Abstract
The expression level of human leukocyte antigens (HLA) is known to influence pathological outcomes: pathogens downregulate HLA to evade host immune responses, host inflammatory reactions upregulate HLA, and differences between people in steady-state expression levels of HLA associate with disease susceptibility. Yet precise quantification of relative expression levels of the various HLA loci is difficult due to the tremendous polymorphism of HLA. We report relative expression levels of HLA-A, HLA-B, HLA-C and HLA-E proteins for the specific haplotype A*02:01, B*44:02, C*05:01, characterized using two independent methods based on flow cytometry and mass spectrometry. Peripheral blood lymphocytes from normal donors showed that HLA-A and HLA-B proteins are expressed at similar levels, which are 13-18 times higher than HLA-C by flow cytometry and 4-5 times higher than HLA-C by mass spectrometry, differences that may reflect variation in the conformation or location of proteins detected. HLA-E was detected at a level 25 times lower than that of HLA-C by mass spectrometry. Primary CD4+ T cells infected with HIV in vitro were also studied since HIV downregulates selective HLA types. HLA-A and -B were reduced on HIV-infected cells by a magnitude that varied between cells in an infected culture. Averaging all infected cells from an individual showed HLA-A to be 1-3 and HLA-B to be 2-5 times higher than HLA-C for different individuals by flow cytometry. These results quantify substantial differences in expression levels of the proteins from different HLA loci, which are very likely physiologically significant on both uninfected and HIV-infected cells.
Introduction
Human Leukocyte Antigens (HLA) are a family of molecules essential for immune function and with diverse clinical implications in infectious disease, autoimmunity, transplantation, cancer and pregnancy [1-5]. This study focuses on class-I HLA, which comprise three classical loci (HLA-A, HLA-B and HLA-C) and the additional non-classical molecule HLA-E, all related by a common ancestral origin and retaining substantial sequence homology. The classical HLA class-I molecules are expressed by almost all human cells. They sample intracellular peptides and present them at the cell surface where they are recognized by cytotoxic T lymphocytes (CTL), which can respond to foreign peptides. A defining feature of the classical HLA class-I is their tremendous polymorphism, concentrated in regions of the HLA molecule involved in peptide binding [6]. Hundreds of distinct protein sequence allotypes are encoded by each of the three classical HLA class-I loci. HLA-E expression at the cell surface is also dependent on binding an intracellular peptide, but HLA-E specifically binds the leader peptide derived from classical HLA class-I molecules. HLA-E has very limited polymorphism and it serves as the ligand for the inhibitory NKG2A receptor expressed by innate natural killer (NK) cells [7]. Both classical and non-classical HLA loci encode an approximately 45kDa heavy chain which associates with a conserved beta-2-microglubulin (β2m) molecule of 12kDa to form the complex that binds and presents small peptides of around 8 amino acids.
Numerous observations in vivo demonstrate that expression level of HLA molecules has an important influence on their function. One of the most striking cellular changes in the inflammatory response is interferon-γ (IFN-γ) mediated up-regulation of HLA expression [8]. In contrast, numerous pathogens downregulate HLA to evade T cell recognition [9-12]. Cells of the innate immune system carry multiple inhibitory receptors for classical HLA in order to detect this pathogen-mediated manipulation. Examples of these inhibitory receptors include leukocyte immunoglobulin-like receptors (LILR), which are expressed by cells of the myeloid lineage and bind all classical HLA class-I, and killer immunoglobulin-like receptors (KIR), which are expressed by NK cells and bind specific HLA allotypes, predominantly from the HLA-C locus. The level of expressed protein at some HLA loci varies between normal individuals. For example, allotypes of the HLA-C locus differ in expression level by up to 3-fold and these differences correlate with clinical outcomes in some disease settings [13]. Individuals with HLA-C allotypes that are expressed at higher levels show better control of viral load during HIV infection. Higher expression may result in more efficient initiation of T cell responses as both HIV-specific CTL responses and viral escape mutation associated more strongly with higher expressed HLA-C alleles [13]. This effect in HIV infection is significant, as expression levels of HLA-C are marked by a single nucleotide polymorphism in the region 5’ of HLA-C, and this polymorphism showed one of the two strongest effects in the human genome on outcome of HIV infection [14-16]. This variant is in strong linkage disequilibrium with a 3’UTR insertion/deletion polymorphism in a microRNA binding site, which associates with HIV outcome and may account in part for differential HLA-C expression levels [17,18].
Although expression level has important consequences for HLA function, relative levels of proteins expressed from the HLA-A, B, C and E loci are not well established. Precise quantitation is made difficult by the extreme polymorphism of classical HLA loci. Most individuals express 6 different classical HLA alleles (i.e. heterozygous at HLA-A, -B, and -C) in addition to HLA-E, so detecting a particular locus with specificity is challenging. Even if antibodies specific to molecules from each locus can be identified, their binding cannot simply be compared due to differences in affinity for their respective antigens. In the 1970’s, analysis of HLA from a B lymphoblastoid cell line (B-LCL) determined that HLA-A/B were expressed at a level which was at least 10-fold higher than HLA-C [19]. HLA heavy chains were quantified indirectly via antibody to β2m, after enrichment for surface membrane proteins and separation of the HLA allotypes by elution from a lectin column. But HLA allotypes were not resolved into fully distinct fractions, limiting the accuracy of the method. For example, HLA-C eluted in two fractions, of which only the first was used for HLA-C quantitation since the second contained both HLA-C and HLA-A. HLA-C was less than a tenth the level of HLA-A/B, but by how much less could not be defined. The experiment tested a single B cell line and did not consider HLA-E, which is thought to be expressed at even lower levels than HLA-C [14]. Expression level of HLA-E is somewhat controversial, with differences in the conformations of HLA detected by antibodies to HLA-E [20] and cross-reaction of HLA-E antibodies with classical HLA allotypes [21] having complicated attempts to determine the level of HLA-E on primary cells.
