Abstract
The importance of specific pathways of purine metabolism for normal brain function is highlighted by several inherited disorders, such as Lesch-Nyhan disease (LND). In this disorder, deficiency of the purine recycling enzyme, hypoxanthine-guanine phosphoribosyltransferase (HGprt), causes severe neurological and behavioral abnormalities. Despite many years of research, the mechanisms linking the defect in purine recycling to the neurobehavioral abnormalities remain unclear. In the current studies, an unbiased approach to the identification of potential mechanisms was undertaken by examining changes in protein expression in a model of HGprt deficiency based on the dopaminergic rat PC6-3 line, before and after differentiation with nerve growth factor (NGF). Protein expression profiles of 5 mutant sublines carrying different mutations affecting HGprt enzyme activity were compared to the HGprt-competent parent line using the method of stable isotopic labeling by amino acids in cell culture (SILAC) followed by denaturing gel electrophoresis with liquid chromatography and tandem mass spectrometry (LC-MS/MS) of tryptic digests, and subsequent identification of affected biochemical pathways using the Database for Annotation, Visualization and Integrated Discovery (DAVID) functional annotation chart analysis. The results demonstrate that HGprt deficiency causes broad changes in protein expression that depend on whether the cells are differentiated or not. Several of the pathways identified reflect predictable consequences of defective purine recycling. Other pathways were not anticipated, disclosing previously unknown connections with purine metabolism and novel insights into the pathogenesis of LND.
Keywords: Lesch-Nyhan disease, dopamine neurons, proteome, hypoxanthine-guanine phosphoribosyltransferase
Introduction
The purines are a class of organic molecules that include adenine-based derivatives (e.g. ATP, ADP, AMP, cAMP, NAD, adenosine), guanine-based derivatives (e.g. GTP, GDP, GMP, cGMP, guanosine), and related metabolites (hypoxanthine, xanthine, and uric acid). Various purines play critical roles in many cellular functions such as serving as cofactors for enzymatic reactions and building blocks for DNA and RNA synthesis. Purines also are involved in many aspects of neuronal development and function. Multiple purines have trophic influences on neurons and glia [1-4]. Neurite extension and synaptic remodeling is regulated by a group of GTP-dependent GTPases [5, 6]. There also are intercellular signaling pathways involving adenine or guanine metabolites, and G-proteins that require cAMP or cGMP.
The importance of purines for normal brain function is highlighted by several rare inherited disorders of purine metabolism [7-10]. For example, defects in purine recycling mediated by the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGprt) cause the severe neurobehavioral problems of Lesch-Nyhan disease (LND). However, the mechanisms by which HGprt deficiency cause the clinical problems are not well understood [11]. Clinical studies have suggested that dysfunction of specific dopaminergic neurons projecting to the basal ganglia underlie many of the neurological and behavioral abnormalities seen in patients with LND [12, 13], and autopsy studies have revealed significant reductions in basal ganglia dopamine and related indices of the dopamine neuron phenotype [14-16]. Prominent abnormalities of dopamine neurons have been documented for an HGprt knockout mouse [17-20], and HGprt− neuron-like cells in culture [21-30]. These findings imply that HGprt deficiency causes a cell-intrinsic defect that can be modeled in vitro, in the absence of complex inter-cellular relationships in the developing nervous system. Although clinical and experimental studies both have pointed towards prominent involvement of dopamine neurons, the mechanism by which HGprt deficiency influences these neurons remains enigmatic, because there are no known biochemical connections linking purine metabolism with dopamine pathways [12].
Prior studies addressing the relationship between HGprt deficiency and dopamine systems have taken a largely hypothesis-driven approach, exploring proposed connections between purine metabolism and the function of dopamine neurons such uric acid toxicity [31], oxidative stress [25, 32], reduced tetrahydropterin synthesis [33], abnormal purinergic neurotransmission [34-37], G-protein dysfunction [38, 39] micro RNAs [40, 41], or dysregulation of neurodevelopmental processes [26, 27, 29, 41-43]. These studies have provided a wealth of conflicting data, so the most relevant pathways for pathogenesis remain to be established. In the current studies, we applied a proteomic approach unencumbered by specific hypotheses to disclose novel potential connections between purine recycling and neuronal dysfunction in a recently developed model of HGprt-deficient dopamine-like neurons. The results revealed that HGprt deficiency has an unexpectedly broad influence on many biochemical pathways. Several of these pathways could be predicted from the known consequences of HGprt deficiency, while others were not anticipated.
Experimental Design & Methods
Cells and culture
The current studies focused on the PC6-3 cell line, a subline of the rat PC12 pheochromocytoma line [44]. This cell line was chosen because it expresses pathways involved in dopamine synthesis and metabolism, and it exhibits rapid and high efficiency neuronal differentiation after exposure to nerve growth factor (NGF), permitting evaluation of both proliferating and post-mitotic neuron-like cells. It is well known that subclones of established cell lines exhibit idiosyncratic properties that differ from the parental line because of genetic drift during cell culture [25, 45-48]. Such idiosyncratic variation was apparent in prior studies of the PC6-3 line used in the current studies [28], raising concerns for studying only a single HGprt− subline. To guard against this problem of idiosyncratic clonal heterogeneity, 5 independent HGprt− PC6-3 subclones were compared against the PC6-3 parent line, a strategy used in prior studies of HGprt− mouse MN9D [25] and human SK-N-BE(2) M17 [48] sublines. The analytical strategy focused on delineating abnormalities that were abnormal across the HGprt− sublines as a group, rather than idiosyncratic abnormalities among individual mutants.
The 5 HGprt− mutant sublines were established and characterized earlier [28]. Each has a different mutation in the HPRT1 gene, but all have <1% residual HGprt enzyme activity. All cells routinely were grown at 37 °C in an atmosphere of 5% CO2 and 95% air in RPMI 1640 medium supplemented with 5% fetal bovine serum (Thermo Fisher Scientific, Logan UT), 10% horse serum (Invitrogen, Carlsbad CA), 1 U/mL penicillin, 2 mM L-glutamine, and 100 μg/mL streptomycin. When cells were to be harvested for studies, they were grown on flasks coated with rat tail collagen type I (BD Biosciences, Bedford MA) to promote adherence as previously described [28]. Because the consequences of HGprt deficiency may be influenced by neuronal differentiation, all cell lines were evaluated before and 4 days after exposure to 50 ng/mL of 2.5S NGF from the mouse submaxillary gland.
Protein measurements
The SILAC method was used for proteomic analyses [49]. The culture medium for labeling consisted of RPMI-1640 without arginine or lysine (89984, Thermo Fisher Scientific, Rockford IL) and dialyzed animal sera as described above. Parent cells were grown for at least 10 doublings in “heavy” medium containing 1.15 mM labeled (U-13C6, U-15N4) arginine (CNLM-539-H, Cambridge Isotope Laboratories, Andover MA) and 0.274 mM labeled (U-13C6, U-15N2) lysine (CNLM-291-H, Cambridge Isotope Laboratories). Mutant cells were grown for at least 10 doublings in “light” medium containing 1.15 mM unlabeled L-arginine (ULM-8347, Cambridge Isotope Laboratories) and 0.274 mM unlabeled lysine (ULM-8766, Cambridge Isotope Laboratories). To compensate for slight differences in growth rates among the lines, the density of initial seeding of the cultures was varied so that all cultures had a similar level of confluency at harvest. Subsequently, the supernatant was removed and cells were detached by trypsinization and washed once in RPMI-1640 without arginine, lysine or animal sera. Cells then were disrupted by suspending in 600 μL of 8M urea plus 50 mM ammonium bicarbonate. Homogenates were sonicated for 30 sec (Branson Sonifier 450, Danbury, CT) and stored at −80°C. Total protein concentrations were estimated with the Pierce BCA kit (Thermo Fisher Scientific, Rockford, IL).
Mass spectrometry (MS) was performed as previously described [50]. Briefly, samples were homogenized and solubilized in 8M urea supplemented with Halt protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). Equal protein weights of light total protein from each of the 5 HGprt− cell lines were mixed separately with heavy SILAC control internal standard protein, and run separately in a 10% SDS-PAGE gel. Five bands were excised separately for each of the 5 cell lines and in-gel digested with trypsin. Peptides were extracted in 50% acetonitrile and 5% formic acid and lyophilized (Thermo Scientific Savant ISS110 SpeedVac concentrator). Lyophilized peptides were resuspended in a loading buffer consisting of 1% acetonitrile, 0.1% formic acid, and 0.03% trifluoroacetic acid.
