collagen-dependent and titin-dependent stiffness (differential extraction assay), collagen assays (biochemistry or histology), or titin isoform and phosphorylation assays. Compared with controls, patients with HTN(-)HFpEF had no change in LV end diastolic pressure (LVEDP), myocardial passive stiffness, collagen, or titin phosphorylation but had an increase in biomarkers of inflammation (CRP, sST2, TIMP-1). Compared with both control and HTN(-)HFpEF, patients with HTN(+)HFpEF had increased LVEDP, left atrial volume, NT-proBNP, total, collagen-dependent and titin-dependent stiffness, insoluble collagen, increased titin phosphorylation on PEVK S11878(S26), reduced phosphorylation on N2B S4185(S469), and increased biomarkers of inflammation.
Conclusions
Hypertension in the absence of HFpEF, did not alter passive myocardial stiffness. Patients with HTN(+)HFpEF had a significant increase in passive myocardial stiffness; collagen-dependent and titin-dependent stiffness were increased. These data suggest that the development of HFpEF is dependent on changes in both collagen and titin homeostasis.
Keywords: heart failure, diastole, hypertension, hypertrophy, collagen, titin
Introduction
Patients with heart failure and a preserved ejection fraction (HFpEF) have been shown to have abnormalities of left ventricular (LV) diastolic function including slowed/incomplete relaxation, decreased suction/recoil, and increased passive chamber stiffness 1-5. However, the myocardial basis for these changes remains incompletely understood. Some recent clinical studies have suggested that development of HFpEF is accompanied by significant changes in the composition and structure of the extracellular matrix (ECM), especially fibrillar collagen 6-8. Other studies and editorials have emphasized the contribution of changes in titin (the giant molecular spring protein that is one important factor responsible for cardiomyocyte passive stiffness) 9-20, 21; specifically, changes in the phosphorylation of titin 22-32. However, in previous studies of patients with HFpEF, the role of titin was examined in isolated, chemically demembranated cardiomyocytes obtained from endocardial myocardial biopsies; these preparations did not include surrounding ECM structures. The aforementioned changes in collagen and/or titin are expected to increase passive myocardial stiffness; however, myocardial stiffness has never been measured directly in myocardium from HFpEF patients. Thus, the magnitude of the assumed increase in myocardial passive stiffness and therefore its importance as a determinant of diastolic dysfunction are unknown. In addition, the role of increased passive stiffness in the development and clinical course of patients with HFpEF and the relative contribution of changes in the ECM versus titin to passive stiffness in HFpEF are also unknown. In the present study, we hypothesized that changes in both collagen and titin occur during the development of HFpEF and that these changes combine to cause a major increase in myocardial passive stiffness that contributes to the development of HFpEF.
To determine the extent to which ECM collagen and titin contribute to changes in myocardial passive stiffness in patients with HFpEF certain experimental conditions must be met. First, since several potential determinants of passive stiffness could change concurrently during the development of HFpEF, the contribution of each must be determined in samples of myocardium which include an integrated, intact composite structure. To satisfy this condition, methods of differential extraction have been developed to determine the separate contributions of collagen and titin to stiffness in demembranated myocardial strips 9, 10. In the current studies these methods were applied to strips prepared from LV myocardial biopsies obtained during coronary artery bypass grafting (CABG). Second, the effects of co-morbidities and antecedent diseases must be distinguished from changes associated with the clinical syndrome of HFpEF. Two of the most common antecedent disease processes that lead to HFpEF are arterial hypertension (HTN) and diabetes mellitus (DM) 33-37. In the current study, patients with HTN (or DM combined with HTN) without HFpEF [HTN(-)HFpEF] were compared to patients with HTN (or DM combined with HTN) with HFpEF [HTN(+)HFpEF]. Third, an appropriate referent control group must be studied. For this purpose, CABG patients with no history of HTN or DM were chosen. There are limitations in using these patients as referent controls in that CAD could have uncertain effects on myocardial stiffness that cannot be distinguished from those related to hypertension. However, CAD is present in a majority of patients with HFpEF (38); thus, a CAD “background” is quite representative of the HFpEF population. Using these methods, the purpose of this study was to determine whether patients with HFpEF and an antecedent history of HTN or HTN/DM have an increase in passive myocardial stiffness and whether changes in stiffness are dependent on changes in collagen and/or titin. Additionally, we sought to determine the relationship between changes in myocardial passive stiffness and echocardiographic measures of LV structure and function and selected plasma biomarkers.
Methods
Study Population
Recruitment
The study cohort consisted of 70 males and females recruited to undergo intraoperative LV myocardial biopsy from amongst those scheduled for CABG at 1) Fletcher Allen Health Care in Burlington, Vermont, the clinical facility of the University of Vermont College of Medicine (UVM), 2) the Ralph H. Johnson Department of Veterans Administration Medical Center and the Medical University of South Carolina Hospital Authority (MUSC) in Charleston, South Carolina, and 3) selected NHLBI Heart Failure Research Network (HFRN) centers (University of Alberta [Alberta, Canada], Intermountain Medical Center [Murray, UT], the Mayo Clinic [Rochester, MN], Minnesota Heart Institute [Minneapolis, MN], University of Utah [Salt Lake City, UT] and the Utah VA Medical Center [Salt Lake City, UT]) between October 1, 2008 and August 6, 2012 who satisfied the inclusion and exclusion criteria specified below. All patients signed consent forms approved by their respective Institutional Review Boards.
Experimental Measurements
Demographic, medication and laboratory data and cardiac catheterization results (coronary anatomy, LV end-diastolic pressure) were tabulated. The severity of coronary artery disease (CAD) was graded based on the number of major vessels (left anterior descending, left circumflex, right coronary arteries) with a stenosis >70%, with left main coronary stenosis considered as two vessels. Patients recruited at UVM and MUSC underwent an echocardiographic-Doppler examination to assess LV chamber structure and function. In addition, in these patients a 10 cc plasma sample was obtained for measurement of biomarkers. Intra-operative LV anterior wall epicardial biopsies were obtained as previously described (39, 40). Patients recruited to this protocol were part of an NIH grant (RO1HL089944) with multiple specific aims; therefore, biopsy samples were allocated to several protocols, data from some of which have been published 39, 40. Given the size of each biopsy, all protocols could not be performed on each biopsy. For the current study, 25 biopsies were used to assess myocardial passive stiffness; 30 were used to measure tissue collagen content; and 14 were used for titin phosphorylation studies (Table 1).
Table 1. Sample Size for Each Endpoint.
| Referent Control | Hypertension | Total | ||
|---|---|---|---|---|
| (-) HFpEF | (+) HFpEF | |||
| Echocardiogram | 17 | 31 | 22 | 70 |
| Plasma Biomarker | 12 | 22 | 18 | 52 |
| Myocardial Stiffness* | 9 | 10 | 6 | 25 |
| Myocardial Collagen* | 7 | 4 | 4 | 15 |
| Collagen Volume Fraction* | 5 | 5 | 5 | 15 |
| Titin* | 4 | 4 | 6 | 14 |
Abbreviations:
Biopsy size limited number of studies/patient, HFpEF = heart failure with a preserved ejection fraction.
General Inclusion Criteria
Patients scheduled to undergo CABG over 21 years of age, with a preserved LVEF (≥50%), normal wall motion and end-diastolic volume index (EDVi, < 75mL/m2), and without evidence of previous myocardial infarction were eligible. Patients were categorized into three groups: control, HTN(-)HFpEF and HTN(+)HFpEF.
Specific Patient Group Inclusion Criteria
Control patients fulfilled the general inclusion criteria, but did not have a history of HTN or DM. HTN(-)HFpEF patients fulfilled the general inclusion criteria, and had a history of HTN documented in their records and/or had been told of this diagnosis by a physician, and were receiving medications for its treatment. These patients had no evidence of heart failure as defined below.
