Abstract
The age of stressor exposure can determine its neurobehavioral impact. For example, exposure of adolescent male rats to resident-intruder stress impairs cognitive flexibility in adulthood. The current study examined the impact of this stressor in female rats. Rats were exposed to resident-intruder stress during early adolescence (EA), mid-adolescence (MA) or adulthood (Adult). They were tested in an operant strategy-shifting task for side discrimination (SD), reversal learning (REV) and strategy set-shifting (SHIFT) the following week. Performance varied with age, stress and coping style. MA and EA rats performed SD and SHIFT better than other ages, respectively. Social stress impaired performance in rats depending on their coping strategy as determined by a short (SL) or long (LL) latency to become subordinate. SL rats were impaired in SD and REV, whereas EA-LL rats were impaired in SHIFT. These impairing effects of female adolescent stress did not endure into adulthood. Strategy set-shifting performance for female adolescents was positively correlated with medial prefrontal cortex (mPFC) activation as indicated by c-fos expression suggesting that this region is engaged during task performance. This contrasts with the inverse relationship between these indices reported for male adolescent rats. Together, the results demonstrate that social stress produces cognitive impairments for female rats that depend on age and coping style but unlike males, the impairing effects of female adolescent social stress are immediate and do not endure into adulthood. Sex differences in the impact of adolescent social stress on cognition may reflect differences in mPFC engagement during the task.
Keywords: resident-intruder, strategy shifting, prefrontal cortex, development, sex difference, reversal learning
1. Introduction
Stress has been implicated in diverse psychiatric disorders including depression, anxiety, post-traumatic stress disorder and substance abuse [1–3]. In an effort to understand these links, research has focused on stress-induced alterations of affective processes. More recently, impairments in cognitive functions have become recognized as core features of stress-related psychiatric diseases that contribute to their debilitating nature. Although it is adaptive for stressors to alter cognitive processes such as arousal state, attention biases, decision-making and working memory to promote survival, chronic or repeated stress can impair these processes and contribute to cognitive symptoms of psychiatric disorders [4–6].
Substantial individual variability exists in the pathological consequences of stress, giving rise to the concepts of stress vulnerability and resilience [7, 8]. Various factors contribute to individual variability including the sex of the subject, genetic factors, environmental or social modulating factors and the developmental stage at which the stress occurs. Given the prevalence of social stress in humans and its negative impact on mental and physical health [9, 10], our laboratory has investigated individual differences in the consequences of the rat resident-intruder model of social stress [11, 12]. We determined that exposure of male rats to repeated resident-intruder stress results in the emergence of two subpopulations based on their coping strategy to exhibit a subordinate defeat posture with either a short latency (SL) vs. long latency (LL) [11]. SL male rats show certain behavioral, endocrine and physiological endpoints of depression, including increased immobility in the Porsolt swim test, decreased sucrose preference, decreased heart rate variability and dysregulation of the hypothalamic-pituitary-adrenal axis [11, 13, 14]. Recently, the effects of resident-intruder stress presented at different ages on cognitive function were investigated in male rats [15]. This social stress presented during early or late adolescence or adulthood had no immediate effects but adolescent social stress impaired strategy shifting, a form of cognitive flexibility, when tested in adulthood and rats with the SL coping strategy were more vulnerable. This study revealed that social stress in male rats has distinct effects on cognitive processes that are dependent on the age at which it occurs, the age at which the endpoint is tested and the coping style of the subject. Notably, strategy-shifting performance in adult male rats was positively correlated to activation of medial prefrontal cortical (mPFC) neurons as indicated by c-fos expression, whereas in adolescent males this correlation was negative, suggesting that different circuits are engaged in adolescent and adult male rats during task performance.
Although social stress is also relevant for females, this has been primarily modeled in the laboratory using social isolation or social instability because neither male nor female rats are typically aggressive to female rats [16–18]. However, lactating females exhibit aggression towards female rats and this has been used as a female resident-intruder social stress model [19–21]. The present study used this model of female resident-intruder stress to investigate the effects of social stress occurring in early and mid-adolescence or adulthood on cognitive function tested shortly after the last stressor or later for adolescent rats, in adulthood. The modifying influence of coping style determined by the latency to become subordinate on stress effects was assessed. Finally, because the mPFC mediates cognitive flexibility and is exquisitely stress-sensitive [22–24], measures of mPFC activity were related to behavioral effects.
2. Materials and Methods
2.1. Animals
Female Sprague Dawley rats (Charles River, Wilmington, Massachusetts) served as social stress “intruder” rats or matched controls. Rats had free access to food and water and were allowed to acclimate to a 12-h light/dark cycle (lights on at 06:00 AM), temperature-controlled room for 4 days prior to the study. Sprague Dawley lactating adult rats that were housed separately were used as resident rats in the resident–intruder test. The care and use of animals was approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia.
2.2. Experimental Design
Stress or control manipulations occurred during early adolescence (PND 28–32, EA), mid-adolescence (PND 42–46, MA), or adulthood (PND 70–74, Adult). These ages were selected to span the social and physical stages of early and mid-adolescence as designated previously [25–27]. In addition a group of EA stressed rats were tested as adults (PND 70–74; EA-Adults) and a group of MA stressed rats were tested as adults (PND 70–74; MA-Adults). Rats were exposed to five consecutive days of social stress or control manipulation. On the last day of social stress or control manipulation EA, MA, and Adult rats began food restriction to maintain 85% free-feeding weight. Training in the operant strategy set-shifting task (OSST) began 3 days after the last experimental manipulation and testing occurred after 3 days of training, 6 days after the final experimental manipulation. EA-Adult and MA-Adult animals were food restricted, trained, and tested in the operant chamber at the same age as Adult animals. These rats were group housed after the last experimental or control manipulation until training and testing as adults.
