Abstract
Programmed Death (PD)-1 promotes T cell tolerance. Despite therapeutically targeting this pathway for chronic infections and tumors, little is known about how different T cell subsets are affected during blockade. We examined PD-1/PD-L1 regulation of self-antigen-specific CD4 and CD8 T cells in autoimmune susceptible models. PD-L1 blockade increased insulin-specific effector CD4 T cells in Type 1 Diabetes. However, anergic islet-specific CD4 T cells were resistant to PD-L1 blockade. Additionally, PD-L1 was critical for induction, but not maintenance, of CD8 T cell intestinal tolerance. Therefore, while PD-L1 blockade enhanced functionality of effector T cells, established tolerant or anergic T cells were not dependent on PD-1/PD-L1 signaling to remain unresponsive. This highlights the existence of antigen-experienced T cell subsets that do not rely on PD-1/PD-L1 regulation. These findings illustrate how positive treatment outcomes and autoimmunity development during PD-1/PD-L1 inhibition is linked to the differentiation state of a T cell.
INTRODUCTION
The inhibitory receptor Programmed Death-1 (PD-1) interacts with PD-Ligand 1 (PD-L1) to regulate T cell function and autoimmunity [1-5]. Prolonged, elevated PD-1 and PD-L1 expression occurs during chronic infections and cancer, and leads to T cell exhaustion [6]. PD-1 blockade can reinvigorate exhausted T cells, providing enhanced anti-viral and anti-tumor responses [7, 8]. These observations led to the development of PD-1 pathway blockers, which are anticipated to revolutionize cancer therapy.
While inhibitory blockade can be successful, not all patients had positive outcomes and some developed autoimmunity. These observations indicate differential susceptibility to PD-1/PD-L1 inhibitors. Recent reports have shown adverse events with anti-PD-1/-PD-L1 in clinical trials for cancer, including vitiligo, colitis, hepatitis, thyroiditis, and Type 1 Diabetes (T1D)[9] . The notable prevalence of these side effects strongly warrants further investigation into biomarkers to identify patients at risk prior to therapy. Therefore, we asked whether T cell activation or differentiation state impacted PD-1/PD-L1 dependence for effector function and loss of tolerance.
The goal of this study was to assess PD-1/PD-L1 regulation of self-Ag-specific CD4 and CD8 T cells to determine autoimmune risk with PD-1/PD-L1 inhibition. We utilized the non-obese diabetic (NOD) model of T1D to investigate CD4 T cells, given their requirement for disease. NOD mice deficient for PD-1 or PD-L1 develop accelerated T1D [1, 3], and selective loss of PD-1 on islet-reactive CD4 T cells enhances proliferation and pancreas infiltration [10]. In order to investigate the role of PD-L1 in regulating mucosal CD8 T cell responses, we utilized the iFABP-Ova transgenic mouse, where transfer of naïve OT-I CD8 T cells leads to Ag-specific tolerance [11, 12]. Using these models, we re-evaluated the role of PD-1/PD-L1 during the induction and maintenance of T cell tolerance. Unexpectedly, PD-1/PD-L1 regulation of autoreactive T cells was dependent on the T cell differentiation state and timing of blockade relative to Ag encounter. While PD-L1 blockade resulted in enhanced functionality of effector T cells, established anergic T cells were not sensitive to PD-L1 inhibition. These data have important clinical implications regarding the use of PD-L1 inhibitors, suggesting productive anti-tumor response and patient autoimmune susceptibility is linked to T cell activation state at the time of treatment.
MATERIALS AND METHODS
Mice
Female mice were housed in specific-pathogen free facilities and all experiments were IACUC approved at the University of Minnesota. NOD mice were purchased from Taconic. OTI, iFABP-Ova, B6.g7, NOD. PD-1−/−, NOD. PD-L1−/− and NOD.BDC2.5 Thy1.1 were generated as described [10, 11].