HTN(+)HFpEF patients fulfilled the general inclusion criteria and had HTN and HFpEF as specified by the European Society of Cardiology and Heart Failure Society of America criteria41,42. These criteria require: 1) signs and/or symptoms of heart failure (Framingham or Boston criteria, exercise testing, quality of life questionnaire), 2) LVEF ≥ 50 %, 3) LVEDVi < 75 mL/m2, 4) evidence of diastolic LV dysfunction obtained invasively (cardiac catheterization) or non-invasively (transmitral or tissue Doppler or left atrial size) and 5) exclusion of non-cardiac diseases that could cause symptoms commonly present in patients with heart failure.
Exclusion Criteria
Patients were excluded if they had a previous transmural myocardial infarction, LVEF < 50%, LVEDVi > 75 mL/m2, significant valvular or other non-coronary heart disease, severe chronic pulmonary disease requiring oral steroids and/or oxygen therapy, any non- cardiac disease or condition known to affect myocardial function, anemia (Hgb < 13.0 g/dl), serum creatinine > 2.0 mg/dL, poorly controlled hypertension (blood pressure > 140/90 mmHg), off-pump or emergency CABG, morbid obesity, history of substance abuse, inability to provide informed consent, poorly controlled diabetes (HbA1c >8.5% within the past 6 months), active malignancy, severe connective tissue disease, severe liver disease, hypertrophic cardiomyopathy, restrictive cardiomyopathy or constrictive pericarditis, HIV or active infection.
Myocardial Biopsy Procedure
Anterior LV free wall epicardial biopsies weighing ∼25-50 mg were obtained during CABG soon after the patient was placed on cardiopulmonary bypass, as previously described 39,40. The biopsy was placed in oxygenated HEPES-based Krebs buffer containing 30 mmol/L 2,3-butanedione monoximine (BDM) at room temperature 39, 40. Small samples (< 5 mg) were removed and frozen for collagen and titin studies or placed in formalin for histology. The remainder of the tissue was processed for stiffness studies. From the section of the biopsy that remained in buffer, tissue was dissected into pieces < 2 mm in length and placed in skinning solution containing Triton-X100 at 4°C. For samples obtained at MUSC and the HFRN centers, the skinning period coincided with overnight transit to UVM at 4°C. After 18-24 hour skinning, strips were dissected to 150- 200 μm diameter and 800-1200 μm length and then underwent measurements of myocardial stiffness as described below.
All patients were followed until discharge, with particular attention to ventricular arrhythmias and bleeding complications. No adverse effects or post-operative complications ascribable to the biopsy procedure were detected and all patients were discharged alive.
Measurement of Passive Myocardial Stiffness
At time of study, aluminum T-clips were attached to the ends of each strip. The strip was mounted between a piezoelectric motor (Physik Instrumente, Auburn, MA) and a strain gauge (Kronex Technologies, Oakland, CA) and initially lowered into a 30 μL droplet of relaxing solution maintained at 37°C. The composition of relaxing solution is specified in previous reports 39, 40. Sarcomere length (SL) was measured by Fourier Transform of digital images (IonOptix Corp, Milton, MA) 9. Measurements of total, collagen-dependent, and titin-dependent stiffness were made using a previously published differential extraction protocol 9, 10. The extraction method removes the anchors of titin within the myofilament, leaving only ECM-based stiffness. This method, originally developed and validated by Granzier and colleagues 9, 10, has been successfully used in other studies 43. In previous control studies in which titin's stiffness was eliminated by protease treatment and the muscle strip was then treated with KCL/KI 12, ECM stiffness was shown to be unaffected by the KCL/KI treatment. Thus, while the differential extraction protocol causes irreversible myocardial damage it does not affect the measurements that are central to the questions addressed in this study.
Echocardiography
Echocardiographic studies performed at UVM and MUSC were interpreted by a core laboratory at MUSC. Studies were de-identified, coded, and interpreted in a blinded fashion. Measurements were made using American Society of Echocardiography criteria 44.
Collagen
Collagen was assessed using both biochemical and histologic methods. Soluble, insoluble, and total collagen content were determined using tissue samples sequentially extracted and assayed directly using the microplate picrosirius red assay 45-47. Collagen volume fraction (CVF) was measured using light microscopy with samples stained with picrosirius red (PSR) to detect collagen and viewed with polarized light under dark field optics to detect birefringence of the fibers 45.
Titin Studies
Titin isoform analysis was performed with 1% agarose gels using a vertical SDS-agarose gel system as previously described 23, 48. LV myocardium co-expresses compliant N2BA titin and stiffer N2B titin isoforms 9; their expression ratio was determined. We also measured titin degradation as the ratio of T2 (∼ 2 MDa degradation product of titin) to T1 (full length titin).
Titin phosphorylation levels were quantified via Western blotting 9, 25. Blots were stained with Ponceau S (Sigma) to visualize the total protein transferred and then probed with phospho-specific rabbit polyclonal antibodies against phosphorylated S11878(S26) and S12022(S170) of the PEVK element 25. These sites are known to be phosphorylated by PKC. In addition, blots were probed with phospho-specific rabbit polyclonal antibodies against phosphorylated S4185(S469) of titin's N2B element. This site is known to be phosphorylated by PKA and PKG 32. Membranes were labeled with secondary antibodies conjugated with fluorescent dyes with infrared excitation spectra (CF680, goat anti-rabbit, Biotium Company, Hayward CA). Blots were scanned using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln NE) and images analyzed using Li-Cor software. Ponceau S scans were analyzed in One-D scan to normalize phosphorylation signal to protein loading.
Plasma Biomarkers
Biomarkers were chosen that reflect changes in ECM homeostasis, specifically matrix metalloproteinases [MMPs] and their tissue inhibitors [TIMPs]. Four classes of MMPs, gelatinases (MMP-2 and MMP-9), collagenase (MMP-1 and 8), stromelysin (MMP-3), and matrilysin (MMP-7), and all 4 tissue inhibitors of MMPs (TIMP-1, -2, -3, -4) were assayed. In addition, N-terminal propeptide of brain naturetic peptide (NT-proBNP) was measured. Finally, biomarkers that reflect a proinflammatory and/or profibrotic state, specifically CRP, IL-6, IL-8, TNF-α, and sST2 were examined.
Statistical analysis
Data are reported as mean ± SD in tables and text and mean ± SE in figures. A t-test was used to detect differences in continuous variables, Pearson's Chi-square test was used to detect differences in categorical variables for demographics, collagen-dependent and titin-dependent tension, collagen content, and titin phosphorylation and isoforms and plasma biomarkers amongst referent controls, HTN(-)HFpEF and HTN(+)HFpEF groups. A general linear mixed model (GLMM) was also employed to compare the relationship between stress and sarcomere length in the three groups (Figure 1); this type of model is ideal for handling repeated measures obtained within the same study subjects 49. Within the GLMM, linear and quadratic relationships between stress and sarcomere length were considered, and an unstructured covariance structure was selected after comparing the model's AIC value to those from models incorporating other covariance structures (e.g. autoregressive, compound symmetry) 50. In addition, stress was compared between the 3 groups at selected common values of SL using ANOVA. Linear correlations were used to examine the relationship between echocardiographic measurements and stiffness measurements and between echocardiographic measurements and plasma biomarkers using a least squares best fit model and a Pearson's correlation coefficient.
