Abstract
The Eph family of receptor tyrosine kinases play key roles in both the patterning of the developing nervous system and neural plasticity in the mature brain. To determine functions of ephrin-A5, a GPI-linked ligand to the Eph receptors, in animal behavior regulations, we examined effects of its inactivation on male mouse aggression. When tested in the resident-intruder paradigm for offensive aggression, ephrin-A5-mutant animals (ephrin-A5−/−) exhibited severe reduction in conspecific aggression compared to wild-type controls. On the contrary, defensive aggression in the form of target biting was higher in ephrin-A5−/− mice, indicating that the mutant mice are capable of attacking behavior. In addition, given the critical role of olfaction in aggressive behavior, we examined the ability of the ephrin-A5−/− mice to smell and found no differences between the mutant and control animals. Testosterone levels in the mutant mice were also found to be within the normal range. Taken together, our data reveal a new role of ephrin-A5 in the regulation of aggressive behavior in mice.
Keywords: Eph receptor, ephrin-A5, aggression, resident-intruder, target biting, testosterone
1. Introduction
Aggressive behavior is defined as behavior that occurs when a conflict between the interest of two individuals exists [1, 2]. Appropriate levels of aggression may be viewed as a universal fitness trait which enables survival, whereas exaggerated levels can inappropriately harm or even cause death of the individual involved [3]. Animal studies classified male aggression into two major categories: offensive and defensive, which differ in their motive, site and intensity of attack, and outcomes [4, 5]. Offensive aggression is also known as inter-male aggression and occurs in response to challenges over resources (i.e., territory). It involves attack toward the back and flank of the opponent [5, 6]. In rodents, offensive aggression is used to gain dominant status and access to sexually active females [1]. In laboratory research, the resident-intruder (RI) model is commonly used to study offensive aggression [7]. Defensive aggression, also known as fear-induced aggression [7], occurs in the presence of a stimulus that is considered dangerous to the animal. Here, the animal will first try to avoid the threat and will attack only if escape is not possible. This type of aggression may elicit submissive posture or, if the threat persists, attacks directed toward the nearest offending body parts, which are usually the head and snout [8]. The target biting test has been used to measure this type of aggressive behavior in rodents [9, 10].
Different brain regions and signaling molecules are linked to aggression including the hypothalamus, medial amygdala (MEA), lateral septum (LAS), periaqueductal grey (PAG) and the bed nucleus of the stria terminalis (BNST) [1, 2, 4]. Studies in rats identified a broadly distributed “hypothalamic attack area” (HAA) from which electrical and pharmacological stimulation elicited attacks, and lesions reduced it [4, 11]. The HAA includes the lateral part of the anterior hypothalamus (AH), the ventromedial nucleus of the hypothalamus (VMN) and the ventral part of the lateral hypothalamus [1]. It has been suggested that under normal conditions, this area controls whether agonist behavior is appropriate or not, but when stimulated the animal will attack even when not suitable [12]. Recently, Lin et al. [13] identified an aggression locus in the mouse ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) that corresponds to the HAA of the rat.
In this study, we found that ephrin-A5, a ligand of the Eph receptor tyrosine kinase family, is important for the development of aggressive behavior in mice. The Eph receptors and their ephrin ligands are the largest family of receptor tyrosine kinases with 14 receptors and 8 ligands in mammals [14]. Members of this family are divided into the EphA/ephrin-A and EphB/ephrin-B subclass based on structural homology and binding affinities [15]. In general A-class receptors bind to all A ligands and B-class receptors bind to all B ligands. However, some exceptions exist, specifically, EphB2 can bind to ephrin-A5 and EphA4 can bind to all the class B ligands [16, 17]. Both receptor and ligand are anchored to the membrane resulting in signal transduction that can propagate into both receptor and ligand-expressing cells. These singling events have been implicated in various biological responses including proper development of the central nervous system and blood vessel formation [17–19]. In addition, several Eph receptors have been shown to regulate the proper development of motor and social behavior in mice [20–22]. We have previously shown that EphA5 inactivation caused a decrease in aggressive behavior in mice [23]. In the current study, we report that inter-male, offensive aggression is severely reduced in male mice lacking ephrin-A5 (ephrin-A5−/−). This does not appear to be related to an inability to attack, since during the target biting test, ephrin-A5−/− mice exhibited increased target biting. In addition, testosterone levels and general olfaction were normal in the null mice indicating that their ability to smell and recognize the presence of the intruder is intact. Taken together our data reveal an important role of ephrin-A5 in aggressive behavior.
2. Materials and Methods
2.1 Animals
Both wild-type and ephrin-A5−/− animals used for this study were generated from litter mates on a mixed background (C57BL/6 and 129/SV) as described previously[24, 25], since backcrossing into pure C57BL/6 background leads to embryonic lethality (data not shown). Mice were maintained on a 12 h light/dark reverse cycle (lights off from 07:00 to 19:00 h), and had free access to food and water. The temperature was maintained at 25 °C. All behavioral experiments were performed during the first phase of the dark cycle.
2.2 Resident-intruder (RI) aggression test
Adult (p>60 days) ephrin-A5−/− and wild-type male mice (n=9 per genotype) were used as the residents and were individually housed for two weeks. Since territoriality is based strongly on the presence of olfactory cues [26], bedding was not changed in the week before testing. Each resident was than confronted in its home cage with a group housed (4–5 mice per cage) male intruder, that was age and genotype matched to the resident, for 10 minutes. During the test, the latency to the first attack as well as the number of attacks were recorded. For resident intruder test, using a zinc sulfate treated intruder, a new set of adult (p>60 days) ephrin-A5−/− and wild-type male mice (n=10 per genotype) were used as the residents and group housed (4–5 mice per cage) zinc-sulfate-treated wild-type males were used as the intruders. In addition, we measured the number of times the resident spent in non-aggressive exploratory face and anogenital sniffing of the intruder.
2.3 Target biting defensive aggression test
Adult (p>60 days) ephrin-A5−/− (n=9) and wild-type (n=10) male mice were tested in the target biting test as described previously [27]. Briefly, mice were confined in a plastic cylinder (2.8 cm inner diameter; 9.8 cm long) with their tails passed through a slot at the rear of the cylinder and taped to 2 brass bar electrodes. The cylinder was placed in a chamber with a biting target in front of the mouse. The test session lasted 20 minutes with 10 two-minute trials. During the test the mice received a tone-conditioned stimulus (CS) for 15 seconds which terminated with the onset of a 2 mA, 0.15 sec tail shock. The number of times the mouse bit the target was collected in eight 15 sec bins over the two-minute trials and cumulated over the 20-minute session. The number of times the animal bit the target was recorded per bin. The number of bites from bins 2–7 was averaged and the data was analyzed as target bites in 3 bins (bin1, bin2–7 and bin8).