Precise quantitation of the HLA-A, B, C and E protein expression levels on normal primary cells would therefore be valuable. Relative expression levels of the classical HLA class-I loci are of particular interest on HIV-infected cells, because HIV encodes the Nef protein, which downregulates HLA-A and HLA-B [9,22]. Nef has multiple functions but specifically downregulation of HLA/MHC has been shown to be significant in vivo [23,24]. As HLA-A and -B are not reduced with equal efficiency by HIV, and Nef does not modulate HLA-C, it is not clear which HLA locus dominates on HIV-infected cells [25,26]. Here we describe two independent approaches, using flow cytometry and mass spectrometry, to determine the relative expression levels of HLA class-I proteins on normal and HIV-infected primary cells.
Materials and Methods
HLA genotyping and monoclonal antibodies
This study was approved by the local Institutional Review Boards and all donors gave informed consent. Peripheral blood leukocytes (PBL) and archived B-LCL were genotyped for HLA-A/B/C to 4-digit resolution by PCR-SSOP (sequence-specific oligonucleotide probing) and PCR-SBT (sequence-based typing) as recommended by the 13th International Histocompatibility Workshop [27]. Only samples homozygous for A*02:01, B*44:02 and C*05:01 were studied further. Monoclonal antibodies (mAb) that bind HLA were characterized for reactivity against 97 common HLA class-I alleles using commercially available beads coated with individual HLA allotypes as previously described [28]. Of numerous antibodies screened, the following were used for further analyses: purified mAbs W6/32 [29], BBM.1 [30] and L31 [31] purchased from Serotec, Santa Cruz Biotechnology and MediaPharma, respectively; hybridoma supernatants of mAbs PA2.1 [32] and 22E-1 [33,34] generously supplied by Drs. N. Holmes and I. Smith; mAb DT9 [14,35] purified from supernatant of a hybridoma kindly provided by Dr. V. Braud.
Primary cell preparation and infection with HIV
Leukocytes were isolated by density gradient separation from peripheral blood freshly-drawn from healthy donors and either used immediately for cytometry, lysed and frozen for immunoprecipitation/mass spectrometry, or selected for HIV infection using anti-CD4 mAb-based magnetic selection (EasySep) to attain a purity exceeding 95% CD4+ with less than 1% CD8+ cells as assessed by cytometry staining with L120-PE and SK1-FITC (both Becton Dickinson). CD4+ cell preparations were expanded for 3-5 days in RPMI supplemented with 100 U/ml IL-2 (Peprotech), anti-CD3/28 beads (Invitrogen), FBS (Lonza) and PSG (Invitrogen) before infection with the HIV strain NL4-3 [36]. NL4-3 was generated by infectious molecular clone transfection of HEK293T, with the infectious titre of culture supernatants determined using TZM-Bl [37]. Infection was performed by incubation of cells with virus at a nominal multiplicity of infection of 0.1 for 4-6hrs at 37°C. Cells were then washed and cultured in RPMI with IL-2 for a further 6 or 7 days before analysis by cytometry.
Flow cytometry
B-LCL from culture, PBL freshly isolated from normal donors, or CD4-selected cells isolated from normal donors and infected in vitro with HIV, were incubated with unlabeled primary antibody to HLA or isotype controls, followed by PE-conjugated anti-mouse IgG (Sigma-Aldrich). Free secondary antibody-binding sites were blocked with murine immunoglobulin before further staining of primary cell samples with directly conjugated mAb to identify specific leukocyte populations on which HLA staining is reported. CD3+ cells were identified in freshly isolated PBL preparations using UCHT1-FITC (Beckman Coulter). Cultures infected in vitro with HIV were stained with CD4-PB (Biolegend), CD8-APC and CD3-APC.Cy7 (both Becton Dickinson) and yellow fluorescent reactive viability dye (Invitrogen). Expression of CD4 was used to discriminate HIV infected cells after gating on CD3+ CD8- cells as previously described [13,38]. Briefly, uninfected cells remained CD4+, whereas infected cells lost CD4 expression, and HLA staining could be compared between these two populations (Fig. 1A). To verify that CD4 downregulation identified infected cells, a parallel stain was performed in which HIV was detected directly. A sample of cells from each culture well in which HLA was assayed were stained with the same panel of conjugated mAbs, but instead of the indirect HLA stain, cells were fixed and permiabilised by incubation with paraformaldehyde and saponin (Becton Dickinson) before staining of intracellular gag with KC57-PE (Beckman Coulter). Within the CD3+ CD8- population, more than 97% of CD4 negative cells were KC57+ and more than 97% of CD4 positive cells were KC57- for every culture from which HLA expression was reported, confirming that CD4 downregulation could be used to discriminate HIV infected from uninfected cells (data not shown). Staining results were acquired using either FACscan or LSRII flow cytometers (both Becton Dickinson) with analysis performed using FlowJo (Tree Star).
