Abstract
Cochlear inflammatory diseases such as tympanogenic labyrinthitis are associated with acquired sensorineural hearing loss. Although otitis media is extremely frequent in children, tympanogenic labyrinthitis is not commonly observed, which suggests the existence of a potent anti-inflammatory mechanism modulating cochlear inflammation. In this study, we aim to determine the molecular mechanism involved in cochlear protection from inflammation-mediated tissue damage, focusing on interleukin-10 (IL-10) and hemoxygenase-1 (HMOX1) signaling. We demonstrated that IL-10 receptors (IL-10Rs) are expressed in the cochlear lateral wall of mice and rats, particularly in the spiral ligament fibrocytes (SLFs). The rat SLF cell line (RSL) was found to inhibit nontypeable H. influenzae (NTHi)-induced up-regulation of monocyte chemotactic protein-1 (MCP-1/CCL2) in response to IL-10. This inhibition was suppressed by silencing IL-10R1 and was mimicked by cobalt protoporphyrin IX (CoPP) and carbon monoxide-releasing molecule-2 (CORM-2). In addition, IL-10 appeared to suppress monocyte recruitment through reduction of NTHi-induced RSL-derived chemoattractants. Silencing of HMOX1 was found to attenuate the inhibitory effect of IL-10 on NTHi-induced MCP-1/CCL2 up-regulation. Chromatin immunoprecipitation (ChIP) assays showed that IL-10 inhibits NTHi-induced binding of p65 NF-κB to the distal motif in the promoter region of MCP-1/CCL2, resulting in suppression of NTHi-induced NF-κB activation. Furthermore, IL-10 deficiency appeared to significantly affect cochlear inflammation induced by intratympanic injections of NTHi. Taken together, our results suggest that IL-10/HMOX1 signaling is involved in modulation of cochlear inflammation through inhibition of MCP-1/CCL2 regulation in SLFs, implying therapeutic potential of a carbon monoxide (CO)-based approach for inflammation-associated cochlear diseases.
Introduction
Over 48 million Americans, 12 years and older, are known to have hearing loss (1). Among the various types of hearing loss, acquired sensorineural hearing loss (SNHL) is clinically important because it is preventable and manageable (2, 3). Since acquired SNHL is frequently associated with inflammation, as observed in acoustic trauma and cisplatin ototoxicity (4, 5), understanding the molecular mechanism involved in cochlear inflammation may provide us with a novel therapeutic approach.
Middle ear infection, one of the most common pediatric diseases, induces inner ear inflammation (6), which may lead to SNHL (7) and vertigo (8). Consistently, we demonstrated that the spiral ligament fibrocytes (SLFs), which are specialized cochlear fibrocytes (9), play a pivotal role in tympanogenic cochlear inflammation through up-regulation of chemokines such as MCP-1/CCL2 and CXCL2 in response to a middle ear pathogen, nontypeable H. influenzae (NTHi) (10–12). However, considering the extremely high incidence of otitis media (OM) in children (13) and potential communication between the middle ear and the cochlea via the round window membrane (14, 15), middle ear infection-induced cochlear complications are clinically less frequent than expected (16, 17). Therefore, we hypothesize that an effective anti-inflammatory and/or protective mechanism exists in the cochlea. Since cochlear damage, like injury of neural tissues, is frequently permanent and irreversible in mammals, the mammalian cochlea should be well protected from inflammation-mediated tissue damage. A blood-labyrinthine-barrier (18) and Hensen’s cell-derived annexin A1, a potent inhibitor of leukocyte migration (19), may protect the cochlea from inflammation-mediated tissue damage, but our understanding about modulation of cochlear inflammation is limited.
Exogenous IL-10 inhibits cisplatin nephrotoxicity (20) that is associated with inflammatory reactions similar to cisplatin ototoxicity (5). Recently, IL-10 deficiency has been reported to exacerbate experimental autoimmune hearing loss (21); however, the molecular mechanism involved in IL-10-mediated inhibition of cochlear inflammation is poorly understood. Therefore, we aim to determine how cochlear fibrocytes modulate chemokine regulation in response to IL-10, focusing on MCP-1/CCL2, a key monocyte chemoattractant. Since we showed that HMOX1, a downstream molecule of IL-10 signaling, attenuates cisplatin ototoxicity through down-regulation of pro-inflammatory cytokines (22), we aim to further determine the involvement of HMOX1 signaling in IL-10-mediated modulation of cochlear inflammation.
Here, we present that IL-10 receptors are expressed in the cochlear lateral wall (CL) and that SLFs attenuate NTHi-induced MCP-1/CCL2 up-regulation in response to IL-10 via HMOX1 signaling. Exogenous and endogenous carbon monoxide (CO) modulated MCP-1/CCL2 regulation through inhibition of p65 NF-κB binding to the promoter region of MCP-1/CCL2. IL-10 deficiency appeared to significantly affect tympanogenic cochlear inflammation in the animal model. Taken together, this study suggests that IL-10 signaling plays a critical role in protection of the cochlea from inflammation-mediated tissue damage.
Materials and Methods
Reagents
Recombinant human IL-10 (rhIL-10) and Protoporphyrin IX cobalt chloride (C34H32CoN4O4Cl, CoPP) were purchased from Sigma-Aldrich (St. Louis, MO). TaqMan primers and probes for rat MCP-1 (Rn00580555_m1), rat IL-10 (Rn99999012_m1), rat IL-10R1 (Rn00589389-ml), rat HMOX1 (Rn01536933_m1), and rat GAPDH (4352338E) were purchased from Life Technologies (Grand Island, NY). Tricarbonyldichlororuthenium(II) dimer ([Ru(CO)3Cl2]2, CORM-2) was purchased from Santa Cruz Biotechnology, Inc (Dallas, Tx).
Animal Experiments
Male C57BL/6 (Jackson Laboratory, Bar Harbor, MA) and IL-10-deficient mice (http://jaxmice.jax.org/strain/002251.html), and Wister Han IGS rats (Charles River Laboratory, Wilmington, MA) were used. All animal experiments were approved by the Institutional Animal Care and Use Committee. 107 cfu of live NTHi was suspended in 10 µl of saline and was transtympanically inoculated into the middle ear of 8 to 10-week old mice using a 30 G needle and syringe under a surgical microscope. As a control, normal saline was inoculated with the same procedure. Animals were sacrificed 2 and 6 days after inoculation, and temporal bones were dissected. After fixation and decalcification, the temporal bones were embedded in paraffin and were serially sectioned through the mid-modiolar plane at a thickness of 5 µm. H & E staining was performed, and an inflammation index was estimated with a mean of the nucleated inflammatory cells across the cochlear turns and compartments at the mid-modiolar section.