2.3. Social Stress
The social stress and matched control methods were a modification of the resident-intruder model [28] and identical to that previously described [15] except that lactating female rats were used as resident rats. Intruders were individually placed in the home-cage of a novel lactating female (resident) whose pups had been removed immediately prior. The resident and intruder were allowed to interact until either the intruder exhibited a submissive defeat posture (>2 s frozen in a supine position) or 15 min elapsed. Upon reaching one of these criteria, the animals were separated by a wire barrier, allowing only auditory, olfactory and visual contact for the remainder of the 30-min test period. Intruders were then returned to their home-cages and lactating mothers were reunited with their pups. This was repeated for 5 consecutive days with the intruder being randomly placed into the cage of a different lactating female each day. Control rats were placed into a novel cage for 30 min/day with the last 15 min spent behind the wire mesh cage for 5 consecutive days. For all rats subjected to resident-intruder stress the latency to assume the defeat posture was recorded for each session and averaged across all 5 sessions for an individual intruder. If the defeat posture was not assumed during the 15 min period, the latency was given the value of 900 s. The mean latencies for all intruder rats of each age group (EA, MA and Adult) were subjected to a K-means cluster analysis (JMP 9.0; SAS, Cary, NC) to define the subpopulations of rats as short latency (SL) or long latency (LL).
2.4. Operant Training and Testing
Training and testing were carried out in two-lever operant chambers (Med-Associates, St. Albans, VT, USA), each within a sound-attenuating box. A stimulus light was positioned above each lever, and a house light was positioned top-center on the wall opposite the levers. Data were recorded and stored onto a PC computer via an interface module.
The operant strategy-shifting task (OSST) was adapted from Floresco et al. [29]. On Day 1, rats were shaped to lever press on a fixed-ratio 1 schedule on one lever (randomly chosen left/right) to a criterion of 50 presses within 30 minutes. On Day 2, rats were trained to the same criterion with a fixed-ratio 1 schedule on the opposite lever. On Day 3, rats were introduced to the trial structure of the task, under conditions with no discernable “rule”. On each trial, the house light and both stimulus lights were illuminated for 15-seconds during which rats could press one of the two levers for food reward. The correct lever was randomly selected to occur one, three, or five times in a row on a particular side, such that over many trials it was equally likely to occur on either side. This encouraged rats to switch sides during training while not allowing them to use spatial or light cues to reliably predict the location of the correct lever. If the correct lever was pressed within 15 seconds of trial initiation, a single reward pellet was delivered, and all lights remained illuminated for 3 seconds followed by darkness for a 5 second timeout before initiation of the next trial. If the incorrect lever was pressed within 15 seconds of trial initiation, no reward was delivered, and all lights were immediately shut off for a 10 second timeout before initiation of the next trial. If neither lever was pressed within 15 seconds of trial initiation, all lights were shut off for a 5 second timeout before initiation of the next trial. Additionally, if either lever was pressed during a dark timeout period, the initiation of the following trial would be reset to occur 5 seconds after the time of this lever press. Trials continued until rats achieved 50 correct trials. Each animal’s side bias was determined to be toward the lever on the side that the animal pressed on the majority of trials during training. On Day 4, behavior was tested in a series of three consecutive discriminations: an initial side discrimination (SD), a side reversal discrimination (REV), and a shift to light discrimination (SHIFT). Animals proceeded from one stage of the task to the next after achieving a criterion of 8 consecutive correct choices, with the stipulation that to proceed from SD to REV or from REV to SHIFT a minimum of 30 trials had been attempted. This minimum of 30 trials performed stipulation was added to ensure that each animal experienced a sufficient number of trials in those stages of the task for the transitions from one type of discrimination to the next to be cognitively meaningful. Regardless of this stipulation, the dependent measure was the number of trials required to reach the criterion of 8 consecutive correct trials. If the animal reached this criterion prior to completing 30 total trials, the task was considered complete and trials and errors during the “extended period” were not counted. The trial structure and timing of light illuminations during each stage of the task were the same as they were during the previous training session, with the exception that only one stimulus light was illuminated. For every pair of trials, on the first trial of the pair the left or right stimulus light was randomly selected to be illuminated, and the opposite stimulus light was illuminated on the following trial. During the SD stage, the lever on the side opposite the animal’s side bias was designated to be the correct lever on every trial, regardless of the location of the stimulus light. During the REV stage, the correct lever on each trial was designated to be the lever opposite the correct lever during the initial side discrimination. During the SHIFT stage, the correct lever was designated as the lever underneath the illuminated stimulus light on each trial. After reaching the criterion of 8 consecutive correct trials in the SHIFT stage, the task was ended. Trials to criterion (TTC) and number of errors were recorded during each stage of the OSST for each rat. Omitted trials during which neither lever was pressed were not included in the TTC measure. Error types were characterized using logistic regression to determine whether treatments impacted perseveration on the previous rule or the acquisition and maintenance of the new rule. For the REV stage, every trial attempted by a particular animal was categorized as “correct” or “incorrect” and regressed by trial number. A logistic curve of best fit, representing the probability of a correct response with respect to trial number, was generated and the trial number after which the value of this curve became greater than or equal to chance performance value of 50% was noted. Errors that occurred on or before this trial were characterized as perseverative errors, as they occurred while the animal was following the old rule with greater than chance probability. Errors that occurred after this trial were characterized as regressive errors, as these errors were made after the animal had disengaged from following the previous rule and was in the process of acquiring the new rule. For the SHIFT stage, trials attempted were split into two categories: (1) trials when the stimulus light was illuminated above the previously correct lever during the REV stage and (2) trials when the stimulus light was illuminated above the opposite lever. Errors from trials of the first category were classified as perseverative or regressive using the same method described above for the REV stage. Errors from trials of the second category were counted as random errors, as they were unrelated to the previously learned rule.