Fig. 1. Representative flow cytometry staining of each HLA locus on CD4 cells purified from normal donor 2 and infected in vitro with HIV.
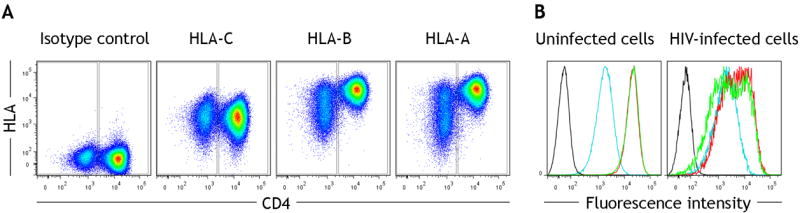
(A) After gating the CD3+ CD8- population, CD4 downregulation was used to discriminate infected (CD4-) from uninfected (CD4+) cells in a culture (x-axis). Staining for each classical HLA class-I locus was then measured (y-axis). (B) Relative staining of HLA-A (green), HLA-B (red), HLA-C (blue) and isotype control (black) is shown in separate histograms for uninfected (left) and infected (right) cells from the culture.
Immunoprecipitation
B-LCL from culture or PBL freshly isolated from normal donors were lysed in RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor (Roche), and HLA class-I molecules were purified by immunoprecipitation. mAb W6/32 or isotype control covalently conjugated to protein G-sepharose (GE Healthcare), as previously described [39], were incubated with lysate from 12.5×106 B-LCL cells or 25×106 PBL for 90mins rotating at 4°C, after preclearing of the lysate against protein G-sepharose beads alone. Bound complexes were washed with RIPA and frozen for subsequent analysis by mass spectrometry. To verify immunoprecipitation efficiency, parallel immunoprecipitations were eluted with LDS sample buffer, resolved by PAGE and transferred to PVDF (all Invitrogen Novex). Blots were probed with mAb L31 (MediaPharma), reactive with HLA-C*05:01 in the haplotype studied [40,41], which was detected by peroxidase-conjugated anti-mouse antibody visualized using enhanced chemiluminescence (both GE Healthcare).
Mass spectrometry (MS)
Target peptides unique to each HLA protein to be used for quantitation were determined empirically after immunoprecipitation of the four HLA proteins from B-LCL. Both “heavy” isotope-labeled (single amino acid at C-terminus of each peptide labeled with C13N15) and “light” non-labeled synthetic sets of the target peptide sequences were obtained (New England Peptide). Proteins were eluted from immunoprecipitation beads using 2% SDS in 50mM pH 8.3 Tris-HCl and subsequently reduced, alkylated, and digested using Trypsin-LyC (Promega) overnight following a filter-aided procedure using FASP digestion kits (Expedeon Inc.). Tryptic peptides generated were combined with synthetic heavy isotope-labeled peptides, desalted using C18 ZipTip (Millipore), lyophilized, and reconstituted in 0.1% TFA. Nanobore reversed-phase liquid chromatography tandem mass spectrometry (nanoRPLC-MS/MS) was performed using an Agilent 1200 nanoflow liquid chromatography (LC) system coupled online with a LTQ Orbitrap XL mass spectrometer. RPLC columns (75 μm i.d. × 10 cm) were slurry-packed in-house with 5 μm, 300Å pore size C-18 stationary phase into fused silica capillaries with a flame pulled tip. After sample injection, the column was washed for 20 min with 98% mobile phase A (0.1% formic acid in water) at 0.5 μl/min. Peptides were eluted using a linear gradient of 2% mobile phase B (0.1% formic acid in ACN) to 35% B in 100 minutes, then to 80% B over an additional 20 minutes. The column flow-rate was maintained at 0.25 μl/min throughout the separation gradient. The mass spectrometer was operated in a data-dependent mode in which each full MS scan was followed by three MS/MS scans wherein the three most abundant molecular ions were dynamically selected for collision-induced dissociation using a normalized collision energy of 35%. To quantify precipitated proteins a calibration curve was generated for each peptide monitored, by analyzing increasing amounts of synthetic light peptide mixed with the fixed amount of heavy peptide added to biological samples, with data processing performed using Xcalibur software (Thermo Scientific).
Results
Sequenced-based HLA typing of 300 healthy donors identified two unrelated individuals who were homozygous for one of the most frequent Caucasian haplotypes: A*02:01, B*44:02, C*05:01. These individuals were studied to simplify the task of comparing relative expression levels from each HLA class-I locus. A B-LCL from a third donor homozygous for this haplotype was also identified. Three mAbs appropriate for measurement of HLA-A, B and C expression levels, respectively, by cytometry were identified by demonstrating specificity to each of the three allotypes of this haplotype and comparable binding strengths (Suppl Fig. 1). The mAb PA2.1 is specific to HLA-A*02:01, mAb 22E.1 is specific to B*44:02 and mAb DT9 is specific to C*05:01 in subjects homozygous for the A*02:01, B*44:02, C*05:01 haplotype. mAb DT9 is known to recognize HLA-E in addition to HLA-C [35], but we and others have previously determined that binding of DT9 to PBL is dominated by reactivity with HLA-C [14,42], likely because expression levels of HLA-E are very much lower than HLA-C. mAb BBM.1 recognizes the β2m molecule with which all HLA 45kDa heavy chains associate [30]. Binding of mAbs PA2.1, 22E.1 and DT9 was normalised to that of BBM.1 in our screening panel, in order to quantify the relative strength of antibody binding corrected for variation in the amount of each HLA allele present (Suppl Fig. 1). This approach indicated that PA2.1, 22E.1 and DT9 bind at very similar levels to their respective antigens in the target haplotype (Table I). Binding of these mAbs to primary cells in cytometry can therefore be directly compared to estimate relative expression levels of the HLA loci present.