Samples were loaded and eluted over a 130 min reverse-phase gradient on a Waters NanoAcquity UPLC fitted with a 15 cm 100 μm ID nanoLC column packed with 1.9 μm ReproSIL Pur C18 beads (Dr. Maisch HPLC GmbH, Ammerbuch-Entringen Germany) and electrospray ionized at 2kV into a hybrid LTQ Orbitrap XL mass spectrometer (Thermo). One survey MS scan was collected on the Orbitrap (300-1600 m/z, 1x106 AGC target, 500 ms maximum ion time, at 60000 resolution followed by 10 data-dependent LTQ MS/MS scans (2 m/z isolation width, 35% collision energy, 5,000 AGC target, 150 ms maximum ion time). Raw files were converted and searched on a Sorcerer 2 IDA (Sage-N Research) with Sorcerer Sequest version 4.0.3. The searches were performed with a concatenated target-decoy rat reference database (REFSEQ version 54, containing 29735 target proteins) from the National Center for Biotechnology Information (NCBI). Search parameters included: partial tryptic restriction, parent ion mass tolerance (± 20 ppm), and dynamic modifications of oxidized methionine (+15.9949 Da), SILAC labeled lysine (+8.014199) or arginine (+10.020909). Peptides were first filtered by a mass accuracy of +/− 10ppm after correction of instrumental mass shift and then grouped into trypticity (full or partial) and charge state. This was followed by a dynamic XCorr and ΔCn filtration algorithm that reduced the protein false discovery rate to less than 1% according to the target-decoy strategy.
Quantitative analyses of the proteome and pathways
Quantitative pair-wise comparisons of parent and mutant cell lines were performed according to previously reported methods [51-53]. Results for proteomics were analyzed by automated extracted ion current (XIC) SILAC ratio comparisons. In brief, the ratio of each peptide pair (light/heavy) was transformed into logarithmic (log2) values and the detection of outliers determined using Dixon's Q test. If a peptide was identified in multiple MW fractions the intensities for the peptide pair (light and heavy) were summed to give a single cumulative peptide ratio (light/heavy). Any proteins with fewer than 2 unique peptides quantified were eliminated (n=142 proteins) to increase the reliability of the final results. The local false discovery rate was controlled at 5% by using locFDR software [54]. The Database for Annotation, Visualization and Integrated Discovery (DAVID) functional annotation chart analysis [55] of the gene symbols was then performed to identify overrepresented biochemical pathways and processes or subcellular compartments.
Results
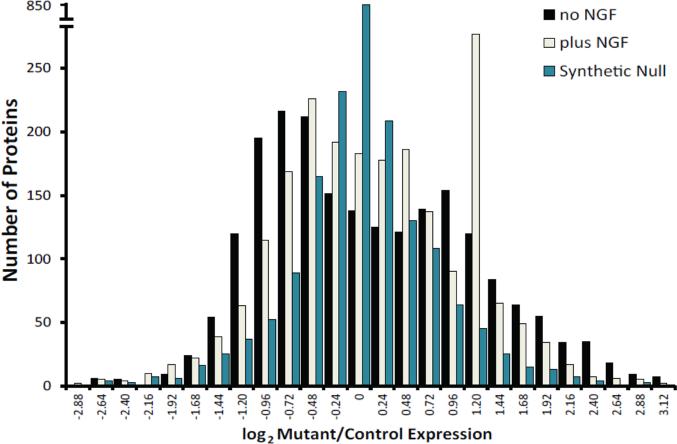
A preliminary LC-MS/MS run of SILAC internal standards derived from total protein from the control PC6-3 cell line, followed by analysis of the population of all proteins identified and quantified for light/heavy peptide ratios revealed approximately 95% efficiency of heavy labeling. After confirming labeling efficiency, light (mutant) and heavy (control) proteins were mixed 1:1, resolved into 5 bands by SDS-PAGE, and tryptic digests analyzed by LC-MS/MS. Despite equal loading of heavy and light proteins, the overall distribution of protein abundance changes in HGprt− cell lines was asymmetric, with a bias toward protein decrease in the mutant lines. The distribution of proteins was bimodal for most of the HGprt− lines, unlike comparative protein distributions from synthetic null comparisons, which display the effects of biological and technical noise from both components of the SILAC mixture, including normal control PC6-3 cells (Figure 1). These results imply an unexpectedly broad abnormality of protein synthesis and metabolism in the HGprt− lines.
Figure 1.
Distribution of altered proteins. A histogram is shown of five biological replicate log2(mutant/control) quantified protein populations at baseline (black bars) and after 4 days NGF exposure (light bars). An additional quantitative population comparison of one mutant (b) versus another mutant (d) at baseline (blue bars) is provided as a synthetic null, which represents experimental noise level contributing to false positive quantitative differences in the actual experiment.
In order to identify the most significantly affected proteins, initial normalization of the total quantified protein population relied upon centering the higher expressed subpopulation of proteins of the bimodal peak in the mutants at zero change (log2 light/heavy ratio of zero). For each experiment, 95% confidence intervals were calculated for variance within the Gaussian fit to the higher expressed subpopulation, and proteins falling outside of this range were considered candidates for significant change in the HGprt− cell lines compared to the parent line. Then, additional steps were applied to enforce a local false discovery rate of <5% across the 5 mutant HGprt− cell lines both before and after NGF-induced differentiation.
Using these criteria, and the additional requirement that reliable quantification was made using 2 or more peptides in at least 3 mutants, a total of 1055 proteins were expressed at significantly abnormal levels in the HGprt− lines in the undifferentiated state. A total of 736 proteins were abnormally expressed in the mutant lines following NGF-mediated differentiation. Many proteins were abnormal in both the undifferentiated and differentiated conditions (n=332), but some were abnormal only before (n=319) or after (n=404) differentiation (Supplemental Table 1).
Signal from canonical HGprt protein sequence was reduced by approximately 95% (1-2−4.2 = 0.95). Since 5% unlabeled signal is essentially equal to the unlabeled fraction of the heavy standard, this result implies an essentially complete loss of HGprt protein from the mutant lines. This finding of nearly complete loss of HGprt protein is consistent with prior studies showing nearly complete loss of HGprt enzymatic activity [28]. The majority of the remaining proteins identified as being abnormal in the mutant cell lines also were decreased, but some were increased. Before differentiation only 8 proteins were significantly increased in the mutants, and most were likely to be artifacts from tissue culture additives devoid of SILAC label (e.g. albumin, trypsin, keratins). After differentiation, 307 proteins were significantly increased in the HGprt− mutants. When considering only the proteins that were significantly increased, DAVID pathway analyses identified over-represented functions in the HGprt− mutants to include mRNA splicing, RNA binding, and mitochondrial function (Table 1).
Table 1.