2.5. Immunohistochemistry and Western blot
Thirty minutes after reaching criterion in the SHIFT stage, some of the rats in each group were anesthetized with isoflurane and transcardially perfused with heparinized saline followed by 4% paraformaldehyde for processing of c-fos in the mPFC as previously described [30]. The specific area in which c-fos was quantified included both prelimbic and infralimbic cortex and was identical to the region described by Snyder et al. [30].
Brains from the remaining rats were removed and the frontal cortex was dissected and frozen for Western blot analysis of ERK1/2 and phosphorylated ERK1/2. Samples were homogenized in a RIPA buffer (Sigma, St Louis, MO) with a protease inhibitor cocktail (Halt, Pierce, Rockford, IL) and centrifuged at 15,000 g for 15 min. Protein content was determined using the BCA method. Samples (50 μg per condition) were subjected to SDS-PAGE gel electrophoresis and proteins transferred to polyvinylidene fluoride membranes as previously described [31]. After blocking the membranes with Odyssey Buffer (1 h, diluted in PBS 1:1), membranes were incubated with mouse monoclonal antibody raised against P-ERK1/2 (1:2,000, Santa Cruz Biotech) at 4°C, overnight. Following rinsing, membranes were incubated with donkey anti-mouse IRDye 800CW, (1:5,000, LiCor, Lincoln, NE) at room temperature for 1 h. Membranes were rinsed and incubated with a rabbit antibody directed against ERK1/2 (1:1000, Cell Signaling) at 4°C, overnight. Following rinsing, membranes were incubated with for 1 h with donkey anti-rabbit IRDye 680CW (1:10,000) for simultaneous detection of both primaries. Membranes were scanned and proteins detected using the Odyssey Infrared Imaging System (LiCor). Odyssey Infrared Imaging software was used to quantify the integrated intensity of each band and to determine the molecular weights of the proteins (based on Biorad Precision Plus Protein Standards). A ratio of P-ERK:ERK1/2 was calculated for each sample. These were run in duplicate and the mean ratios were compared between groups using a two-way ANOVA.
2.6. Statistical Analysis
Task performance in terms of trials to criterion was analyzed by three-way ANOVAs (Age x Stress x Stage) with repeated measures across Stage. Additionally, each stage was analyzed separately by two-way ANOVA for interaction effects of age with stress (Age x Stress) and latency cluster (Age x Latency Cluster). Molecular biomarkers (i.e. c-fos expression, ERK phosphorylation) were also analyzed via two-way ANOVA (Age x Stress). Relationships between task performance in each stage and c-fos expression were assessed using linear regression. Where significant main effects or interactions were found, follow-up post-hoc comparisons were performed using the Holm-Sidak method, unless otherwise noted.
Cluster analyses (JMP 9.0; SAS, Cary, North Carolina) were applied separately to the defeat latencies of animals within each experimental group in order to categorize animals on the basis of their stress-coping strategy as short (SL) or long latency (LL) animals.
3. Results
3.1. Effects of social stress during development on operant strategy set shifting
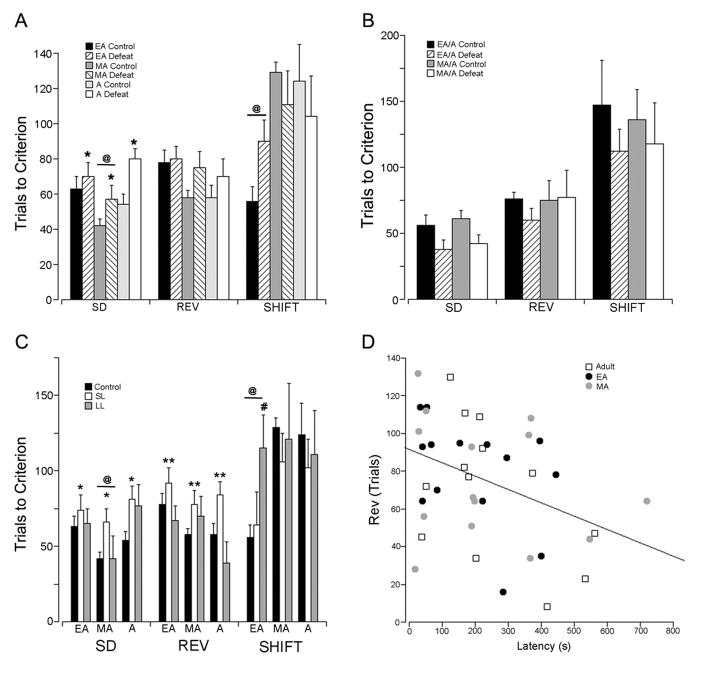
The number of rats that underwent social stress or control manipulation and completed the task was 92 with 27 adults (14 control and 13 stressed), 33 EA (19 control and 14 stressed) and 32 MA rats (18 control and 14 stressed). Some rats did not finish the task including 5 control and 9 stressed EA rats, 3 control and 3 stressed MA rats and 1 control and 3 stressed Adult rats. A three-way repeated measures ANOVA (Age X Stress X Stage) with task stage as the repeated measure showed a trend for an overall effect of stress on task performance (F(1,86)=3.6, p=0.06) and an Age X Stage interaction (F=(4,170)=4.7, p<0.005). Stress impaired SD performance (F(1,90)=7.5, p<0.01) (Fig. 1A). There was also an effect of age on SD performance in that MA rats required fewer trials to reach criterion compared to EA or Adult rats (F(2,89)=4, p<0.05; post-hoc p<0.05). There was a trend for an effect of stress on REV (p=0.09). For the SHIFT stage there was an effect of age (F(2.89)=6.1, p<0.005) in that EA rats performed better than other groups (p<0.01) (Fig. 1A). An analysis of the percentage of omitted trials revealed an effect of age (F(2,87)=4, p<0.05) but no effect of stress (F(1,88)=1.4, p=0.24) or Age X Stress interaction (F(2,89)=0.69, p=0.5). The mean percentage of omitted trials was 18±3, 21±3 and 11±3 for EA, MA and Adult rats, respectively with MA rats having a higher percentage of omitted trials compared to Adult rats (p<0.05).