Cell culture and migration assays
Rat SLF cell line (RSL) (23), primary rat cochlear lateral wall (CL) and primary rat splenocytes were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (0.1 mg/ml) (Life Technologies, Grand Island, NY). Primary SLFs were cultured from explants of the rat CL as described (10). In brief, rat pups (P3-P6) were euthanatized in a CO2 chamber and then decapitated. The cochlea was isolated with preservation of its normal structure after dissecting the inner ear from the skull base. After removal of the bony otic capsule, the CL was dissected away from the surrounding tissue (organ of Corti and Reissner’s membrane) using fine forceps. Primary SLFs proliferated from the explants of CL and primary cells of passage 5 or less were used in this study. THP-1 cells (human acute monocytic leukemia cell line) were purchased from ATCC (Manassas, VA) and maintained in RPMI 1640 medium with 2 mM L-glutamine, 10% FBS. All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. For migration assays (12), the RSL cells were exposed to the NTHi lysate (1 µg/ml) with or without rhIL10 (50 ng/ml) for 24 h and the conditioned medium was collected. Using a 24-well plate with polycarbonate membrane inserts (5 µm pores) (Millipore, Billerica, MA), THP-1 cells and isolated mouse splenocytes were added onto each insert at a density of 5 × 105 cells/insert and the conditioned medium was added to the lower chamber. Cells were allowed to migrate for 16 ~ 18 h. Migrated cells were counted with a hemacytometer.
Bacterial culture and preparation of bacterial lysate
NTHi strain 12, originally a clinical isolate from the middle ear fluid of a child with acute OM, was used in this study (24). NTHi lysates were prepared as described previously (25). In brief, a single colony of NTHi was harvested from a chocolate agar plate, inoculated into 3 ml of brain heart infusion (BHI) broth supplemented with NAD and hemin (both at 10 µg/ml) and placed in a 37 °C CO2 incubator overnight. After addition of 50 ml of fresh BHI broth, bacteria were further grown for 4 h to a mid-log phase (A600 = 0.4 to 0.6). The supernatant was discarded after centrifugation at 6,000 × g for 30 min. The bacterial pellet was resuspended in 5 ml of phosphate-buffered solution (PBS) and sonicated to lyse the bacteria. The lysate was then centrifuged at 12,000 × g for 10 min and the supernatant was collected. Protein concentrations of the NTHi lysates were determined using a BCA™ protein assay kit (Pierce Biotechnologies, Rockford, IL).
Quantitative RT-PCR
Quantitative RT-PCR was performed as described previously (10). In brief, SLFs were exposed to the NTHi lysate (1 µg/ml) with and without rhIL-10 (50 ng/ml), and total RNA was extracted using TRIzol reagent (Life Technologies). After cDNA was synthesized using the TaqMan® reverse transcription kit (Life Technologies), multiplex PCR was performed using the ABI 7500 Real-Time PCR system (Applied Biosystems) with gene-specific primers (FAM-conjugated probes for MCP-1, IL-10, and HMOX1) and control primers (a VIC-conjugated probe for GAPDH). The cycle threshold (CT) values were determined according to the manufacturer’s instructions. The relative quantity of mRNA was determined using the 2−ΔΔCT method (26). CT values were normalized to the internal control (GAPDH), and the results were expressed as a fold change of mRNA, with the mRNA levels in the non-treated group set as 1. For conventional PCR analysis, primers were used as follows: rat MCP-1 (356 bp), 5’-TGCTGTCTCAGCCAGATGCAGTTA-3’ and 5’-AGAAGTGCTTGAGGTGGTTGTGGA-3’; rat IL-10 (330 bp), 5’-GGGAAGCAACTGAAACTTCG-3’ and 5’-GCTTTCGAGACTGGAAGTGG-3’; rat IL-10R1 (345 bp), 5’-ATCCTAAGCACACACAGGATGGCT-3’ and 5’-TAACCACACCCAGGAGTGAGCATT-3’; rat IL-10R2 (494 bp), 5’-TTCTGTCCCGTGGAAGACACCATT-3’ and 5’-AACTCCTTGAGGTGCTGTGGAAGA-3’; rat Aif-1 (219 bp), 5’-AGAGCAAGGATTTGCAGGGAGGAA-3’ and 5’-TCTCCAGCATTCGCTTCAAGGACA-3’ ; rat CD68 (144 bp), 5’-CTGTTGCGGAAATACAAGCA-3’ and 5’-GGCAGCAAGAGAGATTGGTC-3’ and 18S rRNA (200 bp), 5’-GTGGAGCGATTTGTCTGGTT-3’ and 5’-CGCTGAGCCAGTCAGTGTAG-3’. PCR products were analyzed by electrophoresis on 1.5% agarose gels, stained with GelRed Nucleic Acid Stain (Biotium, Inc. Hayward, CA), and viewed and photographed using ChemiDoc™ (BIO-RAD, Hercules, CA).
Plasmid, siRNA, transfection and luciferase assay
The luciferase-expressing vector with 5’ flanking regions of rat MCP-1/CCL2 was kindly provided by Dr. D. L. Eizirik (Brussels University, Brussels, Belgium) (27). Luciferase assays were performed as previously described (28). In brief, cells were seeded into 12-well plates at a density of 1.0 × 105 cells/well and transfected at 60% confluence using the Transit®-LT1 transfection reagent (Mirus, Madison, WI) according to the manufacturer’s instructions. The pRL-TK vector (Promega, Madison, WI) was cotransfected to normalize for transfection efficiency. Transfected cells were then starved overnight in serum-free DMEM, followed by exposure to the NTHi (1 µg/ml) lysate with and without rhIL-10 (50 ng/ml) for 8 h before harvesting. All transfections were carried out in triplicate. After washing with PBS, cells were dissolved in lysis reagent (Promega). Luciferase activity was measured using a luminometer (BD Monolight™, 3010) after adding the necessary luciferase substrate (Promega). Results were expressed as a fold change of luciferase activity, taking the value of the non-treated group as 1. For silencing of IL-10R1 expression, cells were transfected with IL-10R1-specific siRNA (s138796: 5’-CGCUGGAUGUCUAUCCCAAtt-3’, Life Technologies) using siPORT™ NeoFX™ transfection agent (Life Technologies), according to the manufacturer’s instruction. As a control, the negative control siRNA (AM4635, Life Technologies) was transfected in parallel. Silencing of IL-10R1 expression was determined with quantitative RT-PCR analysis.
Immunoblotting and ELISA
After overnight starvation with a basal medium, RSL cells were treated with the NTHi lysate (1 µg/ml) for 8 h with and without rhIL-10 (50 ng/ml). Cells were lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA) supplemented with a protease inhibitor cocktail and 1 mM PMSF (Calbiochem). The lysates were then centrifuged at 12,000 × g for 15 min, and the supernatants were collected. An equivalent amount of 20 µg of protein was loaded onto 10% Tricine gels (Life Technologies). After electrophoresis, the proteins were transferred onto PVDF membranes (Bio-Rad) and washed three times for 5 min each in Tris-buffered saline plus 0.05% Tween 20 (TBST). Membranes were blocked using 5% nonfat dry milk in TBST for 1 h at room temperature and incubated overnight at 4 °C in the presence of 1:200 dilution of a polyclonal antibody against MCP-1/CCL2 (sc-28879) and α-tubulin (sc-53646, Santa Cruz Biotechnologies, Inc). After washing, membranes were incubated with a HRP-conjugated secondary antibody in a blocking buffer. Membranes were then incubated with a SuperSignal substrate (Pierce Biotechnologies, Rockford, IL) for 1 min at room temperature, and chemiluminescence signals were detected by exposure to X-ray films. Protein levels of MCP-1/CCL2 and IL-10 were measured using a rat MCP-1 ELISA kit (BD Bioscience, San Diego, CA) and a mouse IL-10 ELISA kit (R & D systems, Minneapolis, MN), following the manufacturer’s instructions. For silencing of HMOX1 expression, cells were transfected with HMOX1-specific siRNA (s127884, Life Technologies) using siPORT™ NeoFX™ transfection agent.