Significantly increased proteins in HGprt- PC6-3 mutants after differentiation
| Proteins involved in RNA splicing | |
|---|---|
| CDC5L | CDC5 cell division cycle 5-like (S. pombe) |
| DNAJC8 | DnaJ (Hsp40) homolog, subfamily C, member 8 |
| FUS | fusion, derived from t(12;16) malignant liposarcoma |
| GTF2F1 | general transcription factor IIF, polypeptide 1 |
| GTF2F2 | general transcription factor IIF, polypeptide 2 |
| HNRNPA1 | heterogeneous nuclear ribonucleoprotein A1 |
| HNRNPA2B1 | heterogeneous nuclear ribonucleoprotein A2/B1 |
| LOC687575 | Splicing factor U2AF 35 kDa subunit |
| PTBP2 | polypyrimidine tract binding protein 2 |
| SF3A1 | splicing factor 3a, subunit 1 |
| SNRNP70 | U1 small nuclear ribonucleoprotein polypeptide A |
| SNRPA | small nuclear ribonucleoprotein polypeptide A |
| SNRPB2 | small nuclear ribonucleoprotein polypeptide B" |
| SNRPC | small nuclear ribonucleoprotein polypeptide C |
| SNRPD1 | small nuclear ribonucleoprotein D1 |
| SNRPD2 | small nuclear ribonucleoprotein D2 |
| SRRM1 | serine/arginine repetitive matrix 1 |
| YBX1 | Y box binding protein 1 |
| ZRANB2 | zinc finger, RAN-binding domain containing 2 |
| Proteins that bind RNA | |
| RPS15 | 40S ribosomal protein S15 (RIG protein) |
| RPL23A | 60S ribosomal protein L23a |
| CALR | calreticulin |
| CNBP | CCHC-type zinc finger, nucleic acid binding protein |
| CDC5L | CDC5 cell division cycle 5-like |
| CPSF6 | cleavage and polyadenylation specific factor 6 |
| CIRBP | cold inducible RNA binding protein |
| CSDA | cold shock domain protein A |
| ELAVL2 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 2 (Hu antigen B) |
| EIF3G | eukaryotic translation initiation factor 3, subunit G |
| EIF4H | eukaryotic translation initiation factor 4H |
| FUBP1 | far upstream element (FUSE) binding protein 1 |
| HNRNPA1 | heterogeneous nuclear ribonucleoprotein A1 |
| HNRNPA2B1 | heterogeneous nuclear ribonucleoprotein A2/B1 |
| KHDRBS1 | KH domain containing, RNA binding, signal transduction associated 1 |
| MECP2 | methyl CpG binding protein 2 |
| MRPL1 | mitochondrial ribosomal protein L1 |
| NXF1 | nuclear RNA export factor 1 |
| NOLC1 | nucleolar and coiled-body phosphoprotein 1 |
| NCL | nucleolin |
| NPM1 | nucleophosmin (nucleolar phosphoprotein B23, numatrin) |
| PTBP2 | polypyrimidine tract binding protein 2 |
| PURB | purine rich element binding protein B |
| LOC684988 | ribosomal protein S13 |
| SARNP | SAP domain containing ribonucleoprotein |
| SERBP1 | Serpine1 mRNA binding protein 1 |
| SSB | Sjogren syndrome antigen B |
| SNRPC | small nuclear ribonucleoprotein polypeptide C |
| SF3A1 | splicing factor 3a, subunit 1 |
| LOC687575 | Splicing factor U2AF 35 kDa subunit |
| SNRNP70 | U1 small nuclear ribonucleoprotein polypeptide A |
| YBX1 | Y box binding protein 1 |
| ZRANB2 | zinc finger, RAN-binding domain containing 2 |
| Mitochondrial proteins | |
| HSPD1 | 60 kDa heat shock protein 1 (chaperonin) |
| ABHD11 | abhydrolase domain containing 11 |
| ATP5H | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit d |
| ATP5I | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit E |
| ATP5B | ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide |
| ATP5D | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit |
| ATP5C1 | ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 |
| ATPIF1 | ATPase inhibitory factor 1; similar to ATPase inhibitor, mitochondrial precursor |
| ATP6V1E1 | ATPase, H+ transporting, lysosomal V1 subunit E1 |
| BOLA1 | bolA homolog 1 (E. coli) |
| CLU | clusterin |
| COQ9 | coenzyme Q9 homolog (S. cerevisiae) |
| CHCHD3 | coiled-coil-helix-coiled-coil-helix domain containing 3 |
| C1QBP | complement component 1, q subcomponent binding protein |
| CYB5A | cytochrome b5 type A (microsomal) |
| COX5B | cytochrome c oxidase subunit Vb |
| COX5A | cytochrome c oxidase, subunit Va |
| LOC688869 | cytochrome c oxidase, subunit VIb polypeptide 1 |
| DIABLO | diablo homolog (Drosophila) |
| DBI | diazepam binding inhibitor (GABA receptor modulator) |
| FXN | frataxin |
| GCSH | glycine cleavage system protein H (aminomethyl carrier) |
| GM2A | GM2 ganglioside activator |
| GRPEL1 | GrpE-like 1, mitochondrial |
| HSPE1 | heat shock protein 1 (chaperonin 10) |
| HINT2 | histidine triad nucleotide binding protein 2 |
| HAGH | hydroxyacyl glutathione hydrolase |
| HADH | hydroxyacyl-Coenzyme A dehydrogenase |
| ISCU | iron-sulfur cluster scaffold homolog (E. coli) |
| MRPL1 | mitochondrial ribosomal protein L1 |
| MRPL12 | mitochondrial ribosomal protein L12 |
| MRPL23 | mitochondrial ribosomal protein L23 |
| MRPL49 | mitochondrial ribosomal protein L49 |
| MRPS23 | mitochondrial ribosomal protein S23 |
| MRPS36 | mitochondrial ribosomal protein S36 |
| NDUFA2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2 |
| NDUFAF4 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 4 |
| NDUFS8 | NADH dehydrogenase (ubiquinone) Fe-S protein 8 |
| PARK7 | Parkinson disease (autosomal recessive, early onset) 7; similar to DJ-1 |
| PRDX6 | peroxiredoxin 6 |
| PEBP1 | phosphatidylethanolamine binding protein 1 |
| PHB2 | prohibitin 2 |
| PSAP | prosaposin |
| QDPR | quinoid dihydropteridine reductase |
| PHB | similar to prohibitin; prohibitin |
| RGD1306917 | similar to RIKEN cDNA 2900010M23; hypothetical protein LOC687308 |
| STOML2 | stomatin (Epb7.2)-like 2 |
| SOD1 | superoxide dismutase 1, soluble |
| SNCB | synuclein, beta |
| TXN2 | thioredoxin 2 |
| TFAM | transcription factor A, mitochondrial |
| TIMM9 | translocase of inner mitochondrial membrane 9 homolog (yeast) |
| UQCRFS1 | ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 |
| VDAC1 | voltage-dependent anion channel 1 |
When considering the entire population of significantly altered proteins (both increased and decreased), DAVID pathway analysis revealed several partly overlapping functional categories to be abnormal in the HGprt− mutant lines: 1) protein synthesis and metabolism, 2) mitochondrial function, 3) S-adenosyl methionine (SAM) and one-carbon metabolite generation via tetrahydrofolate, 4) monoamines and neurotransmission. Many of these fall into a broader umbrella category of proteins that bind purine nucleotides such as ATP or GTP (annotated in the tables as a superscripted 1). For example, there were a disproportionate number of protein translation elongation-associated factors significantly reduced in the mutant lines (EEF1G, EEF2, LOC498555, LOC684988, RPL6, RPL23A, RPL10A, RPL32, RPL35, RPS5, RPS15, RPS15A), many of which have GTPase activity (Table 2). Some of these showed recovery of expression after NGF differentiation, but many showed no significant recovery (Figure 2). The tRNA synthetases (YARS, KARS, DARS, WARS, MARS, SARS, QARS, VARS, IARS, RARS, GARS, AARS, TARS, NARS, LARS, CARS, and HARS) as a group were decreased significantly and consistently. All are ATP dependent. In addition, ribosome assembly factors such as BOP1 were decreased before differentiation. Also significantly reduced in the mutant cells were proteins involved in protein degradation, including peptidases (ACE, CPD, DNPEP, ERAP1, NPEPPS, RNPEP, XPNPEP1) and proteins related to the proteasome (PSMC2, PSME4, PSMD1, PSMD2, PSMD3, PSMD5, PSMD6, PSMD9, PSMD12, PSMD13, UBQLN1). Many of these proteins remained at significantly depressed levels after differentiation.
Table 2.