Figure 1.
Effect of age, stress and coping strategy on task performance. A) Performance of rats that were exposed to repeated social stress or control manipulation and trained in the operant set shifting task during the week following the last stress/control manipulation. The abscissa indicates task stages of side discrimination (SD), reversal learning (REV) and strategy set-shifting (SHIFT). The ordinate indicates trials to reach criterion. Each bar is the mean of 19 control and 14 stressed EA rats, 18 control and 14 stressed MA rats and 14 control and 13 stressed Adult rats. Vertical lines are S.E.M. @ p<0.05, effect of age; *p<0.05, effect of stress. B) Performance of rats that were exposed to repeated social stress or control manipulation during EA or MA and trained in the operant set shifting task as adults. The abscissa and ordinate are as in A. Each bar is the mean of 7 controls and 7 stressed EA rats and 7 control and 5 stressed MA rats. There were no effects of age or stress on performance at any task stage. C) Performance of rats based on latency clusters. The abscissa and ordinate are as in A. Each bar is the mean of 19 control, 7 SL and 7 LL EA rats, 18 control, 9 SL and 5 LL MA rats and 14 control, 9 SL and 4 LL Adult rats. @ p<0.05, effect of age; *p<0.05, effect of latency cluster, **p<0.005, effect of latency cluster; # p<0.05. D) Correlation between defeat latency and trials to reach criterion in the REV task. Each point represents an individual Adult, EA or MA rat as indicated by the legend. R2=0.15, p<0.02.
The impairing effects of social stress did not endure into adulthood (Fig. 1B). For separate groups of rats that were stressed in EA and MA and were trained and tested in adulthood (EA controls=7, EA stress=7, MA controls=7, MA stressed=5) there was no effect of prior stress (F(1,22)=2.0, p=0.17) or age at which manipulations occurred (F(1,22)=0.08, p=0.8) (Fig. 1B). There was no Age X Stress interaction (F(1,22)=0.22, p=0.64), no Age X Stage interaction (F(2,21)=0.04, p=0.96), no Stress X Stage interaction (F(2,21)=0.3, p=0.74) and no Age X Stress X Stage interaction (F(2,21)=0.35, p=0.7).
3.2. Relationship between coping strategy and behavior
As in our studies of resident-intruder stress in male rats, female rats exposed to social stress clustered into subpopulations based upon their latency to defeat. The ranges and mean latencies for EA, MA and Adult rats for the short latency (SL) cluster and long latency (LL) clusters are shown in Table 1. A repeated measures ANOVA across stage revealed an Age X Stage interaction (F(4,164)=4, p<0.005). Analysis of SD revealed an effect of age (F(2,89)=4, p<0.05) with MA rats performing better than other age groups. There was also an effect of latency cluster (F(2,89)=4.7, p<0.05) indicating that SL rats performed worse than control rats (p<0.05). Latency cluster had a significant effect on REV (F(2,89)=5.7, p<0.005). SL rats required more trials to reach criterion than control or LL rats (p<0.05). For the SHIFT stage of the task there was an effect of age (F(2,89)=3.5, p<0.05) with EA exhibiting better performance. Notably, stress impaired strategy set shifting performance selectively in the LL latency cluster of EA rats. A repeated measures ANOVA across stages for the EA rats alone indicated a Stage X Cluster interaction (F(4,58)=2.8, p<0.05). This effect was a result of a difference in the SHIFT component of the task in which EA-LL rats required more trials to reach criterion than either EA-controls or EA-SL rats (F(2,32)=6.2, p<0.01; p<0.05 Tukey HSD).
Table 1.
Range and average defeat latencies for all age groups
| Short Latency | Long Latency | |
|---|---|---|
|
| ||
| Adult | 152±22 s (38–223 s) | 472±45 s (374–562 s) |
| EA | 67+16 s (33–154 s) | 325+33 s (221–444 s) |
| MA | 105+28 s (19–198 s) | 473+71 s (363–720 s) |
To better identify relationships between coping strategy and task performance the latency to defeat was correlated with trials to criterion for different task stages. The latency to defeat was inversely correlated to the number of trials to reach criterion in the REV stage of the task (r2=0.14, p<0.02) indicating that rats that resist defeat perform better in reversal learning tasks (Fig. 1D). No statistically significant correlations were observed between latency to defeat and performance in any other task stage.
3.3. Relationship between coping strategy and error type
Coping strategy was related to the error type during reversal learning (Fig. 2). A two-way ANOVA (Age x Latency cluster) of regressive errors made during reversal learning showed a significant effect of latency cluster (F(2,89)=7.6, p<0.001) such that LL rats made significantly fewer regressive errors compared to both controls and SL rats (p<0.001). There were no effects on perseverative errors. During the SHIFT stage there was a significant effect of age on regressive errors (F(2,89)=5.0, p<0.01) such that MA rats made more regressive errors compared to EA and Adult rats (p<0.01).