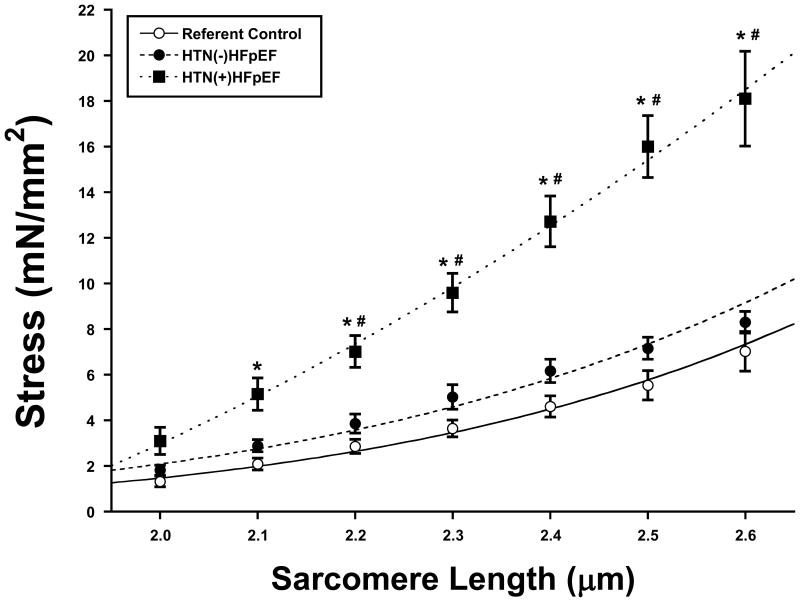
Figure 1.
Total myocardial stiffness expressed as the relationship between myocardial stress (mN/mm2) versus cardiomyocyte sarcomere length (μm) for referent control patients (open circle, solid line), patients with hypertension but without heart failure and a preserved ejection fraction (HTN(-)HFpEF, closed circle, dashed line), and patients with hypertension and HFpEF (HTN(+)HFpEF closed squares, dotted line). As sarcomere length increases, the slope increases most rapidly in the HTN(+)HFpEF group (p<0.0001 when compared to both the control and HTN(-)HFpEF groups). Overall, the curves for the control and HTN(-)HFpEF groups were not significantly different from one another. Patients with HTN(+)HFpEF had an increase in total myocardial stiffness as indicated by a leftward shift in the stress vs. sarcomere length relationship; for any given sarcomere length ≥ 2.1 μm, stress was higher in the HTN(+)HFpEF vs. HTN(-)HFpEF or referent control patients. There were no significant differences between HTN(-)HFpEF vs. referent control patients. * = p < 0.01 vs. referent control, # = p < 0.01 vs. HTN(-)HFpEF.
Results
Demographic and Echocardiographic Data
The mean age of the study group was 65 years; most subjects were male (Table 2). This age and sex distribution is typical of CABG populations. By definition none of the controls had DM or HTN. The prevalence of DM was comparable in both HTN groups [48% in HTN(-)HFpEF and 59% in HTN(+)HFpEF]. The extent of CAD was similar in the three groups (number of arteries with a > 70% obstruction 2.5 ± 0.7). Compared with controls, blood pressure was higher in the two HTN groups but not different from each other and within national guidelines. The mean creatinine values were not statistically different between the 3 groups. There were significant differences in the medications taken by each group (Table 2). As expected, both HTN groups were receiving antihypertensive medications (β-blockers, ACE-I/ARBs and diuretics) more often than the controls. Patients with HTN(+)HFpEF were taking diuretics and nitrates more often than the other groups.
Table 2. Patient Demographics.
| Referent Control | Hypertension | ||
|---|---|---|---|
| (-) HFpEF | (+) HFpEF | ||
| Age (years) | 65 ± 8 | 62 ± 9 | 66 ± 9 |
| BSA (Kg/m2) | 2.0 ± 0.2 | 2.1 ± 0.3 | 2.2 ± 0.3 |
| Sex (% Male) | 88% | 90% | 82% |
| Diabetes Mellitus (%) | 0 | 48%* | 59%* |
| Creatinine (mg/dL) | 0.97 ± 0.21 | 0.94 ± 0.20 | 1.2 ± 0.5 |
| Heart Rate (bpm) | 65 ± 15 | 67 ± 10 | 68 ± 10 |
| Systolic BP (mmHg) | 122 ± 7 | 132 ± 11* | 136 ± 14* |
| Diastolic BP (mmHg) | 73 ± 7 | 70 ± 10 | 70 ± 11 |
| LV EDP (mmHg) | 11 ± 4 | 13 ± 4 | 17 ± 5 |
| Medications (%) | |||
| β-Blocker | 65 | 81 | 82 |
| ACE-I/ARB | 0 | 77 * | 77 * |
| CCB | 0 | 26 * | 27 * |
| Diuretic | 0 | 16 * | 68 *# |
| Lipid Therapy | 65 | 87 | 86 |
| Nitrates | 12 | 13 | 55 *# |
| CAD (# Vessels) | 2.5 ± 0.7 | 2.5 ± 0.7 | 2.6 ± 0.6 |
Abbreviations: Data = Mean ± SD,
= p < 0.05 vs Referent Control,
= p < 0.05 vs Hypertension (-) HFpEF, HFpEF = heart failure with a preserved ejection fraction, BSA = Body surface area, HR = heart rate, BP = blood pressure, β-blocker = beta adrenergic receptor blocker, CCB = calcium channel blocker. ACE-I = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker, CAD = coronary artery disease, LVEDP = left ventricular end diastolic pressure.
The structural and functional data obtained from the echocardiographic studies also confirm the category definitions for each group (Table 3). By definition, LV volumes and EF were normal in each group. Both HTN groups had increased LV mass and RWT. The number in each HTN group with concentric LVH was comparable (39% in HTN(-)HFpEF and 41% in HTN(+)HFpEF); the number with concentric remodeling was also comparable (32% in HTN(-)HFpEF and 30% in HTN(+)HFpEF). Thus, the percentage with either concentric LVH or concentric remodeling (i.e., increased RWT without increased LV mass) was ∼70% in both groups. Measurements that reflect diastolic function and filling pressure (E, E', PCWP, LVEDP, LA volume) were increased in HTN(+)HFpEF. With the exception of a small increase in LA volume, these measurements were normal in HTN(-)HFpEF patients. LA enlargement was also more frequent in the HTN(+)HFpEF group (41%) vs. HTN(-)HFpEF (20%).
Table 3. Echocardiography.
| Referent Control | Hypertension | ||
|---|---|---|---|
| (-) HFpEF | (+) HFpEF | ||
| LV EDVi (mL/m2) | 58 ± 11 | 60 ± 10 | 58 ± 14 |
| LV ESVi (mL/m2) | 20 ± 7 | 21 ± 4 | 21 ± 11 |
| LV EF (%) | 65 ± 7 | 65 ± 6 | 65 ± 14 |
| LV Massi (g/m2) | 88 ± 15 | 107 ± 20* | 116 ± 20* |
| LV Wall Thickness, cm | 0.9 ± 0.1 | 1.1 ± 0.1* | 1.2 ± 0.1* |
| RWT | 0.38 ± 0.04 | 0.45 ± 0.06* | 0.48 ± 0.10* |
| E (cm/s) | 80.1 ± 16.9 | 76.7 ± 19.8 | 83.5 ± 22.2 |
| E' (cm/s) | 10.8 ± 1.8 | 10.1 ± 3.3 | 7.3 ± 2.0*# |
| PCWP (mmHg) | 12.1 ± 2.8 | 12.5 ± 3.3 | 17.6 ± 5.4*# |
| LA Voli (mL/m2) | 20.8 ± 3.6 | 25.7 ± 8.0* | 37.0 ± 11.9*# |
Abbreviations: Data = Mean ± SD,
= p < 0.05 vs Referent Control,
= p < 0.05 vs Hypertension (-) HFpEF, HFpEF = heart failure with a preserved ejection fraction (EF), LV = Left ventricle, BSA = body surface area, EDVi = end diastolic volume indexed (normalized) to BSA, ESVi = end systolic volume indexed (normalized) to BSA, LV Massi = LV mass indexed (normalized) to BSA, PCWP = pulmonary capillary wedge pressure as estimated from E/E' ratio, LA Voli=left atrial volume indexed (normalized) to BSA, RWT = relative wall thickness, E = transmitral early peak filling velocity, E' = Tissue Doppler mitral annular early peak diastolic velocity.
