Abstract
Galectin-3 is a 31 kD lectin that modulates T-cell responses through several mechanisms including apoptosis, T-cell receptor (TCR) cross-linking, and TCR down-regulation. We found patients with pancreatic ductal adenocarcinoma (PDA) responding to a granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic PDA vaccine developed post immunization neutralizing antibodies to galectin-3. We show that galectin-3 binds activated antigen-committed CD8+ T cells only in the tumor microenvironment (TME). Galectin-3-deficient mice exhibit improved CD8+ T-cell effector function, and increased expression of several inflammatory genes. Galectin-3 binds to LAG-3, and LAG-3 expression is necessary for galectin-3-mediated suppression of CD8+ T cells in vitro. Lastly, galectin-3-deficient mice have elevated levels of circulating plasmacytoid dendritic cells (pDC), which are superior to conventional dendritic cells (cDC) in activating CD8+ T cells. Thus, inhibiting galectin-3 in conjunction with CD8+ T cell-directed immunotherapies should enhance the tumor-specific immune response.
Keywords: Galectin-3, LAG-3, plasmacytoid DCs, GM-CSF vaccine, CD8 T cell
INTRODUCTION
We previously reported the clinical outcomes of a phase II study testing an allogeneic granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting pancreatic ductal adenocarcinoma (PDA) vaccine in 60 patients with resected PDA. Twelve of the 60 (20%) vaccinated patients demonstrated a greater than 3-year disease-free survival (DFS) associated with induction of T-cell responses against the PDA-associated antigen, mesothelin (1). In the present study, we used the enriched vaccine-induced antibody responses to probe the vaccine cell lines for PDA-associated antigens that are targets of the humoral immune response with the goal of identifying proteins important to PDA development. Using this approach, we identified 11 new PDA-associated proteins for which specific antibody titers were elevated in the post-vaccination sera (2).
One of the proteins that we identified was galectin-3, a 31-kD lectin that is unique among the galectin family members due to the presence of both a carbohydrate recognition domain (CRD) and an oligomerization domain that enables galectin-3 to cross-link its binding targets. Galectin-3 has been shown in vitro to possess several immunomodulatory functions such as reducing the affinity of the T-cell receptor (TCR) for its cognate major histocompatibility complex (MHC) I-peptide ligand by sequestering the TCR from its CD8+ co-receptor (3), inducing apoptosis (4), and internalization of the TCR (5). Galectin-3 also influences the strength of antigen activation in dendritic cells (DC) (6, 7). Thus, we sought to develop a mouse tumor model that would allow us to evaluate in vivo the role of galectin-3 in shaping the antitumor response in a tolerogenic setting.
We previously used the HER-2/neu transgenic (neu-N) mouse model of mammary tumors to identify mechanisms of tumor tolerance (8). In this study, we crossed the neu-N mice, which develop neu-expressing mammary tumors, and high avidity CD8+ TCR transgenic mice specific for the immunodominant epitope for neu onto galectin-3 knock-out (KO) mice, and analyzed CD8+ T-cell responses following treatment with a neu-targeted vaccine. This approach enabled us to examine genome-wide changes in protein expression caused by galectin-3. Here, we demonstrate that in vivo depletion of galectin-3 increases both the number of functional CD8+ T cells found in the tumor microenvironment (TME) as well as the expression of inflammatory proteins by these T cells, leading to enhanced tumor rejection in galectin-3 KO mice when compared with galectin-3 wildtype (WT) mice. Further, we demonstrate that the effects of galectin-3 extend beyond modulation of T-cell function to include expansion of plasmacytoid dendritic cells (pDC), which we show to be more potent activators of CD8+ T cells than conventional dendritic cells (cDC).
MATERIALS AND METHODS
ELISA
Costar 3690 96-well half-area EIA/RIA plates (Corning) were coated at 4°C overnight with purified recombinant proteins at 5 μg/ml in bicarbonate/carbonate coating buffer. The protein-coated plates were incubated with ELISA Blocker Blocking Buffer (Pierce Biotech) for 1 h at room temperature. The wells were then incubated with serial dilutions (1:100, 1:200, 1:400, and 1:800) of sera for 2 h at room temperature and with 1:200,000 dilution of goat anti-human IgG (γ-chain specific) peroxidase conjugate (Sigma, A8419) for 1 h at room temperature. The wells were washed extensively with TBS-T between incubations. 3,3′5,5′-tetramethylbenzidine liquid substrate (Sigma, T0440) was added to the wells and incubated in the dark for 20 min at room temperature. The color development was stopped by 1 N sulfuric acid. Absorbance at 450 nm (with a reference wavelength of 570 nm) was measured on a PowerWave 340 microplate reader (BioTek).
Mice
HER-2/neu (neu-N) and LGALS3−/− (galectin-3 KO) mice were purchased from Jackson Laboratories (Bar Harbor, ME), bred and housed in the Johns Hopkins animal facility. High-avidity neu-specific TCR transgenic mice were generated as previously described, and avidity was confirmed by tetramer staining (9). Galectin-3 KO mice were backcrossed for 6 generations using a marker-assisted selection (i.e. “speed congenic”) approach. Mouse genomes were assessed at the DartMouse™ Speed Congenic Core Facility at Dartmouth Medical School. Genetic background at the final backcross generation was determined to be 99.75% for the desired FVB/N background. Backcrossed galectin-3 KO mice were bred with neu-N and high-avidity neu-specific TCR transgenic mice to generate galectin-3 KO neu-N and galectin-3 KO high-avidity neu-specific TCR transgenic mouse lines. All experiments were conducted with female mice between 6–12 weeks of age according to protocols approved by the Johns Hopkins Animal Care and Use Committee.
Cell lines and media
The NT2.5 is a neu-expressing tumor cell line generated from spontaneously arising tumors in female neu-N mice as previously reported (10, 11). The 3T3neuGM vaccine line is genetically modified from 3T3 fibroblast cells to secrete GM-CSF and express rat neu (12). The T2-Dq cell line is a TAP-2-deficient cell line that expresses the Dq MHC I allele, and was generated as previously reported (12). All cell lines are cultured and maintained as previously reported (8).