Figure 2.
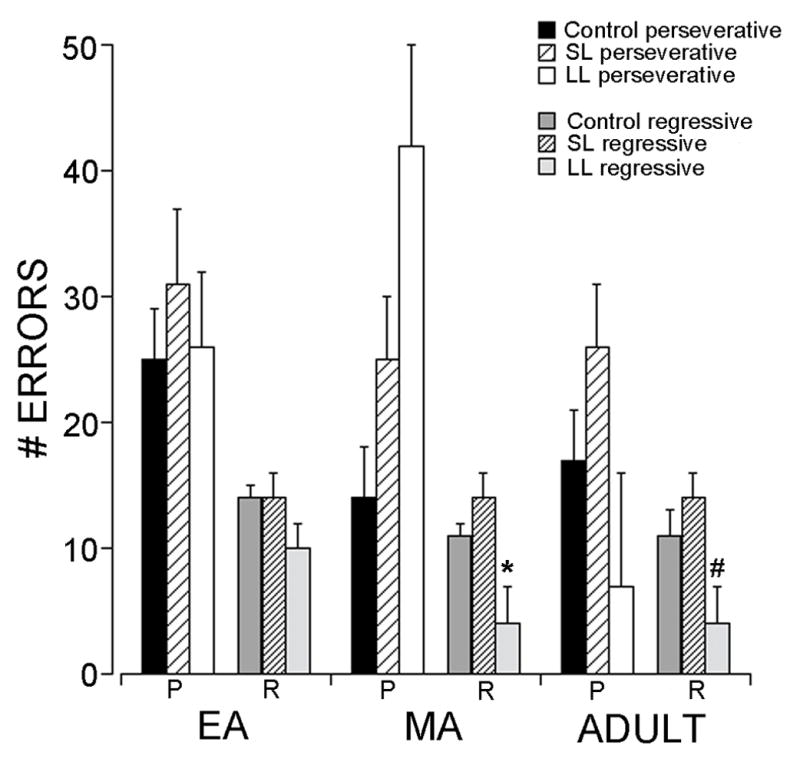
Effect of stress and coping strategy on error type. Bars indicate the number of perseverative (P) and regressive (R) errors made by control, SL or LL rats of different age groups during REV. Vertical lines indicate S.E.M., *p<0.05 compared to both control and SL. #p<0.05 compared to SL.
3.4. Neuronal activation in the prefrontal cortex associated with behavioral performance
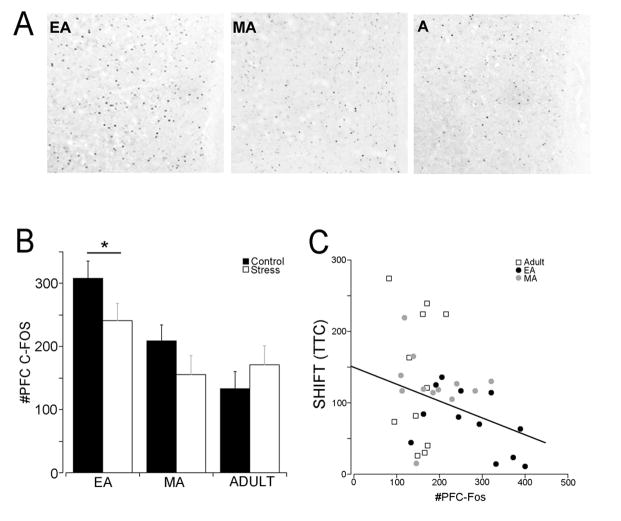
EA rats had more c-fos profiles in the PFC, consistent with their superior performance in strategy set-shifting stage (Fig. 3A,B). A two-way ANOVA (Age x Stress) showed an effect of age (F(2,34)=10.4, p=0.0005) with EA rats having more c-fos profiles compared to both MA and Adult rats (p<0.01). The number of c-fos profiles in the PFC was inversely correlated to trials to criterion in the SHIFT stage (r2=0.15, p=0.02) (Fig. 3C). Interestingly, this correlation improved when data from Adults were deleted and only adolescent rats were used (r2=0.26, p=0.01).
Figure 3.
Effect of social stress and coping strategy on PFC c-fos. A) Representative photomicrographs of the mPFC from EA, MA and Adult rats showing c-fos immunoreactive profiles. B) Mean number of c-fos profiles in the PFC of EA, MA and Adult rats that had social stress or control manipulation. The ordinate indicates the mean number of c-fos profiles. Each bar represents the mean of 6 control and 6 stressed EA rats, 7 control and 5 stressed MA rats and 6 control and 5 stressed Adult rats. Vertical lines indicate S.E.M. *p<0.0005 compared to other ages. C) Correlation between c-fos profiles in the PFC and performance in the SHIFT stage. The abscissa indicates the number of PFC c-fos profiles and the ordinate indicates trials to criterion for the strategy set-shifting stage. Each point represents an individual EA, MA or Adult rat as indicated by the legend. R2=0.15, p<0.02.
Consistent with the c-fos results, the ratio of pERK:ERK was also greater in EA rats compared to MA or Adult rats (Age effect (F(2,46)=7.0, p<0.005; post-hoc p<0.05) (Fig. 4). There was no correlation between the ratio of pERK:ERK and trials to criterion in the strategy set-shifting task (r2=0.07, p=0.26).
Figure 4.
