Abstract
Autonomic responses, including changes in heart rate and respiratory sinus arrhythmia (RSA) can provide indications of emotional reactivity to social stimuli in mammals. We have previously reported that male prairie voles (Microtus ochrogaster) spontaneously care for unfamiliar infants, showing a robust and sustained increase in heart rate in the presence of a pup, thus providing an opportunity to examine the physiology of care-giving in reproductively naïve animals. However, the purpose of such heart rate increases has not been explained by previous efforts. In the present study, we first compared male and female prairie vole cardiac responses in the presence of a pup and found no evidence of sex differences in heart rate or RSA. Using male prairie voles, we then examined the characteristics of pups that were capable of eliciting physiological responses, including age of the pup and pup odors. As prairie vole pups increased in age they vocalized less and there was an associated decline in alloparental cardioacceleration. Exposure to pup-related odors induced cardioacceleration in adult males and this effect also diminished with increasing pup age. Finally, we were able to block the cardioacceleratory effect when the testing environment was warmed to a temperature of 36° C [versus ambient room temperature (approximately 22° C)]. These findings suggest that pup-induced cardioacceleration is a robust phenomenon across alloparental prairie voles of both sexes, and depends on multi-modal processing of different stimuli from the pups. Young pups require care-giving behavior, which appears to drive cardioacceleration in the alloparent. This study also supports the usefulness of autonomic measures in the evaluation of social experiences.
Keywords: Prairie Vole, Alloparenting, Social behavior, Pup, Heart rate, Autonomic, Thermoregulation
1.0 Introduction
Caretaking behavior in response to infants shown by non-reproductive animals, also known as alloparenting, is a prominent characteristic of many socially monogamous species [1, 2]. Alloparenting plays an especially important role in successful reproduction in communal species [3], including humans [4]. The analysis of alloparental behavior has also provided a useful model for discovering the autonomic and neuroendocrine factors capable of influencing the physiology of social behavior, more broadly defined [5-7]. However, the features of an infant that can elicit social approach and allocare have not been well-identified. In previous research, behavioral and endocrine measures during interactions with young animals have been used to assess the features of infants that elicit alloparenting [6]; however, both have limitations. For example, behavioral measures can be difficult to interpret, since animals may show approach to an infant followed by an attack. In small mammals blood sampling used for most endocrine measures is invasive and often only a single sample is possible, restricting repeated sampling and temporal resolution. Measures of autonomic responses, such as heart rate, have the advantage of continuous, non-invasive measurement of physiological state during social engagement. Via radiotelemetry, autonomic measures can be studied in the context of specific behaviors and can be taken repeatedly with minimal disturbance to the subject. In addition, knowledge of the neural control of heart rate provides new insights into the neurobiology of sociality [8].
Prairie voles are a socially monogamous species which has proven a particularly useful model for the analysis of the neurobiology of alloparenting. As in humans, the autonomic regulation of heart rate in prairie voles involves both sympathetic and parasympathetic processes, and prairie voles (like humans) have high levels of vagal activity, indexed by respiratory sinus arrhythmia (RSA) [9]. RSA represents the contribution of the vagal parasympathetic tone in dampening heart rate, and is widely interpreted as an index of emotion, sociability and adaptability to stress [10, 11]. In the presence of a pup, alloparental male prairie voles show a rapid and robust cardioacceleration, but do not show parasympathetic withdrawal which is typically associated with increases in heart rate [5, 7]. Given the seemingly innocuous nature of a helpless pup and gregarious nature of the alloparent, such a response was largely unexpected. This cardioacceleratory response was also novel on account of the behavioral calmness and immobility that accompany alloparental care as well as the decrease observed in circulating glucocorticoid levels [6]. Typically, the activity of the hypothalamic-pituitary-adrenal axis, which releases glucocorticoids, and the activity of the sympathetic nervous system, which regulates heart rate, are positively correlated [12]. It remains to be investigated whether other rodent species show similar cardioacceleratory responses while providing care for pups.
In prairie voles, pup-induced cardioacceleration is not a result of increased locomotor activity [5]; in fact, alloparental prairie voles are relatively less mobile while a pup is present, showing instead a still, arched-back posture over the pups. What's more, the cardioacceleratory response did not habituate to prolonged nor repeated exposure to infants. Additional experiments revealed that pup-induced cardioacceleration was also observed in male vole fathers even after 10 days of paternal experience [7]. These experiments suggested that full expression of pup-induced cardioacceleration depends on physical proximity to the pup and heightened sympathetic drive to the heart [5].
Temperature regulation using the body heat of the offspring has been suggested as a regulatory factor for parenting behavior [13], and possibly could be relevant to the reaction of prairie voles to infants. Likewise, infants from altricial rodent species may require caregiver warmth for their own thermoregulation [14, 15]. However, studies of both prairie vole fathers as well as reproductively naïve prairie vole males, did not reveal a change in core body temperature in the caregiver during acute bouts of alloparental care [5, 7]. Alternatively, specific characteristics of the pup might induce increased vigilance and/or social engagement in the alloparent. Originally, the cardioacceleratory response was interpreted as supporting the notion that an optimal range of autonomic activation (in this case activation of the sympathetic nervous system) was critical for positively engaging with the pup. Since approximately 20% of male prairie voles are non-parental (some even attack the pup), we hypothesized that these responses might represent hypo- and hyper-arousal respectively.
The purpose of the present study, conducted in reproductively naïve adult prairie voles with indwelling transmitters, was to measure autonomic function in response to direct exposure to pups or stimuli from pups to explore the purpose of pup-induced cardioacceleration. In Experiment 1, pup-induced cardioacceleration was compared in females and males. Females and males did not differ; therefore, the remaining experiments were aimed at identifying pup-related stimuli capable of inducing cardioacceleration in males. In Experiments 2 and 3, the effects of the age of the pup on alloparents' behavior and cardioacceleration (Experiment 2) as well as pups' rates of isolation-induced ultrasonic vocalization (Experiment 3) were tested. In Experiment 4, the effect of odors obtained from pups at increasing ages were examined. Finally, we sought to re-evaluate the role of temperature and thermoregulation by examining cardioacceleration in an environment of increased ambient temperature (Experiment 5).