Significant decreases in proteins involved in protein processing HGprt- PC6-3 cells
| tRNA Aminoacylation for protein translation | |
|---|---|
| AARS1 | alanyl-tRNA synthetase |
| DARS1 | aspartyl-tRNA synthetase |
| EPRS1 | glutamyl-prolyl-tRNA synthetase |
| FARSB1 | phenylalanyl-tRNA synthetase, beta subunit |
| GARS1 | glycyl-tRNA synthetase |
| HARS1 | histidyl-tRNA synthetase |
| IARS2 | isoleucyl-tRNA synthetase 2, mitochondrial |
| KARS1 | lysyl-tRNA synthetase |
| LARS1 | leucyl-tRNA synthetase |
| LOC679383 | similar to DNA segment, Chr 5, ERATO Doi 135, expressed |
| MARS1 | methionine-tRNA synthetase |
| NARS1 | asparaginyl-tRNA synthetase |
| QARS1 | glutaminyl-tRNA synthetase |
| RARS1 | arginyl-tRNA synthetase |
| SARS1 | seryl-tRNA synthetase |
| TARS1 | threonyl-tRNA synthetase |
| VARS1 | valyl-tRNA synthetase |
| WARS1 | tryptophanyl-tRNA synthetase |
| YARS1 | tyrosyl-tRNA synthetase |
| Translation initiation Factor Activity | |
| EEF1G | eukaryotic translation elongation factor 1 gamma |
| EEF21 | similar to Elongation factor 2 (EF-2); eukaryotic translation elongation factor 2 |
| EIF2B11 | eukaryotic translation initiation factor 2B, subunit 1 alpha |
| EIF2B3 | eukaryotic translation initiation factor 2B, subunit 3 gamma |
| EIF2B4 | eukaryotic translation initiation factor 2B, subunit 4 delta |
| EIF2B5 | eukaryotic translation initiation factor 2B, subunit 5 epsilon |
| EIF2S3X1 | eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked |
| EIF3C | eukaryotic translation initiation factor 3, subunit C |
| EIF3D | eukaryotic translation initiation factor 3, subunit D |
| EIF3S6IP | eukaryotic translation initiation factor 3, subunit 6 interacting protein |
| EIF4G2 | similar to Eif4g2 protein; eukaryotic translation initiation factor 4, gamma 2 |
| EIF5B1 | eukaryotic translation initiation factor 5B |
| Proteasomal proteins | |
| PSMD1 | proteasome 26S subunit, non-ATPase, 1 (RPN2) |
| PSMD12 | proteasome 26S subunit, non-ATPase, 12 |
| PSMD13 | proteasome 26S subunit, non-ATPase, 13 |
| PSMD2 | proteasome 26S subunit, non-ATPase, 2 (RPN1) |
| PSMD3 | proteasome 26S subunit, non-ATPase, 3 |
| PSMD5 | proteasome 26S subunit, non-ATPase, 5 |
| PSMD6 | proteasome 26S subunit, non-ATPase, 6 |
| PSMD8 | proteasome 26S subunit, non-ATPase, 8 |
| PSME4 | proteasome activator subunit 4 (PA200) |
| PSMG1 | proteasome assembly chaperone 1 |
| Aminopeptidases | |
| DNPEP | aspartyl aminopeptidase |
| DPP3 | dipeptidylpeptidase 3 |
| ERAP1 | endoplasmic reticulum aminopeptidase 1 |
| METAP2 | methionyl aminopeptidase 2 |
| NPEPPS | aminopeptidase puromycin sensitive |
| RNPEP | arginyl aminopeptidase (aminopeptidase B) |
| TPP2 | tripeptidyl peptidase II |
| XPNPEP1 | X-prolyl aminopeptidase (aminopeptidase P) 1, soluble |
| Ribosomal proteins and ribosome biogenesis | |
| BOP1 | ribosome biogenesis protein BOP1 |
| NOP56 | nucleolar protein 56 |
| NOP58 | nucleolar protein 58 |
| RPL10A | 60S ribosomal protein L10a |
| RPS15A | 40S ribosomal protein S15a |
| RPS5 | 40S ribosomal protein S5 |
| RPS6KA31 | ribosomal protein S6 kinase alpha-3 |
proteins that also bind purines
Figure 2.
Altered proteins before and after differentiation. Log2(mutant/control) average quantified protein amounts at 0 days (left) and 4 days NGF exposure (right) are shown for proteins which were significantly down-regulated in HGprt− mutants compared to control cell line. Selected proteins which remained significantly downregulated at 4 days (blue lines) and HGprt itself (thick black line) are listed under the “No Recovery” heading and segregate from proteins which regain expression at 4 days NGF treatment (red lines), that are listed under the “Recovered” heading.
Many mitochondrial proteins were significantly increased or decreased in the HGprt− lines (Table 3). This finding may not be surprising, because many of these proteins are involved in purine synthesis or bind purines or their derivatives. Some of these proteins recovered following NGF differentiation, whereas others did not (Figure 2).
Table 3.
Significant abnormalities of proteins involved in mitochondrial function
| ABCB71 | ATP-binding cassette, sub-family B (MDR/TAP), member 7 |
| ABCD31 | ATP-binding cassette, sub-family D (ALD), member 3 |
| ABCE1 | ATP-binding cassette, sub-family E (OABP), member 1 |
| ABHD112 | abhydrolase domain containing 11 |
| ACACA1 | acetyl-coenzyme A carboxylase alpha |
| ACAD91 | acyl-Coenzyme A dehydrogenase family, member 9 |
| ACLY1 | ATP citrate lyase |
| ACO2 | aconitase 2, mitochondrial |
| ACSL11 | acyl-CoA synthetase long-chain family member 1 |
| ADSL | adenylosuccinate lyase |
| AGPS1 | alkylglycerone phosphate synthase |
| ALDH3A2 | aldehyde dehydrogenase 3 family, member A2 |
| ARMC10 | armadillo repeat containing 10 |
| ASL | argininosuccinate lyase |
| ATAD11 | ATPase family, AAA domain containing 1 |
| ATIC | 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase |
| ATP5B1,2 | ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide |
| ATP5C12 | ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 |
| ATP5D2 | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit |
| ATP5H2 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit d |
| ATP5I2 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit E |
| ATP6V1E12 | ATPase, H+ transporting, lysosomal V1 subunit E1 |
| ATPIF12 | ATPase inhibitory factor 1; similar to ATPase inhibitor, mitochondrial precursor |
| BAX | Bcl2-associated X protein |
| BCAT2 | branched chain aminotransferase 2, mitochondrial |
| BOLA12 | bolA homolog 1 (E. coli) |
| C1QBP2 | complement component 1, q subcomponent binding protein |
| CCT71 | chaperonin containing Tcp1, subunit 7 (eta) |
| CDS2 | CDP-diacylglycerol synthase (phosphatidate cytidylyltransferase) 2 |
| CHCHD32 | coiled-coil-helix-coiled-coil-helix domain containing 3 |
| CLIC4 | chloride intracellular channel 4 (mitochondrial) |
| CLPB1 | ClpB caseinolytic peptidase B homolog (E. coli) |
| CLTC | clathrin, heavy chain (Hc) |
| CLU2 | clusterin |
| COQ92 | coenzyme Q9 homolog (S. cerevisiae) |
| COX2 | COXII |
| COX4I2 | cytochrome c oxidase subunit IV isoform 2 |
| COX5A | cytochrome c oxidase, subunit Va |
| COX5B2 | cytochrome c oxidase subunit Vb |
| CPT1A | carnitine palmitoyltransferase 1a, liver |
| CS | citrate synthase; similar to citrate synthase |
| CTPS21 | CTP synthase II |
| CYB5A | cytochrome b5 type A (microsomal) |
| CYB5R1 | cytochrome b5 reductase 1 |
| CYB5R31 | cytochrome b5 reductase 3 |
| DBI2 | diazepam binding inhibitor (GABA receptor modulator) |
| DIABLO2 | diablo homolog (Drosophila) |
| DNM1L1 | dynamin 1-like |
| DRG21 | developmentally regulated GTP binding protein 2 |
| ETFDH | electron-transferring-flavoprotein dehydrogenase |
| FDPS | farnesyl diphosphate synthase (farnesyl pyrophosphate synthetase, dimethylallyltranstransferase, geranyltranstransferase) |
| FKBP8 | FK506 binding protein 8, 38kDa |
| FXN2 | frataxin |
| GARS1 | glycyl-tRNA synthetase |
| GCSH2 | glycine cleavage system protein H (aminomethyl carrier) |
| GM2A2 | GM2 ganglioside activator |
| GOT2 | glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 (soluble) |
| GPD2 | glycerol-3-phosphate dehydrogenase 2, mitochondrial |
| GRPEL11,2 | GrpE-like 1, mitochondrial |
| HADH2 | hydroxyacyl-Coenzyme A dehydrogenase |
| HADHA | hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), alpha subunit |
| HAGH2 | hydroxyacyl glutathione hydrolase |