Table I.
Antibodies PA2.1, 22E-1 and DT9 bind with similar strength to their respective alleles of the haplotype studied in flow cytometry, when correcting for variation in antigen levels in the screening panel as measured by staining β2m with mAb BBM.1.
| Antibody | Median Fluorescence Intensity | Binding relative to BBM.1 | ||||
|---|---|---|---|---|---|---|
| A*0201 | B*4402 | C*0501 | A*0201 | B*4402 | C*0501 | |
| PA2.1 | 9,927 | 29 | 37 | 1.3 | 0.0 | 0.0 |
| 22E-1 | 29 | 5,563 | 148 | 0.0 | 1.3 | 0.0 |
| DT9 | 27 | 55 | 7,107 | 0.0 | 0.0 | 1.3 |
| BBM.1 | 7,367 | 4,413 | 5,959 | - | - | - |
Staining of the mAbs PA2.1 (HLA-A), 22E.1 (HLA-B) and DT9 (HLA-C) was compared for the samples homozygous for the A*02:01, B*44:02, C*05:01 haplotype. HLA-A was detected at 15 times and HLA-B at 18 times the level of HLA-C on B-LCL (Table II). On freshly isolated PBL from two normal donors, HLA-A was detected at 13-16 times the level of HLA-C and HLA-B at 17-18 times the level of HLA-C (Table II). Staining of normal PBL is reported specifically for CD3+ cells, but relative differences between HLA loci were similar for all major leukocyte populations. CD4+ cells from these donors were activated and then infected with HIV-1 in vitro before staining each HLA allotype on both infected and uninfected cells from the same cultures. Uninfected cells show that in vitro culture marginally reduced HLA-A and -B expression relative to HLA-C to 12-13 times higher than HLA-C for both donors (Table II). In contrast, HLA-A and -B were reduced substantially on HIV infected cells, where the median fluorescence intensity (MFI) of HLA-A staining was reduced to 1-3 fold higher than HLA-C, and HLA-B staining was reduced to 2-5 fold higher than HLA-C (Table II). Specifically for HIV-infected cells, HLA-A/B expression levels were not normally distributed across cells from a given individual. Many infected cells have HLA-A/B expression levels that are only marginally higher than HLA-C, and for some cells, HLA-A is expressed at a level lower than HLA-C in the representative example shown (Fig. 1).
Table II.
Relative expression levels of HLA class-I proteins measured by flow cytometry.
| Sample type | Median Fluorescence Intensity | Level relative to HLA-C | ||||
|---|---|---|---|---|---|---|
| IgG | HLA-A | HLA-B | HLA-C | HLA-A | HLA-B | |
| B cell line | 5 | 1,134 | 1,357 | 76 | 15 | 18 |
| Normal PBL (donor 1) | 4 | 922 | 1,208 | 70 | 13 | 17 |
| Normal PBL (donor 2) | 4 | 806 | 947 | 51 | 16 | 18 |
| Cultured uninfected PBL (donor 1) | 63 | 20,839 | 20,627 | 1,551 | 13 | 13 |
| Cultured uninfected PBL (donor 2) | 33 | 19,844 | 19,629 | 1,678 | 12 | 12 |
| Cultured HIV-infected PBL (donor 1) | 58 | 4,363 | 7,043 | 1,387 | 3 | 5 |
| Cultured HIV-infected PBL (donor 2) | 47 | 2,508 | 4,445 | 1,795 | 1 | 2 |
Staining is reported for total CD3+ cells from normal PBL, and from CD4+ (uninfected) or CD4- (infected) cells within the CD3+ CD8- population for cultured PBL. Expression levels are shown for a single representative replicate of two-three from independently obtained biological samples. The ranges of expression relative to HLA-C in these replicates were: B cell line, n=3, HLA-A (14-16), HLA-B (17-19); normal PBL donor 1, n=3, HLA-A (13-15), HLA-B (17-19); normal PBL donor 2, n=3, HLA-A (13-16), HLA-B (18-18); cultured uninfected PBL donor 1, n=2, HLA-A (13-14), HLA-B (13-13); cultured uninfected PBL donor 2, n=3, HLA-A (12-13), HLA-B (11-13); HIV-infected PBL donor 1, n=2, HLA-A (3-4), HLA-B (5-6); HIV-infected PBL donor 2, n=3, HLA-A (1-3), HLA-B (2-6). Absolute MFIs are higher for all cultured PBL due only to use of a different cytometer.