Lymphocyte transfer, isolation and detection
7,500 NOD.BDC2.5.Thy1.1+ CD4 T cells from 4-6 week old donors were transferred into prediabetic NOD with or without CFSE labeling [10]. 500,000 naïve OT-I CD8 T cells isolated from SPL and LN were transferred i.v. to adult iFABP-Ova mice [11]. Insulin-specific CD4 T cells were detected by double insB10-23r3:I-Ag7 tetramer staining and enrichment [13]. Intraepithelial lymphocytes (IEL) and SPL were isolated as described [11].
Flow cytometry
Surface staining was performed as described [13]. Gating strategies: singlet+, CD3+ lineage− (B220−, CD11b−, CD11c−) CD4+ CD8α−, insB10-23r3: I-Ag7-PE and -APC double positive (ins-specific CD4+ T cells); singlet+, CD3+ lineage− (B220−, CD11b−, CD11c−) CD4+ Thy1.1+ (BDC2.5); singlet+, CD45.1+, CD8+, Kb SIINFEKL+ (OT-I) [11].
Histology
Islet inflammation was scored: 0–no insulitis; 1–perinsulitis; 2–less than 25% of the islet is infiltrated; 3–less than 75% of the islet is infiltrated; 4–less than 25% of the islet mass is intact.
Administration of antibodies
Anti-PD-L1 (M1H6 or 10F.9G2), PD-1 (J43), rat IgG2a, rat IgG2b, or hamster IgG was injected i.p. [10]. For CD4 tolerance, mice received 2-3 doses (250μg) as indicated in each figure, and 250μg every other day for 10 days for T1D incidence. Glucose levels above 250mg/dL are diabetic. For CD8 tolerance induction, iFABP-Ova mice received 200μg of anti-PD-L1 i.p. (clone 10F.9G2) on the day of transfer and day +3. For CD8 tolerance maintenance, mice received 200μg anti-PD-L1 starting at d30 and doses every third day for 15d.
Cytokine assays
IFNγ was measured as described from BDC2.5 cells 4h after 500μg i.v. of acetylated p31 peptide (YVRPLWVRME) (Genemed Sythesis) or OT-I cells after 1μg/ml SIINFEKL ex vivo for 4h (New England Peptide) [10, 11].
Statistics
Unpaired two-tailed Student's t tests or Mann-Whitney tests with a 95% CI were performed using Prism 5 software (GraphPad). Symbols indicating statistical significance correspond to: not significant >0.05 (ns), significant 0.01 to 0.05 (*), very significant 0.001 to 0.01 (**), and extremely significant <0.001 (***).
RESULTS AND DISCUSSION
Loss of PD-1 or PD-L1 results in increased numbers of insulin-specific CD4 T cells in NOD mice
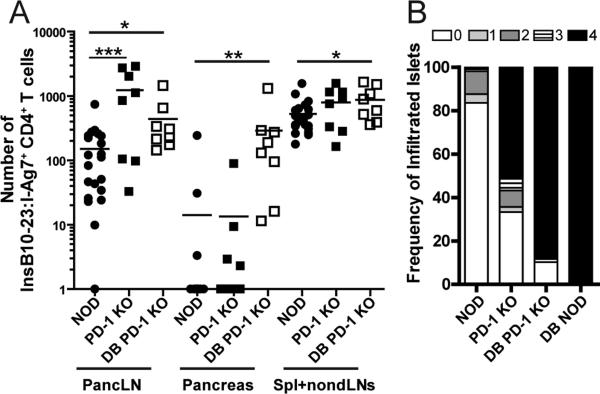