2.4 Zinc sulfate treatment
Intranasal instillation was performed as described previously [28]. Briefly, animals were lightly anesthetized with ketamine and an Eppendorf microloader (Eppendorf Hauppauge, NY) attached to a Hamilton syringe containing 0.15 ul of 5% zinc sulfate (Sigma, St. Louis, MO) was inserted 7 mm into one naris. The mouse was placed on its back for five minutes and then rotated to its side for another 20 min. The procedure was then repeated for the other naris.
2.5 Olfactory-guided foraging test
The test was performed as described previously [29, 30] with some modification. Briefly, individually housed ephrin-A5−/−and wild-type male mice (n=8 per genotype) were provided with flavored cereal in their home cage for 5 days. Food was then withheld and testing began 24 hours later. Each mouse was then transferred to a holding cage and a piece of flavored cereal was placed on the surface of the cage bedding. The latency to locate the cereal was recorded and the mouse was returned to his home cage. This procedure was then repeated three times with the cereal buried in different positions of the cage about 2 cm beneath the bedding.
2.6 Olfactory habituation-dishabituation test
The olfactory habituation-dishabituation test was adapted from previously published reports [29–31]. Briefly, ephrin-A5−/− (n=9), and wild-type (n=10) mice were presented in their home cage with a cotton swab dipped in water. The animal was allowed to explore it for 2 minutes and the procedure was repeated 2 more times at 1 minute intervals. On the fourth trial, the cotton swab was laced with urine from male mice and the procedure was repeated for 2 more times for a total of 6 trials. During each 2 minute presentation the total number of investigatory sniffs (defined as nasal contact with the cotton swab) was recorded. Urine was collected from ten gonadally intact males by holding the mouse by the scruff of the neck over a funnel and applying pressure on the abdomen. Samples were pooled and stored at −80° C until use.
2.7 Male-female recognition test
Male ephrin-A5−/−, and wild-type mice (n=9 per genotype) were tested for their preference to male verses female mice using a social chamber. The chamber was a 40 cm×40 cm×36.6 cm Plexiglas chamber with a stainless steel grid floor. On two opposite corners of the chamber there were two cylinders, 11 cm in diameter and 13 cm tall, made of the same stainless steel grid as the floor. An adult wild-type male mouse was placed in one of the cylinders and an adult wild-type female mouse in the second one. Each mouse was given a 15-minute habituation period to explore the empty chamber before the start of the trial. The test begins when a mouse was placed in the center of the chamber and allowed to explore it for 15 minutes. Each time the subject placed one or both paws on a cylinder a contact was recorded. The number of contacts as well as the time spent near either the male or female-containing cylinder was recorded.
2.8 Testosterone ELISA
Blood samples were collected into 1.5 Eppendorf tubes or BD Vacutainer® SST™ serum separation tubes (BD, Franklin Lakes, New Jersey), by cardiac puncture from adults (p=70 ±5 days) ephrin-A5−/− (n=13), and wild-type (n=15) male mice and allowed to clot. The blood was then centrifuged to isolate the serum, which was then extracted using diethyl ether (Sigma, St. Louis, MO). Testosterone concentrations were measured using a commercially available competitive ELISA kit (Cayman Chemical Ann Arbor, MI, Cat No.582701).
2.9 Statistical analysis
Data were analyzed using Statview statistical software. An unpaired Student's t-test was used for two samples comparisons (resident intruder test, olfactory foraging test, olfactory habituation-dishabituation test, male-female recognition test and testosterone levels) and a repeated-measures analysis of variance (ANOVA) was used to analyze target biting. The results are expressed as mean + SEM and p < 0.05 was considered significant.
3. Results
3.1 Reduced inter-male (offensive) aggression in ephrin-A5−/− mice
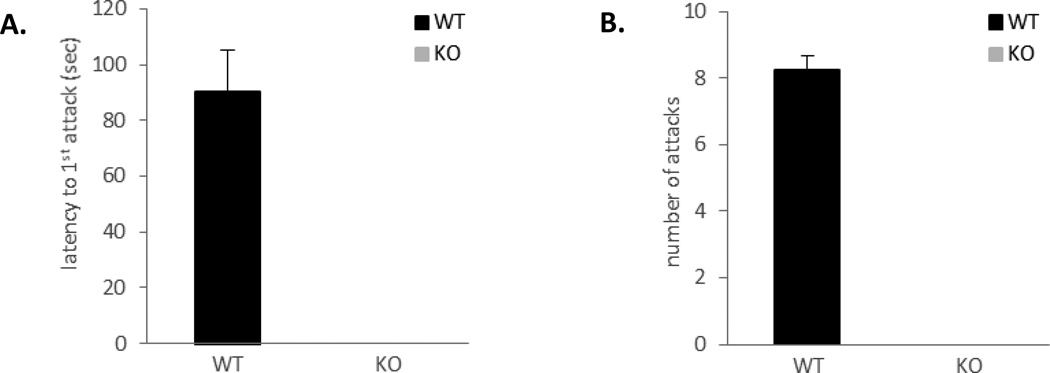
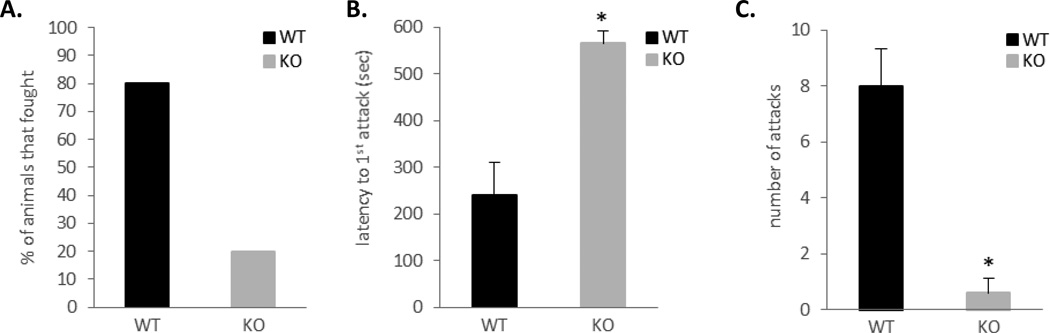
In order to evaluate roles of ephrin-A5 in animal behavior regulations, we examined effects of its inactivation on mouse motor activity, spatial learning, and aggression. We found no defects in motor activity except a mild hyperactivity [25] and no significant differences in spatial learning between ephrin-A5−/− and wild type control mice (data not shown). In contrast, our analysis revealed a striking absence of fighting in ephrin-A5−/− mice (Figure 1). Wild-type mice took an average of 90 seconds to initiate a fight (Figure 1A) and fought about 8 times per 10-min test session (Figure 1B), whereas null mice did not fight at all. Since it has been shown that individual differences can elicit different responses from the resident [7, 32], we wanted to confirm that the lack of aggression is indeed due to behavioral changes in the resident and not differences in the non-test intruders. Therefore, we repeated the RI test using a zinc-sulfate treated anosmic, wild-type mice as an intruder (Figure 2). Supporting our previous results, ephrin-A5−/− mice displayed a large decrease in inter-male aggression toward an anosmic intruder. Only two out of ten null mice engaged in aggressive behavior compared to eight out of ten wild-type mice (Figure 2A) and those that did fight had a significant increase in the latency to the first attack (t=4.31, p=0.0004) (Figure 2B) and a significant decrease in the number of fights (t=−5.20, p<0.0001) (Figure 2C). Taken together, our data show that loss of ephrin-A5 caused significant decrease in inter-male aggression.