The cytometry method relied on comparisons between antibodies that were not identical in affinity and that could not assess HLA-E. We therefore applied a mass spectrometry-based method to differentiate and quantify the classical HLA class I molecules as well as HLA-E. Cells from the single B-LCL or two normal PBL donors homozygous for the A*02:01, B*44:02, C*05:01 haplotype were analysed. mAb W6/32, which binds all HLA class-I alleles equally [28], was used to immunprecipitate total HLA from cell samples (Suppl Fig. 2). Precipitated material was digested with trypsin and analysed by LC-MS. Multiple peptides unique to each HLA heavy chain among the known HLA alleles present were monitored and quantified (Fig. 2). These peptides were selected from those detected in initial analyses of the B-LCL sample on the basis of high signal intensity, distance from mis-cleavage sites, low modification potential and high LC performance. A tandem mass spectrum showing representative identification in a PBL sample of one of the peptides specific to HLA-A is shown (Fig. 3). Peptides were quantified relative to a heavy-isotope labeled synthetic peptide with the same sequence that was added to the biological samples (Suppl. Fig. 3) using a calibration plot generated for each peptide (Suppl. Fig. 4). Amounts of all peptides monitored for each HLA locus are shown for a representative analysis of one PBL donor (Table III). The median of expression levels indicated by all peptides monitored for a locus was taken as the overall measure for expression level at that locus. All peptides were measured in the single B-LCL and two normal PBL donors analysed and results summarized in Table IV. In B-LCL, HLA-A was detected at 6 and HLA-B at 5 times the level of HLA-C, and HLA-C was observed at a level 17 times higher than HLA-E. The two normal PBL donors demonstrated the same results as each other. HLA-A was detected at 5 times and HLA-B at 4 times the level of HLA-C, with HLA-C expressed 25 times higher than the level of HLA-E.
Fig. 2. Peptides unique to each HLA used for quantitation in a mass spectrometry-based assay.

Mature protein sequence of each HLA class-I protein present in the individuals studied is shown. Peptides unique to an HLA locus within this individual that were used for quantitation are highlighted: 3 peptides for HLA-A (yellow), 4 peptides for each of HLA-B (green) and HLA-C (blue) and 2 peptides for HLA-E (red). In the HLA-E sequence X represents either R or G.
Fig. 3. Representative mass spectrometry fragmentation spectrum.
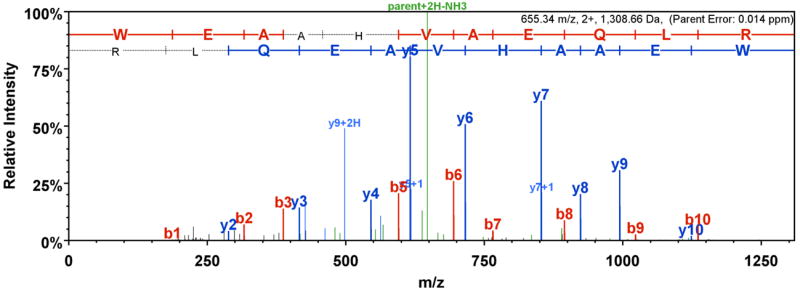
The doubly charged HLA-A specific peptide WEAAHVAEQLR, detected for normal PBL donor 1, is shown visualized using Scaffold software. N-terminal b fragment ions are labeled in red, C-terminal y fragment ions are labeled in blue and other fragment ions are labeled in green.
Table III.
Representative results for the peptides monitored by mass spectrometry at each HLA locus, determined for an immunoprecipitation sample from normal PBL donor 1.
| Target allele | Quantitating peptide | Amount detected (fmol) | Median amount for locus (fmol) |
|---|---|---|---|
| HLA-A*0201 | WAAVVVPSGQEQR | 3,185 | |
| WEAAHVAEQLR | 2,905 | 2,905 | |
| VDLGTLR | 2,504 | ||
| HLA-B*4402 | THVTHHPISDHEVTLR | 2,117 | |
| FITVGYVDDTLFVR | 2,298 | 2,503 | |
| APWIEQEGPEYWDR | 2,977 | ||
| AYLEGLCVESLR | 2,708 | ||
| HLA-C*0501 | THVTHHPVSDHEATLR | 333 | |
| DYIALNEDLR | 836 | 582 | |
| APWVEQEGPEYWDR | 756 | ||
| GYNQFAYDGK | 408 | ||
| HLA-E | AEWSDSAQGSESHSL | 18 | |
| SWTAVDTAAQISEQK | 29 | 23 |
Table IV.
Relative expression levels of HLA class-I loci measured by mass spectrometry.
| Sample type | Amount detected (fmol) | Level relative to HLA-C | |||||
|---|---|---|---|---|---|---|---|
| HLA-A | HLA-B | HLA-C | HLA-E | HLA-A | HLA-B | HLA-E | |
| B cell line | 2,888 | 2,759 | 521 | 32 | 6 | 5 | 0.06 |
| Normal PBL (donor 1) | 2,905 | 2,503 | 582 | 23 | 5 | 4 | 0.04 |
| Normal PBL (donor 2) | 3,424 | 3,137 | 726 | 31 | 5 | 4 | 0.04 |
Amounts detected are medians from the multiple peptides used to quantitate each HLA locus. For each donor and the cell line, expression levels shown are from a single representative experimental replicate. Ranges of expression relative to HLA-C in these replicates were: B cell line, n=8, HLA-A (5.5-6.2), HLA-B (4.8-5.6), HLA-E (0.06-0.07); PBL donor 1, n=4, HLA-A (4.3-5.0), HLA-B (3.7-4.3), HLA-E (0.03-0.06); PBL donor 2, n=4, HLA-A (4.3-5.0), HLA-B (3.6-4.3), HLA-E (0.03-0.05).