Immunolabeling
To determine the translocation of NRF2, cells were cultured on a 4-chamber microscope slide and treated with rhIL-10 (50 ng/ml) for 2 h. After fixation with 4% paraformaldehyde and permeabilization of cell membranes with 0.15% Triton X-100 (Sigma-Aldrich), cells were blocked using 10% goat serum and subsequently incubated in the presence of rabbit anti-NRF2 antibody (1:200, Santa Cruz) overnight at 4 °C. Alexa Fluor 488® goat anti-rabbit IgG (1:500, Life Technologies) was used as the secondary antibody. For paraffin sections, paraffin was removed with a series of washes with xylene, ethanol and phosphate buffered saline. Sections were then blocked with 10% goat serum and incubated with rat anti-IL-10R1 antibody (1:200, Thermo Scientific) overnight at 4 °C and then incubated with rhodamine-conjugated goat anti-rat IgG (1:200, Life Technologies). Sections were then mounted with anti-fade mounting media (Life Technologies). Samples were then viewed and photographed using a TCS SP5 confocal microscope (Leica, Buffalo Grove, IL).
Chromation immunoprecipitation (ChIP)
Binding of p65 NF-κB to the enhancer region of the MCP-1/CCL2 gene was determined as described (11). Briefly, the RSL cells were treated for 2 h with the NTHi lysate in the presence or absence of rhIL-10 and fixed with 1% formaldehyde for 10 min. After lysis of cells with 1 ml of an ice-cold lysis buffer supplemented with a protease inhibitor cocktail and PMSF, the nuclear fraction was collected using Nuclear Extract Kit (Active Motif, Carlsbad, CA). After resuspending of the nuclear extract, enzymatic shearing of chromatins was conducted. The sheared DNA samples were centrifuged at 18,000 × g for 10 min at 4 °C, and the supernatant was collected (Input DNA). Input DNA samples were incubated with 2 µg/ml of the polyclonal rabbit anti-NF-κB p65 antibody (Abcam, Cambridge, MA), and 25 µl of protein G magnetic beads overnight at 4 °C. The samples were placed on the magnetic stand to pellet the beads, and the supernatant was discarded carefully. After washing, the pelleted beads were resuspended and named “ChIP DNA”. To reverse cross-links, ChIP DNA samples and Input DNA samples were incubated at 95 °C for 10 min and then were mixed with 1 µg/µl of proteinase K and incubated at 37 °C for 1 h. Conventional PCR was performed on Input DNA and ChIP DNA samples using the following primer pairs: distal NF-κB motif (223 bp), 5’-AGCATCTGGAGCTCATATTCCAGC-3’ and 5’-CAGTTAGCATACGATGCAACACAGT-3’; proximal NF-κB motif (200 bp), 5’-GCAGCTTCATTTGCTCCCAGTAGT-3’ and 5’-TTATTGTAAGCCAGAGGGTGGAGTCAGG-3’. (29)
Statistics
All experiments were carried out in triplicate and repeated twice independently. For quantitative RT-PCR analysis, luciferase assays and ELISA, results were analyzed using Student’s t-test and ANOVA followed by Tukey’s post hoc test using the R2.14.0 software for Windows (The R Foundation for Statistical Computing). A value of p<0.05 was considered significant. For histological analysis of cochlear inflammation, we performed mixed model analysis and Fisher’s Exact test using SPSS 12.0 (IBM, Armonk, NY). A value of the Bonferroni corrected p<0.025 was considered significant.
Results
IL-10 receptors are expressed in the cochlear lateral wall
SLFs, which reside in the cochlear lateral wall (Fig. 1A), play an important role in cochlear inflammation through up-regulation of chemokines (10–12), but it is unclear if SLFs are able to respond to a potent anti-inflammatory cytokine, IL-10. To determine if IL-10 receptors (IL-10Rs) are expressed in SLFs, we performed RT-PCR analysis using the isolated rat cochlear lateral wall (CL) tissues and the RSL cells. The CL tissues and RSL cells expressed both IL-10R1 and 2 (Fig. 1B). Notably, IL-10R1, not IL-10R2, was found to be up-regulated by treatment with the lysate of NTHi, a middle ear pathogen. Consistently, immunolabeling showed the expression of IL-10R1 in the RSL cells (Fig. 1C). Next, we sought to determine IL-10R1-expressing cochlear cells in the murine temporal bone sections. IL-10R1 was expressed in the spiral ligament area and stria vascularis (Fig. 1D). Particularly, the type II SLFs were found to markedly express IL-10R1 after NTHi injection. In addition to the CL, IL-10R1 was expressed in the organ of Corti and the Reissner's membrane (data not shown), suggesting that the cochlea largely consists of IL-10-responding cells.
Figure 1.
IL-10 receptors are expressed in the cochlear fibrocytes. (A) Schematic illustration showing the cochlear lateral wall (CL) of the right inner ear. AN: auditory nerve, OW: oval window, RW: round window, ST: scala tympani, *: scala vestibuli, L: lateral, P: posterior, S: superior. (B) RT-PCR analysis shows the IL-10 receptors (IL-10R1 and IL-10R2) are expressed in the rat CL tissue and RSL cells. Note that IL-10R1, not IL-10R2, is up-regulated upon exposure to NTHi lysate. 18s: 18s rRNA. (C) Immunolabeling shows the expression of IL-10R1 (red) in the RSL cells. Blue: DAPI. Scale bar: 25 µm. (D) Immunolabeling of the murine CL shows that IL-10R1 is expressed highly in the spiral ligament type II region (#) and the stria vascularis. Con: no injection. Saline: intratympanic injection of saline. NTHi: intratympanic injection of live NTHi. OC: otic capsule. Original magnification: ×40.