Figure 1. Loss of inter-male aggression in ephrin-A5−/− mice.
Inter-male aggression of ephrin-A5−/− and wild-type mice was tested in the resident-intruder paradigm. Ephrin-A5−/− male did not exhibit aggressive behavior toward an intruder male.
(A, B) Wild-type males attacked male intruders with an average latency to the first attack of 90 seconds (A) and an average of 8 attacks per 10 minutes test (B) compared to no attacks by the null male mice.
n= 9 per genotype. Data are presented as mean +SEM.
Figure 2. Decreased aggression toward an anosmic mice in ephrin-A5−/− male mice.
Inter-male aggression of ephrin-A5−/− and wild-type male mice toward a zinc sulfate treated anosmic intruder was measured in the resident intruder test.
(A) Only two out of ten null mice engaged in aggressive behavior compared to eight out of ten wild-type mice. Data are presented as percent of animals that fought.
(B, C) Ephrin-A5−/− mice had increased latency to the first attack (B) as well as reduced number of attacks (C) compared to wild-type control.
n= 10 per genotype. Data are presented as mean +SEM. * indicates significantly different from wild-type; p<0.001.
3.2 Investigatory sniffs during the resident intruder test
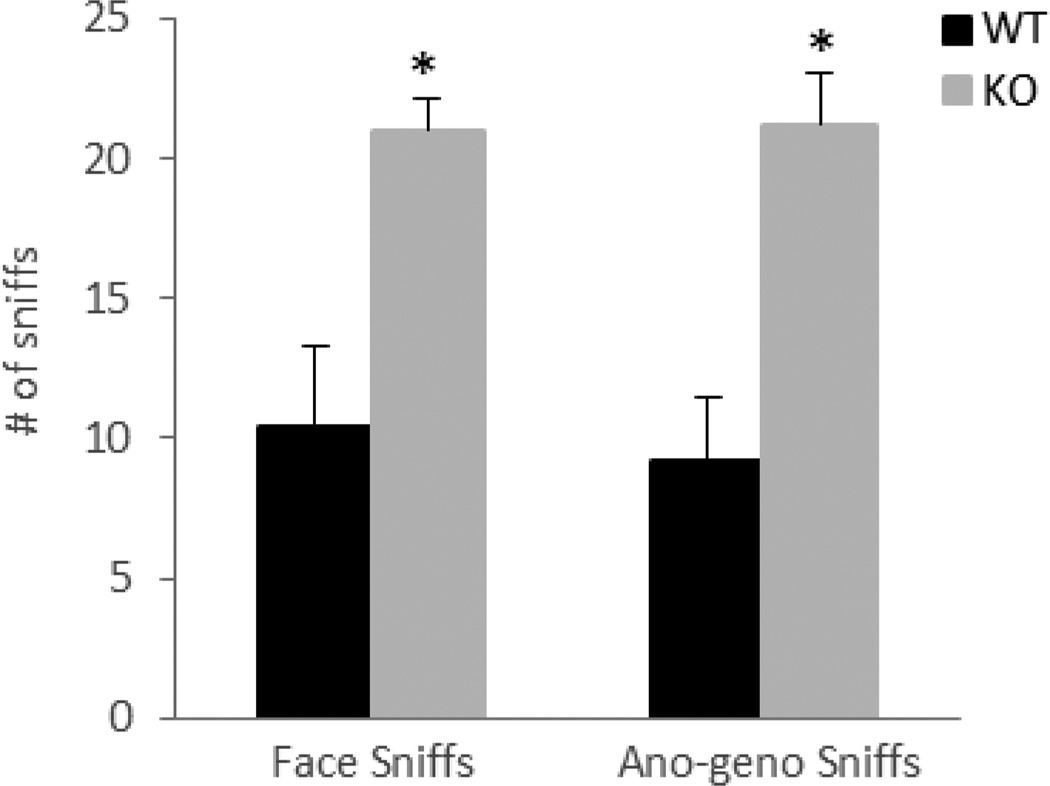
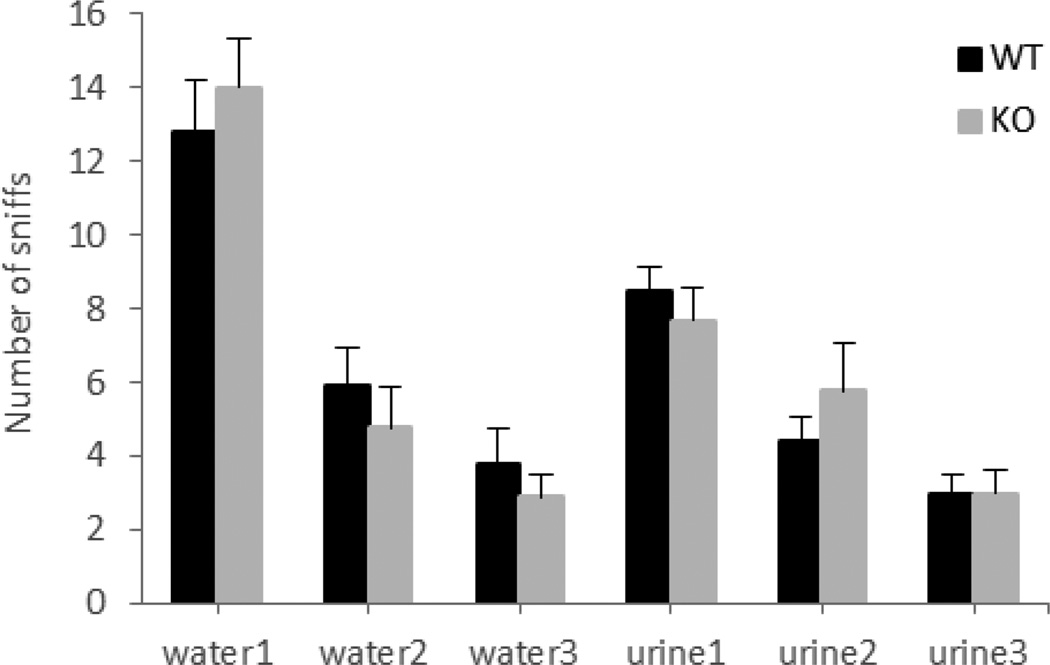
In order to confirm that the null mice are able to recognize the presence of another animal in their home cage, investigatory behavior in the form of face and anogenital sniffs was monitored during the RI test. As illustrated in figure 3, both genotypes had high levels of investigatory sniffs, but the null mice showed a significant increase in sniffs [F(1,18)=16.12, p=0.0008]. This increase in non-aggressive behavior and decrease in aggressive fights were reported previously in TNF-receptor-deficiency mice [33] and might be due to differences in social interactions between the genotypes. While the wild-type mice engaged in aggressive attacks, the null mice were involved in investigatory sniffs.