The PD-1/PD-L1 pathway is essential for tolerance and autoimmunity [1, 3, 14]. We investigated the impact of genetic loss of PD-1 on endogenous insulin-specific CD4 T cells in diabetes-susceptible non-obese diabetic (NOD) mice. Using an insB10-23r3:I-Ag7 tetramer [13, 15], we quantified insulin-specific CD4 T cells in the secondary lymphoid organs (SLO) and pancreas of NOD.PD-1−/− mice. We observed significantly more insB10-23r3:I-Ag7 tetramer-binding cells (ins-specific) in the pancreatic lymph node (pancLN) of pre-diabetic and diabetic NOD.PD-1−/− mice compared to WT control NOD (Fig 1A), and PD-L1−/− mice, but not control HEL11-25:IAg7 tetramer binding cells (Supplemental Fig 1A and 1B). There was not a significant change in the total number of CD4 T cells in PD-1 or PD-L1 KO mice (Supplemental Fig 1C). The number of ins-specific CD4 T cells did not differ in either SLO or pancreas of prediabetic NOD and NOD.PD-1−/− mice, but was significantly higher in diabetic NOD.PD-1−/− mice (Fig 1A). Histological analysis revealed that pre-diabetic NOD.PD-1−/− mice had more severe islet inflammation compared to age-matched non-diabetic NOD mice, and this increased with diabetes (Fig 1B). These results indicate that PD-1 is a central regulator of autoreactive CD4 T cells in autoimmune prone mice.
Figure 1. Loss of PD-1 increases insulin-specific CD4 T cells in NOD mice.
(A) Insulin-specific (insB10-23r3:I-Ag7-tetramer+) CD4 T cells in the pancLN, pancreas, SPL and non-draining LN of pre-diabetic WT (n=21) and PD-1−/− NOD mice (n=8), between 4-7 weeks old, and new onset diabetic NOD.PD-1−/− mice (n=8). (B) Insulitis scores from non-diabetic or diabetic (DB) NOD and NOD.PD-1−/− mice. Data represent >100 islets from each group. Data are compiled from at least 4 experiments.
PD-L1 blockade fails to induce autoimmunity in diabetes-resistant B6.g7 mice
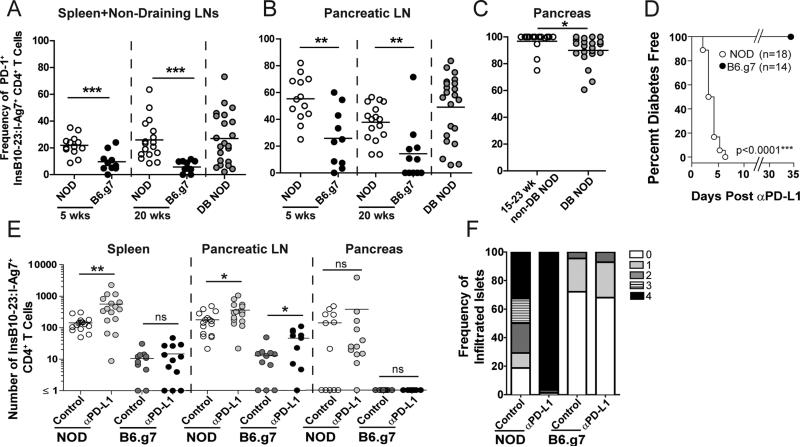
PD-1 loss is known to potentiate autoimmunity in susceptible mice [1]; however, its role in regulating known self-Ag specific T cells in genetically resistant hosts is unclear. We previously examined ins-specific T cells in NOD and B6.g7, because of the known genetic risk of MHC for T1D [16]. We reported that both strains contained ins-specific CD4 T cells. Ins- specific CD4 T cells became activated in the pancLN and infiltrated the pancreas in NOD, but remained naïve in B6.g7 mice [13]. In this study, PD-1+ ins-specific T cells are increased in the SLO of NOD mice compared to B6.g7 (Fig 2A and 2B). In B6.g7, the frequency of ins-specific PD-1+ cells was low, consistent with a naïve phenotype. Interestingly, in NOD mice, the highest frequency of PD-1+ cells was in the pancreas (Fig 2C). This result supports the idea that pancreatic cells are susceptible to PD-1 inhibition, consistent with previous work [10, 17].