Discussion
We report relative expression levels of the HLA-A, HLA-B, HLA-C and HLA-E proteins measured for normal and HIV-infected primary cells. Two independent methods were used, flow cytometry and mass spectrometry. On freshly-isolated PBL from normal donors, the HLA-A/B proteins were expressed at similar levels to each other, but relative to HLA-C they were 13-18 times higher as measured by flow cytometry and 4-5 times higher as measured by mass spectrometry. HLA-E was expressed 25 times lower than HLA-C as measured by mass spectrometry. On HIV-infected cells, HLA-A and -B were reduced by a magnitude that varied between cells in an infected culture. Some infected cells expressed lower HLA-A than HLA-C, but when averaging across all infected cells from an individual, HLA-A/B were between 1 and 5 times higher than HLA-C in measurements made using cytometry. Although we studied only one haplotype common in Caucasians, the differences between certain loci (e.g. HLA-B vs. HLA-C) are large compared with the variation in expression level of alleles that is seen at a single locus [13]. Therefore, we expect that the observed magnitudes of differences in expression levels between HLA loci are broadly applicable across individuals.
Two PBL donors were analysed and yielded almost identical results within each assay on normal cells. For example, HLA-B was detected at 4 times the level of HLA-C for both individuals by mass spectrometry, and between 17-18 times the level of HLA-C for both individuals by flow cytometry. On HIV infected cells HLA-A/B expression levels did differ to some extent between the donors, likely a consequence of variable in vitro infection efficiency between individuals. The cytometry and mass spectrometry assays gave corresponding results, but the former estimated a greater difference between HLA-A/B versus HLA-C expression levels. Multiple differences between the two assays might account for this variation. Cytometry specifically detects surface HLA expression level as compared to measurement of total cellular HLA that is detected by the mass spectrometry-based approach. Characterisation of the mAbs used in cytometry detected HLA molecules expressed in vitro, which may not wholly reflect the reactivity on primary cells. MS analysed HLA purified by immunoprecipitation with mAb W6/32. This mAb binds the predominant functional conformation of HLA, heavy chain associated with β2m and peptide, whereas the cytometry mAbs may also recognize unfolded conformations. Despite these caveats, the overall concordance observed from such different assays as cytometry and mass spectrometry strengthens confidence in the relative HLA expression levels reported.
Our results support the overall findings of Snary et al and address several of the limitations of that approach from 1977 [19]. A major limitation of the Snary approach was that HLA heavy chains were quantified indirectly based on the β2m signal from an elution fraction, but serial elutions from a lectin column did not completely isolate all proteins from different HLA loci into distinct fractions. In contrast, our quantification by cytometry and spectrometry was based on direct detection of the HLA class-I heavy chain proteins themselves. This was achieved by antibody specificity for each heavy chain in the case of cytometry and by quantitation of peptides unique to each HLA heavy chain by mass spectrometry. Further, Snary et al imprecisely defined the magnitude by which HLA-A and B expression was estimated to exceeded HLA-C by, which could only be determined as “at least 10 times” because HLA-C was observed below the threshold for quantitation by β2m detection. Our new measurements were able to quantify the low amount of HLA-C, and thus define an absolute level by which HLA-A/B are more highly expressed. HLA-A and B were detected 13-18 times higher than HLA-C by flow cytometry and 4-5 times by mass spectrometry using PBL, consistent with a lower extreme of the estimate provided by Snary et al.
Our findings extend the previous results in three significant ways: quantifying HLA-E expression level, measuring expression level on primary PBL for each of the HLA types studied, and measuring the levels on HIV-infected cells for classical HLA class-I specifically. mAbs can recognize HLA-E on transfectants, but precisely which antigens these mAbs bind on primary cells is not clear [20,21], so we were unable to quantify HLA-E by cytometry. HLA-E was identified and quantified by the mass spectrometry-based assay and found to be nearly 20 times lower than HLA-C in B-LCL and 25 times lower than HLA-C in PBL. Relative levels of the HLA loci for PBL freshly isolated from normal donors were broadly similar to those for B-LCL. Primary CD4 T cells infected in vitro with HIV showed the expected reduction of specifically HLA-A and -B [22,25]. The magnitude of this HLA-A/B reduction varied between infected cells in any given culture, which may correlate with the time since infection and accumulation of Nef in each cell. On average, HLA-A/B expression was still higher than HLA-C, but only by 1-5 fold on infected cells compared to 12-13 fold on uninfected cells from the same culture. Our results also confirm a previous observation made using HLA transfectants: Nef downregulates HLA-A by a greater amount as compared to HLA-B [26], showing that this is also the case for infection of primary cells. The reduction of HLA-A/B we observed was not as dramatic as that reported by the seminal studies defining Nef modulation of HLA [22,25]. These previous studies used HLA transfectants rather than primary cells and were designed to maximize effects of Nef by employing strains of HIV engineered to over-express Nef. Consequently, the smaller magnitude of HLA reduction we see using primary cells infected with the HIV strain NL4-3 more likely represents the magnitude of HLA reduction by HIV in vivo. This is supported by a similarly modest magnitude of HLA-B*57 downregulation that has been observed by infection of primary cells in vitro with HIV [43]. The locus-specificity and modest magnitude of HLA downregulation by HIV may be precisely selected for an optimal balance of reducing CTL response whilst minimizing induction of innate cells with inhibitory receptors for HLA.
