Abstract
Clinical diagnostics can be improved by faster and more accessible disease detection. Our laboratory has developed a point of service (POS) device capable of rapid, sensitive, automated and multiplexed biomarker detection using human saliva instead of other biofluids. In this article, we review the technology that led to the development of this POS device. This POS technology can advance clinical diagnostics by saving time due to faster diagnosis, saving money due to a shorter hospital stay and ultimately improving clinical care.
Keywords: biomarker, detection, device, diagnostic, disease, protein, saliva
Introduction
Human saliva is comprised of 99.5% water containing electrolytes, proteins, nucleic acids, peptides, polynucleotides, hormones, enzymes, cytokines, antibodies, and other components (1, 2). Saliva is primarily secreted from the parotid, the sub-mandibular and the sublingual salivary glands (3). Saliva also contains serumnal components that are transported from blood capillaries into saliva by diffusion, active transport and/or ultra-filtration via gingival crevices (4); hence, saliva can beconsidered to be a partial filtrate of blood and can provide a window into the health status of an individual. Saliva is an attractive diagnostic biofluid because its collection is relatively non-invasive, stress-free, inexpensive, and requires minimally trained personnel (5). Diagnostic methods that use biomarkers from biofluids such as blood and saliva are essential for clinical analyses. Protein and nucleic acid biomarkers can be used to detect medical conditions rapidly, ideally even before the disease presents symptoms in the patient (6). Many researchers have reported using saliva as a diagnostic fluid. For example, mRNA detection using saliva samples for oral cancer diagnosis was reported by Wong and coworkers (7). It has also been established in multiple studies that various proteins in saliva correlate with the pathophysiological state of certain medical conditions (8–11).
Laboratory testing of clinical samples can be time-intensive and expensive (12). Diagnostic testing is moving towards point-of-service (POS) devices due to the rapid results possible from POS devices where early detection is paramount (13). Multiple POS devices have been developed over the past decade for various diagnostic applications (14–19). Our laboratory has developed a portable POS device that is capable of automated, multiplexed and sensitive detection of biomarkers present in saliva (19) (Fig. 1). This review will describe the benefits and disadvantages of using saliva over blood in a clinical environment, and how a POS device can be used effectively in certain settings. We will briefly describe saliva sample preparation including saliva collection and extraction—a critical aspect of the overall diagnostic process. Next, we discuss the use of protein and nucleic acid biomarkers. We will then describe the principles of sandwich immunoassays, enzyme linked immunosorbent assays (ELISAs), digital ELISAs and microsensor arrays. Finally, we will discuss the multiplexing capabilities of these assays and their applications to salivary diagnostics.
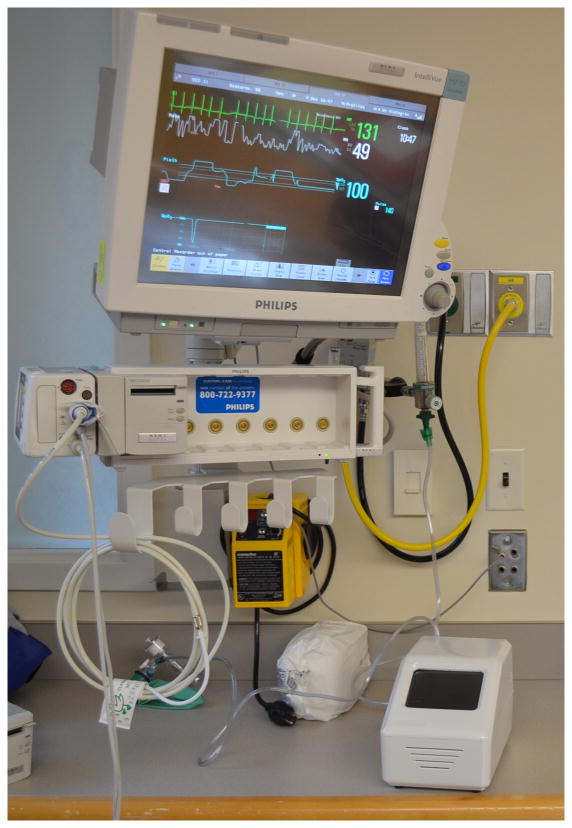
Figure 1. Point of service (POS) device, developed in our laboratory for salivary diagnostics, present at a patient’s hospital bedside.