Figure 3. Ephrin-A5−/− mice showed high levels of non-aggressive investigatory sniffs during the RI test.
Both genotypes were able to recognize the presence of an intruder as revealed by the number of face and ano-geno sniffs during the RI test. Data are presented as the mean number of sniffs +SEM.
n= 10 per genotype. * indicates significantly different from wild-type; p<0.05.
3.3 No differences in olfactory-guided foraging and habituation-dishabituation tasks between ephrin-A5−/− and wild-type mice
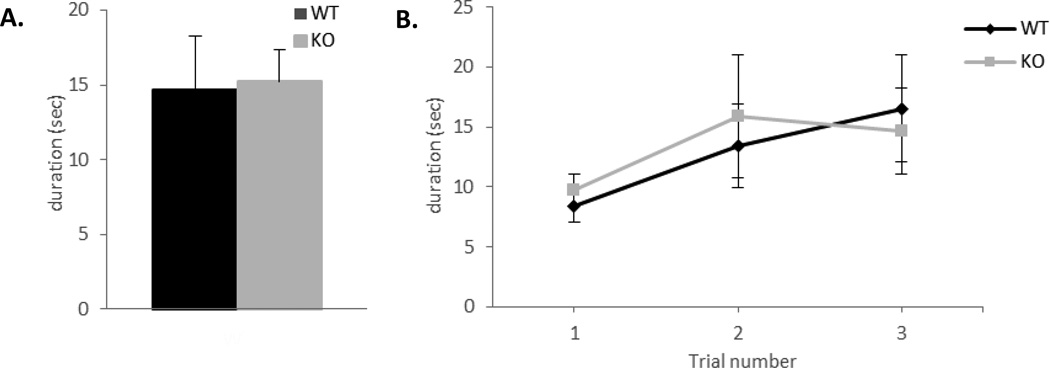
The olfactory-guided foraging test is based on the mouse ability to use olfactory cues for foraging [30]. In this test the animal uses odor cues to locate food hidden underneath the bedding. An inability or a delay in the time it takes to locate the food is used as an indicator of dysfunctional olfactory behavior [30]. The test began when the mouse was transferred to a holding cage and a piece of flavored cereal was placed on the surface of the cage bedding. The latency to locate the cereal was recorded and the mouse was returned to his home cage. This procedure was then repeated three times with the cereal buried in different positions of the cage underneath the bedding. There were no significant differences between the genotypes [F(1,14)=0.19, p=0.67]. Both ephrin-A5−/− and wild-type mice located the food placed on the surface as well as buried underneath the bedding as rapidly (Figure 4A and B), suggesting that the general ability to smell is intact in the null mice.
Figure 4. No differences in the olfactory foraging test between the genotypes.
Olfactory-guided foraging was tested in ephrin-A5−/− and wild-type male mice (n=8 per genotype).
(A, B) Both genotypes were able to locate the flavored cereal when placed on the surface (A) or buried beneath the bedding (B)
n= 8 per genotype. Data are presented as the time required by the mice to locate the flavored cereal (sec) +SEM.
In the habituation-dishabituation test, investigatory sniffs were recorded in six trials consisted of three presentations of water, followed by three presentations of male urine. Upon initial presentation of the cotton swab (dipped in water) both genotype showed high levels of investigatory sniffs (Figure 5). This exploratory activity was induced by the novelty of the cotton swab (since water doesn’t have an odor) and declined across the second and third exposure to the water (habituation). Next, the introduction of male urine on the swab elicited significantly higher number of sniffs (dishabituation) then the water (p<0.0001) suggesting that the animals were able to smell and distinguish between the water and the urine. Finally, both genotyped habituated to the urine odor, indicated by a decline in the number of sniffs over the last three trials. Although there was no genotypic effect across all six trials [F(1,17)=0.003, p=0.96], a significant effect of trial was found [F(5,85)=45.75, p<0.0001] suggesting that both ephrin-A5−/− and wild-type mice were able to recognize the new odor.
Figure 5. No differences in the olfactory habituation-dishabituation test between the genotypes.
Both eprin-A5−/− and wild-type male mice showed increase of sniffing upon the presentation of the new odor, male urine (dishabituation), and a decrease in subsequent presentations (habituation).
Ephrin-A5−/−n=9; wild-type, n=10. Data are presented as the mean number of sniffs +SEM.