| HINT22 | histidine triad nucleotide binding protein 2 |
| HK11 | hexokinase 1 |
| HK21 | hexokinase 2 |
| HSP90AB11 | heat shock protein 90kDa alpha (cytosolic), class B member 1 |
| HSPD11,2 | 60 kDa heat shock protein 1 (chaperonin) |
| HSPE11,2 | heat shock protein 1 (chaperonin 10) |
| IARS2 | isoleucyl-tRNA synthetase 2, mitochondrial |
| IDE1 | insulin degrading enzyme |
| ISCU2 | iron-sulfur cluster scaffold homolog (E. coli) |
| KRT5 | keratin 5 |
| LDHA | lactate dehydrogenase A |
| LETM1 | leucine zipper-EF-hand containing transmembrane protein 1 |
| LOC606294 | hypothetical protein LOC606294 |
| LOC6888692 | cytochrome c oxidase, subunit VIb polypeptide 1 |
| LONP11 | lon peptidase 1, mitochondrial |
| LRPPRC | leucine-rich PPR-motif containing |
| LRRC59 | leucine rich repeat containing 59 |
| MAOA1 | monoamine oxidase A |
| ME1 | malic enzyme 1, NADP(+)-dependent, cytosolic |
| MGEA5 | meningioma expressed antigen 5 (hyaluronidase) |
| MGST1 | microsomal glutathione S-transferase 1 |
| MRPL12 | mitochondrial ribosomal protein L1 |
| MRPL122 | mitochondrial ribosomal protein L12 |
| MRPL232 | mitochondrial ribosomal protein L23 |
| MRPL492 | mitochondrial ribosomal protein L49 |
| MRPS232 | mitochondrial ribosomal protein S23 |
| MRPS362 | mitochondrial ribosomal protein S36 |
| MTCH1 | mitochondrial carrier homolog 1 (C. elegans) |
| MTCH2 | mitochondrial carrier homolog 2 (C. elegans) |
| MTHFD11 | methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1, methenyltetrahydrofolate cyclohydrolase, formyltetrahydrofolate synthetase |
| MTHFD1L1 | methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1-like |
| NARS1 | asparaginyl-tRNA synthetase |
| NDUFA22 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2 |
| NDUFAF42 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 4 |
| NDUFS1 | NADH dehydrogenase (ubiquinone) Fe-S protein 1 |
| NDUFS2 | NADH dehydrogenase (ubiquinone) Fe-S protein 2 |
| NDUFS7 | NADH dehydrogenase (ubiquinone) Fe-S protein 7 |
| NDUFS82 | NADH dehydrogenase (ubiquinone) Fe-S protein 8 |
| NDUFV1 | NADH dehydrogenase (ubiquinone) flavoprotein 1 |
| NLN | neurolysin (metallopeptidase M3 family) |
| NME31 | non-metastatic cells 3, protein expressed in |
| NNT | nicotinamide nucleotide transhydrogenase |
| NRD1 | nardilysin 1 (N-arginine dibasic convertase) |
| OAT | ornithine aminotransferase (gyrate atrophy) |
| OGDH | oxoglutarate (alpha-ketoglutarate) dehydrogenase (lipoamide) |
| OPA11 | optic atrophy 1 homolog (human) |
| PARK72 | Parkinson disease (autosomal recessive, early onset) 7; similar to DJ-1 |
| PC1 | pyruvate carboxylase |
| PCK21 | phosphoenolpyruvate carboxykinase 2 (mitochondrial) |
| PDE12 | phosphodiesterase 12 |
| PEBP11,2 | phosphatidylethanolamine binding protein 1 |
| PHB2 | prohibitin |
| PHB22 | prohibitin 2 |
| PICK1 | protein interacting with PRKCA 1 |
| POR1 | P450 (cytochrome) oxidoreductase |
| PPP1CC | protein phosphatase 1, catalytic subunit, gamma isoform |
| PRDX62 | peroxiredoxin 6 |
| PRKACA1 | protein kinase, cAMP-dependent, catalytic, alpha |
| PRKCD1 | protein kinase C, delta |
| PSAP2 | prosaposin |
| PTCD3 | Pentatricopeptide repeat domain 3 |
| PTPN11 | protein tyrosine phosphatase, non-receptor type 11 |
| QARS1 | glutaminyl-tRNA synthetase |
| QDPR2 | quinoid dihydropteridine reductase |
| RAB11B1 | RAB11B, member RAS oncogene family |
| RAB351 | RAB35, member RAS oncogene family |
| RAB3D1 | RAB3D, member RAS oncogene family |
| RARS1 | arginyl-tRNA synthetase |
| RGD13069172 | similar to RIKEN cDNA 2900010M23; hypothetical protein LOC687308 |
| RGD1309676 | similar to RIKEN cDNA 5730469M10 |
| RGD1309922 | similar to 2610301G19Rik protein |
| RHOA1 | ras homolog gene family, member A; ras homolog gene family, member C |
| RPL10A | ribosomal protein L10A; similar to ribosomal protein L10a |
| RPS15A | ribosomal protein S15a |
| SARS1 | seryl-tRNA synthetase |
| SDHA1 | succinate dehydrogenase complex, subunit A, flavoprotein (Fp) |
| SFXN1 | sideroflexin 1 |
| SHMT1 | serine hydroxymethyltransferase 1 (soluble) |
| SLC16A1 | solute carrier family 16, member 1 (monocarboxylic acid transporter 1) |
| SLC25A1 | solute carrier family 25 (mitochondrial carrier, citrate transporter), member 1 |
| SLC25A11 | solute carrier family 25 (mitochondrial carrier; oxoglutarate carrier), member 11 |
| SLC25A20 | solute carrier family 25 (carnitine/acylcarnitine translocase), member 20 |
| SLC25A3 | solute carrier family 25 (mitochondrial carrier, phosphate carrier), member 3 |
| SLC25A4 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 |
| SLC25A5 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 5 |
| SNCB2 | synuclein, beta |
| SND1 | staphylococcal nuclease and tudor domain containing 1 |
| SOD12 | superoxide dismutase 1, soluble |
| STOML22 | stomatin (Epb7.2)-like 2 |
| STXBP1 | syntaxin binding protein 1 |
| SUCLA21 | succinate-CoA ligase, ADP-forming, beta subunit |
| SURF4 | surfeit 1; surfeit 4 |
| SYNJ2BP | synaptojanin 2 binding protein |
| TBC1D15 | TBC1 domain family, member 15 |
| TFAM2 | transcription factor A, mitochondrial |
| TFRC | transferrin receptor |
| TH | tyrosine hydroxylase |
| TIMM92 | translocase of inner mitochondrial membrane 9 homolog (yeast) |
| TOMM70A | translocase of outer mitochondrial membrane 70 homolog A (S. cerevisiae) |
| TST | thiosulfate sulfurtransferase, mitochondrial |
| TXN22 | thioredoxin 2 |
| UQCRFS12 | ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 |
| VARS1 | valyl-tRNA synthetase 2, mitochondrial (putative); valyl-tRNA synthetase |
| VDAC12 | voltage-dependent anion channel 1 |
| VDAC3 | voltage-dependent anion channel 3 |
Proteins that also bind purines.
Proteins that were significantly increased; all of the rest were decreased.
SAM and folate are cofactors with roles as methyl group donors in multiple biosynthetic pathways, including methylation of protein or DNA. A significant over-representation of proteins in both of these pathways was evident in the HGprt− mutant lines (Figure 3). Notably, methyl CpG binding protein 2 (MeCP2) was significantly elevated after differentiation, potentially balancing a significant decrease in de novo DNA methyltransferase (DNMT1) across the mutant lines.
Figure 3.
Altered proteins involved in single-carbon transfer reactions. Each mutant line is shown separately (A, B, C, D) both before (0, open area) and 4 days after (4, shaded area) NGF-mediated differentiation. All of these proteins were significantly reduced in comparison to the normal controls, and virtually all showed significant recovery after differentiation.
The HGprt− lines also showed multiple abnormalities in proteins involved in neurotransmission (Table 4). For example, tyrosine hydroxylase (TH) is the rate-limiting enzyme for dopamine synthesis and was significantly reduced in the mutant lines before and after differentiation. The vesicle-associated membrane proteins VAMP2 and VAMP3, which are involved in vesicular dopamine release, also were reduced in the mutants. The pheochromocytoma signature secretory vesicle proteins, SCG2 and SCG3, were elevated in the mutant lines after differentiation. Monoamine oxidase A, which degrades dopamine was reduced. Also affected were numerous small GTPases known to be involved in neuronal differentiation and the establishment and formation of neurites and synapses such as 3C, 8A, 27A, RAB3A, RAC1, RHOA, RHOG, and others.
Table 4.