IL-10 inhibits NTHi-induced MCP-1/CCL2 up-regulation in SLFs
We sought to determine if IL-10 is expressed in the CL in response to the NTHi lysate. ELISA showed that IL-10 protein was expressed in the isolated CL tissues (42.03 ± 11.27 pg/ml/µg) and splenocytes (85.30 ± 31.66 pg/ml/µg) of wild-type mice, but not in IL-10-deficient mice (Fig. 2A). RT-PCR analysis showed that IL-10 was up-regulated only in the isolated rat CL tissues, but not in the RSL cells, in response to the NTHi lysate (Fig. 2B), which indicates that IL-10-expressing cells reside in the CL. In contrast, MCP-1/CCL2 was up-regulated in both isolated rat CL tissues and RSL cells. Since macrophages are known to reside in the mouse cochlea (30–32), we performed RT-PCR analysis to determine localization of macrophages in the rat CL. As shown in Figure 2C, CD68 (a marker for monocytes/macrophages) and Aif-1 (a marker for resident macrophages) were found to be expressed in the isolated rat CL tissue, but not in the RSL cells, which suggests that cochlear resident macrophages are a potential source of IL-10 in the CL. However, further studies are necessary to localize and characterize IL-10-producing cells in the cochlea. Next, we sought to determine if IL-10 affects MCP-1/CCL2 regulation in SLFs, which may contribute to modulation of cochlear inflammation. Quantitative RT-PCR analysis and luciferase assays showed that the RSL cells inhibit NTHi-induced transcriptional regulation of MCP-1/CCL2 up-regulation upon exposure to recombinant IL-10 (Fig. 2D, 2E). Interestingly, IL-10 appeared to shift a peak of MCP-1/CCL2 up-regulation to 12 h later, compared with the IL-10-untreated control. Moreover, immunoblot analysis showed the inhibitory effect of IL-10 on NTHi-induced MCP-1/CCL2 production in the RSL cells (Fig. 2F). In contrast to IL-10, IL-1β and TNFα rather enhanced NTHi-induced MCP-1 up-regulation in the RSL cells (Fig. 2G). Since SLFs were found to release monocyte-attracting molecules in response to NTHi (12), we sought to determine if IL-10 affects chemoattraction of RSL-derived molecules. Migration assays showed that recombinant IL-10 suppresses migration of THP-1 cells by reduction of NTHi-induced RSL-derived chemoattractants (Fig. 2H). Moreover, IL-10-deficient splenocytes appeared to migrate more actively in response to NTHi-induced SLF-derived molecules, compared to wild-type splenocytes (Fig. 2I). To further determine the involvement of IL-10R1 in IL-10-mediated inhibition of MCP-1/CCL2 regulation, RSL cells were transfected with a siRNA specific to IL-10R1. Luciferase assays showed that silencing of IL-10R1 significantly suppresses an inhibitory effect of IL-10 on NTHi-induced MCP-1/CCL2 up-regulation (Fig. 3A). Quantitative RT-PCR analysis showed siRNA-mediated silencing of IL-10R1 expression (Fig. 3B). Collectively, these results suggest that the IL-10/IL-10R1 axis is involved in modulation of cochlear inflammation through attenuation of MCP-1/CCL2 expression in SLFs.
Figure 2.
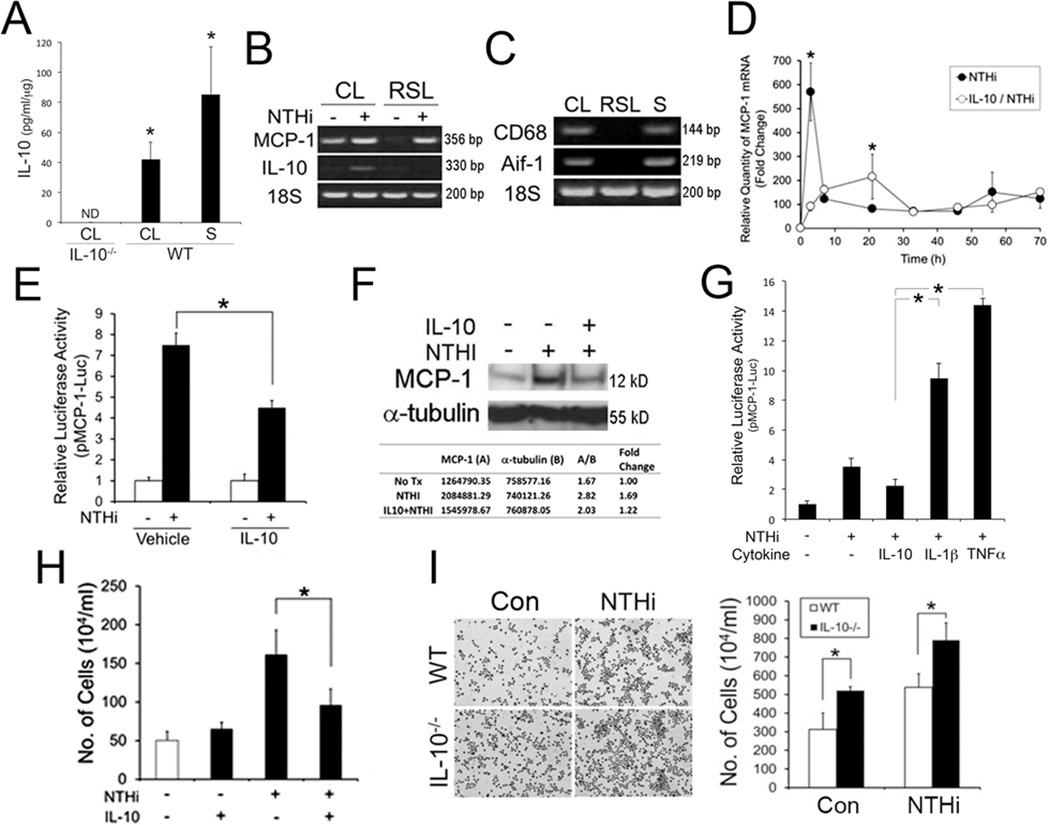
IL-10 inhibits NTHi-induced MCP-1 up-regulation in SLFs. (A) ELISA shows the expression of IL-10 protein in the cochlear lateral wall tissues (CL) and splenocytes (S) of wild-type mice (WT). IL-10 was not detectable (ND) in the CL of IL-10-deficient mice (IL-10−/−). (B) Rat CL tissue, not RSL cells, up-regulated IL-10 expression in response to NTHi. 18S: 18S rRNA. (C) RT-PCR analysis shows that CD68 and Aif-1 are expressed in the isolated rat CL tissue, but not in the RSL cells. S: splenocytes as a positive control. Quantitative RT-PCR analysis (D), luciferase assays (E) and immunoblot analysis (F) show that IL-10 inhibits NTHi-induced MCP-1 up-regulation. pMCP-1-Luc: a luciferase-expressing reporter construct of rat MCP-1. (G) Luciferase assays show that NTHi-induced MCP-1 up-regulation is reduced by IL-10, but is enhanced by IL-1β and TNFα. IL-10: 50 ng/ml. IL-1β: 20 ng/ml. TNFα: 20 ng/ml. (H) Migration assays show that the RSL cells inhibit NTHi-induced release of THP-1-attracting molecules upon exposure to IL-10. (I) Migration assays with Giemsa staining (Left panel) and cytometry (Right panel) show that IL-10-deficient splenocytes (IL-10−/−) migrate more in response to NTHi-induced SLF-derived molecules, compared with the wild-type splenocytes (WT). Con: conditioned medium from the NTHi-unexposed RSL cells, NTHi: conditioned medium from the NTHi-exposed RSL cells. The experiments were performed in triplicate and repeated twice independently. Values are given as the mean ± standard deviations (n = 3). *: p < 0.05.
Figure 3.