Tumor, vaccine, chemotherapy, and adoptive transfer
For tumor clearance studies, mice were tumor challenged with the minimal tumorigenic dose of 5×104 NT2.5 tumor cells injected subcutaneously (s.c.) in the right upper mammary fat pad on day 0, given 100 mg/kg cyclophosphamide (CY) intraperitoneally (i.p.) on day 2, vaccinated with 3 simultaneous s.c. injections of 1×106 3T3neuGM cells in the bottom and right limbs on day 3, and given 2×106 adoptively transferred high-avidity neu-specific CD8+ T cells on day 4. For tumor-infiltrating lymphocytes (TIL) and lymph node experiments, mice were given 2 simultaneous s.c. injections of 2×106 NT2.5 tumor cells in the right and left upper mammary fat pads on day 0, CY on day 8, vaccine on day 9, and 6×106 adoptively transferred CD8+ T cells on day 10. High-avidity neu-specific Thy1.2+ CD8+ T cells were negatively isolated from spleens of female TCR transgenic mice and adoptively transferred as previously described (8)
Peptides and antibodies
RNEU420–429 (PDSLRDLSVF) and the negative control peptide LCMV NP118–126 (RPQASGVYM) were produced in the Johns Hopkins Biosynthesis and Sequence Facility at a purity >95%. Antibodies used for flow cytometry studies were: anti-CD8-FITC (BD Biosciences), anti-CD8-PE (BD Biosciences), anti-CD8-PerCP (BD Biosciences), anti-CD8-APC (BD Biosciences), anti-galectin-3-AF647 (Biolegend), anti-galectin-3-PE (R&D), anti-PD1-PE (eBioscience), anti-LAG-3-PE (eBioscience), anti-Thy1.2-PerCP (Biolegend), anti-CD44-Pacific Blue (eBioscience), anti-CD11b-PE (BD Biosciences), anti-CD11c-FITC (BD Biosciences), anti-B220-APC (BD Biosciences), anti-Ly6C-PerCP-Cy5.5 (eBioscience), anti-IFNγ–PE (BD Biosciences), anti-IFNγ-Pacific Blue (eBioscience), and anti-Granzyme B-APC (BD Biosciences). Cellular division was assessed by labeling of high-avidity neu-specific CD8+ T cells with 1.5 μM CellTrace CFSE cell proliferation kit (Invitrogen) prior to adoptive transfer (8). Permeability was assessed with LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen). Antibody staining was conducted at 4°C for 20 minutes in FACS buffer (PBS, 5%FBS, 0.02% NaAzide).
Flow cytometry and intracellular cytokine staining (ICS)
Lymph nodes were dissected 3 and 5 days after adoptive transfer, and tumors were dissected 5 days after adoptive transfer, and homogenized by mashing through 40 μM nylon cell strainers. Tumors were further processed by enzymatic digestion using collagenase (1mg/mL, Gibco) and hyaluronidase (25 μg/mL, Sigma). After digestion, cells were washed with RPMI before being trypsinized for 2 minutes with 0.25% Trypsin-EDTA (Gibco). Lymphocytes were incubated for 5 hours at 37°C in CTL media with RNEU420–429 or NP118–126 peptide-pulsed T2-Dq target cells in the presence of monensin (GolgiStop, BD Biosciences) at a lymphocyte to target ratio of 4:1. Cells were surface stained for Thy1.2 expression prior to fixation/permeabilization using a mouse ICS kit (BD Biosciences) to stain for intracellular IFNγ and granzyme B.
In vitro activation and suppression of CD8+ T cells with exogenous galectin-3
CD8+ T cells were negatively isolated from total splenocytes using Dynal CD8+ negative isolation kits (Invitrogen), and stimulated for 3 days with anti-CD3/CD28 (Invitrogen) beads at a T cell to bead ratio of 1:1 according to the manufacturer’s recommendations (13). For suppression studies, cells were also incubated with the indicated amount of recombinant mouse or human galectin-3. For human studies, cells were also incubated with a 5-fold molar concentration of purified IgG from patient’s serum, as described in the figures. After 3 days of activation, CD8+ T cells were assessed for IFNγ production by ICS. Beads were removed by magnetic separation and cells were plated with fresh anti-CD3/CD28 beads in a 96-well assay plate at a T cell to bead ratio of 1:1 in the presence of monensin (GolgiStop, Invitrogen) for 5 hours at 37°C. Culture media were also supplemented with fresh galectin-3 at the indicated concentrations. Following the 5-hour incubation period, ICS studies were conducted as above.
Cloning and purification of recombinant mouse galectin-3
Total RNA was isolated from in vitro activated high-avidity neu-specific CD8+ T cells using the RNEasy Mini Kit (Qiagen). Galectin-3 cDNA was amplified with Superscript III First Strand Synthesis System (Invitrogen) and galectin-3-specific primers containing BamHI and NdeI restriction sites: 5′-GGAATTCCATATGGCAGACAGCTTTTCGCTTAACGATG-3′ (Forward) and 5′-CGGGATCCTTAGATCATGGCGTGGTTAGCGCTGGTGAGGG-3′ (Reverse). The galectin-3 cDNA was cloned into the pET-22B bacterial expression vector (Novagen), and protein expression carried out according to manufacturer’s instructions. Galectin-3 was purified from bacterial cell lysate material by binding to lactosyl-agarose beads (Sigma) and eluting with 200 mM lactose. Purified material was dialyzed into PBS, and endotoxin was removed using the ToxinEraser Endotoxin Removal Kit (GenScript). Endotoxin was quantified to be less than 1.0 EU/mL by the LAL assay (Pierce).
Direct Ex Vivo Antigen Detection Assay
Mice were treated as in tumor challenge experiments, but did not receive cyclophosphamide or adoptive transfer. Four days after vaccination, CD8+ DCs and pDCs were isolated from spleen tissue using CD8+ DC and pDC isolation kits (Miltenyi). CD8+ T cells were negatively isolated from high-avidity neu-specific TCR transgenic mice and labeled with CFSE as described above. All cells were co-cultured at a 1:1 ratio in CTL media for 3 days before evaluating CFSE dilution and cytokine production by FACS.
Co-Immunoprecipitation of galectin-3 and LAG-3
10 μg LAG-3-specific (Clone 410C9) (14) or galectin-3-specific (M3/38) antibody and corresponding isotype controls were conjugated to Protein G Dynabeads (Invitrogen) in PBS followed by cross-linking with 10mM BS3. CD8+ T cells were isolated and activated as previously described. Cell surface proteins were cross-linked with 10 mM BS(PEG)9 prior to cell lysis with CelLytic M (Sigma) supplemented with 100 mM lactose and protease inhibitor. Conjugated beads were incubated at 4°C overnight with CD8+ T-cell lysates. After washing beads with TBST (Tris-Buffered Saline + 0.1% Tween-20) the following day, bound proteins were eluted by boiling in sample buffer under reducing conditions. Standard western blotting procedures were followed and protein interactions were shown after developing membranes for 1 hour on high chemiluminescence film.
Gene Expression Analysis
RNA was extracted using the Stratagene Absolutely RNA Nanoprep Kit. Microarray hybridization and analyses were performed by the Johns Hopkins Deep Sequencing and Microarray Core Facility using the NuGen amplification system and an Affymetrix Exon 1.0 ST array. The data discussed in this publication are accessible through GEO Series accession number GSE59454.
Statistical Analysis
Student’s t tests were performed using GraphPad Prism software assuming equal variances. Log-rank tests were used for Kaplan-Meier plots. P values of less than 0.05 were considered to be statistically significant.