The device is powered by a conventional AC adapter providing 12 VDC at 8.5 A (not shown), and the case is 15 cm (wide) × 15 cm (tall) × 25 cm (deep). The weight of the device is 2.7 kg. The concentrations of multiple protein biomarkers in the saliva sample are quantified via sandwich immunoassays conducted on a microfluidic chip present in the device.
Sample Preparation
Two essential components of the overall salivary diagnostic process are the saliva collection and processing steps. Saliva collection can be done by three established ways include suctioning, ejecting whole saliva and swabbing (20). Saliva can be collected under resting or ‘unstimulated’ conditions or can be ‘stimulated’ by varied methods, with gustatory and masticatory stimuli being the most common (21). The method utilizing ejection of whole saliva is recommended for collection of both unstimulated and stimulated whole saliva due to its reproducibility and reliability (21).
Once saliva is collected, it can be analyzed either as whole saliva or separated by centrifugation into the supernatant and pellet and analyzed independently. The supernatant contains dissolved proteins, nucleic acids, organic metabolites, and ions while the pellet contains bacteria and viruses, human cells, debris (such as food particles) and other insoluble components. The composition of whole saliva is complex and, due to its highly proteolytic nature, presents challenges to sample preservation, particularly for disease biomarkers (22). Unless whole saliva is utilized immediately, centrifugation is encouraged to separate the cells from the protein-containing supernatant to delay protein degradation (23). Oftentimes, protease and nuclease inhibitors are added to prevent degradation of proteins and nucleic acids, respectively. In addition, cooling the sample on ice reduces the rate of proteolysis and other degradative processes.
Transcriptional and post-transcriptional mechanisms of gene regulation can cause protein and RNA expression levels to be dissimilar (24). Due to this uncorrelated expression, using proteins as biomarkers for disease detection can sometimes present an incomplete picture. Detecting nucleic acids is not only a potential alternative for clinical biomarker detection in saliva but also a way of providing a more comprehensive picture of the biomarker expression activity by studying the full transcriptome. Numerous studies have successfully detected human mRNA transcripts in cell-free saliva by various methods including oligonucleotide microarray profiling and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) (25–29). Nucleic acid detection can be used to complement protein detection methods and provide additional information about the biomarkers and their post-transcriptional activity.
Proteins are commonly used as clinical biomarkers. The salivary proteome has been characterized and was recently reviewed by Oppenheim and coworkers (22). Analyzing proteins in saliva involves standardizing saliva collection, sample preparation and protein extraction. Extracting and stabilizing proteins for detection typically involves separating saliva supernatant by centrifugation to prevent protein degradation and analyzing the sample immediately or storing it at 0 to 4°C for short-term use or −80°C if the sample can’t be analyzed within a few hours. There are many aspects about saliva collection, storage, and composition that cannot be covered here. The remainder of this review will focus on protein biomarker detection.
Sandwich immunoassays
Sandwich immunoassays are frequently used to detect clinical biomarkers. These assays detect the presence and/or the concentration of proteins of interest using an antibody as a recognition element. Sandwich immunoassays depend on an antibody’s ability to recognize and bind to a specific site, called an epitope present on the antigen of interest. A capture antibody is first attached to a solid surface and the sample containing the protein of interest is then incubated with the immobilized antibody. The bound protein is then detected by using a second antibody, called the detection antibody, which recognizes and binds to a different epitope on the protein. This second antibody carries a label that produces a signal after binding to the protein. Before conducting sandwich immunoassays, the saliva sample is typically diluted with a blocking buffer to prevent nonspecific binding, thereby minimizing background signal (30). One way of implementing the sandwich immunoassay is to immobilize the capture antibodies onto microspheres. Our laboratory has successfully developed numerous microsphere-based immunoassays for various protein targets (30). Using microsphere-based immunoassays has been shown to improve assay sensitivity, reproducibility and analysis times (17, 31–35). In a microsphere-based immunoassay, the target-specific capture antibodies are chemically coupled to microspheres. These antibody-labeled microspheres are then sequentially incubated with a saliva sample to capture the target protein molecules, biotinylated detection antibodies, and a streptavidin-conjugated fluorescent probe (such as phycoerythrin) to label the sandwich complex (Fig. 2). The fluorescence intensities are then quantified to detect concentrations of the protein biomarkers (19, 34).