Figure 2. PD-L1 blockade differentially impacts insulin-specific CD4 T cells in NOD and B6.g7 mice.
Frequency of PD-1+ insulin-specific CD4+ T cells in NOD and B6.g7 mice (A) SPL and non-draining LNs, (B) pancLN, and (C) pancreas. (A-B) Non-diabetic NOD mice (n=13 at 5 weeks, n=17 at 20 weeks), diabetic NOD (n=22 at new onset), non-diabetic B6.g7 (n=10 at 5 weeks, n=11 at 20 weeks). Data for panels A-C are compiled from at least 10 experiments. (D) Diabetes incidence of 15 week old NOD (n=18) or B6.g7 (n=14) mice after anti-PD-L1. Data are compiled from 4 experiments. (E) Enumeration of CD44high ins-specific CD4 T cells in SPL, pancLN and pancreas of 11-16 week old NOD (n=16) or B6.g7 (n=11) mice treated with isotype control or anti-PD-L1. Data compiled from ≥3 experiments. (F) Insulitis scores from NOD (n=11) and B6.g7 (n=12) with or without anti-PD-L1. Data represent >100 islets from each group. Data are representative of ≥3 experiments.
Considering the differences in PD-1 expression, we next injected anti-PD-L1 to NOD and B6.g7 mice. NOD mice rapidly developed T1D (Fig 2D) [2]. However, despite the fact that B6.g7 carry the highest genetic risk factor for T1D [16], anti-PD-L1 did not induce T1D (Fig 2D). We hypothesized PD-L1 blockade in NOD mice, but not B6.g7, would cause ins-specific cells to increase due to PD-1 expression (Fig 2A and 2B) [10]. In NOD mice, CD44high ins-specific CD4 T cells were increased in the spleen and pancLN following anti-PD-L1 (Fig 2E) and in the pancreas following anti-PD-1 but not anti-B7-1 blockade (Supplemental Fig 1D). In B6.g7 mice, PD-L1 blockade failed to increase CD44high cells in the spleen (Fig 2E), but, unexpectedly, resulted in a significant increase in the pancLN. However, ins-specific CD4 T cells did not infiltrate the pancreas, and did not cause T1D (Fig 2E and 2D). Islet histology was examined and anti-PD-L1 caused severe insulitis in NOD, but not in B6.g7 mice (Fig 2F).
Differential susceptibility of islet-reactive CD4 T cell subsets to PD-1 blockade
Our results demonstrated that anti-PD-L1 selectively increased Ag-experienced cells (Fig 2). However, there is notable heterogeneity within the Ag-experienced (CD44highFoxp3−) compartment in pancLN of NOD mice with both effector and anergic cells [13]. These populations have been characterized based on expression of two surface proteins, folate receptor 4 (FR4) and CD73 [13, 18]. FR4+CD73+ anergic cells produce less effector cytokines than FR4- CD73− effector T cells [13, 18]. Using these markers we tested the impact of PD-1 signaling in light of our previous work demonstrating anti-PD-L1 promoted the breakdown of tolerance by inducing the T cell stop signal [17] and reversing tolerance causing diabetes [4].