3.4 No defects in male-female recognition task in ephrin-A5−/− mice
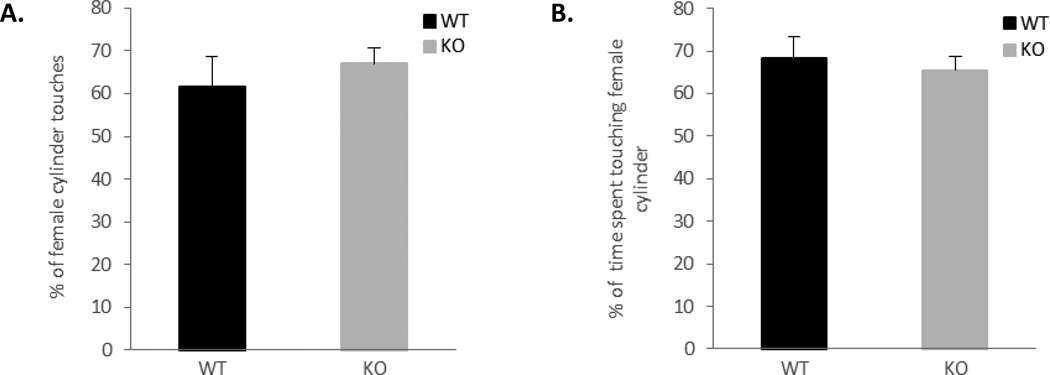
To investigate whether the loss of aggression in ephrin-A5−/− mice is due to loss of sex discrimination, we simultaneously introduce a female and a male mouse to each genotype and monitor their preference using a social chamber. Both ephrin-A5−/− and wild-type mice spent on average about 65 percent of the time investigating a female. There were no significant differences in the percentage of number of female cylinder touches (Figure 6A; t=0.67, p=0.51) nor in the percentage of time touching a female cylinder (Figure 6B; t=−0.47, p=0.65) between the genotypes (Figure 6). These data suggest that both genotypes are able to distinguish between a male and a female.
Figure 6. No defects in male-female recognition task in ephrin-A5−/− mice.
Ephrin-A5−/− and wild-type male mice were tested for their preference toward male or female mice in a social chamber. Both genotypes showed preference toward female mice.
All male animals touched the female containing cylinder more than the one with the male and spent on average about 65 percent of the time investigating it.
n= 10 per genotype. Data are presented as percent of female cylinder touches (left panel) or percentage of time (right panel) +SEM.
3.5 Increased defensive aggression in ephrin-A5−/− mice
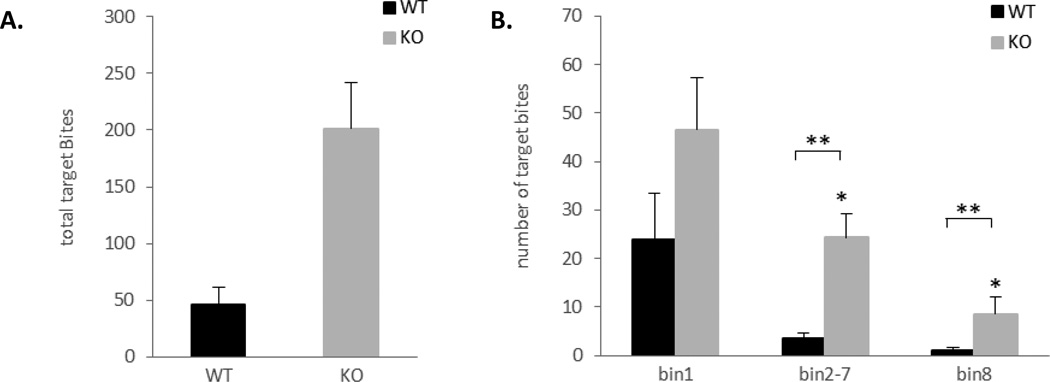
To determine whether the reduction of offensive aggression is due to a general lack of aggression, we measured defensive aggression using the target-biting test (Figure 7). Ephrin-A5−/− mice had significant higher cumulative number of target biting in the 20 min session compared to wild-type mice (t=3.71, p=0.002) (Figure 7A). In addition, a repeated measure ANOVA was used to analyze the average number of target bites over the 3 bins and there was an overall significant effect of genotype [F(1,17)=6.43, p=0.02] and bin [F(2,34)=18.70, p<0.0001] (Figure 7B). Post hoc analysis revealed that null mice bite the target significantly more on bin 2–7 (p=0.0005) and bin 8 (p=0.046) compared to wild-type controls. In addition, target biting following the shock (bin1) was higher than target biting during the inter-shock interval (bin2–8) (p=0.0002) and during the tone CS (bin8) (p<0.0001). These three distinct rates of target biting behavior are in agreement with previously published report [10, 27].
Figure 7. Increased target biting in ephrin-A5−/− mice.
Defensive aggression was measured in ephrin-A5−/− and wild-type male mice in the target biting test.
(A) Ephrin-A5−/− mice had increased cumulative target biting during the 20 minutes test compared to wild-type controls. Data are presented as mean number of bites.
(B) Analysis of target biting per bin. Ephrin-A5−/− mice bite the target significantly more on bin 2–7 and bin 8 compared to wild-type controls. In addition, both genotypes bite the target more in bin 1 compared to bin 2–7 and bin 8. Data are presented as mean number of bites per bin.Ephrin-A5−/−n=9; wild-type, n=10. * indicates significantly different from wild-type; p<0.05.** indicates significantly different from bin 1; p<0.05.