2.0 Methods
2.1 Subjects
Descendants of wild prairie voles (F4 generation) captured near Champaign, Illinois were used in these experiments. Subjects of 60-90 days of age were maintained on a 14/10 hour light/dark cycle on at 06:30 AM in a temperature- and humidity-controlled vivarium. Food (Purina rabbit chow) and water were available ad libitum. Prairie vole offspring remained in their natal group with their parents in large polycarbonate cages (24 × 46 × 15 cm) containing cotton nesting material. Offspring were weaned at 20 days of age, prior to the arrival of the next litter to prevent premature exposure to pups, and then were pair-housed with a same-sex sibling in smaller cages (17.5 × 28 × 12 cm) in a single-sex colony room until testing. Except in Experiment 1 (see below), all test subject cohorts were made up of sexually naïve males that had never been exposed to pups, other than their own littermates, at the beginning of testing. Stimulus pups were drawn from the colony and used for testing immediately following removal from their natal nest.
Experiments 1 and 2 were conducted at the University of Illinois at Chicago and replicated upon moving our laboratory to Northeastern University. Experiments 3, 4 and 5 were conducted at Northeastern University. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Illinois at Chicago and Northeastern University Institutional Animal Care and Use Committees. Experiments began during the lights-on period between 10:00 and 11:00 AM. Throughout all the experiments in which cardiovascular responses were recorded, it is likely that disturbing the home cage and removing the sibling contributed to tachycardia, however we have previously compared the magnitude of this response to that following the introduction of a novel pup [5], which is marked by its magnitude and persistence.
2.2 Experimental Design
Experiment 1
To test for sex differences in the cardiovascular response to pups, naive male and female prairie voles were implanted with radiotelemetry devices and allowed to recover with their siblings (see methods below). Beginning two weeks after surgery, male and female subjects (n=8 for each sex) were tested for behavioral and cardiovascular responses to a pup. As in our previous investigations [5, 7], baseline data were collected over the hour prior to testing, from which 5 min of data were collected while animals was stationary selected as temporally close to stimulus presentation as possible. Immediately prior to testing, the non-implanted sibling cage-mate was removed, and a 1-3 day old pup from an un-related breeder pair was introduced into the subjects' cage. Stimulus pups remained in the subject's cage for 20 min while behavioral and cardiovascular data were recorded.
Experiment 2
Since the age of the pup is likely to contribute to the alloparent's behavioral and cardiovascular responses, we next varied the stimulus pups' age, using male vole subjects. Beginning two after surgery, a separate cohort of male subjects (n=8) was tested for behavioral and cardiovascular responses to a pup of varying ages. Based on earlier studies stimulus pups were used on postnatal (PND) days 1-3, or 10 or 19 (1 day prior to weaning). [5, 7, 16-19]. Stimulus pups remained in the subject's cage for 20 min while behavioral and cardiovascular data were recorded. Stimulus pups were unrelated to the subjects. The order of presentation was randomized and tests were conducted 24-48 hrs apart.
Experiment 3
To examine how pup vocalizations vary with the pup's degree of helplessness, both acutely when a caregiver is either present or not and also developmentally over the course of maturation, we recorded the production of vocalizations [including ultrasonic vocalizations (USVs)] from pups in various conditions. To evaluate the possible effect of pup age on the production of USVs, isolated pups of four different ages were used: PND-1, PND-4, PND-10 and PND-19, (n = 8-14 for each age). To evaluate the role of the alloparent, we also recorded 1-3 day old pups (n = 6) while under the care of an adult male alloparent for min 1-5 and 15-20 of an alloparental test to be compared to isolated 1-3 day old pups (n = 9). Thirdly, upon observing that PND-4 pups produced the most vocalizations, and wishing to test the effect of temperature, we measured USVs in isolated PND-4 pups (n = 8-12 per condition) under conditions of either an ambient 36°C or 22°C. All recordings lasted 5 min. For each recording, a single pup was removed from its natal nest and placed in a 10cm cylindrical plastic container and placed on a cotton pad, with the exception of pups tested in the alloparental condition; in this case a standard mouse cage was used to accommodate the alloparent. No more than 2 pups were used from a given litter.
Experiment 4
Pup exposure is a multisensory experience, and previous experiments suggested the hypothesis that the perception of pup-related auditory and olfactory stimuli could induce cardioacceleratory effects [5]. In this experiment we tested the effect of pup odor on adult males' cardiovascular responses. In a separate cohort of radiotelemetry implanted animals, we paralleled the designs of Experiments 2 and 3 by exposing adult males to air blown through a sealed chamber containing live pups unrelated to the subjects, which permitted the passage of olfactory but neither visual nor auditory stimuli. Following 5 min of stationary baseline cardiovascular recording, a polyethylene tube was introduced into the home cage blowing room air. The tube remained in the home cage for thirty min to allow for habituation to this novel stimulus. After this time, a sealed container with live pups was inserted between the air source and the tube proximate to the subjects' cage, so that odor could be introduced without disturbing the subjects. Pup odors were presented for 5 min, during which time radiotelemetric and cardiovascular parameters were recorded. Corresponding to Experiment 2, three age groups of pups were presented (in randomized, counter-balanced order): PND 3, PND-10 and PND-19. In the PND-3 condition, odors were derived from air passing over 4 pups from the same litter (approximately 12 g total), in the PND-10 condition, odors were derived from 2 pups from the same litter (approximately 18 g total), and in the PND-19 condition, odors were derived from a single pup (approximately 20 g total). Cardiovascular responses to 5 minutes of pup odor presentation was compared to the 5 minutes immediately previous to the onset of pup odors.
Experiment 5
We did not detect an effect of pup exposure on an alloparent's core body temperature in Experiment 3 or even during prolonged bouts of alloparental care [5]. However, thermoregulation in the pup or alloparent could not be ruled out as a potential contributor to the alloparents' cardioacceleratory response. Hence, we tested a separate cohort of radiotelemetry implanted adult males (n=7) for their cardiovascular responses to a 1-3 day old pup under both normal conditions (approximately 22°C, Room Temperature) as well as a warmed condition where ambient temperature was 36°C (Warmed). Based on work in mice [20], these temperatures lie below and above the thermoneutral zone, respectively. For the Warmed condition, a surgical warming pad was placed beneath the testing cage and a small space heater maintained ambient temperature in an enclosed space around the testing cage. Testing was conducted similarly to Experiments 1 and 2. Each session was 20 min long and sessions occurred in a randomized, counter-balanced order. Due to the insulating baffles needed to maintain the Warmed condition, and the physical constraints of recording cardiovascular data within range of the telemetry equipment, we were unable to simultaneously observe behavior in this condition.