The large differences in expression levels observed between HLA class-I loci are likely to be functionally significant. Higher HLA expression levels are known to more efficiently initiate CTL responses [13,44] and also modulate the cytokines CTL secrete [45]. Differential expression levels of MHC class I loci has also been well characterized in chickens, and shown to associate strongly with risk of Marek’s disease [46,47], indicating that this phenomenon may be common across species. Further, several inhibitory receptors (such as LILRB1/2 and KIR3DL1) recognize antigens from multiple HLA loci, so allotype-specific expression levels may affect the innate immune response, as well as the acquired immune response. Given the accumulating data pointing to a significant impact of differential allotype-specific and locus-specific expression levels on the immune response, it is necessary to define this property for each of the HLA loci in order to determine its potential effect across human diseases.
Supplementary Material
Acknowledgments
We are grateful to Troy Kemp, Mac Trubey and Abigail Lara for Luminex and flow cytometry assistance; and Elena Chertova, Rasmi Thomas, Arman Bashirova and Colm O’hUigin for critical review of the manuscript.
Abbreviations used in this article
- B-LCL
B lymphoblastoid cell line
- β2m
beta-2-microglubulin
- KIR
killer immunoglobulin-like receptor
- LC
liquid chromatography
- LILR
leukocyte immunoglobulin-like receptor
- MFI
median fluorescence intensity
- MS
mass spectrometry
Footnotes
This project has been funded in whole or in part with federal funds from the Frederick National Laboratory for Cancer Research, under Contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US government. This research was supported in part by the Intramural Research Program of the NIH, Frederick National Lab, Center for Cancer Research.
References
- 1.Bashirova AA, Thomas R, Carrington M. HLA/KIR Restraint of HIV: Surviving the Fittest. Annu Rev Immunol. 2011;29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough SCL, Simmonds MJ. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr Genomics. 2007;8:453–465. doi: 10.2174/138920207783591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, Yoshida T, Kimura A, Akaza T, Kamikawaji N, Kodera Y, Takaku F. Effect of Matching of Class I HLA Alleles on Clinical Outcome after Transplantation of Hematopoietic Stem Cells from an Unrelated Donor. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 4.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, Gjessing HK, Carrington M, Moffett A. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol. 2014;192:5069–5073. doi: 10.4049/jimmunol.1400577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parham P, Lomen CE, Lawlor DA, Ways JP, Holmes N, Coppin HL, Salter RD, Wan AM, Ennis PD. Nature of polymorphism in HLA-A, -B, and -C molecules. Proc Natl Acad Sci USA. 1988;85:4005–4009. doi: 10.1073/pnas.85.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, López-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94yNKG2A. Proc Natl Acad Sci USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koeffler HP, Ranyard J, Yelton L, Billing R, Bohman R. Gamma-interferon induces expression of the HLA-D antigens on normal and leukemic human myeloid cells. Proc Natl Acad Sci USA. 1984;81:4080–4084. doi: 10.1073/pnas.81.13.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV−1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 10.Jones TR, Hanson LK, Sun L, Slater JS, Stenberg RM, Campbell AE. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett EM, Bennink JR, Yewdell JW, Brodsky FM. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- 12.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of Major Histocompatibility Complex Class I Molecules by Kaposi’s Sarcoma-Associated Herpesvirus K3 and K5 Proteins. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, Brumme CJ, John M, Mallal S, Nelson G, Bosch R, Heckerman D, Stein JL, Soderberg KA, Moody MA, Denny TN, Zeng X, Fang J, Moffett A, Lifson JD, Goedert JJ, Buchbinder S, Kirk GD, Fellay J, McLaren P, Deeks SG, Pereyra F, Walker B, Michael NL, Weintrob A, Wolinsky S, Liao W, Carrington M. Influence of HLA-C expression level on HIV control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas R, Apps R, Qi Y, Gao X, Male V, O’hUigin C, O’Connor G, Ge D, Fellay J, Martin JN, Margolick J, Goedert JJ, Buchbinder S, Kirk GD, Martin MP, Telenti A, Deeks SG, Walker BD, Goldstein D, McVicar DW, Moffett A, Carrington M. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet. 2009;41:1290–1294. doi: 10.1038/ng.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. A Whole-Genome Association Study of Major Determinants for Host Control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, Martin MP, Hunt P, Deeks SG, Telenti A, Pereyra F, Goldstein D, Wolinsky S, Walker B, Young HA, Carrington M. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472:495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni S, Qi Y, O’hUigin C, Pereyra F, Ramsuran V, McLaren P, Fellay J, Nelson G, Chen H, Liao W, Bass S, Apps R, Gao X, Yuki Y, Lied A, Ganesan A, Hunt PW, Deeks SG, Wolinsky S, Walker BD, Carrington M. Genetic interplay between HLA-C and MIR148A in HIV control and Crohn disease. Proc Natl Acad Sci USA. 2013;110:20705–20710. doi: 10.1073/pnas.1312237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snary D, Barnstable CJ, Bodmer WF, Crumpton MJ. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977;7:580–585. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- 20.Apps R, Carrington M. Response to Comment on “Influence of HLA-C expression level on HIV control”. Science. 