3.6 Testosterone levels are comparable between ephrin-A5−/− and wild-type mice
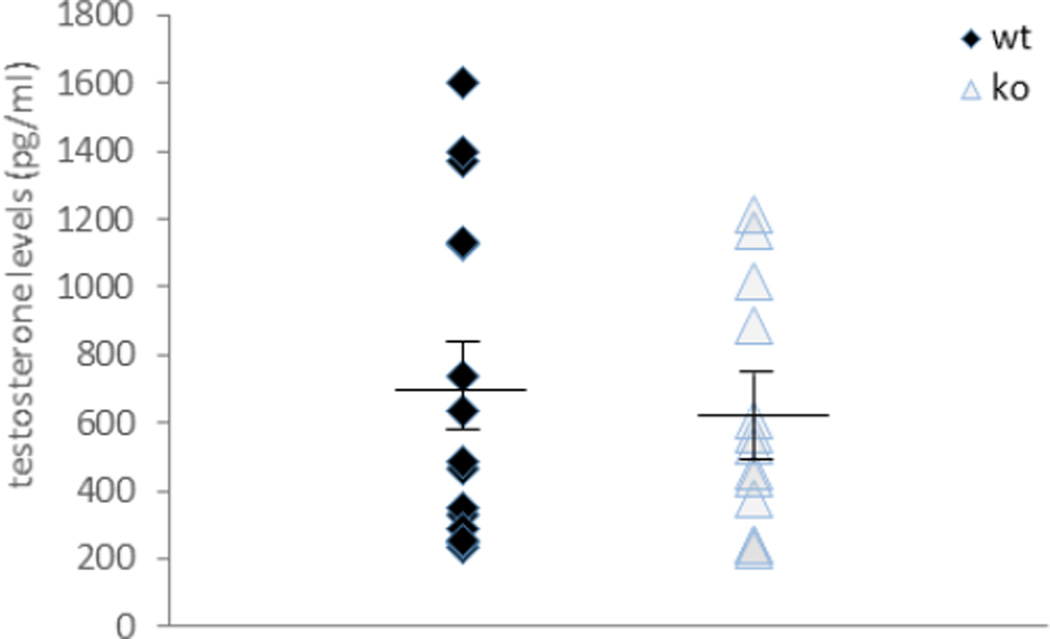
Testosterone is critical to male aggression [34–36]. To examine whether the loss of ephrin-A5 alters testosterone production, we measured testosterone levels in serum of male ephrin-A5−/− and wild-type mice. No differences were found between the two genotype (Figure 8; t=−0.60, p=0.55). However, both null and wild-type mice had high variation in blood testosterone levels, this variation was reported previously in other strains of mice [37–39]. Since hemolysis, the breakdown of erythrocytes with subsequent release of their intracellular contents, might interfere with the test results [40], we repeated the experiment using the BD Vacutainer® SST™ serum separation tubes. These tubes were used in order to minimize the presence of red blood cells in the sample. No differences were found in testosterone levels and/or variation when using these tubes (data not shown). Therefore our results (Figure 8) represent data from both experiments and show that testosterone levels are not affected by loss of ephrin-A5.
Figure 8. Testosterone levels in ephrin-A5−/− mice are within the range of wild-type control mice.
Testosterone levels were measured in serum of ephrin-A5−/− and wild-type male mice. No differences were found in testosterone levels and/or variation between null and wild-type mice.
Ephrin-A5−/−n=13; wild-type, n=15.
Data are presented as individual testosterone levels (pg/ml). Lines represent the mean testosterone levels ± SEM.
4. Discussion
In this study we observed that inactivation of ephrin-A5 in male mice results in a major reduction in offensive aggressive behavior toward an intruder male. When tested with age and genotype matched intruders, none of the ephrin-A5−/− animals engaged in attack behavior. It has been reported that the level of aggressive behavior is influenced by the intruder; changes in social investigation, movement and pheromones led to different responses from the resident [7, 32]. For example, castrated mice do not produce the pheromones that induce aggression and therefore failed to stimulate fighting in the RI test [32]. Thus, it is possible that the lack of aggression in the null mice was due to lack of stimuli from the null intruder that can be either behavioral or hormonal. To eliminate these possibilities, we used zinc sulfate-treated wild-type intruders. Intranasal zinc sulfate application has been shown to cause anosmia by destroying the olfactory epithelium [41]. Rodents treated with zinc sulfate failed to initiate a fight but elicited similar responses from the resident as non-anosmic intruders [42]. Under these conditions, ephrin-A5−/− mice were still less aggressive then wild-type controls, suggesting that the reduced aggression is due to behavioral changes in the resident and not individual differences from the intruders.
Individual recognition and gender discrimination were found to be important for the onset of aggression in rodents. An increase in investigatory sniffing often occurs before aggressive encounters in mice, suggesting that the recognition of the mouse as a “stranger” is important [43]. In addition, the detection of male olfactory cues is essential for sex discrimination and mice that are unable to detect them cannot discriminate males from females and will not engage in inter-male aggression [44]. Thus, activation of the olfactory system by male pheromone has been shown to influence aggression [45]. For example, masking animals' natural odor by artificial scents increased the latency to the first attack and reduced the number of attacks in the RI assay [46]. In addition, surgical removal of the olfactory bulb [46] as well as anosmia [47], produced by intranasal application of zinc sulfate, completely abolished the initiation of aggressive behavior in rodents. Finally, mice lacking functional cyclic nucleotide–gated channel α2 (CNGA2), which is required for the odor-evoked main olfactory epithelium signaling, or TRP2, a putative ion channel that is expressed exclusively in the vomeronasal organ, failed to display inter-male aggression in the RI test [44, 48]. As such, chemosensory cues are required for proper aggressive behavior in animals. These cues are detected by sensory neurons in two olfactory organs: the main olfactory epithelium (MOE) and the vomeronasal organ (VNO), and proceed to the main olfactory bulb and the accessory olfactory bulb respectively [49]. From here the signals are sent to specific brain regions which translate them into the appropriate behavioral response [2]. Recently, one of these regions was identified in the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) [13]. In that study, optogenetic stimulation of the VMHvl initiated aggressive behavior from a resident mouse toward intruders that under unstimulated/normal conditions would not elicit attack, while genetic inhibition of VMHvl neuronal activity prevented attacks even toward an intruder male. Furthermore, cells within the VMHvl that are activated during male aggressive behavior are mostly distinct from those that are activated during mating, suggesting that stimuli from a male intruder processed differently than those from a female intruder, and therefore produced different responses [13]. Further analysis of the VMHvl activity suggested that these neurons play a role in signaling the presence of a male olfactory cues and converting them into the appropriate social behavior, i.e. attack [50].
Ephrin-A5 is expressed in the olfactory system [51–53] and has been shown to play a role in the formation of proper olfactory sensory axon terminal mapping in the main and accessory olfactory bulb [51, 54]. Mice deficient in both ephrin-A3 and ephrin-A5 have a posterior shift in the location of two different glomerular targets in the olfactory bulb, and mice with single mutant for ephrin-A5 have misprojection of the VNO axons to the AOB. In the current study we did not detect significant genotype-dependent differences in the general olfactory behavior. Olfactory-guided foraging and the habituation to a new olfactory stimulus were comparable between the genotypes. In addition, both ephrin-A5−/− and wild-type male mice preferred female mice, suggesting that gender discrimination is intact. Thus the ability to detect chemosensory cues from the environment seems normal in the null mice. However, it is possible that specific connection to the hypothalamic aggression center is disrupted in these mice. This remains to be established in future studies.
In contrast to olfaction, visual cues do not seem to be involved in the development of offensive aggression; blind mice initiated aggressive behavior towards an unfamiliar male similar to a mouse without vision impairments [43] indicating that this behavior is mediated by olfactory cues. However, since previously, we have reported that ephrin-A5−/− mice developed ocular abnormalities [55, 56], we wanted to confirm that the lack of aggression is not due to the inability of the mice to see the intruder. Indeed, ephrin-A5−/− mice, despite the vision impairments, investigated the intruder when introduced into their home cage, suggesting that they were able to sense the presence of another animal in their cage.