RESULTS
Vaccine-induced antibody responses against galectin-3 are associated with improved DFS in surgically resected PDA patients treated with a GM-CSF-secreting PDA vaccine
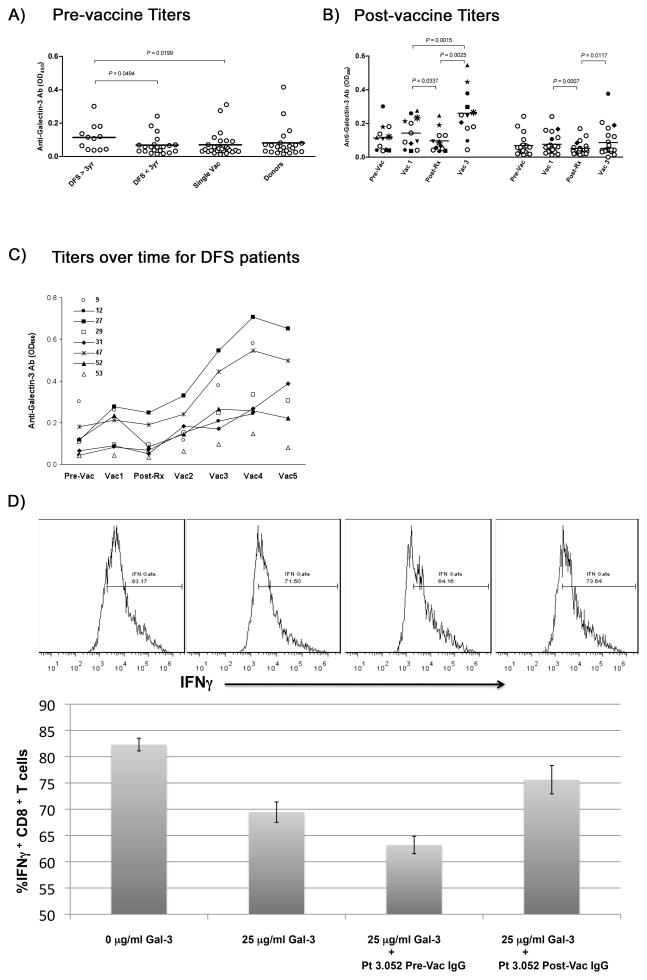
We developed a functional proteomic approach to identify PDA-associated proteins that might serve as targets of the immune response (2). This approach utilizes pre- and post-treatment sera from patients who received two GM-CSF-secreting pancreatic tumor cell lines as vaccine and compared serologic reactive proteins between treatment responders (disease-free survivors or DFS >3 years) and non-responders (early recurrence after a single vaccine or DFS <3 years). Paired sera from 60 subjects treated on a recently reported phase 2 study were evaluated by western blot (Figure S1A–D). Proteins that demonstrated an increase in post-treatment serologic responses were purified by 2-D gel and mass spectrometry. Eleven proteins were identified (Figure S1E–F, Table S1), two of which were serologic targets recognized by multiple patient sera. The first protein, annexin A2, induces an epithelial to mesenchymal transition (EMT) in PDA cells and promotes PDA metastases (2). The second protein, galectin-3, is a galactoside-binding protein and a known cancer-associated protein secreted by a number of tumor types (15). Galectin-3, like annexinA2, induces post-treatment serologic responses that correlate with improved DFS and overall survival (OS) (Figure 1A–C).
Figure 1. Vaccine-induced galectin-3 antibody responses correlate with improved DFS (1:400 dilution of sera were compared from pre- and post-vaccination samples) .
A) Pre-vaccine titers shown for patients who were either disease-free >3 years (DFS>3yr), disease-free less than 3 years (DFS<3yr), patients who received only a single vaccine before disease recurring (single Vac), or healthy donors (Donors). B) Post-vaccine titers for patients divided by DFS >3 years (left panel) or < 3 years (right panel) shown before the first vaccine (pre-Vac), 28 days after the first vaccine (Vac 1), after chemoradiation which was given after the first vaccine (post-Rx), and after two additional vaccines given after completing chemoradiation (Vac 3). C) Titers shown over time for 8 of the 12 patients demonstrated DFS>3 years. These patients received a total of 5 vaccinations (Vac 1, Vac 2, Vac 3, Vac 4, and Vac 5), the first one before chemoradiation, the 2nd, 3rd, and 4th each one month apart beginning one month after completing chemoradiation, and the 5th, 6 months after completing the 4th vaccination. D) Antibodies were purified from patient serum by Protein G isolation, and then incubated with healthy donor T cells and recombinant human galectin-3 as described in materials and methods. TOP) Gating of IFNγ+ CD8+ T cells on histogram plots. BOTTOM) Percentages of IFNγ+ CD8+ T cells after a 3 day anti-CD3/CD28 activation in the presence of various conditions as shown in the graph. All experiments were performed at least 2 independent times with 3 replicates per sample. Bar graph data are represented as mean ± SD.
Galectin-3 is known to inhibit T-cell activation by T-cell binding (16). Thus, we hypothesized that post-treatment serologic responses against galectin-3 improves DFS by enhancing CD8+ T-cell function through inhibition of galectin-3 binding. First, we assessed the ability of recombinant human galectin-3 to inhibit IFNγ production by CD8+ T cells maximally stimulated in vitro with anti-CD3/CD28 beads. The minimal concentration necessary to observe a significant reduction in IFNγ production was 25 μg/mL (data not shown). Next, we tested whether purified IgG from pre-vaccinated and post-vaccinated patient serum could prevent this reduction. IgG from post-vaccine serum but not pre-vaccine serum attenuated the suppression observed at 25 μg/mL of galectin-3 (Figure 1D).
Galectin-3 binds maximally activated CD8+ T cells with a terminally differentiated phenotype
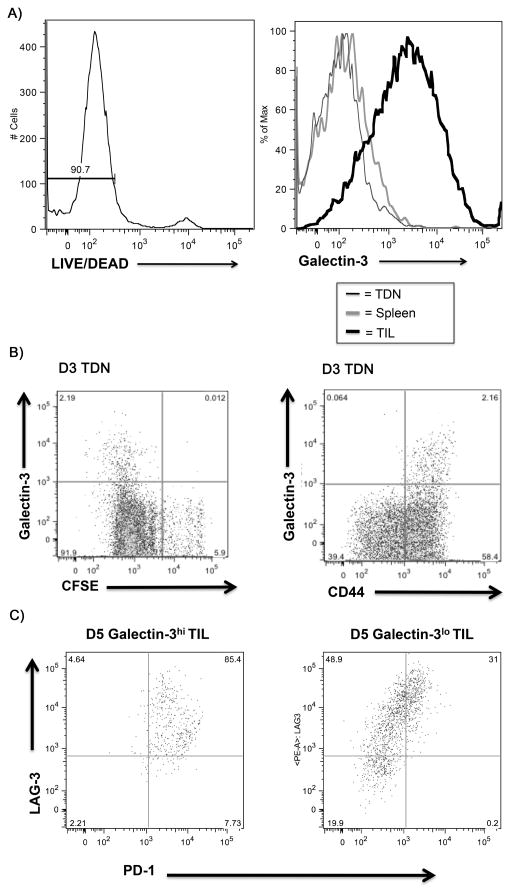
We employed the neu-N mouse model to evaluate the role of galectin-3 in neu-specific effector T-cell responses within the TME. Surface galectin-3 was detected only on adoptively transferred high-avidity neu-specific T cells that had trafficked into the TME (Figure 2A and Figure S2D). These cells have previously been shown to be tumor-antigen committed and activated in contrast to adoptively transferred cells remaining in other T-cell residing sites 5 days after adoptive transfer (8). Because the majority of effector CD8+ T cells in the TME have already been activated and undergone terminal differentiation, we used lymphocytes isolated from tumor-draining lymph nodes (TDN), which contain populations of T cells at different stages of division, to illustrate differences in galectin-3 binding during various stages of T-cell activation in vivo. Carboxyfluorescein succinimidyl ester (CFSE) labeling of adoptively transferred T cells demonstrated that surface galectin-3 was associated with only CFSE dilute and CD44+ cell populations indicating that these are differentiated effector CD8+ T cells (Figure 2B).