2.3 Ultrasonic Vocalization Detection
Vocalizations were captured using an Avisoft UltraSoundGate 116Hme microphone, sampling at 96 KHz. Vocalizations were detected using Avisoft-SASlab Pro version 5.2.07. For each recording, a spectrogram was generated and automatic parameter measurements were used to identify and measure vocalization count and duration. Following automated detection, an observer blind to experimental condition visually inspected the spectrogram and removed any spuriously captured sounds (e.g. claws scraping on plastic or other animal movement artifacts). Prairie vole pups emit calls in a trains of short chirps or mono-syllabic whistles, the complexity of which increases with age. The typical duration of a single call in this study was between 0.02 and 0.05 seconds.
2.4 Radiotelemetry Implantation
Surgical implantation of radiotelemetric devices was conducted according to previously published methods [7]. Subjects were implanted with wireless radiotelemetry transmitters [Model ETA F-10, Data Sciences International (DSI), St. Paul, MN]. These transmitters weigh 1.6 g, with a volume of 1.1 cc. Briefly, telemetric transmitters were implanted subcutaneously under aseptic conditions, following anesthesia with isoflurane. Animals were kept under a warming lamp, and the surgical area was shaved and cleaned before any incisions were made. A subcutaneous pocket was made under the dorsal surface, into which the transmitter was implanted. The leads from the transmitter were pulled rostrally using a trochar and sleeve under the skin, and anchored in place with permanent sutures (DII placement). Skin incisions were sutured closed. Subcutaneous fluids and analgesia (Carprofen) were administered as necessary. All animals then were housed for 7 days in custom-designed cages (24 × 46 × 15 cm) after surgery [9]. These cages included a divider to permit adequate healing of suture wounds in the instrumented animal without socially isolating subjects from their siblings during recovery. Animals were then returned to the standard home cages (with their sibling) for an additional 5–7 days before the onset of experiments.
2.5 Radiotelemetric Recordings
Electrocardiogram (ECG), temperature and activity signals were recorded with a radiotelemetry receiver (DSI; sampling rate 5 kHz for ECG and 256 Hz for activity, 12-bit precision digitizing). This system allows for the recording of heart rate and derived measures of heart rate variability (RSA; respiratory sinus arrhythmia) along with temperature and locomotor activity. Locomotor activity is measured in arbitrary units that report gross locomotor activity. Radiotelemetric data were quantified according to procedures previously described [9].
Heart rate was evaluated using vendor software (Data Sciences International, St. Paul, MN), and R-wave detections were verified with a custom-designed software package (CardioEdit 1.5, Brain Body Center, University of Illinois at Chicago). The R-R intervals were analyzed for variations (heart rate variability) using a custom-designed software package to determine amplitude of RSA [21].
RSA was assessed using time-frequency procedures [22, 23] described in detail elsewhere [9, 24, 25]. RSA was operationally defined as the natural log of the sum of the power within the respiratory bandwidth of 1.0–4.0 Hz. This procedure has been validated in prairie voles [9] and provides the greatest sensitivity to changes in vagal activity [26]. The amplitude of RSA represents the functional vagal impact on the sino-atrial node of myelinated vagal efferent pathways originating in the brainstem. The ECG signal was exported into a data file and examined using CardioEdit, to ensure that all R waves were properly detected. The following procedures were implemented to minimize the possibility of violating the assumption of stationarity which can distort time series analyses of RSA: 1) the R–R intervals (heart period) were time-sampled into equal time intervals with a sampling rate of 20 Hz; 2) the time series were detrended with a moving polynomial filter that removed variance in the series below 1 Hz for RSA (i.e., 21-point cubic polynomial), 3) the spectral analysis identified the peak amplitude of RSA from the de-trended data.
2.6 Behavioral analysis
Animals were considered alloparental if they crouched over the stimulus pup [6, 16, 27]. Pups were returned to their parents after testing. In the rare case of an attack, injured pups were immediately euthanized. The behavior of the adult subjects was videotaped and scored by two trained, experimentally blind observers (inter-rater reliability > 95%, Noldus Observer, Noldus Inc.). Behavioral categories quantified included: first approach toward the stimulus (latency), licking and/or grooming (duration), auto-grooming (duration), crouching over the pup (duration), and contact with the pup (duration) defined as the subject having contact with the pup but not crouching over it nor licking and/or grooming it (duration). In all behavioral experiments, a single pup was used as a stimulus.
2.7 Statistical analysis
The DSI software package records ECG, temperature and gross locomotor activity. Cardiovascular parameters, including R-wave detection and respiratory sinus arrhythmia (RSA) were calculated using in-house software. Temperature, activity and cardiovascular parameters were each averaged across 5 min bins to be compared via repeated measures ANOVA. All statistical comparisons were done using SPSS v19. For pup vocalization measures, litter effects were investigated using repeated measures ANOVA. Behavior was analyzed using Noldus Observer and compared via ANOVA. The post-hoc comparisons for all ANOVA were pair-wise Bonferroni tests. When data were found to be non-normal or heteroschedastic, they were transformed; when data remained non-normal after transformation by either square-root or log transformations (Experiment 3), ANOVA were still conducted on the basis that the ANOVA test is resilient to non-normalcy [28].
In Experiment 1, behavior and cardiovascular/radiotelemetric parameters (including temperature and activity) were compared in males and females. In Experiment 2, a within subjects design was used to compare male alloparents' response to pups of varying ages. In Experiment 3, isolated pups of varying ages were compared in terms of vocalization production relative to PND-1 (ANOVA); pups in the alloparental condition were compared relative to the isolated PND-1 (student's t-test) and also relative to the first 5 min vs. last 5 min of each recording session (paired samples student's t-test); PND-4 pups in the warmed condition were compared to PND-4 pups in the room temperature condition (student's t-test). In Experiment 4, adult males' responses to pup odor during the first 5 min were compared with those of the room air condition in terms of activity, heart rate and temperature. In Experiment 5, adult males' responses were compared between the Room Temperature and Warmed conditions for an entire 20 min alloparental test in terms of activity, heart rate and temperature.
3.0 Results
3.1 Sex differences were not detected
(Experiment 1, Figure 1) Eight out of 8 males (100%) and 6 out of 8 females (75%) responded alloparentally when presented with an unfamiliar 1-3 day old pup. In terms of behavior, cardiovascular parameters, temperature and locomotor activity, there were no differences between males and females, both at rest and while providing alloparental care. Similar to male alloparents, females demonstrated a robust increase in heart rate upon presentation of an unrelated pup. During pup exposure, heart rate in female alloparents averaged 542 ± 22 bpm, as compared to 390 ± 20 bpm.
Figure 1.