IL-10R1 is involved in IL-10-mediated inhibition of NTHi-induced MCP-1 up-regulation in the RSL cells. (A) Luciferase assays show that silencing of IL-10R1 attenuates an inhibitory effect of IL-10 on NTHi-induced MCP-1 up-regulation. (B) Quantitative RT-PCR analysis showing siRNA-mediated inhibition of IL-10R1 expression. NC: a non-specific siRNAs. Results were expressed as a fold induction, taking the value of the non-treated group as 1. Experiments were performed in triplicate and repeated twice independently. Values are given as the mean ± standard deviations (n = 3). *: p<0.05.
HMOX1 mediates IL-10-dependent inhibition of NTHi-induced MCP-1/CCL2 regulation in SLFs
The anti-inflammatory effect of IL-10 is mainly mediated by SOCS3 (33, 34) and HMOX1 (35). In our previous studies, HMOX1 was found to protect the cochlea from cisplatin ototoxicity through down-regulation of pro-inflammatory cytokines (22), which led us to further determine the involvement of HMOX1 in modulation of cochlear inflammation. First, we sought to determine if SLFs activate NRF2, a basic leucine zipper transcription factor regulating HMOX1 (36), in response to IL-10. As shown in Figure 4A, immunolabeling showed that NRF2 is translocated into the nucleus upon exposure to IL-10 in the RSL cells. To determine the involvement of HMOX1 in IL-10 signaling in the cochlea, quantitative RT-PCR analysis was performed using the primers specific to HMOX1. IL-10 was found to up-regulate HMOX1 expression in the primary rat SLFs (Fig. 4B). Furthermore, silencing of HMOX1 suppressed an inhibitory effect of IL-10 on NTHi-induced MCP-1/CCL2 regulation in the RSL cells (Fig. 4C). ELISA analysis showed that the RSL cells reduce NTHi-induced MCP-1/CCL2 production when HMOX1 expression is depleted (Fig. 4D). Taken together, these findings suggest that HMOX1 is required for the IL-10-mediated modulation of cochlear inflammation.
Figure 4.
HMOX-1 is involved in IL-10-mediated inhibition of NTHi-induced MCP-1 up-regulation in SLFs. (A) Confocal microscopic images show that NRF-2 is translocated into the nucleus by IL-10 in the RSL cells. Con: a negative control without IL-10 treatment. Original magnification: ×630. (B) Quantitative RT-PCR analysis shows that primary rat SLFs up-regulate HMOX1 in response to IL-10. Quantitative RT-PCR analysis (C) and ELISA analysis (D) show that silencing of HMOX1 suppresses an inhibitory effect of IL-10 on NTHi-induced MCP-1 up-regulation. NC: a non-specific siRNA. Results were expressed as a fold induction, taking the value of the non-treated group as 1. Experiments were performed in triplicate and repeated twice independently. Values are given as the mean ± standard deviations (n = 3). *: p< 0.05.
Carbon monoxide is involved in IL-10/HMOX1 signaling in SLFs
HMOX1, which catalyzes the initial and rate-limiting step in heme metabolism, degrades heme into carbon monoxide (CO), free iron and biliverdin (37). Since CO gas (10 to 500 ppm) has a potent anti-inflammatory effect (38, 39), we determined if CO serves as a key molecule in IL-10/HMOX1 signaling in SLFs. Quantitative RT-PCR analysis showed that CoPP, an HMOX1 inducer augmenting endogenous CO generation, markedly inhibits NTHi-induced MCP-1/CCL2 regulation (~70%), mimicking the inhibitory effect of IL-10 (Fig. 5A). Furthermore, we found that exogenous CO generated by CORM-2, a ruthenium-based CO releaser, inhibits NTHi-induced MCP-1/CCL2 up-regulation in a dose-dependent manner (Fig. 5B). Consistently, ELISA analysis showed that RSL cells significantly reduce NTHi-induced MCP-1/CCL2 production (~50%) upon exposure to CORM-2 (Fig. 5C). Altogether, it is suggested that HMOX1/CO signaling is critically involved in IL-10-mediated modulation of cochlear inflammation.
Figure 5.
NTHi-induced MCP-1 up-regulation is inhibited by CoPP and CORM-2. (A) Quantitative RT-PCR analysis shows that RSL cells suppress NTHi-induced MCP-1 up-regulation upon exposure to CoPP. (B) Luciferase assays show that CORM-2 inhibits NTHi-induced MCP-1 up-regulation in a dose-dependent manner. (C) ELISA analysis shows that RSL cells inhibit NTHi-induced MCP-1 production in response to IL-10 and CORM-2. Experiments were performed in triplicate and repeated twice independently. Values are given as the mean ± standard deviations (n = 3). *: p< 0.05.
IL-10 inhibits NTHi-induced binding of p65 NF-κB to distal NF-κB motifs of the MCP-1/CCL2 gene
In our previous study, we demonstrated that p65 NF-κB binds to the distal NF-κB binding motif of the rat MCP-1/CCL2 gene in response to the NTHi lysate (10), but it is unclear if IL-10 affects NTHi-induced NF-κB activation in SLFs. Luciferase assays using a reporter construct of NF-κB activity showed that RSL cells reduce NTHi-induced NF-κB activation upon exposure to IL-10 (Fig. 6A). Consistently, exogenous CO generated by CORM-2 was found to inhibit NTHi-induced NF-κB activation (Fig. 6B). Analysis of transcription factor binding sites in the 3’-flanking region (−3000 base pairs) of the MCP-1/CCL2 gene predicted six NF-κB binding motifs in humans, but only three in mice and rats (Fig. 6C). Interestingly, multiple sequence alignment analysis with Clustal Omega showed that the distal NF-κB binding motifs (D-κB1 and D-κB2) between −3000 and −2000 base pairs of the MCP-1/CCL2 gene are highly preserved in humans, mice and rats (Fig. 6D). This finding led us to further determine if IL-10 affects NTHi-induced binding of p65 NF-κB to the promoter region of the MCP1/CCL2 gene. ChIP analysis showed that RSL cells inhibit NTHi-induced binding of p65 NF-κB to D-κB1 and D-κB2 of the MCP-1/CCL2 gene (Fig. 6E, 6F). In contrast, IL-10 appeared to insignificantly affect NF-κB binding to the proximal NF-κB motif (P-κB). Collectively, it is suggested that IL-10/CO signaling attenuates NTHi-induced MCP-1/CCL2 regulation in the SLFs through inhibition of p65 NF-κB binding to the distal NF-κB motifs of the MCP-1/CCL2 gene.
Figure 6.

IL-10 inhibits NTHi-induced NF-κB binding to the promoter of the MCP-1 gene. Luciferase assays show that IL-10 (A) and CORM-2 (B) inhibit NTHi-induced NF-κB activation in the RSL cells. Results were expressed as a fold induction, taking the value of the non-treated group as 1. Experiments were performed in triplicate and repeated twice independently. Values are given as the mean ± standard deviations (n = 3). *: p<0.05. (C) NF-κB binding motifs (solid bars) in the 5’-flanking region of the MCP-1 gene in humans, mice and rats. (D) The distal NF-κB binding motifs (D-κB1 and D-κB2) between −3000 and −2000 base pairs are preserved in humans, mice and rats. (E, F) Chromatin Immunoprecipitation analysis shows that IL-10 inhibits NTHi-induced binding of p65 NF-κB to D-κB1 and D-κB2 of the rat MCP-1 gene. In contrast, IL-10 insignificantly affects NTHi-induced binding of p65 NF-κB to the proximal NF-κB binding motifs (P-κB).