Myocardial Stiffness
Relationships between myocardial stress and SL between 2.0-2.6 μm for the three groups, which reflect total passive myocardial stiffness, are shown in Figure 1. Results of the general linear mixed model (GLMM) indicated that a model assuming a quadratic relationship between stress and SL provided a superior fit compared to a linear model. The GLMM indicated that as SL increased, the slope increased most rapidly in the HTN(+)HFpEF group (p<0.0001 compared with both control and HTN(-)HFpEF), and that the control and HTN(-)HFpEF curves were not significantly different from one another. Significant (p<0.01) differences were noted between the HTN(+)HFpEF group and the other groups at 2.1μm and at each SL assessed up to and including 2.6μm. Passive myocardial tension was also examined at SL 2.6μm before and after differential extraction to estimate collagen-dependent and titin-dependent stiffness (Figure 2). There were no significant differences in collagen- and titin-dependent tension between control and HTN(-)HFpEF. However, both collagen- and titin-dependent tension was significantly increased in HTN(+)HFpEF compared with the other groups. Collagen-dependent stiffness was increased by 220% and titin-dependent stiffness was increased by 92% in the HTN(+)HFpEF group.
Figure 2.
Collagen-dependent and titin-dependent myocardial stress at a sarcomere length of 2.6 μm for referent control patients (white column), patients with hypertension but without heart failure and a preserved ejection fraction (HTN(-)HFpEF, cross-hatched column), and patients with hypertension and HFpEF (HTN(+)HFpEF black column). Total stress is the numerical sum of collagen and titin specific data. Patients with HTN(+)HFpEF had an increase in collagen-dependent and titin-dependent myocardial stress. There were no significant differences between HTN(-)HFpEF or referent control patients. * = p < 0.01 vs. referent control, # = p < 0.01 vs. HTN(-)HFpEF.
There were significant correlations between in vitro measurements of passive myocardial tension and in vivo echocardiographic measurements of diastolic function (Figure 3). Thus, there was a statistically significant direct relationship between collagen-dependent tension and left atrial diameter (r2 = 0.42, p = 0.006) and PCWP (r2 = 0.46, p = 0.002) and between titin-dependent tension and left atrial diameter (r2 = 0.43, p = 0.006) but not PCWP (r2 = 0.16, p = 0.11).
Figure 3.
Relationship between in vivo echocardiographic derived assessment of LV diastolic dysfunction (left atrial diameter and echocardiographically estimated pulmonary capillary wedge pressure [PCWP]) and in vitro measures of myocardial diastolic dysfunction (collagen-dependent and titin-dependent myocardial stiffness) for all patients studied with both measures available. There was a statistically significant direct relationship between collagen-dependent stiffness and left atrial diameter (r2 = 0.42, p = 0.006) and PCWP (r2 = 0.46, p = 0.002) and between titin-dependent stiffness and left atrial diameter (r2 = 0.43, p = 0.006) but not PCWP (r2 = 0.16, p = 0.11).
Collagen
Myocardial collagen content was measured using both biochemical and histologic methods. Biochemical studies assessed soluble, insoluble and total collagen (Figure 4A). CVF was estimated by light microscopy of PSR stained sections (Figure 4B-F). There were no differences in soluble collagen across the three groups. There was a significant increase in insoluble and total collagen (by∼100%) and CVF (by ∼130%) in the HTN(+)HFpEF group compared with the controls and HTN(-)HFpEF groups.
Figure 4.
Myocardial collagen content in patients with hypertension (HTN) with(+) and without(-) heart failure with a preserved ejection fraction (HFpEF). Referent control patients (white column), patients with HTN(-)HFpEF (cross-hatched column), and patients with HTN(+)HFpEF (black column). * = p < 0.01 vs. referent control, # = p < 0.01 vs. HTN(-)HFpEF. Panel A: Soluble, insoluble, and total collagen measured biochemically. Patients with HTN(+)HFpEF had an increase in insoluble and total collagen. There were no significant differences between HTN(-)HFpEF or referent control patients. Panel B: Examples of picrosirius stained myocardial sections. Patients with HTN(+)HFpEF had an increase in collagen. There were no significant differences between HTN(-)HFpEF or referent control patients. Panel C: Collagen volume fraction (CVF) measured from histologic sections. Patients with HTN(+)HFpEF had an increase in CVF. There were no significant differences between HTN(-)HFpEF or referent control patients.
Titin
Titin N2B and N2BA isoforms and phosphorylation were examined in the 3 patient groups. Three phosphorylation sites in titin's spring region were examined: S11878(S26) and S12022(S170) of the PEVK element, sites phosphorylated by protein kinase C (PKC) and calcium/calmodulin dependent protein kinase II (CaMKII), and S4185(S469) of the N2B element, a site phosphorylated by protein kinase A (PKA) and protein kinase G (PKG) (Figure 5). There were no differences in the N2BA/N2B titin ratio between the three groups (0.53±0.14 in control, 0.50±0.13 in HTN(-)HFpEF and 0.59±0.14 in HTN(+)HFpEF) and no changes in T2 (titin degradation product): T1 (full length titin) ratio amongst the groups. Patients with HTN(+)HFpEF had a 31% higher phosphorylation value at the S11878(S26) PKC/CaMKII site, no change at the S12022(S170) PKC CaMKII site and a 28% lower value at the S4185(S469) PKA/PKG site compared with HTN(-)HFpEF. There were no significant differences between HTN(-)HFpEF and control patients at any of the phosphorylation sites.
Figure 5.
Titin phosphorylation state in referent control patients (white column), patients with hypertension but without heart failure and a preserved ejection fraction (HTN(-)HFpEF, cross-hatched column), and patients with hypertension and HFpEF (HTN(+)HFpEF black column). Three titin sites were examined: S11878(S26) and S12022(S170), sites known to be phosphorylated by protein kinase c (PKC) and S4185(S469), a site known to be phosphorylated by protein kinase a (PKA). Patients with HTN(+)HFpEF had an increase in S11878(S26), no change in S12022(S170) and a decrease in S4185(S469). There were no significant differences between HTN(-)HFpEF or referent control patients at any of the three sites. Insert: examples of the phospho-blot (top) and total protein (bottom) for each of the patient groups. There are two bands, the top band represents N2BA and the bottom band represents N2B. Data from both N2B and N2BA bands were summed. * = p = 0.05.
Plasma Biomarkers
Of the 16 biomarkers measured, four were significantly altered in the HTN patients compared with the controls (Table 4). CRP, sST2, TIMP-1 and NT-proBNP were higher in the HTN(-)HFpEF patients compared with the controls. Levels of sST2, TIMP-1 and NT-proBNP increased further in the HTN(+)HFpEF group. There were no differences in MMP-1, 2, 3, 7, 9 or TIMP-2, 3, 4 or IL-6, 8 or TNF-α between groups.