Figure 2. Activation of CD8+ T cells increases surface bound galectin-3.
A) Tissues were processed according to the procedure outlined in materials and methods 5 days after adoptive transfer into neu-N mice. Histogram overlays demonstrate comparison of surface galectin-3 mean fluorescence intensity (MFI) from Thy1.2+ high-avidity CD8+ T cells from TIL, TDN, and Spleen. A LIVE/DEAD permeability stain was included to gate exclusively on nonpermeabilized cells identified by low LIVE/DEAD staining B) High-avidity CD8+ T cells were labeled with 1.5μM CFSE prior to adoptive transfer into neu-N mice. Galectin-3 surface staining is shown with CFSE dilution and CD44 expression for cells gated on Thy1.2, 3 days post adoptive transfer. C) TILs were extracted 5 days after adoptive transfer. Cells were gated on Thy1.2 and divided into galectin-3hi and galectin-3lo staining populations based on isotype staining controls. A comparison of PD-1 and LAG-3 co-expression is shown for galectin-3hi and galectin-3lo population. Hi=high; lo=low; TDN=tumor-draining lymph nodes; gal=galectin-3; TIL=tumor-infiltrating lymphocytes. All experiments performed at least 2 independent times with 3–5 mice per group.
Next, we evaluated TILs for phenotypic differences associated with increased galectin-3 binding. High-avidity neu-specific CD8+ T cells in TILs were separated into galectin-3 high (galectin-3hi) versus galectin-3 low (galectin-3lo) populations and analyzed for co-expression of programmed death 1 (PD-1) and lymphocyte activation gene 3 (LAG-3) because these two markers are often involved in T-cell tolerance or terminal activation (17–19). PD-1 and LAG-3 were increased on galectin-3hi CD8+ T cells when compared with galectin-3lo cells (Figure 2C). Thus, galectin-3 binding on CD8+ T cells occurs almost exclusively in the TME on T cells displaying a terminally differentiated or tolerized phenotype with elevated expression of PD-1 and LAG-3.
LAG-3 is necessary for galectin-3-mediated suppression of T-cell secreted IFNγ in vitro
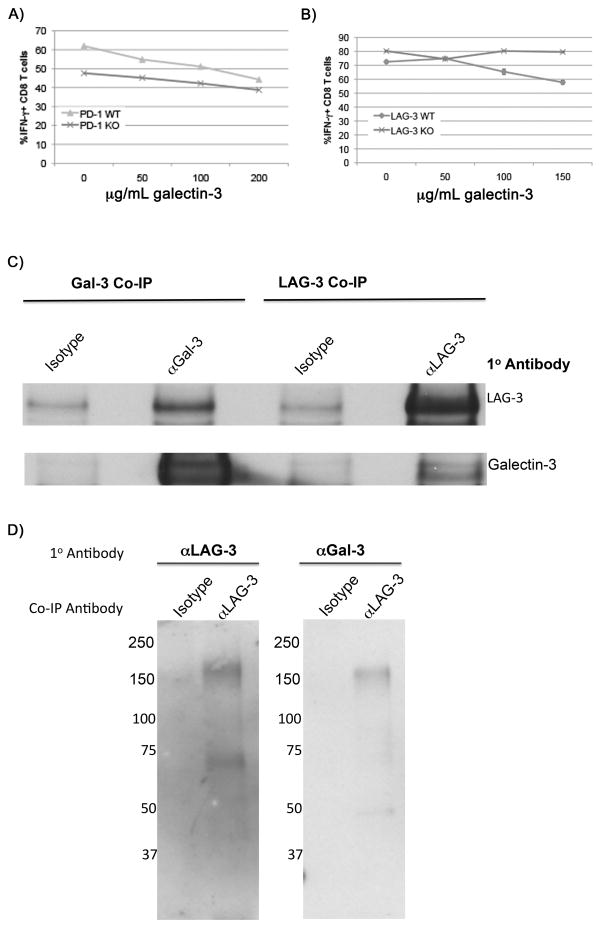
We next evaluated whether PD-1 and LAG-3 are involved in mediating the downstream effects of galectin-3’s on T-cell function. These molecules are heavily glycosylated and possess several binding sites for galectin-3 (20, 21). While WT CD8+ T cells exhibited a 20% reduction in IFNγ production at exogenous galectin-3 concentrations of 150 μg/mL or higher, LAG-3 KO CD8+ T cells produced maximum IFNγ at all concentrations. However, eliminating PD-1 in CD8+ T cells had minimal effect in blocking galectin-3 suppression (Figure 3A and 3B). Co-immunoprecipitation studies using activated CD8+ T-cell lysates further demonstrated a physical interaction between LAG-3 and galectin-3 (Figure 3C). Next, cross-linking of cell surface proteins prior to cell lysis allowed us to immunoprecipitate a LAG-3/galectin-3 complex migrating between 150 and 250 kD with a LAG-3-specific antibody (Figure 3D). Importantly, we were not able to co-immunoprecipitate PD-1 with galectin-3 (data not shown). These data establish a link between LAG-3 and galectin-3 on antigen-committed CD8+ T cells, and suggests a new mechanism by which galectin-3 may regulate CD8+ T-cell function.
Figure 3. LAG-3 but not PD-1 expression is required for galectin-3 suppression of IFNγ production by T cells.
The effect of increasing concentrations of extracellular galectin-3 on the percentage of IFNγ-producing CD8+ T cells is shown for A) PD-1 knockout versus wild type T cells in and B) LAG-3 knockout versus wild type T cells. Experiments performed at least 2 independent times with 3 replicates per sample with errors bars representing SD. C) Co-immunoprecipitation of LAG-3 and galectin-3 from activated CD8+ T-cell lysates with either a galectin-3-specific antibody (M3/38) or a LAG-3-specific antibody (410C9). D) Co-immunoprecipitation of a LAG-3/galectin-3 complex with LAG-3-specific antibody after cross-linking of cell surface proteins on activated CD8+ T cells prior to cell lysis as described in materials and methods.
Depletion of galectin-3 leads to improved tumor-specific CD8+ T-cell function
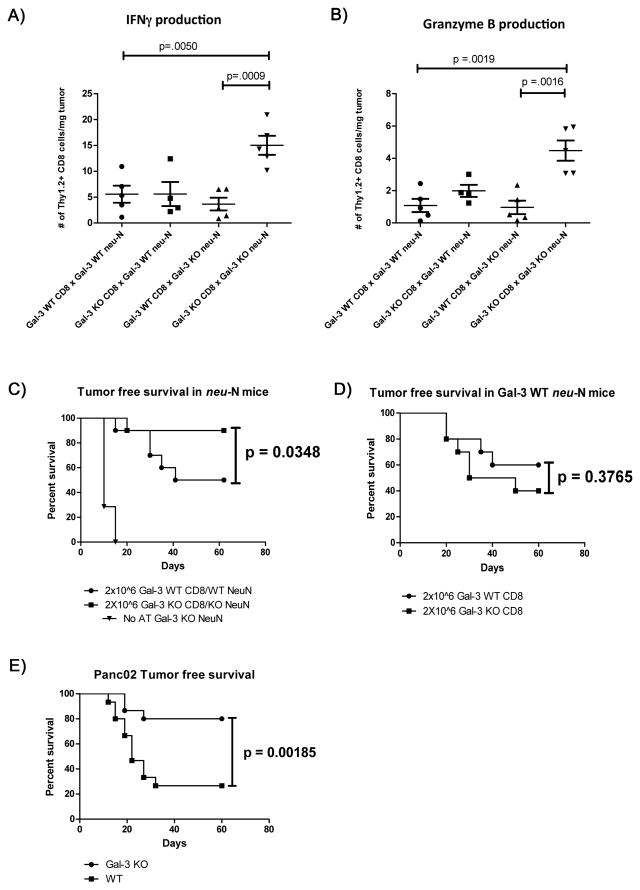
To determine a functional role for galectin-3 in vivo, we bred the neu-N and high-avidity neu-specific (referred to from hereon as simply “high-avidity”) TCR transgenic mice onto a galectin-3 KO background. We observed an increase in the total number of IFNγ- and granzyme B-producing T cells only when galectin-3 is knocked out in both the T cell and the recipient mouse. Depletion of galectin-3 in CD8+ T cells alone or in the recipient mouse alone was insufficient to cause an improvement in CD8+ T-cell effector function as measured by cytokine production (Figure 4A and 4B). This observation is consistent with the finding that galectin-3 elimination is required in both the recipient mouse and the adoptively transferred T cell to completely remove galectin-3 from the cell surface (Figure S2A–C).