IL-10 deficiency affects cochlear inflammation secondary to middle ear infection
Since our result showed that IL-10 inhibits NTHi-induced MCP-1/CCL2 regulation in the SLFs, we sought to determine if IL-10 is involved in modulation of cochlear inflammation in vivo. Live NTHi was injected into the middle ear cavity of IL-10-deficient mice, and histological analysis was conducted. H & E staining of the mid-modiolar sections of the temporal bone showed that intratympanic injection of live NTHi leads to accumulation of serous substances and infiltration of inflammatory cells in the scala tympani of the mouse cochlea, particularly in the cochlear basal turn (Fig. 7A). According to the number of infiltrated inflammatory cells, cochlear samples were divided into 4 groups from 0 to 3+. Fisher’s Exact test showed that inflammatory cells infiltrate into the cochlea more in IL-10-deficient mice than wild-type mice on post-injection day 2, but not on post-injection day 6 (Table 1). To further quantify cochlear inflammation, an inflammation index was determined through the counting of nucleated inflammatory cells across the cochlear turns and compartments at the mid-modiolar sections (Fig. 7B). The mixed model analysis showed more severe cochlear inflammation in IL-10-deficient mice than wild-type mice on both post-injection day 2 and 6 (p=0.016). Altogether, this study suggests that IL-10/HMOX1-mediated CO production plays a critical role in modulation of tympanogenic cochlear inflammation through inhibition of MCP-1/CCL2 regulation in the SLFs (Supplemental Figure).
Figure 7.
IL-10 deficiency affects tympanogenic cochlear inflammation. (A) H & E staining of the representative temporal bone sections shows that intratympanic injection of live NTHi induces more severe inflammatory responses in the cochlea of IL-10-deficient (IL-10−/−) mice (n = 7), compared with wild type (WT) mice (n = 7). Serous substances (#) were accumulated in the scala tympani of the cochlear basal turn in both IL-10−/− and WT mice. In contrast, massive infiltration of the inflammatory cells (arrows) was noted only in the IL-10−/− mice. Ac: an acoustic nerve, M: a middle ear cavity, T: scala tympani, V: scala vestibuli, *: scala media. Original magnification: ×100. (B) Inflammation index of the cochlea is significantly higher in IL-10−/−mice than WT mice. Inflammation index: a mean of the nucleated inflammatory cells across the cochlear turns and compartments at the mid-modiolar section. #: significant interactions of the mouse group and time, estimated from the mixed model analysis. Values are given as the mean ± standard error.
Table 1.
Cochlear inflammation induced by intratympanic injection of live NTHi.
| Wild type | IL-10−/− | Fisher’s Exact Test Value |
Exact Significance (2-sided)# |
|||||
|---|---|---|---|---|---|---|---|---|
| Day 2 | 13/15* | − | 2 | 10/10* | − | 0 | 12.891 | p=0.001 |
| + | 10 | + | 1 | |||||
| ++ | 2 | ++ | 2 | |||||
| +++ | 1 | +++ | 7 | |||||
| Day 6 | 1/6* | − | 5 | 4/8* | − | 4 | 2.744 | p=0.329 |
| + | 0 | + | 3 | |||||
| ++ | 0 | ++ | 0 | |||||
| +++ | 1 | +++ | 1 | |||||
: Values are given as the number of ears showing cochlear infiltration of inflammatory cells per total number of ears. −: no cochlear infiltration of inflammatory cells; +: cochlear infiltration of 11–30 inflammatory cells; ++: cochlear infiltration of 31–50 inflammatory cells; +++: cochlear infiltration of 51 and more inflammatory cells.
: corrected for 2 Fisher’s exact tests, p value must be less than 0.025.
Discussion
In our previous studies, we showed that SLFs play a pivotal role in cochlear inflammation through regulation of chemokines (10–12). In this study, we aimed to determine if IL-10 modulates cochlear inflammation via down-regulation of a model chemokine, MCP-1/CCL2, in SLFs. We found that SLFs down-regulate NTHi-induced MCP-1/CCL2 expression in response to IL-10 through IL-10R1/HMOX1-mediated signaling. Exogenous and endogenous CO inhibited NTHi-induced MCP-1/CCL2 up-regulation through suppression of NF-κB activation. Interestingly, IL-10 inhibited NTHi-induced binding of p65 NF-κB to the distal NF-κB motifs of the MCP-1/CCL2 gene, but not to the proximal motif. Moreover, we found that IL-10 deficiency exaggerates tympanogenic cochlear inflammation in the murine model, implying the protective role of IL-10 against inflammation-mediated cochlear damage.
Cochlear inflammation is frequently associated with acquired SNHL such as cisplatin ototoxicity (5) and acoustic trauma (4). However, like the brain and the retina, the cochlea is protected from immune-mediated tissue damage because the cochlea is an essential organ for survival in mammals. For example, the cochlea is relatively isolated from the immune system by a blood-labyrinthine barrier (BLB) (18). Cochlear Hensen’s cells were found to release a large amount of annexin A1, a potent inhibitor of leukocyte migration, in response to glucocorticoids (19). Recently, it has been reported that CX3CR1-expressing cochlear macrophages play a protective role in aminoglycoside ototoxicity (40). In addition to these cochlear protections, our study introduces the molecular mechanism involved in IL-10-mediated modulation of cochlear inflammation. Interestingly, IL-10 is known to inhibit CD80/CD86 expression in the CX3CR1-expressing myeloid cells, resulting in prevention of T-cell-dependent colitis (41), but further studies are needed to determine if cochlear protection by CX3CR1-expressing cochlear macrophages are mediated by IL-10.
SLFs, the most abundant inner ear cell type, express a variety of ion channels serving as a part of the potassium recycle pathway required for normal hearing (42, 43). In our prior studies, we have demonstrated that SLFs are involved in cochlear inflammation through TLR2/NF-κB-mediated MCP-1/CCL2 regulation and ERK2/c-Jun-mediated CXCL2 regulation (10, 11). However, our understanding about negative regulation of chemokines in SLFs is limited. This study provides us with an insight into IL-10-mediated modulation of chemokine regulation via HMOX1/CO signaling. Furthermore, we found that IL-10R1 is expressed in the cochlear lateral wall, particularly in the area of type II SLFs. The type II SLFs are positive for Na+, K+-ATPase (9) and selectively activate NF-κB in response to inflammatory stress (44). In contrast, the type I SLFs, negative for Na+, K+-ATPase, are known to respond to acoustic stress, resulting in NF-κB activation. An immunological role of each type of SLF remains to be revealed.