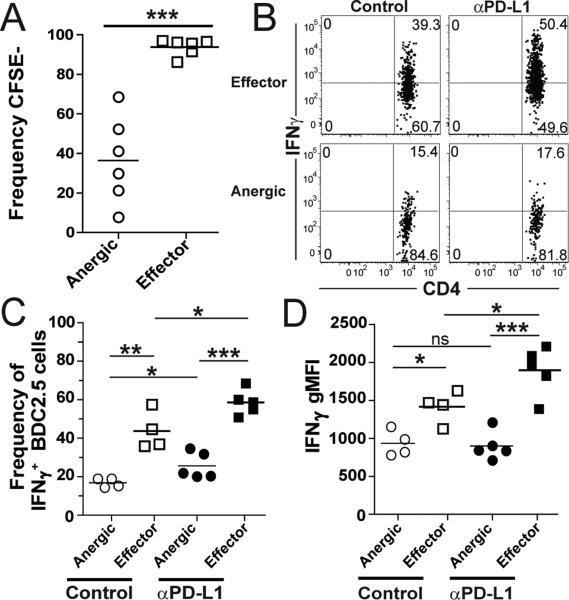
To investigate the role of PD-1 regulation on anergic and effector T cell subsets, we utilized our recently characterized adoptive transfer model of diabetes. In this model, a low number of naive BDC2.5 TCR transgenic T cells specific for an islet antigen were transferred into prediabetic NOD mice to mimic the endogenous response [10]. Using this model, 70% of the BDC2.5 CD4 T cells develop an anergic phenotype and 30% become effector T cells in the pancLN (Supplemental Fig 1E). Not surprisingly, these subsets differ in their proliferative capacity, with anergic subsets dividing less than effector T cells (Fig 3A). Interestingly, PD-1 levels are very similar on anergic and effector T cells (Supplemental Fig 1H, 1I). We tested if anti-PD-L1 altered the frequency of anergic or effector T cell subsets. Anti-PD-L1 decreased the frequency of anergic cells (Supplemental Fig 1E, 1F). However, the decrease was not due to cell loss, but rather, a threefold expansion of effector T cells (Supplemental Fig 1G). We next measured the effect of PD-L1 blockade on anergic cells and predicted these cells would be reinvigorated, given our previous findings with antigen-coupled cell tolerance [4]. Contrary to prediction, anergic cells were blunted in their ability to produce IFNγ. We measured a significant increase in IFNγ production by effector T cells with anti-PD-L1 (Fig 3B-D). The frequency of IFNγ+ anergic cells rose from 16.9 to 25.6% after anti-PD-L1, but remained lower than effector cells with and without PD-L1 blockade (43.7 and 58.4%, respectively)(Fig 3C). If anti-PD-L1 released the anergic CD4 T cells, we would have observed equal levels of IFNγ, but this was not the case indicating there is a subset of anergic CD4 T cells that are not reinvigorated by PD-L1 blockade. Even though the percentage of IFNγ+ cells changed in the anergic population, the amount of produced IFNγ did not increase above baseline following anti-PD-L1 (Fig 3D). Anergic cells made 1.5 fold less IFNγ than effector cells in control animals and 2 fold less than effectors after anti-PD-L1 on a per cell basis (Fig 3D). Lastly, we measured CXCR3 expression on anergic and effector cells and determined CXCR3 was higher on CD4 effector cells compared to anergic cells which could mechanistically explain a differential ability of these cells to traffic to sites of inflammation during autoimmunity (Supplemental Fig 1J). We have previously reported that CXCR3 expression increased following PD-L1 blockade and did not detect any CXCR3+ anergic cells in the pancreas[13]. This is consistent with the idea that anergic cells remain in the periphery and the effector cells traffic to the pancreas to cause T1D [13]. Taken together, our results suggest PD-L1 blockade has the greatest impact on the effector T cell subset allowing enhanced proliferation and IFNγ production over anergic cells (Fig 3D). This is consistent with reports of PD-L1-mediated restoration of exhausted CD8 subsets [19].
Figure 3. Blocking PD-1 preferentially induces IFNγ expression by effector but not anergic BDC2.5 T cells.
(A) Frequency of CFSE− BDC2.5 CD4 T cells in pancLN 3 weeks post transfer. (B) Concatenated FACS plots showing the frequency of IFNγ+ anergic and effector BDC2.5 cells in isotype control (n=4) and anti-PD-L1 treated mice (n=5). (C) Frequency and (D) IFNγ geometric mean fluorescence intensity (gMFI) of IFNγ+ anergic and effector BDC2.5 CD4 T cells in the pancLN 49 days post-transfer. Data are representative of 3 experiments.