Figure 4. Removal of surface galectin-3 from high-avidity CD8+ T cells leads to improved effector function and tumor clearance.
Effector function of TILs was assayed after genetic elimination of galectin-3 in the CD8 T cell, the tumor stroma, or in both. TILs were isolated and cytokine expression assessed according to the protocol outlined in materials and methods. The absolute number is shown for Thy1.2+ CD8+ T cells/mg tumor expressing either A) IFNγ or B) Granzyme B. All experiments were performed at least 2 independent times with 3–5 mice per group. Dot plot data are represented as mean ± SEM. C) All neu-N recipient mice were treated as outlined in materials and methods. Adoptive transfer groups (n=10) received 2×106 galectin-3 WT or KO high-avidity CD8+ T cells as indicated above on day 4. The control group of galectin-3 KO mice (n=7) did not receive adoptive transfer to assess endogenous antitumor response. Mice were followed every 5 days for palpable tumor formation. D) Galectin-3 WT neu-N mice received adoptive transfer of either 2×106 galectin-3 WT high-avidity CD8+ T cells (n=10) or 2×106 galectin-3 KO high-avidity CD8+ T cells (n=10) after receiving treatment as outlined in materials and methods. Mice were followed every 5 days for palpable tumor formation. E) WT C57Bl/6 mice (n=15) or galectin-3 KO C57Bl/6 mice (n=15) received 2.5×105 Panc02 tumor cells on day 0, 100 mg/kg CY and 50 μg PC61 on day 2, and whole-cell GM-CSF vaccine on day 3. Mice were followed every 5 days for development of palpable tumor formation at the injection site. One-tailed p-values are shown.
Next we tested the ability of high-avidity CD8+ T cells to promote long-term tumor control in a galectin-3-null environment. We previously determined that a minimum of 4×106 high-avidity CD8+ T cells is required to achieve tumor control in approximately 75% of neu-N mice (8). To illustrate the efficacy of CD8+ T cells in galectin-3-depleted mice, we tested if 2×106 galectin-3 KO high-avidity CD8+ T cells would be sufficient to mediate a similar level of tumor control. We compared long-term tumor control in WT neu-N mice receiving WT high avidity CD8+ T cells, galectin-3 KO neu-N mice receiving galectin-3 KO high-avidity CD8+ T cells, and galectin-3 KO neu-N mice without adoptive transfer (Figure 4C). Consistent with our functional data, we observed improved tumor-free survival in galectin-3 KO mice that received galectin-3 KO CD8+ T cells with 90% of mice remaining tumor-free at the end of 60 days versus 50% of control mice. Furthermore, because 100% of galectin-3 KO mice that did not receive high-avidity CD8+ T cells rapidly developed tumors by Day 20, we concluded that the survival benefit is mediated by tumor-specific high-avidity CD8+ T cells. We also compared tumor-free survival in galectin-3 WT mice adoptively transferred with either galectin-3 KO or galectin-3 WT high-avidity CD8+ T cells. Consistent with our functional studies, eliminating galectin-3 in high-avidity CD8+ T cells alone did not result in improved survival because this does not eliminate surface galectin-3 that occurs due to secretion of galectin-3 in the TME (Figure 4D).
To validate these findings in endogenous antigen-specific T-cell responses, we used the Panc02 tumor model to compare endogenous antitumor T-cell responses in galectin-3 KO mice versus WT mice in response to whole-cell GM-CSF vaccination (22). Sixty days following treatment, a significantly higher percentage of galectin-3 KO mice were tumor-free versus that of control WT mice (Figure 4E). Together, data from these two mouse models provide evidence that galectin-3 can affect vaccine efficacy, and may potentially do so via modulation of CD8+ T cells.
Galectin-3-depletion leads to increased expression of pro-inflammatory genes in CD8+ T cells
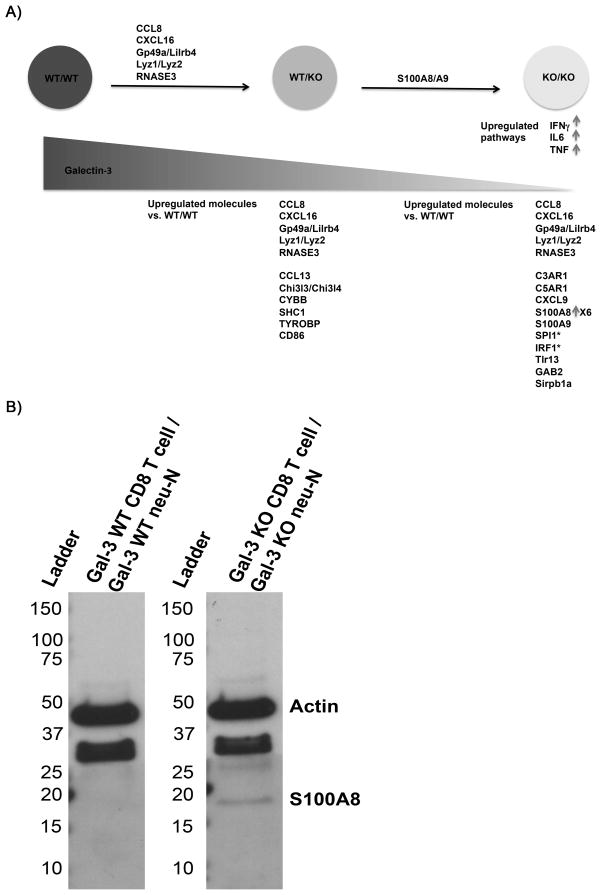
Since galectin-3-depletion results in increased numbers of IFNγ– and granzyme B-producing high-avidity CD8+ T cells, we were interested in identifying other inflammatory signals that may be affected by galectin-3. We used whole genome microarray analysis to compare galectin-3 WT CD8+ TILs isolated from galectin-3 WT neu-N mice (WT/WT), galectin-3 WT CD8+ TILs isolated from galectin-3 KO neu-N mice (WT/KO), and galectin-3 KO CD8+ TILs isolated from galectin-3 KO neu-N mice (KO/KO). Adoptively transferred CD8+ T cells in TILs were purified from endogenous T cells by FACS sorting on Thy1.2 and global gene expression patterns were compared between groups. We found that in both KO/KO and WT/KO CD8+ T cells, molecules associated with inflammatory response processes were increased relative to that in WT/WT cells (Figure 5A). Upstream regulator pathway analyses predicted activation of TNFα, IFNγ, and IL6 signaling pathways in the KO/KO CD8+ T cells but not in WT/KO CD8+ T cells (Table S2).