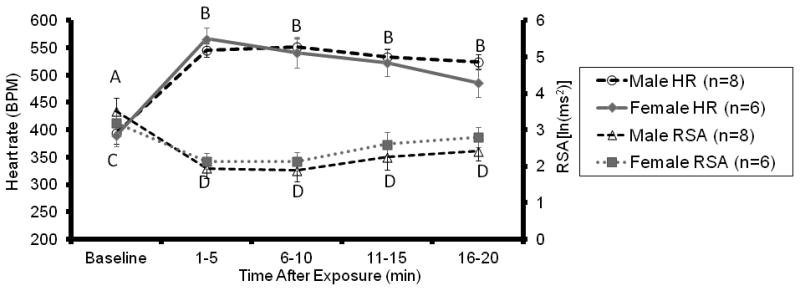
Cardiovascular responses in adult male and female alloparents during exposure to a novel, unrelated pup, 1-3 days old. Similar to adult males, females showed a sustained increase in heart rate while caring for a pup, which was accompanied by a decrease in RSA. There were no sex differences in terms of either heart rate or RSA while caring for a pup. Different letters denote significant differences from baseline (p<0.05), such that heart rate increased (A,B) and RSA decreased (C,D) following presentation of a pup in both sexes.
3.2 Pup age strongly affected heart rate
(Experiment 2, Figure 2A) Heart rate was lower when males were caring for older pups. Eight out of eight males (100%) responded alloparentally when presented with pups at all three ages. There was a main effect of pup age on heart rate [F(2,6) = 83.041, p < 0.001], with both the PND-10 Pup (495 ± 22 bpm) and PND-19 Pup (445 ± 15 bpm) conditions having lower heart rates than the PND-3 Pup condition (538 ± 14 bpm) (p < 0.013 for both comparisons). Heart rate in the PND-19 Pup condition was also significantly lower than in the PND-10 Pup condition (p < 0.001). Analysis of RSA yielded a main effect of the pup age [F(2,6) = 5.206, p = 0.049], with both the PND-10 Pup (2.841 ± 0.315 ln(msec2)) and the PND-19 Pup (3.020 ± 0.288 ln(msec2)) conditions having higher RSA than the PND-3 Pup condition (2.402 ± 0.259 ln(msec2)), (p < 0.019 for both comparisons). RSA in the PND-19 Pup condition was also significantly higher than in the PND-10 Pup condition (p = 0.044). Over the course of the pup exposure, there was a significant time by pup age interaction effect on activity [F(8,56) = 2.576, p = 0.018], with the PND-3 Pup condition producing significantly lower activity during the first 5 minutes of testing (p < 0.005 for both comparisons). No differences were observed as a function of pup age for body temperature (p > 0.05).
Figure 2.
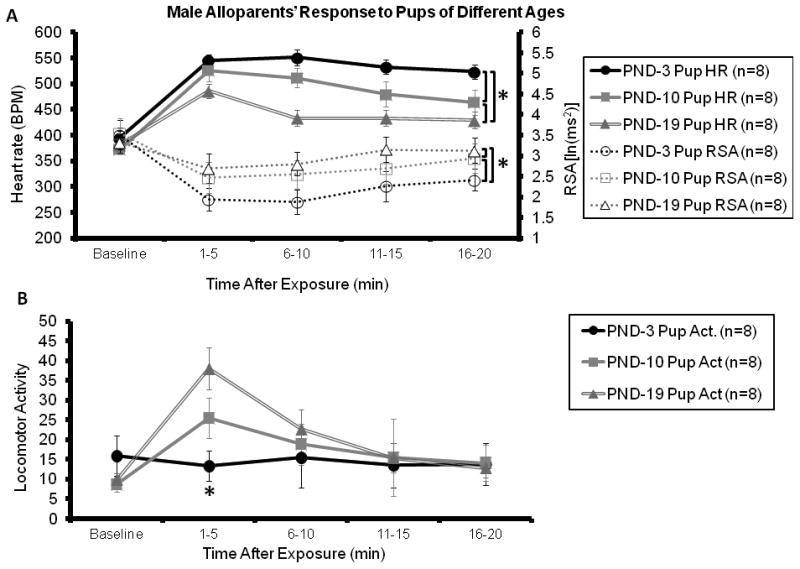
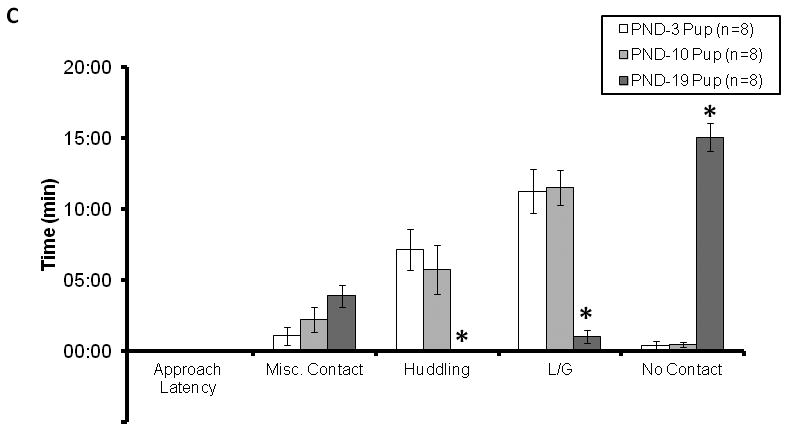
Telemetric/cardiovascular parameters (2A-B) and behavioral responses (2C) in male alloparents as a function of pup age. (2A) With increasing pup age, the alloparents' cardioacceleratory response diminished, resulting in lower heart rate and increased RSA throughout the test. (2B) At the same time, locomotor activity increased along with increasing pup age. (2C) There were no significant differences between PND-3 and PND-10 in terms of any behavior measured, however, PND-19 produced significantly less huddling, less licking/grooming and more time spent not in contact relative to both other conditions (* denotes p < 0.05 post-hoc comparisons).