Table 4. Biomarkers.
| Referent Control | Hypertension | ||
|---|---|---|---|
| (-) HFpEF | (+) HFpEF | ||
| CRP, μg/mL | 2.4 ± 1.8 | 4.8 ± 2.9* | 4.2 ± 2.1* |
| IL-6, pg/mL | 3.7 ± 3.5 | 3.3 ± 1.9 | 4.1 ± 4.5 |
| IL-8, pg/mL | 8.3 ± 6.4 | 8.4 ± 7.3 | 12.9 ± 15.3 |
| TNF- α, pg/mL | 3.4 ± 2.1 | 4.4 ± 2.1 | 4.3 ± 2.6 |
| sST2, ng/mL | 25.6 ± 19.8 | 82.0 ± 35.7* | 105.5 ± 31.4*# |
| NT-proBNP, pg/mL | 897 ± 629 | 1,507 ± 976* | 2,355 ± 1,394*# |
| MMP-1, pg/mL | 383 ± 180 | 551 ± 403 | 372 ±362 |
| MMP-2, ng/mL | 326 ± 106 | 318 ± 69 | 353 ± 115 |
| MMP-3, ng/mL | 9.4 ±7.3 | 7.8 ± 2.9 | 8.1 ± 5.6 |
| MMP-7, pg/mL | 228 ± 132 | 468 ± 288* | 212 ± 210# |
| MMP-8, pg/mL | 1110 ± 656 | 1257 ± 716 | 1852 ± 2198 |
| MMP-9, ng/mL | 120 ± 73 | 114 ± 82 | 152 ± 160 |
| TIMP-1, ng/mL | 64 ± 27 | 101 ± 27* | 128 ± 34*# |
| TIMP-2, ng/mL | 71 ± 15 | 79 ± 11 | 78 ± 13 |
| TIMP-3, ng/mL | 2.6 ± 1.1 | 3.5 ± 2.6 | 3.5 ± 1.6 |
| TIMP-4, ng/mL | 1.7 ± 0.8 | 1.7 ± 0.8 | 2.1 ±0.7 |
Abbreviations: HFpEF = heart failure with a preserved ejection fraction, MMP = matrix metalloproteinase, TIMP = tissue inhibitor of MMP, CRP = C-reactive protein, Il = Interleukin, TNF = tissue necrosis factor, sST2 = soluble ST2, NT-proBNP = N-terminal propeptide of brain natriuretic peptide, Data = Mean ± SD,
= p < 0.05 vs Referent Control,
= p < 0.05 vs Hypertension (-) HFpEF.
There were significant correlations between plasma biomarkers and echocardiographic measurements of diastolic function (Figure 6); there was a statistically significant direct relationship between PCWP and NT-proBNP (r2 = 0.32, p = 0.001) sST2 (r2 = 0.26, p = 0.005) and TIMP-1 (r2 = 0.36, p < 0.001).
Figure 6.
Relationship between echocardiographically derived assessment of LV diastolic dysfunction (estimated pulmonary capillary wedge pressure [PCWP]) and plasma biomarkers of filling pressure (NT-proBNP, N-terminal propeptide of brain natriuretic peptide) and profibrotic factors (soluble ST2 [sST2], tissue inhibitor of matrix metalloproteinase 1 [TIMP-1]). There was a statistically significant direct relationship between PCWP and NT-proBNP (r2 = 0.32, p = 0.001), sST2 (r2 = 0.26, p = 0.005), and TIMP-1 (r2 = 0.36, p < 0.001).
Correlations between NTpro-BNP and titin phosphorylation at S4185(S469), S11878(S26) and S12022 (S170) and NTpro-BNP and titin-dependent stiffness were tested. While the sample size was limited, there was no clear relationship between NTpro-BNP and S4185(S469) (r2=0.009) or S12022 (S170) phosphorylation (r2= 0.04). However, there was a trend with S11878(S26) (r2 = 0.32, p=0.2). There was also a trend between NTpro-BNP and titin-dependent stiffness, r2 =0.22, p=0.08.
Finally, we also tested for correlations between sST2 and CVF and collagen-dependent stiffness. There were trends for both relationships, with sST2 increasing with increased CVF, r2=0.58, p=0.047 and increased collagen-dependent stiffness, r2=0.40, p=0.011.
Effects of Diabetes Mellitus
In the two HTN groups, the presence of DM did not affect echocardiographic parameters of hypertrophy or diastolic function, in vitro measures of stiffness, collagen or titin assays or biomarkers. DM also did not alter the differences in any of these parameters between HTN without or with HFpEF.
Discussion
The results of this study support several novel conclusions. The current study showed for the first time that patients with HTN(+)HFpEF have a significant increase in passive myocardial stiffness measured directly in left ventricular myocardial strips. Although consistent with previous studies in animal models of HFpEF 43, 45, 51, 52, this has never previously been documented in patients with HFpEF. Previous studies in HFpEF patients have examined the passive properties of the composite LV chamber 4 or the passive properties of isolated cardiomyocytes 19, 20, but not the passive properties of the intact myocardium. Making direct measurements in intact myocardium was a pivotal step for several reasons, including defining the relative mechanistic contributions of changes in titin and collagen to the increased passive myocardial stiffness seen in patients with HFpEF and the ability to differentiate the effects of co-morbid conditions on myocardial properties from the effects of HFpEF itself. The techniques used to make direct measurements of passive myocardial stiffness in left ventricular myocardial strips were developed using samples from patients with heart failure and a reduced ejection fraction (HFrEF) but these techniques have not been previously applied to patients with HFpEF.
Previous studies in patients with HFpEF examined the contribution of changes in titin to the resting tension (passive stiffness) of isolated cardiomyocytes obtained from LV endomyocardial biopsies; these studies also showed that collagen volume fraction was increased 19, 20. However, no previous study in patients with HFpEF has shown the relative contributions of changes in both collagen and titin to passive myocardial stiffness. The current study showed for the first time that the increase in myocardial stiffness in HFpEF patients was caused by changes in both ECM fibrillar collagen and cardiomyocyte titin. Specifically, HFpEF patients had increased collagen-dependent stiffness in association with increased fibrillar collagen content. HFpEF patients also had increased titin-dependent stiffness in association with significant changes in the phosphorylation state of titin, with decreased phosphorylation of a PKA/PKG site in the N2B element and increased phosphorylation of one of the PKC sites in the PEVK element.
While all of the HFpEF patients examined in the current study had antecedent HTN and ∼ 50% had DM, the presence of these co-morbid antecedent disease processes alone, in the absence of heart failure, did not alter total myocardial stiffness or its titin and collagen-dependent components. This is the first clinical study in patients with HFpEF to examine and differentiate the effects of a major antecedent disease substrate, such as hypertension, on myocardial structure and function before the clinical syndrome of HFpEF has actually developed. There has been significant controversy regarding the ability to differentiate or distinguish patients with hypertension from those with HFpEF. It has been proposed that HFpEF is simply the aggregate of a number of co-morbid factors such as hypertension, diabetes, CAD, obesity, etc. The current study clearly demonstrates that there are specific structural and functional differences between patients with hypertension without and with HFpEF. Similar conclusions are likely to be applicable to other co-morbid or antecedent disease processes.
The current study also shows for the first time that the development of collagen and titin based changes in diastolic function in patients with HFpEF occur in association with proinflammatory and profibrotic stimuli as measured by plasma biomarkers. These data provide additional support for the overall schematic hypotheses proposed by Paulus and Tschope (i.e., that proinflammatory and profibrotic signaling play a significant mechanistic role in the development of HFpEF) and constitute new mechanistic insights into the pathophysiology underlying HFpEF 13.