Figure 5. Removal of galectin-3 increases expression of inflammatory molecules in high-avidity CD8+ T cells.
A) Summary of microarray findings comparing high-avidity galectin-3 WT CD8+ TILs from galectin-3 WT neu-N mice (WT/WT) (n=4), high-avidity galectin-3 WT CD8+ TILs from galectin-3 KO neu-N mice (WT/KO) (n=4), and high-avidity galectin-3 KO CD8+ TILs from galectin-3 KO neu-N mice (KO/KO) (n=4). Analysis was performed using Ingenuity Pathway Analysis (IPA) software using a filter for linear fold-changes ≥ 2, and p ≤ .05. Molecules listed above the arrows between each group are immune-associated genes found to be upregulated between groups. Molecules listed below each group are immune-associated genes found to be upregulated in comparison to the WT/WT control. CCL8, CXCL16, Gp49a/Lilrb4, Lyz1/Lyz2, and RNASE3 were found to be upregulated in both WT/KO and KO/KO groups in comparison with WT/WT. (*) denotes transcription factors. B) Confirmation of S100A8 expression in KO/KO TILs by western blot with S100A8-specific monoclonal antibody (R&D). Total protein concentration quantified by measuring absorbance at 280 nm. 6 μg of total protein was loaded per sample.
We also show that S100A8 expression is increased almost 7-fold in galectin-3 KO/KO CD8+ T cells when compared with that in WT/WT cells (Figure 5B). S100A8 and S100A9 are EF-hand Ca2+ binding proteins that are heavily involved in several inflammatory processes and possess tumoricidal properties in vitro. These inflammatory response genes are expressed by neutrophils, but have not been reported to be expressed by T cells (23).
Inhibition of galectin-3 expression leads to an increase in pDCs in galectin-3 KO mice
The increased expression of several inflammatory proteins in WT high-avidity CD8 T cells adoptively transferred into galectin-3 KO recipient mice suggests that other cell types are impacted by galectin-3 signaling. Galectin-3 has been reported to play a regulatory role in neutrophils and DCs (6, 7, 24).
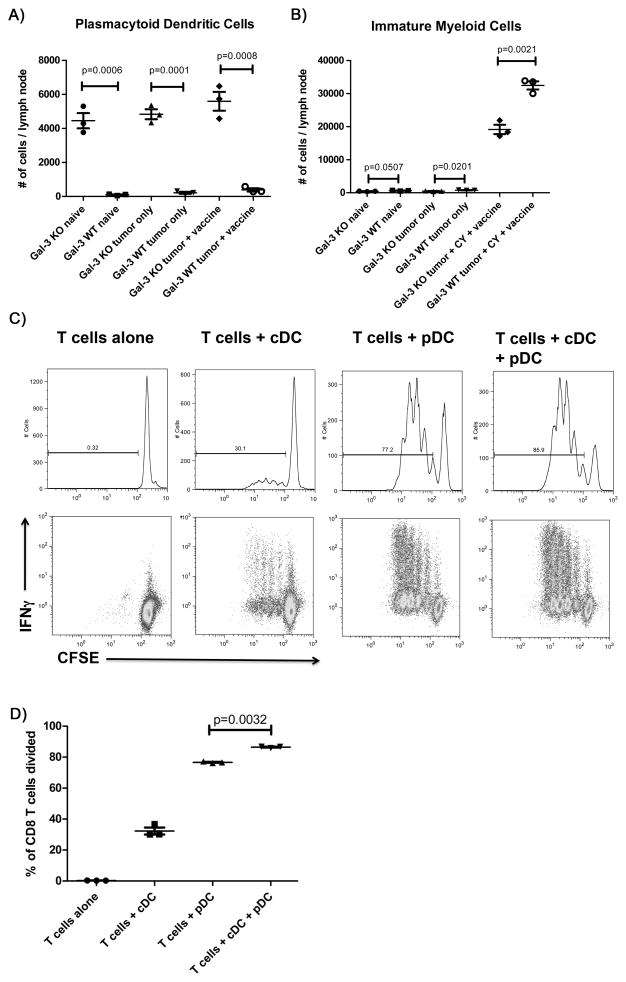
We observed a significant increase in the numbers of CD11c+ B220+ Ly6C+ cells in the galectin-3 KO versus in the galectin-3 WT neu-N mice corresponding with pDCs. Furthermore, the overall number of pDCs found in lymph nodes did not seem to vary significantly between naïve mice given tumor alone, and mice with tumors that are also treated with CY and a GM-CSF vaccine (Figure 6A). This finding indicates that galectin-3 may instead play an intrinsic role in pDC homeostasis rather than solely during immune stimulation, as was the case for CD8+ T cells.
Figure 6. An increase in pDCs in galectin-3 KO neu-N mice is associated with lower numbers of immature myeloid cells.
Galectin-3 KO and WT neu-N mice were left untreated (n=3), challenged with 5×104 NT2.5 tumor cells (n=3), or challenged with 5×104 NT2.5 tumor cells and treated with CY on day 2 and whole cell GM-CSF vaccine on day 3 (n=4). All mice were sacrificed on day 6, and axillary and inguinal lymph nodes were dissected and pooled for each mouse. Tissues were processed according to that described in the materials and methods section, and cells stained for expression of CD11b, CD11c, B220, and Ly6C. The total number of cells was averaged for each lymph node, and is shown for A) pDCs (CD11c+ B220+ Ly6C+) and B) immature myeloid cells (CD11b+ Ly6C+). C) Cells were co-cultured at a 1:1 ratio as specified in the figure prior to being assessed for either CFSE dilution or IFNγ production by flow cytometry. Cytokine expression was assessed by intracellular cytokine staining (ICS) as outlined in materials and methods. D) Statistical comparison of cell division in T cells + pDC vs. T cells + pDC + cDC. Data are represented as mean ± SEM. This experiment was repeated once with similar results. All experiments were performed at least 2 independent times with 3–5 mice per group in A and B, and with 3 replicates per group in C and D.
A reciprocal relationship has been shown to exist between pDCs and myeloid-derived suppressor cells (MDSC) (25). Because pDCs appear to be significantly elevated in the absence of galectin-3, we determined if there was a corresponding decrease in the number of immature myeloid cells, which have the potential to develop into MDSCs. In contrast to what was observed with pDCs in galectin-3 KO mice, the number of immature myeloid cells found in lymph nodes is minimal in naïve and tumor-only mice. GM-CSF will induce MDSCs and this is seen in mice with tumors receiving the GM-CSF vaccine regardless of whether galectin-3 is knocked out. However, even under these conditions, the galectin-3 KO mice have significantly fewer immature myeloid cells than the WT mice indicating that galectin-3 plays a role in GM-CSF recruitment of immature myeloid cells, and potential development into MDSCs (Figure 6B).