Figure 2. Workflow for the protein sandwich immunoassay.
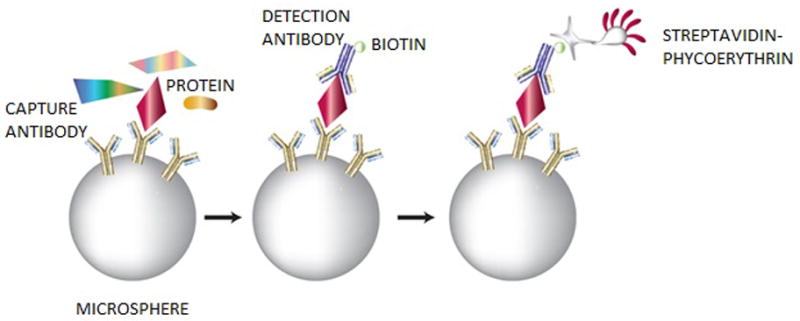
Microspheres are coupled with capture antibodies, incubated with a saliva sample and then biotinylated detection antibodies, and finally a streptavidin-conjugated fluorescent probe is added to the complex for a quantified readout.
The microsphere-based protein sandwich immunoassay described above can be carried out on optical fiber arrays (36). This array platform uses an optical fiber bundle containing approximately 50,000 individual 3.1 μm fibers. The distal ends of these optical imaging fibers are chemically etched with an acid solution to create microwells for depositing the capture microspheres (37, 38). Microspheres are randomly deposited in the etched wells before sample exposure. The assembled arrays are then exposed to the sample to capture the target molecules and the remaining steps in the assay are performed as described above (37). Fluorescent signals emitted from the individual microspheres in the array are detected using a conventional fluorescence microscope (38). This array technology, which is highly sensitive and capable of multiplexed detection, has been successfully used to detect protein biomarkers for different medical conditions such as asthma and other respiratory and inflammatory diseases (36, 39, 40).
Multiplexing Capabilities
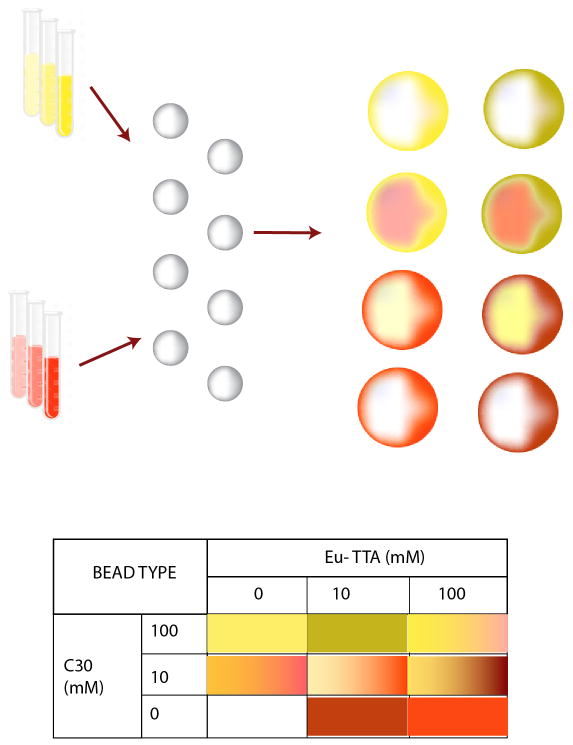
Oftentimes, a single biomarker is not sufficient for diagnosis. In these situations, detection of multiple biomarkers is required to ensure that a definitive diagnosis can be made. By encoding the microspheres to differentiate them from one another, multiplexed immunoassays can be developed where several biomarkers are detected simultaneously. There are a variety of methods for microsphere encoding including physical encoding, electronic encoding, graphical encoding, and spectrometric encoding (41). Physical encoding differentiates between different microspheres by their physical properties such as shape, composition and density (42). Electronic encoding uses radiofrequency memory tags for encoding the microspheres (43). Graphical encoding is where two dimensional patterns, such as barcodes, are encoded in the microspheres (44). Finally, spectral encoding methods encode microspheres with chemical tags that can be decoded by spectrometry (45). Chemical tags can include different luminescent materials such as lanthanides, quantum dots (QDs), fluorescent dyes, and combinations of these materials that emit light at different wavelengths to generate uniquely identifiable signatures (46). Fluorescent dyes are most commonly used for microsphere-based protein microarrays (47). The fluorescent dye encoding mechanism involves staining the microspheres with various concentrations of multiple dyes and decoding the microspheres according to their distinct spectral signatures (Fig. 3). The staining can be done either by labeling the surface of the microsphere with the dye or by entrapping the dye in the microsphere via solvent swelling (30).