IL-10 is mainly produced by immune cells such as macrophages, T regulatory cells and dendritic cells (45). Non-immune cells such as keratinocytes are able to express IL-10 (46), but it is unclear if cochlear cells express IL-10. Since bone marrow-derived cells, including Iba-1-positive cells (31) and perivascular macrophage-like melanocytes (30), are found in the cochlear lateral wall, we hypothesize that these cells may serve as a cochlear source of IL-10 before recruitment of inflammatory cells. We found that the isolated CL tissue constitutively expresses IL-10 protein and up-regulates IL-10 mRNA upon exposure to the NTHi lysate. However, immunolabeling hardly localized IL-10-expressing cells in the CL (data not shown), suggesting a paucity of cochlear IL-10-producing cells and a narrow window of IL-10 induction. We plan to further localize and characterize IL-10-producing cochlear cells using IL-10 reporter animals.
IL-10-deficient mice spontaneously develop a chronic inflammatory bowel disease due to uncontrolled cytokine production (47, 48). They are abnormally sensitive to bacterial lipopolysaccharide (49) and to infections with Psuedomonas aeruginosa (50) and Toxoplasma gondii (51). Since IL-10 is required for resolution of inflammatory reactions, we expected that IL-10 would affect cochlear inflammation in a relatively later phase. Accordingly, SLFs appeared to delay and suppress a peak of MCP-1/CCL2 regulation upon exposure to IL-10 in vitro. However, IL-10 deficiency was found to significantly exacerbate cochlear inflammation in an early phase in vivo, suggesting an important anti-inflammatory role of cochlear resident immune cells before recruitment of inflammatory cells. Further studies are necessary to determine an immunological difference in IL-10 derived from cochlear resident immune cells and recruited inflammatory cells.
IL-10 exerts a dual effect (i.e., stimulation and inhibition) on p38 MAPK signaling (52), which is involved in cytokine production and HMOX1 induction (35). Therefore, we hypothesize that there is an alternative pathway contributing to IL-10-induced HMOX1 up-regulation. Previously, we have shown that NRF2-mediated HMOX1 regulation is involved in protection from cisplatin ototoxicity (22, 36), which led us to focus on NRF2 signaling. NRF2 (or NFE2L2), a leucine zipper transcription factor, translocates to the nucleus in response to oxidative stress and binds to the antioxidant response element, resulting in transcription of various anti-oxidative genes such as HMOX1 (53). Moreover, NRF2 is involved in protection of hearing from aging and aminoglycosides (54). This study demonstrated that NRF2 translocates into the nucleus in response to IL-10 in SLFs, but it is still unclear if NRF2 deficiency affects IL-10-mediated modulation of cochlear inflammation.
Recombinant IL-10 has been clinically tested for the management of various chronic inflammatory diseases (55, 56), but further clinical application has been hindered by a number of obstacles such as induction of neutralizing antibodies and its short half-life in vivo (57). Therefore, targeting of specific molecules downstream of IL-10 signaling such as HMOX1 and CO may represent better “drugable” approaches. Despite a potential risk of prenatal CO exposure (58), CO has been emerging as a promising drug target for the treatment of vascular dysfunction and immune-mediated diseases (38, 39). Ruthenium-based CO-releasing molecules (CORMs) are able to directly liberate a small quantity of CO in biological systems (59, 60). Our result demonstrated that CORM-2 inhibits MCP-1/CCL2 up-regulation via suppression of NTHi-induced NF-κB activation. Since CORM-2 is hardly soluble in water, we will further determine therapeutic potential of a water-soluble CORM-2 derivative, CORM-3 (61), for the management of cochlear inflammation.
In conclusion, we demonstrated that IL-10/HMOX1 signaling is protective for cochlear inflammation through inhibition of MCP-1/CCL2 regulation. This study is expected to provide us with a scientific basis for a novel non-steroidal approach to manage inflammation-associated cochlear diseases.
Supplementary Material
Acknowledgements
We would like to thank Dr. Laurel M. Fisher (University of Southern California) for providing the statistical analysis and David Phak for animal husbandry and manuscript preparation.
This work was supported in part by grants DC005025 and DC011862 from National Institutes of Health, National Institute Deafness and Other Communication Disorders.
Abbreviations used in this paper
- ChIP
Chromation immunoprecipitation
- CL
cochlear lateral wall
- CO
carbon monoxide
- CORM-2
carbon monoxide-releasing molecule-2
- HMOX1
hemoxygenase-1
- IL-10Rs
IL-10 receptors
- NTHi
nontypeable H. influenzae
- RSL
rat SLF cell line
- SLFs
spiral ligament fibrocytes
References
- 1.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers B, Meyer D, Summey C, Scheessele D, Atwell T, Ostendorf J, Randolph SA, Buckheit K. What makes a successful hearing conservation program? AAOHN J. 2009;57:321–335. doi: 10.3928/08910162-20090729-07. quiz 336-327. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjea D, Rybak LP, Sheehan KE, Kaur T, Ramkumar V, Jajoo S, Sheth S. The design and screening of drugs to prevent acquired sensorineural hearing loss. Expert Opin Drug Discov. 2011;6:491–505. doi: 10.1517/17460441.2011.562887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. The Journal of comparative neurology. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- 5.So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paparella MM, Goycoolea MV, Meyerhoff WL. Inner ear pathology and otitis media. A review. Ann Otol Rhinol Laryngol Suppl. 1980;89:249–253. doi: 10.1177/00034894800890s358. [DOI] [PubMed] [Google Scholar]
- 7.Paparella MM, Morizono T, Le CT, Mancini F, Sipila P, Choo YB, Liden G, Kim CS. Sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol. 1984;93:623–629. doi: 10.1177/000348948409300616. [DOI] [PubMed] [Google Scholar]
- 8.Casselbrant ML, Furman JM, Rubenstein E, Mandel EM. Effect of otitis media on the vestibular system in children. Ann Otol Rhinol Laryngol. 1995;104:620–624. doi: 10.1177/000348949510400806. [DOI] [PubMed] [Google Scholar]
- 9.Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-z. [DOI] [PubMed] [Google Scholar]
- 10.Moon SK, Woo JI, Lee HY, Park R, Shimada J, Pan H, Gellibolian R, Lim DJ. Toll-like receptor 2-dependent NF-kappaB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infect Immun. 2007;75:3361–3372. doi: 10.1128/IAI.01886-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S, Woo JI, Lim DJ, Moon SK. ERK2-dependent activation of c-Jun is required for nontypeable Haemophilus influenzae-induced CXCL2 upregulation in inner ear fibrocytes. J Immunol. 2012;188:3496–3505. doi: 10.4049/jimmunol.1103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo JI, Pan H, Oh S, Lim DJ, Moon SK. Spiral ligament fibrocyte-derived MCP-1/CCL2 contributes to inner ear inflammation secondary to nontypeable H. influenzae-induced otitis media. BMC Infect Dis. 2010;10:314. doi: 10.1186/1471-2334-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluestone CD, Klein JO. Otitis Media in Infants and Children. Philadelphia: W.B. Saunders Company; 2000. [Google Scholar]