2013;341:1175. doi: 10.1126/science.1241854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravindranath MH, Pham T, El-Awar N, Kaneku H, Terasaki PI. Anti-HLA-E mAb 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: Web-tools validate the immunogenic epitopes of HLA-E recognized by the antibodies. Mol Immunol. 2011;48:423–430. doi: 10.1016/j.molimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 23.Swigut T, Alexander L, Morgan J, Lifson J, Mansfield KG, Lang S, Johnson RP, Skowronski J, Desrosiers R. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J Virol. 2004;78:13335–13344. doi: 10.1128/JVI.78.23.13335-13344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaunders J, Dyer WB, Churchill M. The Sydney Blood Bank Cohort: implications for viral fitness as a cause of elite control. Curr Opin HIV AIDS. 2011;6:151–156. doi: 10.1097/COH.0b013e3283454d5b. [DOI] [PubMed] [Google Scholar]
- 25.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 26.Rajapaksa US, Li D, Peng YC, McMichael AJ, Dong T, Xu XN. HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc Natl Acad Sci USA. 2012;109:13353–13358. doi: 10.1073/pnas.1204199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.13th IHWS Technology Joint Report. Section A: HLA typing and Informatics. In: Hansen JA, editor. Immunobiology of the Human MHC; Proceedings of the 13th International Histocompatibility Workshop and Conference; Seattle, WA: IHWG Press; 2006. pp. 179–480. [Google Scholar]
- 28.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens- new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 30.Brodsky FM, Bodmer WF, Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979;9:536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- 31.Beretta A, Grassi F, Pelagi M, Clivio A, Parravicini C, Giovinazzo G, Andronico F, Lopalco L, Verani P, Buttò S. HIV env glycoprotein shares a cross-reacting epitope with a surface protein present on activated human monocytes and involved in antigen presentation. Eur J Immunol. 1987;17:1793–1798. doi: 10.1002/eji.1830171218. [DOI] [PubMed] [Google Scholar]
- 32.Parham P, Bodmer WF. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978;276:397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 33.Tahara T, Yang SY, Khan R, Abish S, Hammerling GJ, Hammerling U. HLA antibody responses in HLA class I transgenic mice. Immunogenetics. 1990;32:351–360. doi: 10.1007/BF00211650. [DOI] [PubMed] [Google Scholar]
- 34.Lutz CT, Smith KD, Greazel NS, Mace BE, Jensen DA, McCutcheon JA, Goeken NE. Bw4-reactive and Bw6-reactive antibodies recognize multiple distinct HLA structures that partially overlap in the alpha-1 helix. J Immunol. 1994;153:4099–4110. [PubMed] [Google Scholar]
- 35.Braud VM, Allan DS, Wilson D, McMichael AJ. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr Biol. 1998;8:1–10. doi: 10.1016/s0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 36.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morcock DR, Thomas JA, Sowder RC, 2nd, Henderson LE, Crise BJ, Gorelick RJ. HIV-1 inactivation by 4-vinylpyridine is enhanced by dissociating (Zn2+) from nucleocapsid protein. Virology. 2008;375:148–158. doi: 10.1016/j.virol.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis ZB, Ward JP, Barker E. Preparation and use of HIV-1 infected primary CD4+ T-cells as target cells in natural killer cell cytotoxic assays. J Vis Exp. 2011;49:e2668. doi: 10.3791/2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M, Lucas DA, Chan KC, Issaq HJ, Petricoin EF, 3rd, Liotta LA, Veenstra TD, Conrads TP. An investigation into the human serum “interactome”. Electrophoresis. 2004;25:1289–98. doi: 10.1002/elps.200405866. [DOI] [PubMed] [Google Scholar]
- 40.Marozzi A, Meneveri R, De Santis C, Robbioni P, Molteni E, Beretta A, Siccardi AG, Ginelli E. Expression of distinct conformations of free HLA-Cw4 heavy chains in transfected neuroblastoma cells. Immunogenetics. 1996;43:289–95. doi: 10.1007/BF02440996. [DOI] [PubMed] [Google Scholar]
- 41.Jones DC, Kosmoliaptsis V, Apps R, Lapaque N, Smith I, Kono A, Chang C, Boyle LH, Taylor CJ, Trowsdale J, Allen RL. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol. 2011;186:2990–2997. doi: 10.4049/jimmunol.1003078. [DOI] [PubMed] [Google Scholar]
- 42.Corrah TW, Goonetilleke N, Kopycinski J, Deeks SG, Cohen MS, Borrow P, McMichael A, Brackenridge S. Reappraisal of the relationship between the HIV-1-protective single-nucleotide polymorphism 35 kilobases upstream of the HLA-C gene and surface HLA-C expression. J Virol. 2011;85:3367–3374. doi: 10.1128/JVI.02276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nou E, Zhou Y, Nou DD, Blankson JN. Effective Downregulation of HLA-A*2 and HLA-B*57 by Primary Human Immunodeficiency Virus Type 1 Isolates Cultured from Elite Suppressors. J Virol. 2009;83:6941–6946. doi: 10.1128/JVI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Müller S, Valitutti S. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci USA. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman J, Völk H, Wallny HJ. A “minimal essential Mhc” and an “unrecognized Mhc”: two extremes in selection for polymorphism. Immunol Rev. 1995;143:63–88. doi: 10.1111/j.1600-065x.1995.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman J, Salomonsen J. The “minimal essential MHC” revisited: both peptide-binding and cell surface expression level of MHC molecules are polymorphisms selected by pathogens in chickens. Hereditas. 1997;127:67–73. doi: 10.1111/j.1601-5223.1997.t01-1-00067.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