We have also shown that ephrin-A5 deletion caused increased target biting compared to wild-type controls. The presence of normal and even exaggerated levels of defensive aggression demonstrate that ephrin-A5−/− mice are capable of attack behavior and therefore the lack of aggression toward an intruder male is not due to potential neuromuscular defects [57]
The influence of testosterone on male aggression has been studied extensively [2, 34–36]. In human, males between the ages of 12 to 25 are more likely to commit a crime, a pattern that was referred to as the “Young Male syndrome” [58], and occurs in concert with puberty and the rise of testosterone levels in the blood [58]. In rodents, castration reduced inter-male aggression, while testosterone supplementation restored it [6, 59–62]. In addition, testosterone treatment of wild-type mice but not mice that lack 5α-reductase, the enzyme that convert testosterone to its metabolite, increased aggressive behavior toward an intruder male [35], and conditional inactivation of the androgen receptor in the nervous system reduced it [63]. Finally, administration of anabolic androgenic steroids (AAS), a synthetic derivative of testosterone, increased aggression in animals [64–66]. However, we observed no significant differences in serum testosterone between wild-type and ephrin-A5 null mice.
In conclusion, this study identifies ephrin-A5, as a regulator of aggressive behavior in mice. In the absence of ephrin-A5, inter-male, offensive aggression is severely reduced and defensive aggression in the form of target biting is exaggerated. These observations are consistent with our previous findings that inactivation of EphA5, a receptor of ephrin-A5, also reduced inter-male aggression [23], suggesting that ephrin-A5/EphA5 signaling modulates neural pathways in brain regions that control aggression. Ephrin-A5 has been shown to regulate neural progenitor cell production [67], interneuron migration [68] and neuronal dendritic arborization [69]. Future studies will reveal which specific functions are required for the regulation of aggressive behavior.
Highlights.
-
➢
Offensive and defensive aggression were studied in ephrin-A5 mutant (ephrin-A5−/−) mice
-
➢
In the resident-intruder test for offensive aggression, ephrin-A5−/− mice exhibit severe reduction in aggression toward an intruder male.
-
➢
Defensive aggression in the form of target biting was higher in ephrin-A5−/− mice.
-
➢
Olfaction and testosterone levels were comparable between mutant and wild-type controls.
Acknowledgements
Research partially supported by 2PO1HD023315 (RZ), RO1EY019012 to RZ, P30ES005022, T32007148, and R01ES015991 to JRR; and Charles and Johanna Busch Memorial Fund to GCW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Numan M. Neurobiology of social behavior: toward understanding of the prosocial and antisocial brain. elsevier; 2014. [Google Scholar]
- 2.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 3.Anholt RR, Mackay TF. Genetics of aggression. Annual review of genetics. 2012;46:145–164. doi: 10.1146/annurev-genet-110711-155514. [DOI] [PubMed] [Google Scholar]
- 4.Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neuroscience and biobehavioral reviews. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Hormones and behavior. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RJ. Biology of aggression. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 7.Nelson RJ, Chiavegatto S. Aggression in knockout mice. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2000;41:153–162. doi: 10.1093/ilar.41.3.153. [DOI] [PubMed] [Google Scholar]
- 8.Siegel A. The neurobiology of aggression and rage. Boca Raton: CRC Press; 2005. [Google Scholar]
- 9.Miyakawa T, Yagi T, Takao K, Niki H. Differential effect of Fyn tyrosine kinase deletion on offensive and defensive aggression. Behav Brain Res. 2001;122:51–56. doi: 10.1016/s0166-4328(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 10.Johnson SK, Carlson KM, Lee J, Burr LE, Wagner GC. Effects of nicotine on target biting and resident-intruder attack. Life sciences. 2003;73:311–317. doi: 10.1016/s0024-3205(03)00289-3. [DOI] [PubMed] [Google Scholar]
- 11.Kruk MR, van der Poel AM, de Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70:292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- 12.Kruk MR. Ethology and pharmacology of hypothalamic aggression in the rat. Neuroscience and biobehavioral reviews. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nature reviews Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nature reviews Molecular cell biology. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 16.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nature neuroscience. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 17.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nature neuroscience. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 18.Hruska M, Dalva MB. Ephrin regulation of synapse formation, function and plasticity. Molecular and cellular neurosciences. 2012;50:35–44. doi: 10.1016/j.mcn.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends in cell biology. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Savelieva KV, Rajan I, Baker KB, Vogel P, Jarman W, Allen M, et al. Learning and memory impairment in Eph receptor A6 knockout mice. Neuroscience letters. 2008;438:205–209. doi: 10.1016/j.neulet.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP, et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 22.Gerlai R, Shinsky N, Shih A, Williams P, Winer J, Armanini M, et al. Regulation of learning by EphA receptors: a protein targeting study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:9538–9549. doi: 10.1523/JNEUROSCI.19-21-09538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamiya PC, Hennesy Z, Zhou R, Wagner GC. Changes in attack behavior and activity in EphA5 knockout mice. Brain research. 2008;1205:91–99. doi: 10.1016/j.brainres.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisen J, Yates PA, McLaughlin T, Friedman GC, O'Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 25.Sheleg M, Yochum CL, Wagner GC, Zhou R, Richardson JR. Ephrin-A5 deficiency alters sensorimotor and monoaminergic development. Behavioural brain research. 2013;236:139–147. doi: 10.1016/j.bbr.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. Journal of visualized experiments : JoVE. 2013:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner GC, Nabert DR, Tolbert RK. The Effects of Tail Shock on Target-Biting Behavior of Confined Mice. Aggressive Behav. 1983;9:309–313. [Google Scholar]
- 28.Czarnecki LA, Moberly AH, Rubinstein T, Turkel DJ, Pottackal J, McGann JP. In vivo visualization of olfactory pathophysiology induced by intranasal cadmium instillation in mice. Neurotoxicology. 2011;32:441–449. doi: 10.1016/j.neuro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 30.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience / editorial board. 2009. p. 24. Chapter 8:Unit 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nature neuroscience. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- 32.Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;226:967–968. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- 33.Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain, behavior, and immunity. 2010;24:1276–1280. doi: 10.1016/j.bbi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiology & behavior. 1996;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- 35.Frye CA, Rhodes ME, Walf A, Harney JP. Testosterone enhances aggression of wild-type mice but not those deficient in type I 5alpha-reductase. Brain research. 2002;948:165–170. doi: 10.1016/s0006-8993(02)03076-7. [DOI] [PubMed] [Google Scholar]