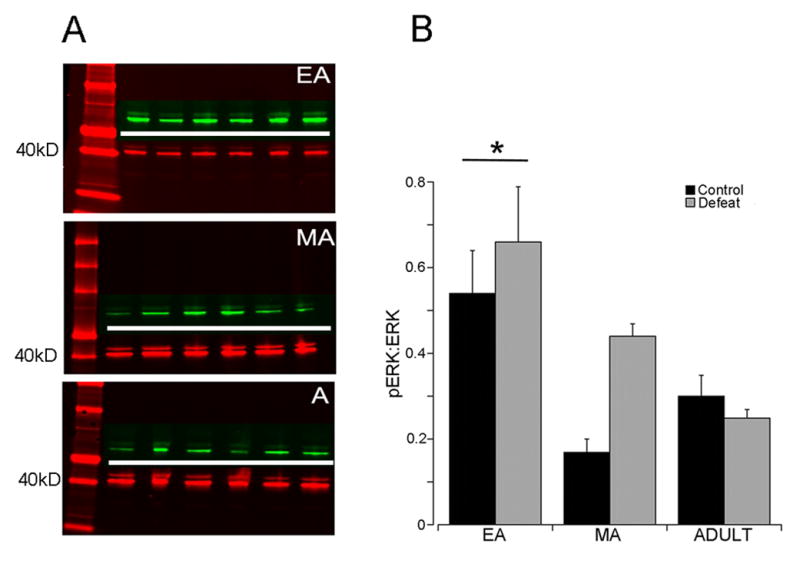
Effect of social stress on ERK phosphorylation in the PFC. A shows representative Western blots from EA, MA or Adult rats. ERK represented in red fluorescence and P-ERK in green. ERK and P-ERK are detected at the same molecular weight (near 42,44 kD) and simultaneous visualization of both appears as one yellow blot. In order to distinguish both proteins in the figure ERK and P-ERK bands were imaged separately and the P-ERK image was placed above the line of ERK bands and separated by a white line. B) Quantification of P-ERK:ERK ratio. The ordinate indicates the ratio of P-ERK:ERK. Each bar is the mean of 10 control and 12 stressed EA rats, 4 control and 4 stressed MA rats and 9 control and 8 stressed Adult rats. *p<0.005 compared to MA and Adult rats.
Although there was no significant correlation between c-fos profiles in the PFC and trials to criterion in the REV stage, the line describing this relationship suggested a positive rather than an inverse relationship as seen with trials to criterion in the SHIFT stage. Therefore, the relationship between REV and SHIFT performance was examined (Fig. 5). There was a statistically significant inverse correlation between performance in REV and SHIFT stages of the task (p<0.01, r2=0.08) that strengthened when the data were constricted to just adolescents (p<0.005, r2=0.12).
Figure 5.
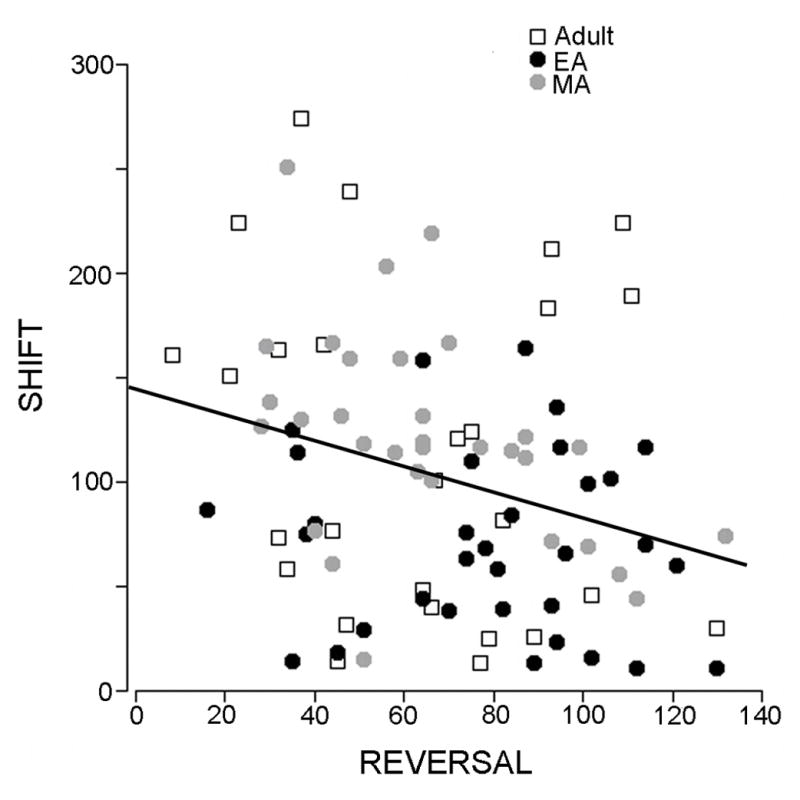
Inverse correlation between reversal learning and strategy shifting. The abscissa indicates the number of trials to reach criterion during REV and the ordinate indicates the number of trials to reach criterion in the SHIFT stage. Each point represents an individual Adult, EA or MA rat as indicated in the legend.
4. Discussion
The present study demonstrated that social stress has relatively immediate impairing effects on cognition of female rats that are a function of the age at which stress occurs, the specific cognitive task and the coping strategy. In general, exposure to social stress was associated with impaired side discrimination and reversal learning in rats that exhibited a propensity towards subordination. Strategy set-shifting performance was optimal in early adolescence and this was preserved in stress-exposed rats that had a subordinate coping style but impaired in rats that resist subordination. The finding of an inverse correlation between performance in reversal learning and strategy set-shifting supports the notion that these processes interfere with each other. Together these findings suggest that stress may impair different cognitive processes (reversal learning or strategy set-shifting) in individuals with different coping strategies. The finding that strategy set-shifting performance of female adolescent rats was positively related to mPFC activation stands in direct contrast with the recent finding that this is inversely correlated to mPFC activation in male adolescent rats [15]. Thus, performance of the same cognitive task can engage different brain regions in adolescent males and females. This can account for differences in the timing of social stress consequences between adolescent female rats that express impairments immediately and adolescent male rats that exhibit protracted impairments.