- 14.Kawauchi H, DeMaria TF, Lim DJ. Endotoxin permeability through the round window. Acta Otolaryngol Suppl. 1989;457:100–115. doi: 10.3109/00016488809138892. [DOI] [PubMed] [Google Scholar]
- 15.Goycoolea MV. The round window membrane under normal and pathological conditions. Acta Otolaryngol Suppl. 1992;493:43–55. [PubMed] [Google Scholar]
- 16.Arjmand EM, Webber A. Audiometric findings in children with a large vestibular aqueduct. Arch Otolaryngol Head Neck Surg. 2004;130:1169–1174. doi: 10.1001/archotol.130.10.1169. [DOI] [PubMed] [Google Scholar]
- 17.Vartiainen E, Vartiainen J. Age and hearing function in patients with chronic otitis media. J Otolaryngol. 1995;24:336–339. [PubMed] [Google Scholar]
- 18.Juhn SK, Rybak LP, Prado S. Nature of blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol Laryngol. 1981;90:135–141. doi: 10.1177/000348948109000208. [DOI] [PubMed] [Google Scholar]
- 19.Kalinec F, Webster P, Maricle A, Guerrero D, Chakravarti DN, Chakravarti B, Gellibolian R, Kalinec G. Glucocorticoid-stimulated, transcription-independent release of annexin A1 by cochlear Hensen cells. Br J Pharmacol. 2009;158:1820–1834. doi: 10.1111/j.1476-5381.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou B, Kermany MH, Cai Q, Cai C, Zhou Y, Nair U, Liu W, Yoo TJ. Experimental autoimmune hearing loss is exacerbated in IL-10-deficient mice and reversed by IL-10 gene transfer. Gene Ther. 2012;19:228–235. doi: 10.1038/gt.2011.88. [DOI] [PubMed] [Google Scholar]
- 22.So H, Kim H, Kim Y, Kim E, Pae HO, Chung HT, Kim HJ, Kwon KB, Lee KM, Lee HY, Moon SK, Park R. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yian C, Moon SK, Jin S, Webster P, Rhim JS, Andalibi A, Lim DJ. Characterization of rat spiral ligament cell line immortalized by adenovirus 12-simian virus 40 hybrid virus. Ann Otol Rhinol Laryngol. 2006;115:930–938. doi: 10.1177/000348940611501213. [DOI] [PubMed] [Google Scholar]
- 24.Barenkamp SJ, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon SK, Lee HY, Pan H, Takeshita T, Park R, Cha K, Andalibi A, Lim DJ. Synergistic effect of interleukin 1 alpha on nontypeable Haemophilus influenzae-induced up-regulation of human beta-defensin 2 in middle ear epithelial cells. BMC Infect Dis. 2006;6:12. doi: 10.1186/1471-2334-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 28.Moon SK, Lee HY, Li JD, Nagura M, Kang SH, Chun YM, Linthicum FH, Ganz T, Andalibi A, Lim DJ. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochim Biophys Acta. 2002;1590:41–51. doi: 10.1016/s0167-4889(02)00196-9. [DOI] [PubMed] [Google Scholar]
- 29.Bluestone CD. Pediatric otolaryngology: past, present, and future. Arch Otolaryngol Head Neck Surg. 1995;121:505–508. doi: 10.1001/archotol.1995.01890050005001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388–10393. doi: 10.1073/pnas.1205210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86:1758–1767. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- 32.Sato E, Shick HE, Ransohoff RM, Hirose K. Repopulation of cochlear macrophages in murine hematopoietic progenitor cell chimeras: the role of CX3CR1. The Journal of comparative neurology. 2008;506:930–942. doi: 10.1002/cne.21583. [DOI] [PubMed] [Google Scholar]
- 33.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–6411. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 35.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 36.So HS, Kim HJ, Lee JH, Lee JH, Park SY, Park C, Kim YH, Kim JK, Lee KM, Kim KS, Chung SY, Jang WC, Moon SK, Chung HT, Park RK. Flunarizine induces Nrf2-mediated transcriptional activation of heme oxygenase-1 in protection of auditory cells from cisplatin. Cell Death Differ. 2006;13:1763–1775. doi: 10.1038/sj.cdd.4401863. [DOI] [PubMed] [Google Scholar]
- 37.Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 38.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 40.Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol. 2010;11:223–234. doi: 10.1007/s10162-009-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, Yamamoto M, Yagita H, Takeda K. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci U S A. 2012;109:5010–5015. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem. 1997;45:773–778. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- 43.Qu C, Liang F, Hu W, Shen Z, Spicer SS, Schulte BA. Expression of CLC-K chloride channels in the rat cochlea. Hear Res. 2006;213:79–87. doi: 10.1016/j.heares.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Adams JC, Seed B, Lu N, Landry A, Xavier RJ. Selective activation of nuclear factor kappa B in the cochlea by sensory and inflammatory stress. Neuroscience. 2009;160:530–539. doi: 10.1016/j.neuroscience.2009.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 46.Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92–95. [PubMed] [Google Scholar]
- 47.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 49.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chmiel JF, Konstan MW, Knesebeck JE, Hilliard JB, Bonfield TL, Dawson DV, Berger M. IL-10 attenuates excessive inflammation in chronic Pseudomonas infection in mice. Am J Respir Crit Care Med. 1999;160:2040–2047. doi: 10.1164/ajrccm.160.6.9901043. [DOI] [PubMed] [Google Scholar]
- 51.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 52.Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M, Davis R, Flavell R, Kollias G. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–3770. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AM, Burow ME, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 54.Hoshino T, Tabuchi K, Nishimura B, Tanaka S, Nakayama M, Ishii T, Warabi E, Yanagawa T, Shimizu R, Yamamoto M, Hara A. Protective role of Nrf2 in age-related hearing loss and gentamicin ototoxicity. Biochem Biophys Res Commun. 2011;415:94–98. doi: 10.1016/j.bbrc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Friedrich M, Docke WD, Klein A, Philipp S, Volk HD, Sterry W, Asadullah K. Immunomodulation by interleukin-10 therapy decreases the incidence of relapse and prolongs the relapse-free interval in Psoriasis. J Invest Dermatol. 2002;118:672–677. doi: 10.1046/j.1523-1747.2002.01731.x. [DOI] [PubMed] [Google Scholar]
- 56.Herfarth H, Scholmerich J. IL-10 therapy in Crohn's disease: at the crossroads. Treatment of Crohn's disease with the anti-inflammatory cytokine interleukin 10. Gut. 2002;50:146–147. doi: 10.1136/gut.50.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 58.Lopez IA, Acuna D, Beltran-Parrazal L, Espinosa-Jeffrey A, Edmond J. Oxidative stress and the deleterious consequences to the rat cochlea after prenatal chronic mild exposure to carbon monoxide in air. Neuroscience. 2008;151:854–867. doi: 10.1016/j.neuroscience.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 59.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 60.Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909–918. doi: 10.1053/jhep.2003.50386. [DOI] [PubMed] [Google Scholar]
- 61.Motterlini R, Mann BE, Johnson TR, Clark JE, Foresti R, Green CJ. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr Pharm Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