Significant decreases involved in proteins for neural transmission in HGprt- PC6-3 cells
| Amine biosynthesis | |
| ASL1 | argininosuccinate lyase |
| ASNS | asparagine synthetase |
| GOT2 | glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) |
| MRI1 | methylthioribose-1-phosphate isomerase homolog (S. cerevisiae) |
| MTHFD11 | methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1, methenyltetrahydrofolate cyclohydrolase, formyltetrahydrofolate synthetase |
| PHGDH | 3-phosphoglycerate dehydrogenase |
| PSAT1 | phosphoserine aminotransferase 1 |
| SHMT1 | serine hydroxymethyltransferase 1 (soluble) |
| TH | tyrosine hydroxylase |
| Amine binding | |
| AARS1 | alanyl-tRNA synthetase |
| FASN | fatty acid synthase |
| GCLC1 | glutamate-cysteine ligase, catalytic subunit |
| GOT2 | glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2) |
| KARS1 | lysyl-tRNA synthetase |
| MAOA1 | monoamine oxidase A |
| NEDD4 | neural precursor cell expressed, developmentally down-regulated gene 4 |
| RARS1 | arginyl-tRNA synthetase |
| SHMT1 | serine hydroxymethyltransferase 1 (soluble) |
| SLC1A5 | solute carrier family 1 (neutral amino acid transporter), member 5 |
| TH | tyrosine hydroxylase |
| Neurotransmitter transport | |
| NSF1 | N-ethylmaleimide-sensitive factor |
| RAB141 | RAB14, member RAS oncogene family |
| RAB3C1 | RAB3C, member RAS oncogene family |
| SLC25A4 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 |
| STX1A | syntaxin 1A (brain) |
| STX1B | syntaxin 1B |
| STX7 | syntaxin 7 |
| SV2A | synaptic vesicle glycoprotein 2a |
| SYT1 | synaptotagmin I |
| VAMP2 | vesicle-associated membrane protein 2 |
| Neurotransmitter regulation | |
| MAOA1 | monoamine oxidase A |
| NSF1 | N-ethylmaleimide-sensitive factor |
| RAB141 | RAB14, member RAS oncogene family |
| RAB3C1 | RAB3C, member RAS oncogene family |
| SLC25A4 | solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 |
| STX1A | syntaxin 1A (brain) |
| STX7 | syntaxin 7 |
| SYT1 | synaptotagmin I |
| TH | tyrosine hydroxylase |
| VAMP2 | vesicle-associated membrane protein 2 |
| Neurotransmitter secretion/release | |
| NSF1 | N-ethylmaleimide-sensitive factor |
| RAB141 | RAB14, member RAS oncogene family |
| RAB3C1 | RAB3C, member RAS oncogene family |
| STX1A | syntaxin 1A (brain) |
| STX7 | syntaxin 7 |
| SYT1 | synaptotagmin I |
| VAMP2 | vesicle-associated membrane protein 2 |
| CADPS | calcium-dependent secretion activator 1; required for Ca++-dependent exocytosis of synaptic vesicles |
| SCAMP3 | secretory carrier-associated membrane protein 3 |
Proteins that also bind purines.
Discussion
Results from the current studies reveal that loss of HGprt-mediated purine recycling has an unexpectedly broad influence on protein expression in dopaminergic PC6-3 cells. The most significantly and consistently affected biological pathways included protein synthesis and metabolism, mitochondrial function, single methyl donor pathways involving SAM or folate, and monoamine neurotransmission.
Proteomic-metabolomic relationships
Prior studies of the PC6-3 model have shown that the loss of HGprt has a relatively small influence on total purine metabolite levels that varies according to differentiation status [28]. Among the major bioactive purines found in mammalian cells, the mutant cells showed only ~20% loss of AMP in the undifferentiated stated and ~50% loss of IMP in the differentiated state. However, there were no apparent decrements in any downstream purine metabolites such as ATP or GTP. Despite the relatively small and circumscribed changes in purine pools, the current studies reveal widespread alterations in the proteome. The extent of the changes in protein expression is surprising, given the specific role of HGprt in recycling purine bases and the relatively minor changes in purine nucleotides in HGprt− PC6-3 cells previously reported.
One potential explanation for the apparent discrepancy between the minor influence of HGprt deficiency on purine metabolites and the much broader impact on protein expression relates to the cell cycle. A relatively minor decrement of purine nucleotides may slow cell division, when there is a heavy demand for purine nucleotides for DNA replication and RNA transcription. This idea is consistent with a number of prior observations. First, cell proliferation is tightly linked with purine biosynthesis, and the demand for synthesis varies with the cell cycle,[56-59]. Second, protein synthesis is among the most sensitive of all cellular processes to ATP deficits [58, 59]. In fact, HGprt− cells tend to grow more slowly than their HGprt-competent counterparts in culture,[25, 48] and prior transcriptomic studies of HGprt-deficient cells have pointed to abnormalities of cell cycling [60]. Thus when purines may be limiting during cell division, cells may down-regulate protein expression to conserve energy and purine nucleotides. This down-regulation of protein synthesis may then only partly recover in the few days it takes for PC6-3 cells to cease cell division during differentiation. This hypothesis could explain why many of the significantly reduced proteins in the current studies appear to recover at least partly after being exposed to NGF for 4 days, when most cells are no longer actively dividing.
An alternative explanation for the minor changes in purines versus broader changes in protein synthesis in the HGprt− cells is that purines play so many essential roles in so many different pathways. Many of the pathways affected in the HGprt− cells, including those involved in protein synthesis, are directly or indirectly dependent upon adenine or guanine nucleotides. For example, there is broad dysregulation of many pathways that depend on ATP to drive energy-dependent reactions, kinases that require ATP as the phosphate donor, or proteins that utilize adenine-based cofactors such as NAD or cAMP. There is similar dysregulation of GTP-dependent pathways including those that involve small GTPases, guanine nucleotide exchange factors, or G-protein dependent signaling. Even release of nascent proteins from signal recognition particle to the endoplasmic reticulum is GTP-dependent [61]. Thus minor decrements in purine levels may have a very broad impact on protein synthesis, especially if purine-dependent regulatory proteins are affected, or if purine limitation is transiently exaggerated during a specific stage in the cell cycle.
Proteomic-transcriptomic relationships
There are no prior proteomic studies of HGprt-deficient cells for comparisons with the current studies, but there are several published transcriptomic studies involving mouse MN9D neuroblastoma cells [26], human NT2 neuroblastoma cells [43], human SH-SY5Y neuroblastoma cells [27], mouse ESD3 embryonic stem cells [30], and human fibroblasts [60]. A common finding in all of these studies was an unexpectedly broad array of gene expression abnormalities. The prominent disruption of SAM- and folate-related single carbon donor reactions may explain these prior findings. SAM contributes to the methylation of histones, a process that has a dramatic influence on chromatin structure and DNA transcription. Folate contributes to methylation of DNA CpG islands, a process central to silencing of DNA transcription.
Involvement of either the SAM or folate pathways could result in substantial and widespread changes in the expression of both mRNA and miRNA species. These changes, in turn, could have a major impact on cell division, when specific genes must be turned on or off in a carefully orchestrated series. They also could have a significant impact on the differentiation of specific cell types such as neurons, where highly regulated changes in gene expression must occur.
Proteomic-dopaminergic relationships
The current studies confirm a prominent influence of HGprt deficiency on the dopaminergic phenotype of PC6-3 cells. These findings are consistent with prior studies showing prominent abnormalities of dopamine neurons in human LND brains collected at autopsy [14-16], HGprt knockout mice [17-19], and other dopaminergic neural cells in culture [16, 20-22, 24, 25, 29].
The mechanisms responsible for dysfunction of dopamine neurons in HGprt deficiency remain enigmatic. Previously we proposed that HGprt influences dopamine neurons via dysregulation of developmental pathways involving neuronal differentiation [26]. This proposal received support from microarray studies of HGprt− MN9D neuroblastoma cells pointing to dysregulation of the engrailed genes En1 and En2 [26]. Later studies involving human NT2 neuroblastoma cultures in which HGprt was knocked down using short hairpin RNAs also revealed dysregulation of the developmental transcription factors Lmx1a and Msx1 [43]. Another study involving the shRNA knockdown strategy applied to human SH-SY5Y neuroblastoma cultures suggested abnormalities of the Wnt/beta-catenin pathways [27], and another showed changes in microRNAs that may influence levels of these transcription factors [40, 41]. A recent study of the transcriptome using the shRNA strategy to knock down HGprt in ESD3 mouse embryonic stem cells differentiated towards the dopaminergic phenotype confirmed prominent dysregulation of the dopaminergic phenotype and associated developmental transcription factors [30].