While pDCs are known to be involved in the promotion of inflammatory processes important for reversing microbial infections (26), they are also known to be immunosuppressive in the TME when galectin-3 is present (natural environment) (27–29). To clarify the function of pDCs following GM-CSF vaccination in the absence of galectin-3, we evaluated their capacity to induce proliferation of naïve high-avidity CD8+ T cells compared with CD11c+ CD8+ conventional dendritic cells in vitro using a direct ex-vivo antigen detection (DEAD) assay. Our data indicate that naïve T cells proliferate to a greater extent and produce more IFNγ when co-cultured with both pDCs and cDCs than when co-cultured with either DC subtype alone (Figure 6C–D). Furthermore, co-culture of naïve T cells with pDCs alone activated a significantly greater number of T cells than with cDCs alone. These findings support a new role for galectin-3 as a regulator of pDCs via inhibiting their function as T-cell activators in the promotion of anticancer inflammatory responses.
DISCUSSION
Our data describe four novel findings supporting galectin-3 as a regulator of antigen-specific T-cell activation within the TME. First, galectin-3 binds T cells only after they have been activated and trafficked into the TME. In addition, T cells and host-derived cells within the TME, but not tumor cells, are the major sources of galectin-3 that mediate T-cell suppression. Second, galectin-3 regulation of activated T cells leads to genome-wide changes in inflammatory gene expression. Third, galectin-3 complexes with LAG-3 on activated terminally differentiated T cells, and functional LAG-3 is required for galectin-3-mediated T-cell suppression. Fourth, inflammatory pDCs that enhance antigen-specific T-cell activation are increased in the absence of galectin-3.
We show that galectin-3 binding on T cells occurs primarily in the TME, and only to tumor-specific T cells. One leading hypothesis is that activation of CD8+ T cells leads to changes in the glycosylation machinery that increases the number and accessibility of LacNAc motifs available for galectin-3 binding (30). However, glycan remodeling fails to explain why only a small percentage of activated CD8+ T cells in peripheral lymphoid tissue bind galectin-3 while the majority of activated CD8+ T cells in the tumor bind galectin-3. Thus, the TME must provide other conditions that are not satisfied elsewhere to promote galectin-3 binding.
The leading assumption is that tumor cells are the major source for galectin-3 (31). While this may be the case for certain tumor types, our data demonstrate that galectin-3 expression by predominantly tumor-specific CD8+ T cells and stromal cells within the TME, but not tumor cells, result in cancer-specific CD8+ T-cell suppression. Fibroblasts have been shown in models of fibrosis to secrete large amounts of galectin-3, and are also known to be important for establishing the TME (32). Our findings highlight the importance of targeting not just tumor cells, but also stromal cells for effective cancer immunotherapy. Future studies should focus on identifying the specific tumor stromal cell types that secrete galectin-3 as well as what other additional factors provided by the TME promote galectin-3 binding to T cells.
We also performed genome-wide analysis to evaluate changes that occur in tumor-specific CD8+ T cells resulting from binding of extracellular galectin-3 in an in vivo setting. The data demonstrate that removal of galectin-3 leads to increased activation of proinflammatory pathways within CD8+ T cells. Recent data from several groups demonstrate the capacity for galectin-3 to suppress T-cell function by inducing T-cell anergy via TCR clustering, and that these T cells can be rescued by removing surface galectin-3 (3, 33). Our gene array findings demonstrate that the mechanisms of T-cell regulation by galectin-3 extends beyond TCR signaling, and includes altering T-cell fate at the gene expression level. These findings indicate that the addition of galectin-3-targeted therapy to existing cancer vaccines may need to occur before such alterations become irreversible. Additional studies should also further elucidate the role of the S100A8/9 signaling pathway as a potential mediator of T-cell lytic activity.
Our in vivo data also demonstrate improved tumor-specific CD8+ T-cell effector function and tumor-free survival only when galectin-3 is deleted from both adoptively transferred T cells and the recipient mouse, which corresponds to our microarray findings demonstrating upregulation of proinflammatory pathways under the same conditions. These conditions are associated with the complete absence of galectin-3 on the CD8+ T-cell surface at the time they are infiltrating the tumor. Therefore, these data suggest a role for galectin-3 in modulating CD8+ T-cell function by engaging surface glycoproteins. However, the absence of endogenous galectin-3 may also enhance antitumor immunity by affecting other cell types involved with T-cell activation. Galectin-3 KO DCs have been shown to increase both T-cell number and cytokine production in helminthic infections, and promote greater Th17 responses to fungal antigens (6,7,34). Furthermore, intracellular galectin-3 can also promote TCR down-regulation in CD4+ T cells via interactions with the protein Alix at the immunological synapse (5), which would be expected to result in less CD8+ T-cell activation.
PD-1 and LAG-3 are two major co-receptors that have been shown to modulate T-cell function, and their co-expression has been shown to be associated with regulating terminal T-cell activation/exhaustion (17). Our finding that LAG-3 expression is associated with galectin-3 binding, and further, is required for galectin-3 suppression in vitro provides a new mechanism through which LAG-3 can regulate CD8+ T cells. LAG-3 is capable of negatively regulating the function of CD8+ T cells despite the fact that CD8+ T cells do not interact with MHC II, a known ligand of LAG-3. Anti-LAG-3 antibody therapy was shown to reverse this effect in CD4+-depleted mice, which indicates a direct role for LAG-3 on CD8+ T cells (35). The mechanism by which LAG-3 mediates CD8+ T-cell activity is unknown. LAG-3 can be extensively glycosylated, and as a result, would be a likely target for galectin-3 binding. Our in vitro data suggest that CD8+ T-cell suppression can be induced by galectin-3 cross-linking of LAG-3; however, whether this mechanism exists in vivo remains to be elucidated.
Our data also support a link between galectin-3 and activation of inflammatory pDCs. Interestingly, LAG-3 has been reported to negatively regulate pDC homeostasis (36). Thus, steady state levels of extracellular galectin-3 may be necessary to regulate pDC expansion via LAG-3. Further, because pDCs are either directly or indirectly involved with the homeostasis of both MDSCs and Tregs, dysregulation of pDCs may have downstream consequences on these cell types (25). Indeed, our findings that immature myeloid cells proliferate significantly less in response to GM-CSF vaccination in galectin-3 KO mice are consistent with the notion of reciprocal regulation between pDCs and MDSCs.
The role of pDCs in tumor immunity remains unclear. While they are important for antiviral immunity, they have also largely been associated with tolerance induction, and poor prognosis in cancer. It may be possible that pDCs have a stimulatory role in the absence of extracellular galectin-3. Our microarray finding that the expression of several inflammatory genes in T cells is increased as a result of galectin-3 deficiency in only host-derived cells helps support this hypothesis. Although these results may be due simply to a reduction of surface galectin-3 on the T cell, a more compelling argument is that the expansion of host-derived pDCs in the absence of galectin-3 helps to skew the immune response toward inflammation. Further studies will be necessary to characterize differences in cytokine production and functionality of pDCs in the presence and absence of galectin-3.