Figure 3. Depiction of encoding beads with multiple fluorescent dyes to produce numerous bead types for multiplexed arrays.
Microspheres are encoded with different concentrations of two fluorescent dyes, Coumarin (C30) and Europium (Eu-TTA) to produce distinct bead types to be used for detection of multiple biomarkers simultaneously.
Integration into a POS Device
POS devices ideally use small sample volumes, detect multiple biomarkers, require minimal training and perform the analysis in an automated fashion. These devices are designed for portability and rapid diagnosis. Portable POS devices have many advantages over larger laboratory devices, including providing clinical care in remote locations (48) and convenient in-home testing such as the self-monitoring of blood glucose (49). Using saliva as a biofluid in conjunction with a POS device ensures better patient compliance because of the simple collection method.
Our laboratory has developed multiple POS diagnostic studies using human saliva. In 2008, we used colorimetric test strips to monitor the effect of hemodialysis on salivary nitrite and uric acid in renal disease patients (50). Using the test strips, reductions in salivary nitrite and uric acid were successfully observed in renal disease patients during dialysis. This assay was useful for evaluating dialysis progress and monitoring renal disease progress noninvasively.
Our lab has also been developing multiplexed assays for detecting inflammatory protein biomarkers in human saliva that have led to the development of a portable POS device. In 2009, our laboratory studied inflammatory cytokines in human saliva relevant to pulmonary inflammatory diseases (40). A multiplexed cytokine array was developed to examine endogenous mediator patterns in saliva supernatants from patients with pulmonary inflammatory diseases such as chronic obstructive pulmonary disease and asthma. This assay was useful in inflammatory disease research and diagnostics and led to a study in which a multiplexed assay was developed to measure a panel of six salivary biomarkers for diagnosing asthma and cystic fibrosis exacerbation (39). This successful assay demonstrates the potential to use the multiplexed protein array for respiratory disease diagnosis, which may be applicable to other protein biomarkers and diseases. Last year, our lab migrated these assays onto an automated, integrated POS platform (19). Six inflammatory protein biomarkers were used for the multiplexed assay. Ten μl of a saliva sample is loaded onto a microfluidic chip (containing all the necessary reagents required for the immunoassay) and the chip is inserted into the automated POS device. The results are read optically and are available within 70 minutes. This POS device was used in two hospitals on over 250 human saliva samples collected from different individuals to successfully diagnose various respiratory conditions. This study demonstrated the POS device’s potential to assist with the diagnosis of respiratory diseases and potentially provide a platform to diagnose additional medical conditions.