Hypertensive Heart Disease
HTN results in LV pressure-overload (PO) and causes a spectrum of structural, functional and clinical outcomes that have collectively been called hypertensive heart disease (HHD). Data from animal models of PO suggest that the myocardium responds to increased load by undergoing cardiomyocyte hypertrophy which results in an increase in myofibrillar content 9, 10, 45, 51. In addition to the myofibrils, other structures and processes within both the cardiomyocyte and the ECM are altered in response to increased load. In animal models these events have a temporal sequence in which the additional changes in the cardiomyocyte and ECM occur only after myofibrillar hypertrophy is well under way or even complete 45, 51. For example, in murine and feline models PO during the first 1-2 weeks of PO, there are no significant changes in CVF or diastolic filling pressures and no evidence of heart failure; however, after 4-8 weeks CVF and filling pressures are increased and evidence of heart failure develops. Data from the current study appear to parallel these temporal changes. Patients with HHD comprise a spectrum: HTN without LVH, HTN with LVH/concentric remodeling but no heart failure [HTN(-)HFpEF], and HTN with heart failure [HTN(+)HFpEF]. In the current study HHD patients were not followed in a serial manner; data were obtained by cross-sectional analysis. However, it was only in the patients with HTN(+)HFpEF that changes in collagen and titin were detected. Although in the absence of sequential data in the same patients we cannot prove the concept that sequential temporal changes occur in the cellular and molecular mechanisms that underlie HHD, our data clearly support the idea that changes in collagen and titin constitute mechanisms are associated with the transition from HTN to HFpEF.
Overall, there is good concordance between the current study and previous clinical studies in pressure-overload and HFpEF 16, 19, 20, 26, 28. Van Heerebeck et al 19 and Borbely et al 20 showed that patients with pressure-overload induced HFpEF had increased cardiomyocyte stiffness; however, because these mechanical studies were performed in isolated cardiomyocytes with the ECM removed, the relative contribution of these changes in collagen and titin to myocardial stiffness could not be determined.
Our results indicate that at SL 2.6 μm increases in ECM collagen account for more than 2/3 of the increase in resting tension in HFpEF. However, the relative contributions of titin and collagen to resting tension are SL-dependent; collagen accounts for a larger proportion at longer SLs and titin for a larger proportion at short SLs. Their relative contributions are therefore ultimately determined by the actual operating SL range in our patients, which is unknown. Based on studies performed in pigs 53, we do know that in large mammals the upper end of the operating range extends to 2.5-2.6μm. To the extent that the actual operating range is lower, the increase in total resting tension in HFpEF will be more evenly divided between the two.
Data from the current study significantly advance our understanding of how these mechanisms contribute to changes in myocardial stiffness by examining intact myocardial samples that allowed integrated physiologic studies to selectively examine the individual contributions of collagen and titin. The current study showed hypophosphorylation of PKG/PKA sites on titin in HFpEF patients, consistent with the reduction in passive tension detected when cardiomyocytes from HFpEF patients are treated with PKA/PKG 19, 20 and consistent with phosphorylation studies in animal models 43, 54. In contrast to Van Heerebeck et al 19 and Borbely et al 20, we did not detect changes in the N2BA/N2B ratio in HFpEF patients. In some but not all of the studies from these investigators, the N2BA/N2B ratio was increased in HFpEF, which would be expected to result in a potentially compensatory decrease in titin stiffness 16-20, 26, 28. It is worth noting that the HFpEF patients studied by these investigators had overtly decompensated heart failure and were therefore very likely at a more advanced stage of the syndrome. Perhaps more advanced disease is required to elicit such a compensatory change. The current study also showed for the first time that patients with HFpEF have hyperphosphorylation of one of the PKC/CaMKII sites on titin. Previous animal studies have demonstrated that hyperphosphorylation at these sites results in increased titin stiffness 23; however, the current study is the first clinical study to show that this contributes to increased myocardial stiffness in patients with HFpEF. In addition, while not yet studied in HFpEF, studies in HFrEF have suggested that CaMKII-dependent phosphorylation of S12022 (S170) may reduce passive stiffness 54.
The finding that both changes in collagen and titin may play a pivotal role in the development of HFpEF in HHD suggests the possibility that there may be common upstream mechanisms resulting in both changes 13. The presence of a HTN induced proinflammatory, profibrotic, and/or redox stress state is supported by the plasma biomarker profiles found in the current study. The increase in CRP, sST2 and TIMP-1 in the HFpEF patients supports this hypothesis. Clearly, more investigation is needed in this area.
Diabetes Mellitus
Diabetes mellitus has been shown in both animal studies and clinical disease to cause abnormal diastolic function that may contribute to the development of HFpEF 14, 16, 18. DM is reported to cause changes in collagen homeostasis (by altering cross-linking) and titin phosphorylation. Approximately 50% of the patients in the current study had DM as well as HTN. The presence of DM in addition to HTN did not appear to alter myocardial stiffness, collagen or titin to an extent greater than that of HTN alone. Because of their rarity in the CABG population, we were unable to identify sufficient numbers of patients with DM without HTN for analysis. This lack of effect differs from previous studies of pressure-overload (caused by aortic stenosis) in which the presence of DM caused a significant increase in cardiomyocyte stiffness over and above that caused by pressure-overload alone. This may be explained by patient selection differences. Thus, previous studies did not specifically identify whether patients with pressure-overload or DM had HFpEF or selectively examine patients with LVH without HFpEF 14, 16, 18. Additionally, the current study may not have been powered sufficiently to examine the separate effects of DM, there may be differences between pressure-overload caused by HTN versus aortic stenosis, and the duration, severity and management of DM may differ between studies. The current study does not necessarily bring into question the importance of DM in HFpEF, but it does raise the question of whether HTN and DM together have a combinatorial effect on passive stiffness. In addition, we did not examine the effects of DM on other determinants of diastolic function that could affect passive stiffness such as calcium homeostasis and contractile proteins, key determinants of relaxation rate and extent.
Effects of changes in collagen and titin homeostasis on diastolic function
Changes in collagen content, geometry, and composition are associated with abnormal diastolic function in HHD and other comorbidities common in patients with HFpEF 55. Collagen content is the product of the balance between collagen synthesis, post-synthetic processing, post-translational modification and degradation. The current study was not designed to examine the determinants of collagen homeostasis or mechanisms that alter it. However, the changes in plasma biomarkers detected in the HFpEF patients may provide some insights 56. Increases in sST-2 and TIMP-1 suggest that a profibrotic stimulus was present that would be expected to increase collagen synthesis and decrease collagen degradation. TIMP-1 has been shown to inhibit MMPs, the major degradation enzymes present in the ECM. ST2 is a member of the interleukin 1 receptor family. Its functional ligand is interleukin 33 (IL-33), a cardiac fibroblast protein. Binding of IL-33 to membrane ST2 elicits an antihypertrophic and antifibrotic response. This cardioprotective effect is negated by soluble ST2 which prevents binding of IL-33 to membrane bound ST2. Thus, increased TIMP-1 and sST-2 provide two potential mechanisms for the profibrotic state in HHD.
Titin's I-band segment serves as a molecular spring that develops passive force when extended57. Alternative splicing results in either the shorter and stiffer N2B or the longer and more compliant N2BA isoforms, which are co-expressed within the sarcomere. When the N2BA/N2B isoform ratio increases, cardiomyocyte and myocardial stiffness decrease as reported in heart failure with reduced EF 58, 59. In contrast, Borbely et al reported an increase in the N2BA/N2B isoform ratio in HFpEF patients but cardiomyocyte stiffness was increased 28, implying that changes in phosphorylation outweigh the isoform shift. The N2BA/N2B ratio did not change in the current study. As discussed above, it is possible that the isoform switch occurs at a later stage of HFpEF. In addition, the N2BA:N2B ratio did not change in at least one study of human HCM and DCM 60.