The current proteomic studies provide some insights into the mechanisms that may underlie abnormal development and differentiation. Specifically, the HGprt− PC6-3 lines showed a significant reduction in multiple small GTPases and related guanine-nucleotide exchange factors (GEFs). Some of these proteins are known to play a prominent role in neurite extension and synaptic remodeling [5, 6], and others play important roles as transcription factors during early development. Thus disruption of one or more of the GTPases involved in dopamine neuron development and differentiation, potentially combined with abnormalities of SAM- or folate-mediated regulatory processes that control gene expression, could explain the abnormalities associated with HGprt deficiency in dopamine neurons.
Study limitations
The current studies provide novel insights into the biology of HGprt deficiency, but they also have some limitations that should be acknowledged. The main limitation is that the PC6-3 cell line used in these studies was derived from a rodent pheochromocytoma. Whether the results found here can be extrapolated to human brain dopamine neurons remains to be established. Unfortunately, large-scale proteomic studies of brain dopamine neurons from LND brains are not technically feasible. Despite the potential species differences, it is interesting to note that some of the abnormalities detected using the PC6-3 cells are similar to those previously reported for immunostains of specific proteins in human autopsy material, such as reductions in TH [16].
Another limitation is that the unexpectedly broad abnormalities in protein synthesis and the bimodal distribution of changes in some of the HGprt− lines required the application of a statistical strategy that might be overly conservative. This strategy means that we most likely revealed the most robust protein changes in the mutant PC6-3 cells. However, we may have inadvertently missed many more proteins that are significantly affected. The final limitation of our study is that we have not empirically validated the majority of the proteomic findings obtained from the PC6-3 cells. Instead, we relied on existing information from prior studies that have shown reductions in HGprt and TH in HGprt− PC6-3 cells, human LND brains or the HGprt knockout mouse [16, 62, 63].
To address some of these limitations, proteomic studies of autopsy material from LND brains may be useful. Additionally, proteomic studies of differentiating neurons from induced pluripotent stem cells prepared from LND fibroblasts could also be useful. Such cells also could be useful for determining if correcting the presumed purinergic deficits with exogenously supplied purines might reverse some of the abnormalities found in recent proteomic or transcriptomic studies.
Supplementary Material
Highlights.
Loss of purine salvage causes a broad disruption of the proteome in cultured cells.
Changes in individual proteins depend on the state of neuronal differentiation.
Many protein changes may be explained by chronic, mild purine deficiency.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (R24 DK082840 and HD053312) and the Proteomics Core of the Emory Neuroscience NINDS Core Facilities grant, (P30NS055077). We thank Shan Randall and David C. Muddiman for scientific advice.
Abbreviations
- DAVID
Database for Annotation, Visualization and Integrated Discovery, and HGprt, hypoxanthine-guanine phosphoribosyltransferase
- LC
liquid chromatography
- LND
Lesch-Nyhan Disease
- MS
mass spectrometry
- N/A
not available
- NGF
nerve growth factor
- SILAC
stable isotopic labeling by amino acids in cell culture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22:194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32:189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Rathbone MP, Middlemiss PM, Gysbers JW, Andrew C, Herman MAR, Reed J, K., Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purine in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 5.de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci. 2008;30:47–58. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- 6.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyhan WL. Disorders of purine and pyrimidine metabolism. Mol Genet Metab. 2005;86:25–33. doi: 10.1016/j.ymgme.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Jinnah HA, Sabina RL, Van Den Berghe G. Metabolic disorders of purine metabolism affecting the nervous system. Handb Clin Neurol. 2013;113:1827–1836. doi: 10.1016/B978-0-444-59565-2.00052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurecka A. Inborn errors of purine and pyrimidine metabolism. J Inherit Metab Dis. 2009;32:247–263. doi: 10.1007/s10545-009-1094-z. [DOI] [PubMed] [Google Scholar]
- 10.Camici M, Micheli V, Ipata PL, Tozzi MG. Pediatric neurological syndromes and inborn errors of purine metabolism. Neurochem Int. 2010;56:367–378. doi: 10.1016/j.neuint.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Jinnah HA. Lesch-Nyhan disease: from mechanism to model and back again. Dis Model Mech. 2009;2:116–121. doi: 10.1242/dmm.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan syndrome and the basal ganglia. Brain Res. Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 13.Baumeister AA, Frye GD. The biochemical basis of the behavioral disorder in the Lesch-Nyhan syndrome. Neurosci. Biobehav. Rev. 1985;9:169–178. doi: 10.1016/0149-7634(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N. Engl. J. Med. 1981;305:1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain Dev. 2000;22(Suppl 1):S122–S131. doi: 10.1016/s0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 16.Goettle M, Prudente CN, Fu R, Sutcliffe D, Pang H, Cooper D, Veledar E, Glass JD, Gearing M, Visser JE, Jinnah HA. Loss of neurotransmitter phenotype among midbrain dopamine neurons in Lesch-Nyhan disease. Ann. Neurol. 2014;76:95–107. doi: 10.1002/ana.24191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, Breese GR. Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J. Neurochem. 1999;72:225–229. doi: 10.1046/j.1471-4159.1999.0720225.x. [DOI] [PubMed] [Google Scholar]
- 18.Jinnah HA, Langlais PJ, Friedmann T. Functional analysis of brain dopamine systems in a genetic mouse model of Lesch-Nyhan syndrome. J. Pharmacol. Exp. Ther. 1992;263:596–607. [PubMed] [Google Scholar]
- 19.Jinnah HA, Wojcik BE, Hunt MA, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ, Friedmann T. Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J. Neurosci. 1994;14:1164–1175. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith D, Friedmann T. Characterization of the dopamine defect in primary cultures of dopaminergic neurons from hypoxanthine phosphoribosyltransferase knockout mice. Mol. Ther. 2000;1:486–491. doi: 10.1006/mthe.2000.0057. [DOI] [PubMed] [Google Scholar]
- 21.Bitler CM, Howard BD. Dopamine metabolism in hypoxanthine-guanine phosphoribosyltransferase-deficient variants of PC12 cells. J. Neurochem. 1986;47:107–112. doi: 10.1111/j.1471-4159.1986.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 22.Yeh J, Zheng S, Howard BD. Impaired differentiation of HPRT-deficient dopaminergic neurons: a possible mechanism underlying neuronal dysfunction in Lesch-Nyhan syndrome. J. Neurosci. Res. 1998;53:78–85. doi: 10.1002/(SICI)1097-4547(19980701)53:1<78::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Boer P, Brosh S, Wasserman L, Hammel I, Zoref-Shani E, Sperling O. Decelerated rate of dendrite outgrowth from dopaminergic neurons in primary culture from brains of hypoxanthine phosphoribosyltransferase-deficient knockout mice. Neurosci. Lett. 2001;303:45–48. doi: 10.1016/s0304-3940(01)01716-5. [DOI] [PubMed] [Google Scholar]
- 24.Egami K, Yitta S, Kasim S, Lewers JC, Roberts RC, Lehar M, Jinnah HA. Basal ganglia dopamine loss due to defect in purine recycling. Neurobiol. Dis. 2007;26:396–407. doi: 10.1016/j.nbd.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewers JC, Ceballos-Picot I, Shirley TL, Mockel L, Egami K, Jinnah HA. Consequences of impaired purine recycling in dopaminergic neurons. Neuroscience. 2008;152:761–772. doi: 10.1016/j.neuroscience.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceballos-Picot I, Mockel L, Potier MC, Dauphinot L, Shirley TL, Torero-Ibad R, Fuchs J, Jinnah HA. Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: implications for Lesch-Nyhan disease pathogenesis. Hum Mol Genet. 2009;18:2317–2327. doi: 10.1093/hmg/ddp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang TH, Guibinga GH, Jinnah HA, Friedmann T. HPRT deficiency coordinately dysregulates canonical Wnt and presenilin-1 signaling: a neuro-developmental regulatory role for a housekeeping gene? PLoS One. 2011;6:e16572. doi: 10.1371/journal.pone.0016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goettle M, Burhenne H, Sutcliffe D, Jinnah HA. Purine metabolism during neuronal differentiation: The relevance of purine synthesis and recycling. J. Neurochem. 2013;127:805–818. doi: 10.1111/jnc.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristini S, Navone S, Canzi L, Acerbi F, Ciusani E, Hladnik U, de Gemmis P, Alessandri G, Colombo A, Parati E, Invernici G. Human neural stem cells: a model system for the study of Lesch-Nyhan disease neurological aspects. Hum Mol Genet. 2010;19:1939–1950. doi: 10.1093/hmg/ddq072. [DOI] [PubMed] [Google Scholar]