Future Directions
Our laboratory has demonstrated a POS device capable of automated, multiplexed and sensitive biomarker detection. To enhance our POS device’s sensitivity, we can improve the detection limit by implementing digital ELISAs using a single molecule array (SiMoA) technology. The SiMoA platform, originating from our lab, has previously been used to capture and analyze individual target molecules in the microwell optical fiber arrays (38). Briefly, SiMoA involves adding capture antibody microspheres to a sample containing the target protein molecules but where there are more microspheres than molecules. After sandwich formation with an enzyme-labeled detection antibody, the microspheres are loaded into femtoliter reaction wells and sealed with a fluorogenic substrate. Determining the number of wells that display increased fluorescence after incubation with the fluorogenic substrate quantifies the protein concentration from the original sample (51). This SiMoA technology is capable of detecting biomarkers at the attomolar (aM) to femtomolar (fM) range (52). These detection limits are hundreds to thousands of times more sensitive than conventional ELISAs, enabling the detection of target proteins at concentrations that have not been measured before. SiMoA technology was developed in our laboratory and recently extended to nucleic acids, thereby providing a sensitive detection alternative to existing technologies that utilize amplification, such as the polymerase chain reaction (PCR) (53). To summarize, SiMoA technology is being used to detect virus particles, proteins, and nucleic acid biomarkers (52, 54) and has the potential to improve the sensitivity of our POS device for future applications.
Currently, our laboratory is implementing the POS device to assess oral feeding maturity in the newborn. Premature births affect an estimated 11.5% of all pregnancies in the United States resulting in medical costs exceeding $26 billion annually (55). Most of these infants do not have the developmental maturity to successfully feed by mouth and must confront the challenges of learning to feed orally before they can be discharged from the hospital. Currently, there are no strategies available to objectively assess oral feeding maturity in newborns (56). This lack of an objective assessment tool has resulted in missed opportunities to feed infants with mature oral feeding skills, while placing immature oral feeders at risk for significant feeding-associated morbidities (57). Each failed approach results in a prolonged length of stay with an aggregated loss of millions of dollars over the neonatal population in associated health care costs (56). We are using the POS platform to provide a sensitive and multiplexed readout of the multiple biomarkers responsible for oral feeding maturity (58). This project represents an important opportunity to integrate advances in salivary molecular diagnostics into the neonatal population to enhance clinical decisions and improve patient outcomes.
The POS device developed in our laboratory using saliva has the potential to be beneficial outside the hospital environment in venues such as remote resource limited locations, areas where blood collection is not possible because of cultural issues, locations where biohazard containment is not possible and venues for individual monitoring. Future improvements to this POS device will include battery power as an option and manual methods that avoid using a pipet to make it more accessible to other venues outside the hospital.
Conclusion
A completely automated and portable POS device using noninvasively collected samples is an ideal diagnostic platform. The ability to noninvasively assess patients rapidly at the point of care is a major goal of modern medicine. Saliva is an attractive bio-fluid to assess health, disease and development (59). Furthermore, saliva is preferred over other types of biological samples due to its convenience and ease of collection. It may be collected repeatedly, even in the most vulnerable patients, without risk of harm (60).
Technological advances are now permitting the high-throughput analysis of saliva for thousands of genes, proteins and metabolites from a single sample source (2, 36). Recently, our laboratory developed an integrated automated diagnostic platform based on an antibody-based multiplexed protein microarray (19, 30). This platform fulfills the requirements of a POS device, including integration, automation, multiplexed detection ability, small sample size, fast analysis, and minimal training. The next step for this technology is translating this device into clinical care for relevant correlates requiring real-time noninvasive assessment such as for oral feeding maturity in the neonatal population.