4.1. Stress and cognitive function
Stressor exposure is thought to contribute to psychiatric disorders through its effects on the neural substrates of affect and cognition. Whereas studies of the effects of different stressors on rodent models of affect are numerous, effects on executive functions that are core features of stress-related psychiatric disorders have been less well explored [32]. Most studies report impairments in cognitive flexibility resulting from stress effects on the mPFC [33–35]. For example, acute tail pinch, chronic restraint and chronic unpredictable stress impair attentional set shifting or strategy set-shifting performance [35–38]. However, this is not a general effect of all stressors as chronic cold stress selectively impairs reversal learning and acute restraint and repeated swim stress enhance reversal learning [39–41]. These differences reflect distinct effects of certain stressors on multiple brain regions that bias the regulation of cognitive processes towards certain strategies [39].
Much human evidence for a role of stress in psychopathology is based on the effects psychosocial stress. Although the resident-intruder model of social stress is used extensively to model psychiatric disease in rodents, there are surprisingly few studies of its impact on cognitive functions [15, 42]. An important determinant of the impact of stress is the developmental stage during which it occurs. The influence of a stressor on cognition is greatest when it occurs within critical windows during which brain structures and circuits underlying cognitive functions are developing [6]. In humans, early life stress is associated with impairments in sustained attention, inhibitory control and cognitive flexibility [5, 6, 43–45] and these are features of the psychiatric disorders that early life stress has been implicated in, including depression, anxiety, post-traumatic stress disorder, personality disorders and schizophrenia [3, 46]. To begin to assess the impact of social stress on cognitive functions at different stages of development, we recently investigated the effects of resident-intruder stress presented to early adolescent, mid-adolescent and adult male rats on performance in the OSST task [15]. Because many stress-related disorders are more prevalent in females [47–50], the present study was run in parallel in female rats.
4.2. Effects of age on OSST performance of female rats
As previously reported for males [15], there were age differences in task performance, irrespective of stressor exposure, that generally indicated that younger female rats performed better. MA females had better side discrimination performance and EA females had better strategy set-shifting performance. The study in males demonstrated that EA and MA rats had better strategy set-shifting performance compared to adults [15]. These findings are consistent with other reports of better attentional set shifting performance in adolescents [51, 52] although one study reported poorer performance in adolescent males [53].
4.3. Effects of stress and coping style on OSST performance of female rats
Female social stress had immediate effects to generally impair side discrimination and reversal learning and to impair strategy set-shifting in a subpopulation of EA rats. These impairments did not endure into adulthood. Although it could be argued that the number of subjects in studies of the protracted effects of female adolescent stress may not have been sufficient to see effects, the tendency in these animals was towards improved rather than impaired performance. The immediate effects of social stress on adolescent female cognitive function contrasts with the reported protracted effects in adolescent males, which are not expressed until adulthood [15]. Sex differences in the timing of stress-induced impairments in cognitive processes imply temporal differences in the development of circuits that subserve these processes. The sex differences in task-related mPFC activation discussed below are consistent with this notion.
Previous studies underscore the importance of coping style in the consequences of male resident-intruder stress [11–13]. In general, male rats that exhibit the subordinate defeat posture with a short latency express certain behavioral, neuroendocrine and physiological endpoints that model what is observed in certain depressed patients. Female rats also clustered into subpopulations based on latency to defeat. Although behavioral or neuroendocrine differences between these two female subpopulations have yet to be thoroughly characterized, the present study demonstrated a distinct association between coping strategy and the cognitive process that was sensitive to stress. Thus, SL rats were vulnerable to impairments in side discrimination and reversal learning but protected from stress-induced impairments in strategy set-shifting, whereas LL rats were sensitive to stress-induced impairments in strategy set-shifting and exhibited better reversal learning performance. The ability of stress to selectively affect one cognitive process and not the other depending on coping strategy may reflect the inverse relationship between the two processes. Chronic stress has been proposed to bias cognitive strategies away from goal directed strategies and towards slow habitual processes by producing opposite morphological effects on neural substrates that underlie these processes [35,54]. For example, chronic stress promotes atrophy of mPFC neurons that project to the associative striatum and hypertrophy of neurons in the lateral orbitofrontal cortex and dorsolateral striatum [54]. The current findings suggest that these neural substrates may be differentially vulnerable to stress in SL and LL rats. It is interesting to speculate that in SL rats, assuming the defeat posture represents a strategy shift and that neural substrates that underlie this may be relatively more prominent and resistant to impairments. In LL rats activity may be biased towards the competing orbitofrontal cortex-dorsolateral striatal circuits that mediate habitual processes such as reversal learning.
The relationship between coping strategy and performance in the OSST in females contrasts the findings in males, for whom coping strategy was only a factor in strategy set-shifting performance and unlike females the SL coping strategy was associated with impaired strategy shifting in males. Additionally, this was only expressed in stressed adolescents that were tested in adulthood. This indicates that optimal coping strategies are sex specific.