The phosphorylation state of 2 elements within titin's spring segment also modulates cardiomyocyte stiffness. Sites within the N2B element (e.g., S4185[S469]) are phosphorylated by PKA and PKG, which decreases passive force 25, 32. Sites within the PEVK element (S11878[S26] and S12022 [S170]) are phosphorylated by PKCα (other PKC isoforms have not yet been studied), which increases passive force. Additionally our data and the data of other investigators suggest that these sites on titin are also a target of CaMKII 54, 61, 62. In both the current and previous studies in HFpEF patients 28, hypophosphorylation of the N2B element has been observed. Moreover, treatment of skinned cardiomyocytes from HFpEF patients with PKG decreased cardiomyocyte stiffness 28, indicating that hypophosphorylation of PKA/PKG sites contributes to elevated passive force. Importantly, PKG treatment did not lower stiffness to the level present in control cardiomyocytes. It has been speculated 22 that this residual elevation in tension is due to hyperphosphorylation of PKC sites. In the current study we demonstrate that hyperphosphorylation of the S11878(S26) PKC/CaMKII site in the PEVK segment is associated with increased myocardial stiffness in HFpEF patients. This constitutes a novel mechanism of increased stiffness in HFpEF.
The increased PKCα activity that is suggested by the increased S26 phosphorylation did not result in phosphorylation of S12022 (S170). Previous studies indicate that PKCα is preferably active at sites with N-terminal and C-terminal basic residues 63. These studies also indicate that PKC preferentially phosphorylates serines with basic residues within three amino acids of both the C-and N-termini. Based on these results, PKC should have a stronger affinity for S11878(S26) than S170 because of the closer proximity of the neighboring basic amino acids (lysine [K] and arginine [R]). Finally, increased PKCα activity has been shown to directly increase protein phosphatase inhibitor-1 (PP1) activity 64, an effect which should facilitate hypophosphorylation of PKA/PKG sites in the N2B element (its effect on PKCα sites might be negated by the increased PKC activity). Thus, increased PKCα levels might increase stiffness directly through phosphorylation of PEVK S11878(S26) and indirectly through PP1 activation causing hypophosphorylation of PKA/PKG sites.
Limitations
The demographic characteristics of the current study population were typical of a CABG population. However, our HFpEF patients were slightly younger and more often male than typical patients in epidemiologic studies or randomized clinical trials. From the viewpoint of LV structure and function, however, they were typical of previous HFpEF studies.
What constitutes an appropriate control group is a challenging issue in all research using human myocardium. The degree of angiographic CAD was similar in all three study groups; therefore, CAD was not likely to be a confounding variable across groups. Alternative choices for controls are limited and less than ideal. Unused explanted donor hearts are usually from young subjects who have been subjected to high levels of stress for varying durations. Open heart procedures in patients without CAD or LV remodeling (e.g., ASD closure) are now rare and usually performed in younger patients. Samples from endomyocardial biopsies cannot be used for mechanical tissue studies because of tissue trauma. On the other hand, CAD, much of which is likely sub-clinical, is very common in HHD and HFpEF patients. Indeed, a recent report indicates that a majority of HFpEF patients have epicardial coronary stenosis 38. Thus, the presence of CAD can be considered a “real world” background in HTN patients and controls. The limitation imposed by the presence of CAD, however, is that we cannot exclude possible CAD effects on myocardial stiffness and/or distinguish them from the effects of hypertension or diabetes.
The current study focused specifically on the contribution of collagen and titin to the development of HFpEF. As in heart failure with a reduced ejection fraction (HFrEF), in HFpEF other mechanisms also contribute to the development of heart failure. Thus, changes in calcium homeostasis, energetics, and actin-myosin cross-bridge dynamics, which govern the speed and completeness of relaxation, all may play a role in HFpEF in addition to changes in passive stiffness.
Titin is a large and complex molecule with many phosphorylation sites outside the mechanically relevant spring region; however, assessing all of them may not be required in the context of the current study. The current study focused on three sites in the N2B and PEVK elements that have been documented in several studies for which we have made and characterized phospho-site specific antibodies. Two additional PKA/PKG sites in the N2B element were published recently by Kotter et al (S4010 and S4099). In their recent study Kotter et al showed that all three sites on the N2B element are hyophosphorylated in heart failure, indicating that they change in concert 60. Thus, assessing the established Ser4185/485 site is likely to reveal the general phosphorylation status of the N2B element.
It is important to acknowledge other limitations of the current study. We did not systematically study all titin phosphorylation sites. The protein has at least 70 phosphorylation sites, as shown by in-vivo phosphoproteomics using a SILAC approach 54. Moreover, recent work has suggested that the N2B domain contains several more conserved serines which are differentially phosphorylated in human HFrEF and in animal models of HFpEF 32, 43, 52. Phosphospecific antibodies against the respective N2Bus sites were used in human HCM and DCM hearts 60, 54. These studies underscore the complexity of titin phosphorylation schemes and the need for further research in patients with HFpEF as well as other forms of heart failure that will allow a more complete understanding of the contribution of titin to passive stiffness.
The current study also did not focus on upstream regulatory mechanisms effecting titin phosphorylation such as changes in the expression, abundance and activity of relevant protein kinases (e.g., PKA, PKG, CaMKII, PKC) and phosphatases (PP1, PP2a). Changes in kinase and phosphatase activity have been shown to be important in other forms of heart failure 26, 43; changes in HFpEF may represent important targets for novel therapies and important areas for future research.
Several other important mechanisms influencing the ECM were not examined in the current study, e.g., collagen isoform expression and content, troponin I and T, and galectin-3. Unfortunately, the size of the LV biopsy limited the total number of experimental measurements obtainable from each sample. Each of these additional mechanisms represents important directions for future research.
Finally, the effects of medications taken by the patients prior to surgery on titin phosphorylation and ECM homeostasis could not be assessed.
Clinical Implications
HTN is commonly a progressive process that leads to adverse cardiovascular remodeling, abnormal diastolic function, and the development of heart failure, particularly HFpEF 65-67. Once HFpEF has developed, subsequent morbidity and mortality rates are very high 67-76. Treatment of HHD is thus a critical unmet challenge. One clear answer is prevention of HHD by early treatment of high blood pressure, but prevention alone is insufficient. LV hypertrophy, concentric remodeling, and diastolic dysfunction commonly develop without concomitant symptoms, often before HTN has been detected. Furthermore, once LV remodeling and/or HFpEF develop, even guideline prescribed blood pressure control does not reduce morbidity and mortality. Randomized clinical trials in HFpEF of beta-blockers, angiotension converting enzyme inhibitors, angiotension II receptor blockers, aldosterone blockers and PDE-5 inhibitors, in which >90% of the patients had hypertension, have failed to show improvement in morbidity or mortality 70-76. Therefore, there is a need for novel treatments beyond blood pressure control.
The development of novel therapies must overcome several critical barriers. Most importantly, the cellular, extracellular, and molecular mechanisms that cause the progression from HHD to HFpEF must be defined in man. Although animal models are useful, they do not capture all of the key elements of complex and chronic human disease processes. Once proven operative in patients, each mechanism can serve as a target for successful therapy. The current study identifies two of these mechanisms; changes in collagen content and titin phosphorylation, each of which is a potential target. In addition, plasma biomarkers that reflect these changes in collagen, titin and the profibrotic milieu could be used to improve diagnostic criteria for HFpEF and prognostic assessment. Thus, changes in biomarkers such as TIMP-1 and sST-2 may detect the earliest transition from HTN to HFpEF. Finally, changes in these biomarkers could possibly be used to monitor treatment efficacy before changes in myocardial structure and function or clinical status are evident.