Footnotes
Conflict of interest statement
The authors PK and DW declare that no other relationships/conditions/circumstances present a potential conflict of interest with this submitted work. There were no study sponsors involved in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
References
- 1.Carpenter GH. The Secretion, Components, and Properties of Saliva. Annual Review of Food Science and Technology. 2013;4(1):267–76. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 2.Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc. 2006;137(3):313–21. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 3.Mandel I. The role of saliva in maintaining oral homeostasis. The Journal of the American Dental Association. 1989;119(2):298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. The Journal of Prosthetic Dentistry. 2001;85(2):162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 5.Mandel ID, Wotman S. The salivary secretions in health and disease. Oral sciences reviews. 1976;(8):25–47. [PubMed] [Google Scholar]
- 6.Shipp G. Ultrasensitive measurement of protein and nucleic Acid biomarkers for earlier disease detection and more effective therapies. Biotechnol Healthc. 2006;3(2):35–40. [PMC free article] [PubMed] [Google Scholar]
- 7.Matse JH, Yoshizawa J, Wang X, Elashoff D, Bolscher JGM, Veerman ECI, et al. Discovery and Prevalidation of Salivary Extracellular microRNA Biomarkers Panel for the Noninvasive Detection of Benign and Malignant Parotid Gland Tumors. Clinical Cancer Research. 2013;19(11):3032–8. doi: 10.1158/1078-0432.CCR-12-3505. [DOI] [PubMed] [Google Scholar]
- 8.Smith DJ, Joshipura K, Kent R, Taubman MA. Effect of Age on Immunoglobulin Content and Volume of Human Labial Gland Saliva. Journal of Dental Research. 1992;71(12):1891–4. doi: 10.1177/00220345920710120701. [DOI] [PubMed] [Google Scholar]
- 9.Huang C-M. Comparative proteomic analysis of human whole saliva. Archives of Oral Biology. 2004;49(12):951–62. doi: 10.1016/j.archoralbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Nagler RM, Hershkovich O, Lischinsky S, Diamond E, Reznick AZ. Saliva analysis in the clinical setting: revisiting an underused diagnostic tool. J Investig Med. 2002;50(3):214–25. doi: 10.2310/6650.2002.33436. [DOI] [PubMed] [Google Scholar]
- 11.Nagler RM, Hershkovich O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Archives of Oral Biology. 2005;50(1):7–16. doi: 10.1016/j.archoralbio.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Mascini M, Tombelli S. Biosensors for biomarkers in medical diagnostics. Biomarkers. 2008;13(7–8):637–57. doi: 10.1080/13547500802645905. [DOI] [PubMed] [Google Scholar]
- 13.Weigl B, Domingo G, LaBarre P, Gerlach J. Towards non- and minimally instrumented, microfluidics-based diagnostic devices. Lab on a Chip. 2008;8(12):1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann M, Schrenk M, Döttinger A, Nagel S, Roeraade J, Joos TO, et al. Expanding Assay Dynamics: A Combined Competitive and Direct Assay System for the Quantification of Proteins in Multiplexed Immunoassays. Clinical Chemistry. 2008;54(6):956–63. doi: 10.1373/clinchem.2007.099812. [DOI] [PubMed] [Google Scholar]
- 15.Hartwell S, Grudpan K. Flow based immuno/bioassay and trends in micro-immuno/biosensors. Microchim Acta. 2010;169(3–4):201–20. [Google Scholar]
- 16.Sia SK, Whitesides GM. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. ELECTROPHORESIS. 2003;24(21):3563–76. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 17.Derveaux S, Stubbe BG, Braeckmans K, Roelant C, Sato K, Demeester J, et al. Synergism between particle-based multiplexing and microfluidics technologies may bring diagnostics closer to the patient. Anal Bioanal Chem. 2008;391(7):2453–67. doi: 10.1007/s00216-008-2062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spisak S, Guttman A. Biomedical Applications of Protein Microarrays. Curr Med Chem. 2009;16(22):2806–15. doi: 10.2174/092986709788803141. [DOI] [PubMed] [Google Scholar]