4.4. Strategy shifting and mPFC activation
Attentional set-shifting and strategy set-shifting in the OSST are regulated by the mPFC, as they are impaired by lesion or chemical inactivation of this region [22, 23, 29, 55]. Consistent with engagement of the mPFC in strategy shifting, mPFC activation as assessed by the number of mPFC c-fos profiles was positively correlated to strategy shifting performance in the female rats in the present study. The higher p-ERK:ERK ratio in EA rats was also consistent with this, given that EA females out-perform the other age groups in the strategy shifting stage of the task. A similar relationship between mPFC c-fos or p-ERK-immunoreactive neurons and extradimensional set shifting performance in an attentional set shifting task and between mPFC c-fos and strategy set-shifting performance in the OSST was reported for adult male rats [15, 30]. Notably, in adolescent male rats, although strategy shifting is superior to adult males, the relationship between mPFC activation and performance is inverted and indices of increased mPFC activation are associated with increased trials to reach criterion or poorer performance [15]. In the present study, limiting the data to just female adolescents improved the correlation between mPFC c-fos profiles and strategy shifting performance. These findings underscore an important developmental sex difference in the neural substrates engaged during strategy shifting that may explain sex differences in timing of the consequences of stress. We speculate that in adolescent males, the PFC is still in development and vulnerable to stress but not on-line for performance of strategy shift and alternate circuits facilitate this task. Because the stress has impacted the mPFC during its development the deficit is expressed in adulthood, at a time when the mPFC typically takes over this task in males. In adolescent females the PFC may be more highly developed compared to males and on-line at the time of testing so that it is more vulnerable to immediate effects of social stress. However, these effects do not endure because the stress occurred at a time when the PFC was more fully developed and less vulnerable to long term effects.
Together these findings emphasize the importance of age and coping style on the impact of repeated social stress on cognitive function, as well as the specific cognitive task. Taken with previous studies in males, the present findings suggest that females may be more resilient to adolescent social stress in that the effects do not endure into adulthood and this may be a function of a developmentally earlier stage at which the PFC comes on-line to perform tasks requiring cognitive flexibility.
Highlights.
We compared social stress effects on cognition in adolescent and adult female rats
Stress-coping strategy determined the impact of adolescent stress on cognition
Consequences of female adolescent social stress do not endure into adulthood
Female adolescent cognitive flexibility correlates to prefrontal cortex activity
Acknowledgments
This work was supported by National Institute of Health Grants MH093981 and T32 MH14654 (KS).
Abbreviations
- EA
early adolescent
- LL
long latency
- mPFC
medial prefrontal cortex
- MA
mid adolescent
- OSST
operant strategy set-shifting task
- PND
post natal day
- REV
reversal learning
- SL
short latency
- SD
side discrimination
- SHIFT
strategy shifting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kevin Snyder, Email: ksny@mail.med.upenn.edu.
Mark Barry, Email: mbarry91@gmail.com.
Zachary Plona, Email: zachary.plona@gmail.com.
Andrew Ho, Email: truho@sas.upenn.edu.
Xiao-Yan Zhang, Email: xyzhang23@hotmail.com.
Rita J. Valentino, Email: rjv@mail.med.upenn.edu.
References
- 1.Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011 doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. The effects of stressful life events on depression. Annual review of psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 3.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–20. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 4.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Front Hum Neurosci. 2013;7:167. doi: 10.3389/fnhum.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–73. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. The American psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 11.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–94. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl) 2012;222:325–36. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood SK, Wood CS, Lombard CM, Lee CS, Zhang X-Y, Finnell JE, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psych. doi: 10.1016/j.biopsych.2014.10.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder KP, Barry M, Valentino RJ. Cognitive impact of social stress and coping strategy throughout development. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller J, Fuchs E, Halasz J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain research bulletin. 1999;50:33–9. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 17.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–33. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 18.Hatch AM, Wiberg GS, Zawidzka Z, Cann M, Airth JM, Grice HC. Isolation syndrome in the rat. Toxicology and applied pharmacology. 1965;7:737–45. doi: 10.1016/0041-008x(65)90132-8. [DOI] [PubMed] [Google Scholar]
- 19.Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. The European journal of neuroscience. 2001;13:1016–24. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl) 2011;218:271–9. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and Long-term Effects of Repeated Social Defeat During Adolescence or Adulthood in Female Rats. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behavioural brain research. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforuk A, Popik P. Neurochemical modulation of stress-induced cognitive inflexibility in a rat model of an attentional set-shifting task. Pharmacological reports : PR. 2013;65:1479–88. doi: 10.1016/s1734-1140(13)71508-1. [DOI] [PubMed] [Google Scholar]
- 25.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 26.McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:756–65. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35:1704–12. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 29.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural brain research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–30. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 96–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graybeal C, Kiselycznyk C, Holmes A. Stress-induced impairments in prefrontal-mediated behaviors and the role of the N-methyl-D-aspartate receptor. Neuroscience. 2012;211:28–38. doi: 10.1016/j.neuroscience.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jett JD, Morilak DA. Too much of a good thing: blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:585–95. doi: 10.1038/npp.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 38.Butts KA, Floresco SB, Phillips AG. Acute stress impairs set-shifting but not reversal learning. Behavioural brain research. 2013;252:222–9. doi: 10.1016/j.bbr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nature neuroscience. 2011;14:1507–9. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cognitive, affective & behavioral neuroscience. 2013;13:164–73. doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapiz-Bluhm MD, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 2009;202:329–41. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patki G, Solanki N, Atrooz F, Ansari A, Allam F, Jannise B, et al. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav. 2014;130:135–44. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–44. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J Neuropsychiatry Clin Neurosci. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 45.Hostinar CE, Stellern SA, Schaefer C, Carlson SM, Gunnar MR. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc Natl Acad Sci U S A. 2012;109 (Suppl 2):17208–12. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- 48.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 49.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 51.Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–59. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- 53.Newman LA, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dias-Ferreira D, Sousa JC, Melo I, Morgado P Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–25. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 55.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]