- 30.Kang TH, Park Y, Bader JS, Friedmann T. The housekeeping gene hypoxanthine guanine phosphoribosyltransferase (HPRT) regulates multiple developmental and metabolic pathways of murine embryonic stem cell neuronal differentiation. PLoS One. 2013;8:e74967. doi: 10.1371/journal.pone.0074967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorpe WP. The Lesch-Nyhan syndrome. Enzyme. 1971;12:129–142. doi: 10.1159/000459527. [DOI] [PubMed] [Google Scholar]
- 32.Visser JE, Smith DW, Moy SS, Breese GR, Friedmann T, Rothstein JD, Jinnah HA. Oxidative stress and dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. Dev. Brain Res. 2002;133:127–139. doi: 10.1016/s0165-3806(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 33.Hyland K, Kasim S, Egami K, Arnold LA, Jinnah HA. Tetrahydrobiopterin and brain dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J. Inherit. Metab. Dis. 2004;27:165–178. doi: 10.1023/B:BOLI.0000028728.93113.4d. [DOI] [PubMed] [Google Scholar]
- 34.Mastrangelo L, Kim JE, Miyanohara A, Kang TH, Friedmann T. Purinergic signaling in human pluripotent stem cells is regulated by the housekeeping gene encoding hypoxanthine guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 2012;109:3377–3382. doi: 10.1073/pnas.1118067109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia MG, Puig JG, Torres RJ. Abnormal adenosine and dopamine receptor expression in lymphocytes of Lesch-Nyhan patients. Brain Behav Immun. 2009;23:1125–1131. doi: 10.1016/j.bbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Torres RJ, DeAntonio I, Prior C, Puig JG. Adenosine transport in peripheral blood lymphocytes from Lesch-Nyhan patients. Biochem. J. 2004;377:733–739. doi: 10.1042/BJ20031035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertelli M, Cecchin S, Lapucci C, Jacomelli G, Jinnah HA, Pandolfo M, Micheli V. Study of the adenosinergic system in the brain of HPRT knockout mouse (Lesch-Nyhan disease) Clin. Chim. Acta. 2006;373:104–107. doi: 10.1016/j.cca.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Kinast L, von der Ohe J, Burhenne H, Seifert R. Impairment of adenylyl cyclase 2 function and expression in hypoxanthine phosphoribosyltransferase-deficient rat B103 neuroblastoma cells as model for Lesch-Nyhan disease: BODIPY-forskolin as pharmacological tool. Naunyn Schmiedebergs Arch Pharmacol. 2012 doi: 10.1007/s00210-012-0759-6. [DOI] [PubMed] [Google Scholar]
- 39.Deutsch SI, K.D. L, Rosse RB, Mastropaolo J, Eller J. Hypothesized deficiency of guanine-based purines may contribute to abnormalities of neurodevelopment, neuromodulation, and neurotransmission in Lesch-Nyhan syndrome. Clin. Neuropharmacol. 2005;28:28–37. doi: 10.1097/01.wnf.0000152043.36198.25. [DOI] [PubMed] [Google Scholar]
- 40.Guibinga GH, Murray F, Barron N, Pandori W, Hrustanovic G. Deficiency of the purine metabolic gene HPRT dysregulates microRNA-17 family cluster and guanine-based cellular functions: a role for EPAC in Lesch-Nyhan syndrome. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guibinga GH, Hrustanovic G, Bouic K, Jinnah HA, Friedmann T. MicroRNA-mediated dysregulation of neural developmental genes in HPRT deficiency: clues for Lesch-Nyhan disease? Hum Mol Genet. 2011;21:609–622. doi: 10.1093/hmg/ddr495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messina E, Micheli V, Giacomello A. Guanine nucleotide depletion induces differentiation and aberrant neurite outgrowth in human dopaminergic neuroblastoma lines: a model for basal ganglia dysfunction in Lesch-Nyhan disease. Neurosci. Lett. 2005;375:97–100. doi: 10.1016/j.neulet.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 43.Guibinga GH, Hsu S, Friedmann T. Deficiency of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) dysregulates neurogenesis. Mol Ther. 2010 doi: 10.1038/mt.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittman RN, Wang S, DiBenedetto AJ, Mills JC. A system for characterizing cellular and molecular events in programmed neuronal cell death. J Neurosci. 1993;13:3669–3680. doi: 10.1523/JNEUROSCI.13-09-03669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- 46.Clementi E, Racchetti G, Zacchetti D, Panzeri MC, Meldolesi J. Differential expression of markers and activities in a group of PC12 nerve cell clones. Eur. J. Neurosci. 1992;4:944–953. doi: 10.1111/j.1460-9568.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 47.Koike T, Takashima A. Clonal variability of PC12 pheochromocytoma cells with respect to catecholamine biosynthesis. J. Neurochem. 1984;42:1472–1475. doi: 10.1111/j.1471-4159.1984.tb02812.x. [DOI] [PubMed] [Google Scholar]
- 48.Shirley TL, Lewers JC, Egami K, Majumdar A, Kelly M, Ceballos-Picot I, Seidman MM, Jinnah HA. A human neuronal tissue culture model for Lesch-Nyhan disease. J. Neurochem. 2007;101:841–853. doi: 10.1111/j.1471-4159.2007.04472.x. [DOI] [PubMed] [Google Scholar]
- 49.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nat Protoc. 2006;1:2650–2660. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 50.Dammer EB, Duong DM, Diner I, Gearing M, Feng Y, Lah JJ, Levey AI, Seyfried NT. Neuron enriched nuclear proteome isolated from human brain. J Proteome Res. 2013;12:3193–3206. doi: 10.1021/pr400246t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dammer EB, Fallini C, Gozal YM, Duong DM, Rossoll W, Xu P, Lah JJ, Levey AI, Peng J, Bassell GJ, Seyfried NT. Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination. PLoS One. 2012;7:e38658. doi: 10.1371/journal.pone.0038658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seyfried NT, Gozal YM, Dammer EB, Xia Q, Duong DM, Cheng D, Lah JJ, Levey AI, Peng J. Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Mol Cell Proteomics. 2010;9:705–718. doi: 10.1074/mcp.M800390-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seyfried NT, Gozal YM, Donovan LE, Herskowitz JH, Dammer EB, Xia Q, Ku L, Chang J, Duong DM, Rees HD, Cooper DS, Glass JD, Gearing M, Tansey MG, Lah JJ, Feng Y, Levey AI, Peng J. Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards. J Proteome Res. 2012;11:2721–2738. doi: 10.1021/pr2010814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efron B. Large-scale simultaneous hypothesis testing. J Am Statistical Assoc. 2004;99:96–104. [Google Scholar]
- 55.Huang DAW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 56.Kondo M, Yamaoka T, Honda S, Miwa Y, Katashima R, Moritani M, Yoshimoto K, Hayashi Y, Itakura M. The rate of cell growth is regulated by purine biosynthesis via ATP production and G(1) to S phase transition. J. Biochem. 2000;128:57–64. doi: 10.1093/oxfordjournals.jbchem.a022730. [DOI] [PubMed] [Google Scholar]
- 57.Chan CY, Zhao H, Pugh RJ, Pedley AM, French J, Jones SA, Zhuang X, Jinnah H, Huang TJ, Benkovic SJ. Purinosome formation as a function of the cell cycle. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1423009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312:163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dauphinot L, Mockel L, Cahu J, Jinnah HA, Ledroit M, Potier MC, Ceballos-Picot I. Transcriptomic approach to Lesch-Nyhan disease. Nucleosides Nucleotides Nucleic Acids. 2014;33:208–217. doi: 10.1080/15257770.2014.880477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connolly T, Gilmore R. GTP hydrolysis by complexes of the signal recognition particle and the signal recognition particle receptor. J Cell Biol. 1993;123:799–807. doi: 10.1083/jcb.123.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jinnah HA, Hess EJ, Wilson MC, Gage FH, Friedmann T. Localization of hypoxanthine-guanine phosphoribosyltransferase mRNA in the mouse brain by in situ hybridization. Mol. Cell. Neurosci. 1992;3:64–78. doi: 10.1016/1044-7431(92)90010-y. [DOI] [PubMed] [Google Scholar]
- 63.Jinnah HA, Page T, Friedmann T. Brain purines in a genetic mouse model of Lesch-Nyhan disease. J. Neurochem. 1993;60:2036–2045. doi: 10.1111/j.1471-4159.1993.tb03488.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.