- 19.Nie S, Henley WH, Miller SE, Zhang H, Mayer KM, Dennis PJ, et al. An automated integrated platform for rapid and sensitive multiplexed protein profiling using human saliva samples. Lab Chip. 2014;14(6):1087–98. doi: 10.1039/c3lc51303c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michishige F, Kanno K, Yoshinaga S, Hinode D, Takehisa Y, Yasuoka S. Effect of saliva collection method on the concentration of protein components in saliva. The Journal of Medical Investigation. 2006;53(1,2):140–6. doi: 10.2152/jmi.53.140. [DOI] [PubMed] [Google Scholar]
- 21.Navazesh M. Methods for Collecting Saliva. Annals of the New York Academy of Sciences. 1993;694(1):72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 22.Helmerhorst EJ, Oppenheim FG. Saliva: a Dynamic Proteome. Journal of Dental Research. 2007;86(8):680–93. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 23.Thomadaki K, Helmerhorst EJ, Tian N, Sun X, Siqueira WL, Walt DR, et al. Whole-saliva Proteolysis and Its Impact on Salivary Diagnostics. Journal of Dental Research. 2011;90(11):1325–30. doi: 10.1177/0022034511420721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proudfoot NJ, Furger A, Dye MJ. Integrating mRNA processing with transcription. Cell. 2002;108(4):501–12. doi: 10.1016/s0092-8674(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 25.Hong J, Leung E, Fraser A, Krissansen GW. Nucleic acid from saliva and salivary cells for noninvasive genotyping of Crohn’s disease patients. Genet Test. 2008;12(4):587–9. doi: 10.1089/gte.2008.0027. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, St John MAR, Zhou X, Kim Y, Sinha U, Jordan RCK, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10(24):8442–50. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Zhou X, St John MAR, Wong DTW. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83(3):199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 28.St John MAR, Li Y, Zhou X, Denny P, Ho C-M, Montemagno C, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(8):929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 29.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56(11):3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie S, Benito-Peña E, Zhang H, Wu Y, Walt DR. Multiplexed fluorescent microarray for human salivary protein analysis using polymer microspheres and fiber-optic bundles. J Vis Exp. 2013;(80) doi: 10.3791/50726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jason AT, Xiaoguang D, Joseph MG, Michael GS, Haim HB. Polymeric microbead arrays for microfluidic applications. Journal of Micromechanics and Microengineering. 2010;20(11):115017. [Google Scholar]
- 32.Barbee KD, Hsiao AP, Roller EE, Huang X. Multiplexed protein detection using antibody-conjugated microbead arrays in a microfabricated electrophoretic device. Lab on a Chip. 2010;10(22):3084–93. doi: 10.1039/c0lc00044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derveaux S, Stubbe BG, Roelant C, Leblans M, De Geest BG, Demeester J, et al. Layer-by-layer coated digitally encoded microcarriers for quantification of proteins in serum and plasma. Anal Chem. 2008;80(1):85–94. doi: 10.1021/ac071212i. [DOI] [PubMed] [Google Scholar]
- 34.Rissin DM, Walt DR. Duplexed sandwich immunoassays on a fiber-optic microarray. Anal Chim Acta. 2006;564(1):34–9. doi: 10.1016/j.aca.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JA, Bau HH. Microfluidic, bead-based assay: Theory and experiments. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(2):228–36. doi: 10.1016/j.jchromb.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walt DR. Protein measurements in microwells. Lab Chip. 2014;14(17):3195–200. doi: 10.1039/c4lc00277f. [DOI] [PubMed] [Google Scholar]
- 37.Szurdoki F, Michael KL, Walt DR. A duplexed microsphere-based fluorescent immunoassay. Anal Biochem. 2001;291(2):219–28. doi: 10.1006/abio.2001.5041. [DOI] [PubMed] [Google Scholar]
- 38.Pantano P, Walt DR. Ordered Nanowell Arrays. Chemistry of Materials. 1996;8(12):2832–5. [Google Scholar]