The differences between anergic and effector cells may be explained by two potential mechanisms. Previous reports implicate other inhibitory receptors such as CTLA-4, LAG-3, 2B4, and TIM-3 may contribute to the establishment of T cell exhaustion or anergy via nonredundant signaling pathways [20]. Alternatively, incomplete TCR stimulation (TCR stimulation without costimulation) leading to a calcium influx-mediated altered gene expression program that includes up-regulation of several E3 ubiquitin ligases and T cell anergy may explain the differences between anergic and effector cells [21]. What was not known from these studies was if PD-L1 blockade can reverse this state of anergy and allow for T cell reinvigoration. We conclude from our data that PD-L1 blockade results in differential responsiveness for anergic and effector CD4 T cells.
PD-L1 blockade enhances effector functions of self Ag-specific CD8 T cells during the induction, but not the maintenance, of tolerance
We next asked if there was differential PD-L1 dependence of self-Ag-specific CD8 T cells. We transferred naïve ovalbumin (Ova) specific OT-I CD8 T cells to iFABP-Ova mice expressing Ova as an intestinal self-Ag [11, 12]. This system eliminates recent thymic emigrants and synchronizes Ag encounter. Importantly, we evaluated both the induction and maintenance of CD8 T cell tolerance, which has previously been difficult, as deletion of transferred T cells is common. This model overcomes this barrier and results in long-term maintenance of self-specific CD8 T cells, indicating that T cell deletion is not necessarily the outcome of tolerogenic interactions [22].
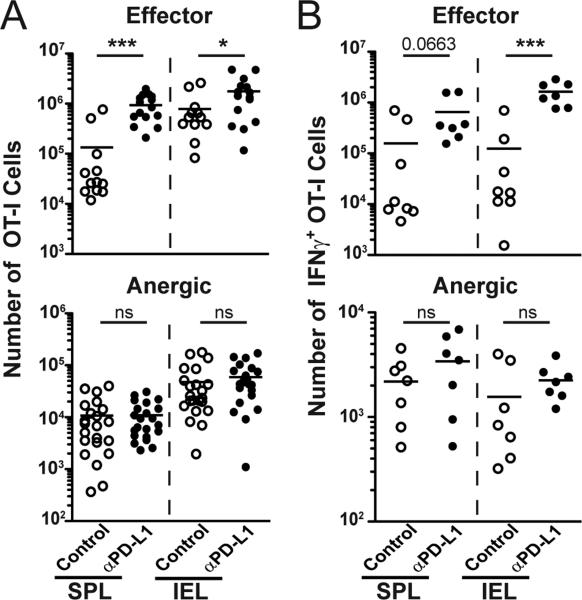
Previous work using this model suggested a role for PD-1/PD-L1 in the initiation of mucosal CD8 T cell tolerance [23]. Here, when PD-L1 was blocked early, tolerance induction was prevented. Anti-PD-L1 caused an increase in the frequency and number of OT-I T cells in the spleen and small intestine (IEL) of iFABP-Ova mice and enhanced IFNγ production from OT-I IEL and granzyme B (Fig 4A and 4B, Supplemental Fig 2A, 2B). As a result of PD-L1 blockade, mice died from severe intestinal inflammation by day 10 (data not shown). OT-I cells examined 30 days after transfer had characteristics of anergic T cells: they were Ag-experienced, but did not exhibit effector functions and could be found in Ag-rich locations, including the IEL (Fig 4, Supplemental Fig 2A, 2B) [11]. Similar to BDC2.5 T cells, CXCR3 expression was increased on effector OT-I CD8 T cells compared to anergic cells (Supplemental Fig 2C). Surprisingly, once anergy was established, PD-L1 blockade did not increase OT-I cell numbers or restore effector functions (Fig 4B). This resistance to treatment was not due to down-regulation of the receptor PD-1, since these cells retain expression (Supplemental Fig 2D). Importantly, anti-PD-L1 during tolerance maintenance did not promote intestinal pathology (data not shown). These data demonstrate critical differences in the temporal requirement for PD-L1 in CD8 T cell tolerance.