3.3 Pup age also influenced alloparental behaviors (Experiment 2, Figure 2C)
Alloparents spent less time licking/grooming and huddling the oldest pups. The increasing age of the pup was not associated with changes in latency to approach the pup or non-alloparental social contact (e.g. sniffing, sitting side by side, p > 0.05 for each comparison). However, there was a main effect of pup age on duration of time spent huddling [F(2,6) = 10.512, p = 0.011], since alloparents tested with PND-19 pups did not huddle over the pup (0.00 ± 0.0 sec). In contrast, time spent huddling over the pup was high in both the PND-3 Pup condition (429.0 ± 86.6 sec, p = 0.002) and PND-10 Pup condition (344.0 ± 101.9 sec, p = 0.012). Additionally, there was a main effect of the age of the pup on time spent licking/grooming [F(2,6) = 38.905, p < 0.001], with alloparents in the PND-19 Pup condition spending less time licking/grooming (59.5 ± 27.2 sec) than either the PND-10 Pup (691.0 ± 74.0 sec, p < 0.001) or the PND-3 Pup condition (675.2 ± 94.8 sec, p < 0.001). Finally, there also was an effect of pup age on time spent not in contact with the pup [F(2,6) = 9.475, p = 0.014], with the PND-19 condition having spent significantly more time not in contact with the pup (903.4 ± 60.3 sec) than either the PND-10 Pup (27.1 ± 10.5 sec, p = 0.004) or the PND-3 Pup condition (24.3 ± 17.9 sec, p = 0.006).
3.4 Pup vocalizations varied with pup age (Experiment 3, Figure 3A)
Figure 3.
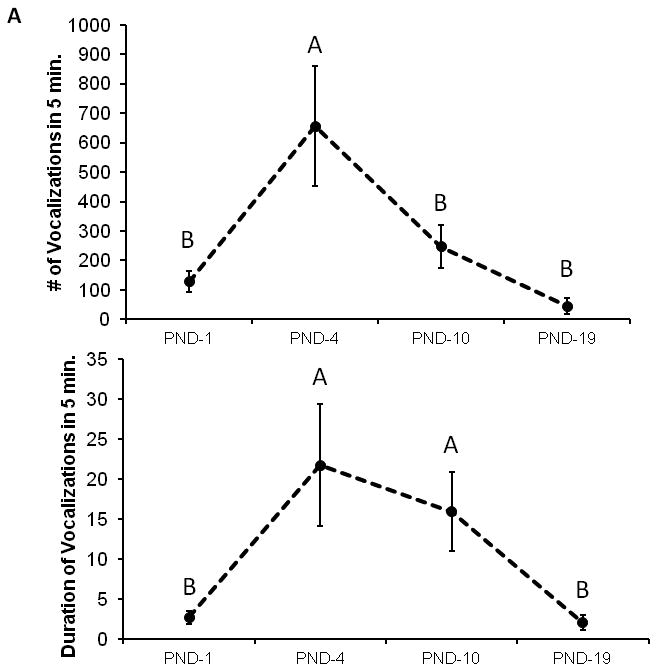
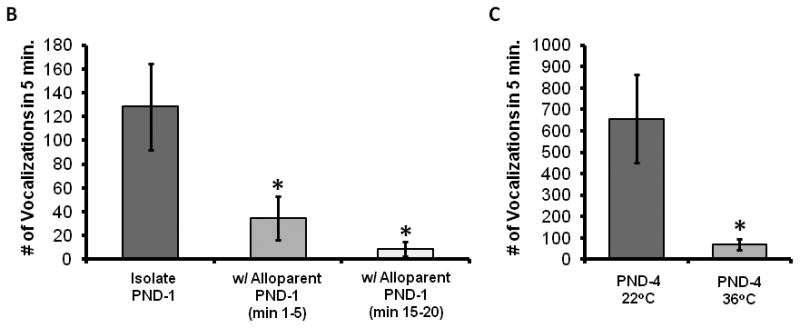
Pup vocalization count and duration varies with advancing pup age (3A). PND-4 pups produced the most vocalizations. Different letters denote significantly different groups. In PND-1 pups, the presence of an alloparent providing care was associated with significantly fewer vocalizations both during the first and last 5 min (3B). In PND-4 pups warming the ambient temperature to 36°C also significantly decreased the number of vocalizations (3C) * denotes p < 0.05 post-hoc comparisons.
Isolated vole pups reliably produced vocalizations, the number and duration of which varied with pup age. Vocalization production was highest at PND-4 and then gradually declined with age. Over the 5 min of recorded time, PND-4 pups produced the greatest number and longest durations of USVs. The number of calls at PND-4 was 656.6 ± 204.1 for a total duration of 21.7 ± 7.6 seconds (significant effect of age on the number of USVs [F(3,48) = 8.307, p < 0.001] and total duration [F(3,48) = 5.350, p = 0.003]). Post-hoc tests revealed a higher number of calls on PND-4 compared to all other ages (p ≤ 0.002 for all comparisons) and a greater total duration of calls compared to PND-1 and PND-19 (p ≤ 0.004 compared to PND-1 and PND-19, p < 0.1 for PND-10).
3.5 Isolated pups vocalized more than those with an alloparent (Experiment 3, Figure 3B)
The presence of an alloparental adult male reduced the number of pup vocalizationsduring both the first and last 5 min of the 20 min testing period (p < 0.05 for both comparisons) and reduced the duration of calls during the last 5 min of testing (p < 0.05); there was also a trend towards a reduction in total duration during the first 5 min (p = 0.08). In the first 5 min pups in the alloparental condition emitted 34.8 ± 18.1 calls, and 8.7 ± 5.9 calls in the last 5 min (p > 0.05). Similarly, total duration of calling declined from 0.7 ± 0.4 sec in the first 5 min of testing to 0.3 ± 0.2 in the last 5 min (p > 0.1).
3.6 Warmed pups vocalized less than those that were isolated (Experiment 3, Figure 3C)
Increasing ambient temperature to 36oC reduced call number and total duration of calling. PND-4 pup vocalizations fell from 656.6 ± 204.1 calls and a total duration of 21.7 ± 7.6 seconds in the room temperature condition, to 68.2 ± 25.9 calls and a total duration of 2.0 ± 0.8 sec in the Warmed condition (p < 0.01 for both comparisons). Throughout Experiment 3, pups from the same litter tended to vocalize to a similar degree, and thus a litter effect is suggested; however, all effects of age and temperature remained significant when data were averaged within the two pups of each litter (p < 0.03 for all comparisons).
3.7 Pup odors elicit heart rate increases in adult alloparents (Experiment 4; Figure 4)
Figure 4.