- 39.Nie S, Benito-Peña E, Zhang H, Wu Y, Walt DR. Multiplexed salivary protein profiling for patients with respiratory diseases using fiber-optic bundles and fluorescent antibody-based microarrays. Anal Chem. 2013;85(19):9272–80. doi: 10.1021/ac4019523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blicharz TM, Siqueira WL, Helmerhorst EJ, Oppenheim FG, Wexler PJ, Little FF, et al. Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva. Anal Chem. 2009;81(6):2106–14. doi: 10.1021/ac802181j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braeckmans K, De Smedt SC, Leblans M, Pauwels R, Demeester J. Encoding microcarriers: present and future technologies. Nat Rev Drug Discov. 2002;1(6):447–56. doi: 10.1038/nrd817. [DOI] [PubMed] [Google Scholar]
- 42.Trau M, Battersby BJ. Novel Colloidal Materials for High-Throughput Screening Applications in Drug Discovery and Genomics. Advanced Materials. 2001;13(12–13):975–9. [Google Scholar]
- 43.Service RF. CHEMISTRY - RADIO TAGS SPEED COMPOUND SYNTHESIS. Science. 1995;270(5236):577. [Google Scholar]
- 44.Xiao X-y, Zhao C, Potash H, Nova MP. Combinatorial Chemistry with Laser Optical Encoding. Angewandte Chemie International Edition in English. 1997;36(7):780–2. [Google Scholar]
- 45.Czarnik AW. Encoding methods for combinatorial chemistry. Current Opinion in Chemical Biology. 1997;1(1):60–6. doi: 10.1016/s1367-5931(97)80109-3. [DOI] [PubMed] [Google Scholar]
- 46.Gerver RE, Gómez-Sjöberg R, Baxter BC, Thorn KS, Fordyce PM, Diaz-Botia CA, et al. Programmable microfluidic synthesis of spectrally encoded microspheres. Lab Chip. 2012;12(22):4716–23. doi: 10.1039/c2lc40699c. [DOI] [PubMed] [Google Scholar]
- 47.Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19(7):631–5. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 48.Tideman PA, Tirimacco R, Senior DP, Setchell JJ, Huynh LT, Tavella R, et al. Impact of a regionalised clinical cardiac support network on mortality among rural patients with myocardial infarction. Med J Aust. 2014;200(3):157–60. doi: 10.5694/mja13.10645. [DOI] [PubMed] [Google Scholar]
- 49.Olansky L, Kennedy L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948–9. doi: 10.2337/dc10-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blicharz TM, Rissin DM, Bowden M, Hayman RB, DiCesare C, Bhatia JS, et al. Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: a proof of principle. Clin Chem. 2008;54(9):1473–80. doi: 10.1373/clinchem.2008.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595–9. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, Song L, et al. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem. 2011;83(6):2279–85. doi: 10.1021/ac103161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song L, Shan D, Zhao M, Pink BA, Minnehan KA, York L, et al. Direct detection of bacterial genomic DNA at sub-femtomolar concentrations using single molecule arrays. Anal Chem. 2013;85(3):1932–9. doi: 10.1021/ac303426b. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Nie S, Etson CM, Wang RM, Walt DR. Oil-sealed femtoliter fiber-optic arrays for single molecule analysis. Lab Chip. 2012;12(12):2229–39. doi: 10.1039/c2lc21113k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin JA, Osterman MJK, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010;39:1–8. [PubMed] [Google Scholar]
- 56.Crowe L, Chang A, Wallace K. Instruments for assessing readiness to commence suck feeds in preterm infants: effects on time to establish full oral feeding and duration of hospitalisation. Cochrane Database Syst Rev. 2012;4:CD005586. doi: 10.1002/14651858.CD005586.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Muraskas J, Parsi K. The Cost of Saving the Tiniest Lives: NICUs versus Prevention. Virtual Mentor. 2008;10(10):655–8. doi: 10.1001/virtualmentor.2008.10.10.pfor1-0810. [DOI] [PubMed] [Google Scholar]
- 58.Maron JL, Johnson KL, Dietz JA, Chen ML, Bianchi DW. Neuropeptide Y2 receptor (NPY2R) expression in saliva predicts feeding immaturity in the premature neonate. PLoS One. 2012;7(5):e37870. doi: 10.1371/journal.pone.0037870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Forde MD, Koka S, Eckert SE, Carr AB, Wong DT. Systemic assessments utilizing saliva: part 1 general considerations and current assessments. Int J Prosthodont. 2006;19(1):43–52. [PubMed] [Google Scholar]