Figure 4. PD-L1 signaling is critical for the induction, but not the maintenance, of CD8 T cell tolerance to intestinal self-antigen.
Naïve OT-I cells were transferred to iFABP-Ova mice and anti-PD-L1 injections began either the day of cell transfer (d0) or at least 30 days later. Cells were analyzed at day 5 (Effector) or at day 45 (Anergic) following control or anti-PD-L1. (A) Enumeration of OT-I cells from SPL and IEL (Effector cells (d5) isotype n=12, anti-PD-L1 n=14) (Anergic cells (d45) isotype n=22, anti-PD-L1 n=20). (B) Enumeration of IFNγ producing OT-I cells isolated from SPL and IEL (Effector (d5) isotype n=8, anti-PD-L1 n=7) (Anergic (d45) isotype n=7, anti-PD-L1 n=7). Data are compiled from ≥ 3 experiments.
The inhibitory receptor PD-1 has long been considered a central mediator of peripheral tolerance, facilitating T cell inhibition and preventing effector function. In this study, however, we report the unexpected finding that the inhibitory effects of PD-1/PD-L1 signaling and subsequent blockade act on specific subsets of CD4 and CD8 T cells. We found that PD-1/PDL1 blockade preferentially acts to restore or potentiate the function of effector cells, rather than broadly reversing tolerance in all T cell subsets. These results suggest that effector T cells are restrained via PD-1 inhibitory signals, whereas anergic T cells are not released through PD-L1 blockade alone. These data have important implications for patients receiving PD-1 pathway inhibitors for treatment of cancer or chronic infections, as it suggests the functional state of T cells will impact patient outcomes. This highlights the importance of autoreactive T cell activation status as an indicator for study exclusion criteria. In addition, our results indicated that PD-L1 blockade alone did not reverse tolerance of self-reactive anergic T cells to cause autoimmunity. Better clinical efficacy of PD-1 pathway inhibitors may be possible by combining blockade with effective therapeutic vaccination to reverse the tolerant or exhausted state of tumor- or microbe-specific T cells and produce synergistic effects [24]. Understanding how PD-1 blockade impacts autoreactive T cells in hosts with varying autoimmune susceptibility is critically important as PD-1 inhibitor use moves forward in the clinic.
Supplementary Material
Footnotes
Supported by NIH R01AI106791 (BTF), P01AI35296 (MJK and BTF), DP2OD006473 (VV), JDRF 2-2011-662 (BTF).
References
- 1.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NODPdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102(33):11823–8. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H, Jr., Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–9. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203(12):2737–47. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 8.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes. 2013;62(8):2859–69. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12(5):505–14. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 12.Vezys V, Lefrancois L. Cutting edge: inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J Immunol. 2002;169(12):6677–80. doi: 10.4049/jimmunol.169.12.6677. [DOI] [PubMed] [Google Scholar]
- 13.Pauken KE, Linehan JL, Spanier JA, Sahli NL, Kalekar LA, Binstadt BA, Moon JJ, Mueller DL, Jenkins MK, Fife BT. Cutting edge: type 1 diabetes occurs despite robust anergy among endogenous insulin-specific CD4 T cells in NOD mice. J Immunol. 2013;191(10):4913–7. doi: 10.4049/jimmunol.1301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 15.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A. 2011;108(40):16729–34. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 17.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188(1):170–81. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105(39):15016–21. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–19. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186(2):239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, Freeman GJ, Turley SJ. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182(4):2102–12. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205(3):543–55. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.