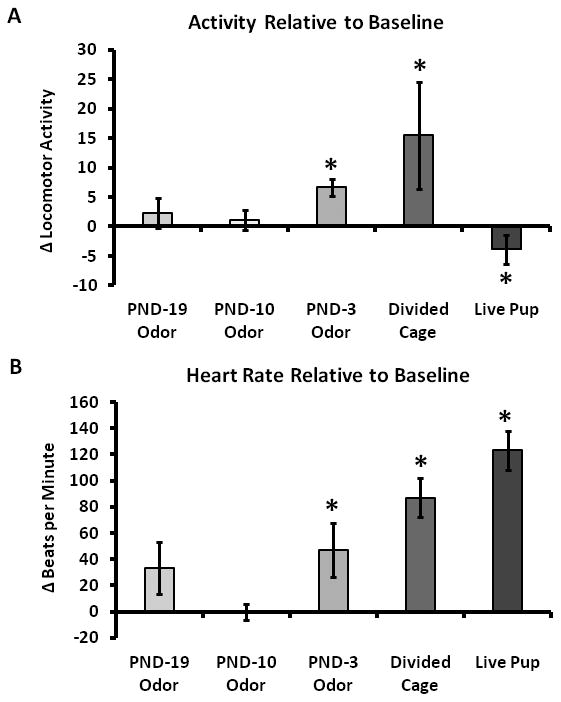
The effect of pup odors on changes in alloparents' activity (4A) and heart rate (4B) relative to baseline prior to pup exposure (* denotes significant difference from baseline, p < 0.05). Odors from pups ≥ PND-10 produced no significant effect on either activity of heart rate, whereas odors from PND-3 pups produced significant increases in both activity and heart rate. For the sake of comparison, the effects of a pup across a barrier in a Divided Cage are reproduced from [5] and the Live Pup data is reproduced from Experiment 1. Note that free access to a Live Pup produced a significant decrease in activity.
The introduction of PND-3 pup odors produced a modest cardioacceleration in adult males. All subjects responded to the introduction of a tube blowing clean room air with an increase in heart rate and locomotor activity, which subsided within 30 min in all cases (data not shown). During the first 5 min of PND-3 pup odor exposure, heart rate increased from 359 ± 23 bpm to 407 ± 21 bpm and locomotor activity increased from 1.8 ± 0.7 to 8.4 ± 1.4 (p < 0.05 for both comparisons). There were no differences in terms of temperature or RSA in response to PND-3 pup odor. We found no effects of PND-10 pup odor presentation on any parameter, but interestingly, there was a trend towards an increase in heart rate in the PND-19 condition (p = 0.08). Differences were no longer present after 5 min for any condition (data not shown).
3.8 Warming the test area reduced cardioacceleration in response to a pup (Experiment 5, Figure 5)
Figure 5.
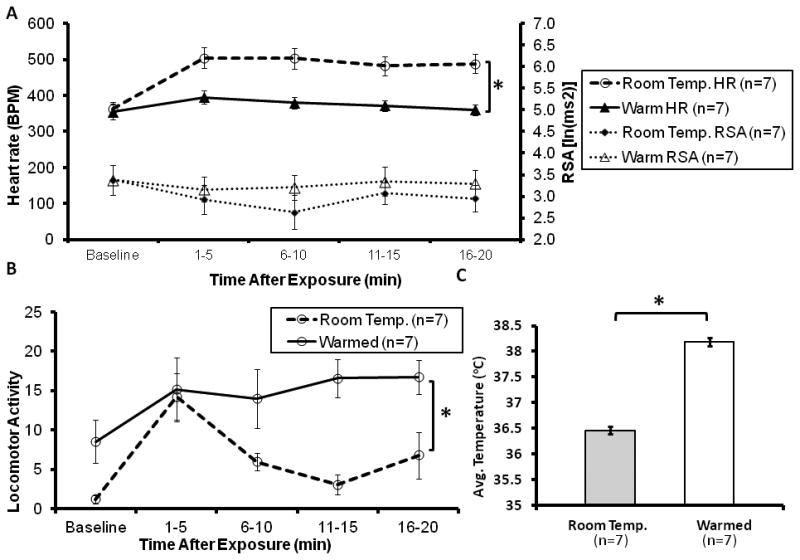
(5A) An increase in ambient temperature(from 22° Room Temperature, to 36°C -Warm) was associated with a reduction in pup-induced cardioacceleration in the alloparent, but no change in RSA. (5B) In the Warm condition, locomotor activity tended to be higher (p = 0.09) and core body temperature of the alloparent was significantly increased (* p < 0.05 post-hoc comparisons).
Testing in a warmed environment greatly reduced the cardioacceleratory response to a pup. When presented with an unfamiliar 1-3 day old pup, 7 out of 8 males (87.5%) responded alloparentally. The single pup attacker occurred at 22°C. (This animal was not tested further and the pup was euthanized). At rest in the home cage, increasing the ambient temperature to 36°C did not affect heart rate (p > 0.1), though it did lead to an increase in activity (1.2 ± 0.5 to 8.5 ± 2.7, p < 0.001) and an increase in core body temperature (36.6 ± 0.4°C to 37.7 ± 0.2 °C, p < 0.001). Ambient temperature significantly contributed to adult alloparents' responses to a pup. In the warmed condition, there was a significant increase in locomotor activity (presumably indicative of less time spent huddling over the pup) from 7.5 ± 1.8 to 15.6 ± 0.5 [F(1,12) = 10.73, p = 0.007]. The warmed condition also reduced heart rate in response to the pup from 494 ± 5 bpm to 376 ± 7 bpm [F(1,12) = 13.245, p = 0.003] and increased temperature from 36.5 ± 0.07 °C to 38.2 ± 0.08 °C [F(1,12) = 32.325, p < 0.001]. There was no effect of warming on RSA.
4.0 Discussion
The present experiments support the notion that pup-induced cardioacceleration is a important aspect of alloparental care in the prairie vole, and that radiotelemetry is a useful approach for studying such behavior. Here, we were able to show that this response is also seen in alloparental virgin females, which extends previous work in virgin males [5] and fathers [7]. Our previous studies have shown that the pattern of sustained cardioacceleration is specific to interacting with a pup [5, 7]. In this work, we observed no differences between male and female alloparental responses, which parallels the similarities among prairie voles with differing reproductive experience [7, 29, 30]. Such consistency between various types of alloparenting points to specificity in the purpose(s) of the behavioral and physiological responses and suggests the cardioacceleratory response serves a purpose, which we now propose to be thermoregulatory in nature.
The cardioacceleratory properties of alloparental care depend on the age of the pup, as increasing pup age produced diminishing cardioacceleration and complementary changes in RSA. Correspondingly, the alloparent's behavior towards the pup also changes along with increasing pup age. Alloparents responded to a PND-19 pup (1 day away from weaning) in behavioral and cardiovascular parameters that were roughly equivalent to the response to a novel adult [5]. Interestingly, while there was no difference in terms of behavior between PND-3 and PND-10, there were cardiovascular differences, which suggests that specific behaviors are not responsible for driving the pup-induced heart rate increase. Taken together, these findings suggest that the altricial nature of prairie vole pups contributes to the alloparent's cardiovascular response, which gradually fades in intensity as the pup becomes more self sufficient.