- 36.Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neuroscience and biobehavioral reviews. 1998;23:325–336. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 37.Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovascular research. 2005;65:148–157. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Klomberg KF, Garland T, Jr, Swallow JG, Carter PA. Dominance, plasma testosterone levels, and testis size in house mice artificially selected for high activity levels. Physiology & behavior. 2002;77:27–38. doi: 10.1016/s0031-9384(02)00767-9. [DOI] [PubMed] [Google Scholar]
- 39.Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. The Journal of endocrinology. 2007;194:153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- 40.Snyder JA, Rogers MW, King MS, Phillips JC, Chapman JF, Hammett-Stabler CA. The impact of hemolysis on Ortho-Clinical Diagnostic's ECi and Roche's elecsys immunoassay systems. Clinica chimica acta; international journal of clinical chemistry. 2004;348:181–187. doi: 10.1016/j.cccn.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 41.McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chemical senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- 42.J FK, H TD. Territorial behavior of laboratory rats under conditions of peripheral anosmia. Animal Learning & Behavior. 1976;4:337–340. [Google Scholar]
- 43.Doty RL. Odor-guided behavior in mammals. Experientia. 1986;42:257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- 44.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 45.Guillot PV, Chapouthier G. Olfaction, GABAergic neurotransmission in the olfactory bulb, and intermale aggression in mice: modulation by steroids. Behavior genetics. 1996;26:497–504. doi: 10.1007/BF02359754. [DOI] [PubMed] [Google Scholar]
- 46.Ropartz P. The relation between olfactory stimulation and aggressive behaviour in mice. Animal behaviour. 1968;16:97–100. doi: 10.1016/0003-3472(68)90117-6. [DOI] [PubMed] [Google Scholar]
- 47.Slotnick B, Restrepo D, Schellinck H, Archbold G, Price S, Lin W. Accessory olfactory bulb function is modulated by input from the main olfactory epithelium. The European journal of neuroscience. 2010;31:1108–1116. doi: 10.1111/j.1460-9568.2010.07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 49.Stowers L, Cameron P, Keller JA. Ominous odors: olfactory control of instinctive fear and aggression in mice. Current opinion in neurobiology. 2013;23:339–345. doi: 10.1016/j.conb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D. Decoding ventromedial hypothalamic neural activity during male mouse aggression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5971–5984. doi: 10.1523/JNEUROSCI.5109-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, et al. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 52.Deschamps C, Morel M, Janet T, Page G, Jaber M, Gaillard A, et al. EphrinA5 protein distribution in the developing mouse brain. BMC Neurosci. 2010;11:105. doi: 10.1186/1471-2202-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St John JA, Pasquale EB, Key B. EphA receptors and ephrin-A ligands exhibit highly regulated spatial and temporal expression patterns in the developing olfactory system. Brain research Developmental brain research. 2002;138:1–14. doi: 10.1016/s0165-3806(02)00454-6. [DOI] [PubMed] [Google Scholar]
- 54.Knoll B, Zarbalis K, Wurst W, Drescher U. A role for the EphA family in the topographic targeting of vomeronasal axons. Development. 2001;128:895–906. doi: 10.1242/dev.128.6.895. [DOI] [PubMed] [Google Scholar]
- 55.Son AI, Sheleg M, Cooper MA, Sun Y, Kleiman NJ, Zhou R. Formation of persistent hyperplastic primary vitreous in ephrin-A5-/- mice. Investigative ophthalmology & visual science. 2014;55:1594–1606. doi: 10.1167/iovs.13-12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yates NJ, Martin-Iverson MT, Rodger J. The role of ephrin-A2 and ephrin-A5 in sensorimotor control and gating. Behavioural brain research. 2014;275:225–233. doi: 10.1016/j.bbr.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 58.Craig IW, Halton KE. Genetics of human aggressive behaviour. Human genetics. 2009;126:101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- 59.Barfield RJ, Busch DE, Wallen K. Gonadal influence on agonistic behavior in the male domestic rat. Hormones and behavior. 1972;3:247–259. doi: 10.1016/0018-506x(72)90038-4. [DOI] [PubMed] [Google Scholar]
- 60.Barkley MS, Goldman BD. The effects of castration and Silastic implants of testosterone on intermale aggression in the mouse. Hormones and behavior. 1977;9:32–48. doi: 10.1016/0018-506x(77)90048-4. [DOI] [PubMed] [Google Scholar]
- 61.Lofgren JL, Erdman SE, Hewes C, Wong C, King R, Chavarria TE, et al. Castration eliminates conspecific aggression in group-housed CD1 male surveillance mice (Mus musculus) Journal of the American Association for Laboratory Animal Science : JAALAS. 2012;51:594–599. [PMC free article] [PubMed] [Google Scholar]
- 62.Luttge WG. Activation and inhibition of isolation induced inter-male fighting behavior in castrate male CD-1 mice treated with steroidal hormones. Hormones and behavior. 1972;3:71–81. doi: 10.1016/0018-506x(72)90009-8. [DOI] [PubMed] [Google Scholar]
- 63.Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, et al. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson S, Penatti CA, Clark AS. The role of the androgen receptor in anabolic androgenic steroid-induced aggressive behavior in C57BL/6J and Tfm mice. Hormones and behavior. 2012;61:67–75. doi: 10.1016/j.yhbeh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 65.McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Hormones and behavior. 2002;41:101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- 66.Breuer ME, McGinnis MY, Lumia AR, Possidente BP. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Hormones and behavior. 2001;40:409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- 67.Gerstmann K, Pensold D, Symmank J, Khundadze M, Hubner CA, Bolz J, et al. Thalamic afferents influence cortical progenitors via ephrin A5-EphA4 interactions. Development. 2015;142:140–150. doi: 10.1242/dev.104927. [DOI] [PubMed] [Google Scholar]
- 68.Steinecke A, Gampe C, Zimmer G, Rudolph J, Bolz J. EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence. Development. 2014;141:460–471. doi: 10.1242/dev.101691. [DOI] [PubMed] [Google Scholar]
- 69.Guellmar A, Rudolph J, Bolz J. Structural alterations of spiny stellate cells in the somatosensory cortex in ephrin-A5-deficient mice. The Journal of comparative neurology. 2009;517:645–654. doi: 10.1002/cne.22198. [DOI] [PubMed] [Google Scholar]