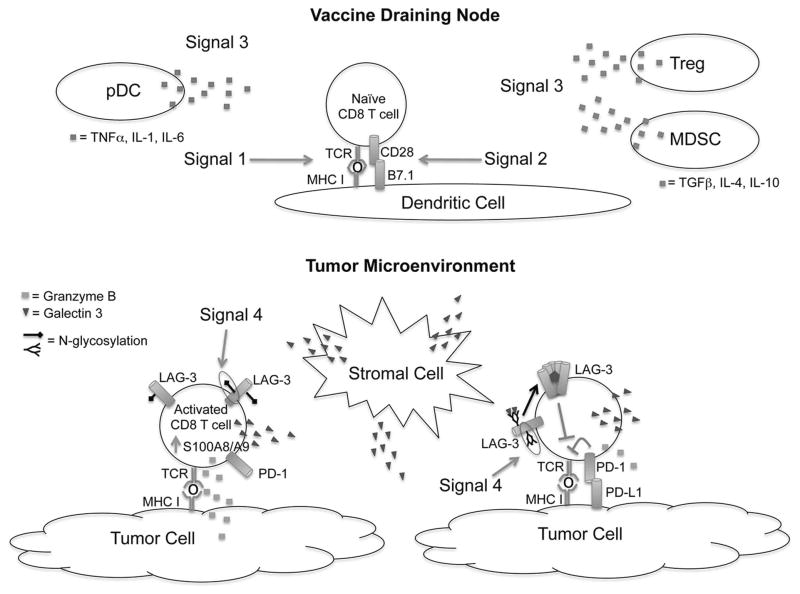
In summary, these findings develop a comprehensive profile of the extensive impact that galectin-3 plays at every stage of an antitumor immune response in a tolerogenic mouse model. We propose that patients who develop anti-galectin-3 antibody titers in response to vaccination are able to neutralize the immunosuppressive effects of galectin-3 either directly or by disruption of galectin-3/receptor lattices, and as a result, mount a more effective cytotoxic CD8+ T-cell response against tumor cells. TCR engagement, co-receptor activation, and the cytokine milieu have long been considered the three signals that determine T-cell fate. However, consideration of protein glycosylation as a “Signal 4” may now help to uncover a new class of molecules for immunomodulation (Figure 7).
Figure 7. N-glycosylation of co-regulatory molecules serves as the signal 4 required for optimal T-cell activation.
In the vaccine draining node (VDN) where naïve T-cell priming occurs, activating signals are delivered via TCR recognition of peptide-MHC I complexes presented by dendritic cells (Signal 1) and interactions between co-stimulatory molecules and their ligands (Signal 2). Furthermore, cytokines secreted by regulatory cell populations also impact cellular differentiation (Signal 3). Plasmacytoid dendritic cells (pDC) are increased in number in the absence of extracellular galectin-3, and the cytokines they secrete may skew T-cell differentiation in the direction of a more pro-inflammatory phenotype. Activation of CD8 T cells leads to increased expression of the co-regulatory molecules PD-1 and LAG-3 by terminally differentiated effector CD8 T cells. In the tumor microenvironment, expression of PD-L1 by tumor cells can suppress T-cell function via PD-1. Changes in glycosylation of LAG-3 (Signal 4) make it a more favorable target for galectin-3 binding, which in turn leads to cross-linking and activation of the LAG-3 signaling complex. Cross-linking of LAG-3 results in suppression of effector CD8 T-cell function. Lack of galectin-3 increases S100A8/9 expression, which may also lead to optimization of lytic function.
Supplementary Material
Acknowledgments
Financial Support: This work was funded by NIH P30CA006973 – William Nelson, Cancer Center Support Grant.
Footnotes
Conflict of interest Statement: Through a licensing agreement between Aduro Biotech and JHU, Dr. Jaffee has the ability to receive royalties in the future on GVAX and Listeria vaccines for cancer. Dr. Jaffee receives grant funding from ROCHE and Aduro Biotech. Dr. Jaffee has served as a consultant for Medimmune.
References
- 1.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng L, Foley K, Huang L, Leubner L, Mo G, Olino K, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demotte N, Wieers G, Van Der Smissen P, Moser M, Schmidt C, Thielemans K, et al. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–88. doi: 10.1158/0008-5472.CAN-10-0761. [DOI] [PubMed] [Google Scholar]
- 4.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–11. [PubMed] [Google Scholar]
- 5.Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:14496–501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breuilh L, Vanhoutte F, Fontaine J, van Stijn CM, Tillie-Leblond I, Capron M, et al. Galectin-3 modulates immune and inflammatory responses during helminthic infection: impact of galectin-3 deficiency on the functions of dendritic cells. Infect Immun. 2007;75:5148–57. doi: 10.1128/IAI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SY, Yu JS, Liu FT, Miaw SC, Wu-Hsieh BA. Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J Immunol. 2013;190:3427–37. doi: 10.4049/jimmunol.1202122. [DOI] [PubMed] [Google Scholar]
- 8.Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 11.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–76. [PubMed] [Google Scholar]
- 12.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, et al. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–80. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 13.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–5. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 14.Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, et al. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur J Immunol. 2010;40:1768–77. doi: 10.1002/eji.200939874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cay T. Immunhistochemical expression of galectin-3 in cancer: a review of the literature. Turk Patoloji Derg. 2012;28:1–10. doi: 10.5146/tjpath.2012.01090. [DOI] [PubMed] [Google Scholar]
- 16.Peng W, Wang HY, Miyahara Y, Peng G, Wang RF. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008;68:7228–36. doi: 10.1158/0008-5472.CAN-08-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–65. [PubMed] [Google Scholar]
- 20.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 21.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–39. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lood C, Stenstrom M, Tyden H, Gullstrand B, Kallberg E, Leanderson T, et al. Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R60. doi: 10.1186/ar3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–22. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 25.Ioannou M, Alissafi T, Boon L, Boumpas D, Verginis P. In vivo ablation of plasmacytoid dendritic cells inhibits autoimmunity through expansion of myeloid-derived suppressor cells. J Immunol. 2013;190:2631–40. doi: 10.4049/jimmunol.1201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–71. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Labidi-Galy SI, Treilleux I, Goddard-Leon S, Combes JD, Blay JY, Ray-Coquard I, et al. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1:380–2. doi: 10.4161/onci.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisirak V, Faget J, Vey N, Blay JY, Menetrier-Caux C, Caux C, et al. Plasmacytoid dendritic cells deficient in IFNalpha production promote the amplification of FOXP3 regulatory T cells and are associated with poor prognosis in breast cancer patients. Oncoimmunology. 2013;2:e22338. doi: 10.4161/onci.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonopoulos A, Demotte N, Stroobant V, Haslam SM, van der Bruggen P, Dell A. Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation. J Biol Chem. 2012;287:11240–51. doi: 10.1074/jbc.M111.320820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Polanska UM, Orimo A. Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol. 2013;228:1651–7. doi: 10.1002/jcp.24347. [DOI] [PubMed] [Google Scholar]
- 33.Gordon-Alonso M, Demotte N, van der Bruggen P. Sugars boost exhausted tumor-infiltrating lymphocytes by counteracting immunosuppressive activities of galectins. Oncoimmunology. 2014;3:e28783. doi: 10.4161/onci.28783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fermin Lee A, Chen HY, Wan L, Wu SY, Yu JS, Huang AC, et al. Galectin-3 modulates th17 responses by regulating dendritic cell cytokines. Am J Pathol. 2013;183:1209–22. doi: 10.1016/j.ajpath.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–91. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.