Pup-induced cardioacceleration is under multi-modal sensory regulation. Pup vocalizations also diminished with increasing pup age, which supports the idea that vocalizations function as part of a multisensory experience to elicit care when the pup is young and especially vulnerable [31]. When the pup is actively receiving care from an alloparent, vocalizations are dramatically reduced. The presentation of pup odors in the absence of contact with the pup, on the other hand, did elicit a modest increase in heart rate. We have previously observed a substantially greater heart rate increase when male alloparents could see, smell and hear a live pup across a barrier [5]. Together, these results speak to pup-induced cardioacceleration as a response to multi-modal sensory inputs. In a similar manner, maternal behavior in the rat relies on multisensory processing [31-33].
Direct physical contact would be a necessary condition for the complete cardioacceleratory response (see Fig. 4B and [5]) if the purpose of this response is to generate body heat to warm the pup. Previous work examining several vole species has highlighted the importance of thermoregulation in the production of vocalizations and identified the second week of postnatal life as the peak of vocalization production [34]. Similar results have been found in rats [14, 35] and mice [36], in which the relationship between ambient temperature and pup vocalization has been studied in detail. The results of the present study support this earlier work by showing that prairie vole pup vocalizations are diminished when ambient temperature is raised to 36°C. However, the warmed environment did not completely abolish vocalization in isolated prairie vole pups, a result similar to that reported in rats [14, 35].
To the best of our knowledge, pup-induced cardioacceleration has thus far only been observed in prairie voles [5, 7]. We have put forth several hypotheses about the purpose of this response based on the circumstances under which we were able to observe it. Originally, this response was thought to perhaps signify heightened vigilance in the alloparent and/or an aroused state of social engagement. While both of these interpretations could still prove to be valid, the most parsimonious explanation of the cardioacceleratory effect of the pup may be found in temperature regulation. There remains work to be done on the affective state of the alloparent during care-giving.
Rodent pups may vocalize to elicit caregiving behavior, possibly to help them regulate their temperature. In the absence of a need for warming (i.e. at 36°C), pups vocalize substantially less. When tested for alloparental care in a warmed environment, the alloparents' heart rate response also was substantially diminished and locomotor activity increased, indicating the adult spent less time huddled over the pup. We have previously observed that vagal tone is preserved during alloparental care, and that blocking sympathetic adrenergic tone abolishes pup-induced cardioacceleration [5]. The observation here that RSA was not affected by temperature, while heart rate was, supports these earlier findings.
Based on the results of these experiments, we conclude that pup-induced cardioacceleration may serve to help provide the pup with warmth. The results of this work support earlier investigations in rats, which described the importance of temperature in regulating maternal behavior [37, 38]. The radiotelemetric methods used here allow for non-invasive temperature measurement and suggest that cardioacceleration may play a role during care-giving in mothers, paralleling what we have observed here in alloparents and previously in fathers [7].
It is worth pointing out that previous studies did not find any change in core body temperature in prairie vole alloparents upon presentation with a pup, even for longer exposures (i.e. 60 minutes) [5, 7]. During the course of these studies, we have used two different transmitter placement protocols: originally, transmitters were implanted within the peritoneal cavity, but relatively close to the pup when the alloparent huddled over it; we later switched to subcutaneous implantation when smaller transmitters became available, however this method necessitated implantation on the dorsal surface. Future efforts are needed to explore whether ventral skin surface temperature corresponds to pup-induced cardioacceleration.
The experiments described here relied on warming the testing environment, which warmed both pup and alloparent. It is experimentally difficult to keep pups warm without also introducing warmth as a confound with regards to the alloparent. Therefore, it is possible that the warmed condition reduced pup-induced cardioacceleration by acting on the alloparent directly. The warmed condition did represent a hyperthermic state for the alloparent, which could have influenced the behavior of the alloparent, as engaging in pup-directed care giving could have impaired efforts at heat dissipation. While this may be true, it appears the cardioacceleratory aspect of alloparental care was indeed due to the pups' thermoregulatory needs. Additionally, as evidenced in Experiment 3, pups in the warmed condition emitted fewer vocalizations -indicative of less thermoregulatory distress.
Both oxytocin and vasopressin have been implicated in male parental responses and regulation of the autonomic nervous system in prairie voles [6, 16]. However, the effects of these peptides on cardioacceleratory and thermoregulatory aspects of prairie vole alloparental care are not well understood. Peripheral oxytocin administration leads to a decrease in heart rate and core body temperature in rats [39] -results which we have confirmed in the prairie vole (unpublished observations). In our earlier studies of prairie vole alloparenting, pup exposure was associated with increased activity in oxytocin neurons and a transient increase in peripheral oxytocin levels [6]; however, heart rate also increases [5]. It is important to keep in mind, however, that oxytocin is a vasodilator [40, 41] and as such also might elicit a compensatory increase in heart rate. If the release of oxytocin produces sufficient vasodilation, then a compensatory increase in heart rate might occur. Concurrently, peripheral temperature might rise as more blood becomes more available in superficial capillaries, as the organism sheds heat to the environment and/or a nearby pup. Testing this hypothesis would require simultaneous measurement of behavior, peripheral oxytocin, heart rate, blood pressure and peripheral temperature.
The quality of caregiving experienced by a developing mammal influences a wide range of physical and mental health outcomes. Exposure to adverse childhood experiences predisposes children to a lifetime of negative consequences [42, 43]. Across multiple species, early-life adversity has been found to lead to atypical development of hypothalamic-pituitary-adrenal axis functioning, which is believed to contribute to psychiatric vulnerability in adulthood [44, 45]. Lastly, the quality and style of received parental care shapes the offspring's own expression of care-giving in adulthood [46], thereby extending the phenotype to subsequent generations.
Although the consequences of care-giving (or the lack thereof) have been extensively studied, less is known regarding the neurobiological factors which contribute to the production of care-giving behavior in parents or alloparents. Alloparenting is an important element of both prairie vole as well as human social behavior. Presentation with a young, un-related pup reliably elicits caregiving behavior as well as cardioacceleration from adult prairie voles. In so doing, alloparents provide the vulnerable pup with warmth and safety. Maintaining this state of autonomic arousal and high sympathetic tone, while at the same time engaging in prosocial behavior, suggests well-regulated systems. In turn, the dysregulation of these systems and associated hyper-arousal may be responsible for a neglectful or attack response towards a pup seen in a minority of cases in prairie voles. A better understanding of the behavioral neurobiology of pup attackers might inform the human condition and contribute to the prevention of child neglect and abuse, thereby averting the deleterious consequences resultant from early-life adversity.