Conclusions
HTN in the absence of HFpEF was not associated with increased passive myocardial stiffness. However, we show for the first time that patients with hypertension and HFpEF have markedly increased passive myocardial stiffness due to increases in the contribution of both collagen and titin. These results suggest that the development of HFpEF is linked to a major increase in passive stiffness caused by changes in both collagen and titin homeostasis.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grants R56HL123478 (Zile), RO1HL089944 (LeWinter), U10 HL110342 (LeWinter), R01HL06288 (Granzier), and UL1TR000062 (Nietert), Merit Awards from the Veterans' Affairs Health Administration and the NHLBI Heart Failure Research Network. Dr. Michael Zile is supported by the Research Service of the Department of Veterans Affairs (5101CX000415-02 and 5101BX000487-04).
Footnotes
Disclosures: None
References
- 1.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD, TOPCAT Investigators Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Bennett TD, John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Warner-Stevenson L, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiologic insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction. Is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 6.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss HP, Tschöpe C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–85. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Aoki T, Fukumoto Y, Sugimura K, Oikawa M, Satoh K, Nakano M, Nakayama M, Shimokawa H. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. -Comparison between preserved and reduced ejection fraction heart failure. Circ J. 2011;75:2605–13. doi: 10.1253/circj.cj-11-0568. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–87. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 9.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128:19–28. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung CS, Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol. 2011;50:731–9. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. J Mol Cell Cardiol. 2007;42:186–95. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32:2151–62. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 13.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 14.Hamdani N, Franssen C, Lourenço A, Falcão-Pires I, Fontoura D, Leite S, Plettig L, López B, Ottenheijm CA, Becher PM, González A, Tschöpe C, Díez J, Linke WA, Leite-Moreira AF, Paulus WJ. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–49. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 15.Hamdani N, Paulus WJ. Myocardial titin and collagen in cardiac diastolic dysfunction: partners in crime. Circulation. 2013;128:5–8. doi: 10.1161/CIRCULATIONAHA.113.003437. [DOI] [PubMed] [Google Scholar]
- 16.Falcão-Pires I, Hamdani N, Borbély A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJ, Niessen HW, Leite-Moreira AF, Paulus WJ. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–9. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 17.Borbély A, Papp Z, Edes I, Paulus WJ. Molecular Determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–45. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 18.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 19.van Heerebeek L, Borbély A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 20.Borbély A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 21.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 22.LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127:938–44. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson BD, Hidalgo CG, Gotthardt M, Granzier Excision of titin's cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol. 2010;48:972–8. doi: 10.1016/j.yjmcc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–95. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–8. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 26.van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–9. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 27.Borbély A, van Heerebeek L, Paulus WJ. Transcriptional and posttranslational modifications of titin: implications for diastole. Circ Res. 2009;104:12–4. doi: 10.1161/CIRCRESAHA.108.191130. [DOI] [PubMed] [Google Scholar]
- 28.Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 29.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 30.van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbély A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJ. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006;69:876–87. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res. 2014;114:1052–68. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 32.Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 33.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 34.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O'Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in heart failure with preserved ejection fraction study (I-PRESERVE) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–27. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson C, Taatjes DJ, Zile MR, Palmer B, VanBuren P, Spinale F, Maughan D, Von Turkovich M, Bishop N, LeWinter MM. Combined immunoelectron microscopic and compter-assisted image analyses to detect advanced glycation end-products in human myocardium. Histochem Cell Biol. 2010;134:23–30. doi: 10.1007/s00418-010-0706-x. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson C, Palmer BM, Zile MR, Maughan DW, Ikonomidis JS, Granzier H, Meyer M, VanBuren P, LeWinter MM. Myosin cross-bridge dynamics in patients with hypertension and concentric left ventricular remodeling. Circ Heart Fail. 2012;5:803–11. doi: 10.1161/CIRCHEARTFAILURE.112.968925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 42.Heart Failure Society of America. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 44.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, John Sutton M, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Baicu CF, Li J, Zhang Y, Kasiganesan H, Cooper G, Zile MR, Bradshaw AD. Time course of right ventricular pressure-overload induced myocardial fibrosis: relationship to changes in fibroblast dependent post-synthetic procollagen processing. Am J Physiol. 2012;303:H1128–34. doi: 10.1152/ajpheart.00482.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh BJ, Thornton SC, Penny R, Breit SN. Microplate reader-based quantitation of collagens. Anal Biochem. 1992;203:187–190. doi: 10.1016/0003-2697(92)90301-m. [DOI] [PubMed] [Google Scholar]
- 47.Marotta M, Martino G. Sensitive spectrophotometric method for the quantitative estimation of collagen. Anal Biochem. 1985;150:86–90. doi: 10.1016/0003-2697(85)90443-9. [DOI] [PubMed] [Google Scholar]
- 48.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 49.McCulloch C, Searle SR. Generalized, linear, and mixed models. New York: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 50.Akaike Hirotugu. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 51.Zile MR, Baicu CF, Stroud RE, Van Laer A, Arroyo J, Mukherjee R, Jones JR, Spinale FG. Pressure-overload dependent membrane-type 1 matrix metalloproteinase induction: relationship to lv remodeling and fibrosis. Am J Physiol. 2012;302:H1429–37. doi: 10.1152/ajpheart.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr, Linke WA, Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–91. doi: 10.1161/CIRCULATIONAHA.111.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewinter MM, Popper J, McNabb M, Nyland L, Bell SB, Granzier H. Extensible behavior of titin in the miniswine left ventricle. Circulation. 2010;121:768–74. doi: 10.1161/CIRCULATIONAHA.109.918151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamdani N, Krysiak J, Kreusser MM, Neef S, dos Remedios CG, Maier LS, Krüger M, Backs J, Linke WA. Crucial role for Ca2+/calmodulin-dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112:664–674. doi: 10.1161/CIRCRESAHA.111.300105. [DOI] [PubMed] [Google Scholar]
- 55.Spinale FG, Zile MR. Integrating the myocardial matrix into heart failure recognition and management. Circ Res. 2013;113:725–738. doi: 10.1161/CIRCRESAHA.113.300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with preserved ejection fraction. J Cardiovasc Trans Res. 2013;6:501–515. doi: 10.1007/s12265-013-9472-1. [DOI] [PubMed] [Google Scholar]
- 57.Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10:211–23. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- 58.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 59.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 60.Kötter S, Gout L, Von Frieling-Salewsky M, Müller AE, Helling S, Marcus K, Dos Remedios C, Linke WA, Krüger M. Differential changes in titin domain phosphorylation increase myofilament stiffness in failing human hearts. Cardiovasc Res. 2013;99:648–56. doi: 10.1093/cvr/cvt144. [DOI] [PubMed] [Google Scholar]
- 61.Hidalgo CG, Chung CS, Saripalli C, Methawasin M, Hutchinson KR, Tsaprailis G, Labeit S, Mattiazzi A, Granzier HL. The multifunctional Ca2+/calmodulin-dependend protein kinase II delta (CaMKIIδ) phosphorylates cardiac titin's spring elements. J Mol Cell Cardiol. 2013;54:90–97. doi: 10.1016/j.yjmcc.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gladden JD, Linke WA, Redfield MM. Heart failure with preserved ejection fraction. Eur J Physiol. 2014;466:1037–1053. doi: 10.1007/s00424-014-1480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.House C, Wettenhall RE, Kemp BE. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987;262:772–7. [PubMed] [Google Scholar]
- 64.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–54. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 65.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 66.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure. The Framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 67.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 69.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 70.Yusef S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J, for the CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 73.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 74.van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD, SENIORS Investigators Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with heart failure) J Am Coll Cardiol. 2009;53:2150–8. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 75.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 76.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]