Both male and female prairie voles show cardioacceleration while caring for pups
Pup-induced cardioacceleration depended on the pup's age
Pup vocalizations decreased with pup age and temperature
Pup-induced cardioacceleration is blocked by increasing temperature
Acknowledgments
The authors would like to thank Naomi Zingman-Daniels, Ami Kristl, Jessica Amacker, Ian Moore and Emily Grimsley for their assistance, Drs. Maria Davila and Gregory Lewis for developing heart-rate variability analysis software and Sean Sullivan for his support in maintaining our animal colony.
Support: This research was supported by the National Institutes of Mental Health grant MH72935 (CSC), the National Institute of Child Health and Human Development grant HD38490 (CSC) and the National Institute of Child Health and Human Development grant HD075750 (CSC).
Footnotes
Conflict of Interest: The authors wish to report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RL, Carter CS. Intraspecific variation and the presence of a father can influence the expression of monogamous and communal traits in prairie voles. Ann N Y Acad Sci. 1997;807:559–62. doi: 10.1111/j.1749-6632.1997.tb51968.x. [DOI] [PubMed] [Google Scholar]
- 3.Solomon NG, French JA. Cooperative Breeding in Mammals. Cambridge, MA: Cambridge University Press; 1997. [Google Scholar]
- 4.Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press of Harvard University Press; 2009. [Google Scholar]
- 5.Kenkel WM, Paredes J, Lewis GF, Yee JR, Pournajafi-Nazarloo H, Grippo AJ, Porges SW, Carter CS. Autonomic substrates of the response to pups in male prairie voles. PLoS One. 2013;8(8):e69965. doi: 10.1371/journal.pone.0069965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24(6):874–86. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenkel WM, Suboc G, Sue Carter C. Autonomic, Behavioral and Neuroendocrine Correlates of Paternal Behavior in Male Prairie Voles. Physiol Behav. 2014 doi: 10.1016/j.physbeh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porges SW, Furman SA. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90(2-3):386–93. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisler FC, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biol Psychol. 2013;93(2):279–86. doi: 10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(2):S86–90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42(4):111–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Jans JE, Leon M. Determinants of mother-young contact in Norway rats. Physiol Behav. 1983;30(6):919–35. doi: 10.1016/0031-9384(83)90258-5. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: the primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol. 1992;25(4):229–50. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Alberts JR. Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev Psychobiol. 2011;53(8):813–27. doi: 10.1002/dev.20565. [DOI] [PubMed] [Google Scholar]
- 16.Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45(5):354–61. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiol Behav. 2006;87(2):424–9. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141(2):559–68. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49(5):681–7. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology. 2012;37(8):654–685. [Google Scholar]
- 21.Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent? Biol Psychol. 2012;89(2):349–64. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porges SW. US Patent Office, Patent # 4,510,944. United States: 1985. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. [Google Scholar]
- 23.Porges SW, Bohrer RE. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Pres; New York: 1990. pp. 708–753. [Google Scholar]
- 24.Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–53. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson JB, Lewis G, Grippo AJ, Lamb D, Harden E, Handleman M, Lebow J, Carter CS, Porges SW. Autonomic predictors of recovery following surgery: a comparative study. Auton Neurosci. 2010;156(1-2):60–6. doi: 10.1016/j.autneu.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187(1):9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feir-Walsh BJ, Toothaker LE. An empirical comparison of the ANOVA f-test, normal scores test and kruskal-wallis test under violation of assumptions. Educational and Psychological Measurement. 1974;34:789–799. [Google Scholar]
- 29.Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) J Comp Psychol. 2001;115(1):53–61. doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- 30.Roberts RL, Miller AK, Taymans SE, Carter CS. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Canadian Journal of Zoology. 1998;(76):1862–1868. [Google Scholar]
- 31.Farrell WJ, Alberts JR. Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol. 2002;116(3):297–307. doi: 10.1037/0735-7036.116.3.297. [DOI] [PubMed] [Google Scholar]
- 32.Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behav Neurosci. 1995;109(1):135–49. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- 33.Stern JM. Multisensory regulation of maternal behavior and masculine sexual behavior: a revised view. Neurosci Biobehav Rev. 1990;14(2):183–200. doi: 10.1016/s0149-7634(05)80219-2. [DOI] [PubMed] [Google Scholar]
- 34.Blake BH. Ultrasonic vocalization and body temperature maintenance in infant voles of three species (Rodentia: Arvicolidae) Dev Psychobiol. 1992;25(8):581–96. doi: 10.1002/dev.420250805. [DOI] [PubMed] [Google Scholar]
- 35.Blumberg MS, Efimova IV, Alberts JR. Thermogenesis during ultrasonic vocalization by rat pups isolated in a warm environment: a thermographic analysis. Dev Psychobiol. 1992;25(7):497–510. doi: 10.1002/dev.420250704. [DOI] [PubMed] [Google Scholar]
- 36.Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33(3):249–56. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Leon M, Croskerry PG, Smith GK. Thermal control of mother-young contact in rats. Physiol Behav. 1978;21(5):790–811. doi: 10.1016/0031-9384(78)90021-5. [DOI] [PubMed] [Google Scholar]
- 38.Adels LE, Leon M. Thermal control of mother-young contact in Norway rats: factors mediating the chronic elevation of maternal temperature. Physiol Behav. 1986;36(1):183–96. doi: 10.1016/0031-9384(86)90094-6. [DOI] [PubMed] [Google Scholar]
- 39.Hicks C, Ramos L, Reekie T, Misagh GH, Narlawar R, Kassiou M, McGregor IS. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats. Br J Pharmacol. 2014;171(11):2868–87. doi: 10.1111/bph.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grewen KM, Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol Psychol. 2011;87(3):340–9. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: findings in six cases. Int J Obstet Anesth. 2008;17(3):247–54. doi: 10.1016/j.ijoa.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Karatsoreos IN, McEwen BS. Annual Research Review: The neurobiology and physiology of resilience and adaptation across the life course. J Child Psychol Psychiatry. 2013;54(4):337–47. doi: 10.1111/jcpp.12054. [DOI] [PubMed] [Google Scholar]
- 43.Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38(9):1850–7. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 44.McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(10):1079–95. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 46.Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Maternal sensitivity to infants in various settings predicts harsh discipline in toddlerhood. Attach Hum Dev. 2012;14(2):101–17. doi: 10.1080/14616734.2012.661217. [DOI] [PubMed] [Google Scholar]


