Abstract
The small- and intermediate-conductance Ca2+-activated potassium (SK/IK) channels play important roles in the regulation of excitable cells in both the central nervous and cardiovascular systems. Evidence from animal models has implicated SK/IK channels in neurological conditions such as ataxia and alcohol use disorders. Further, genome-wide association studies have suggested that cardiovascular abnormalities such as arrhythmias and hypertension are associated with single nucleotide polymorphisms that occur within the genes encoding the SK/IK channels. The Ca2+ sensitivity of the SK/IK channels stems from a constitutively bound Ca2+-binding protein: calmodulin. Small-molecule positive modulators of SK/IK channels have been developed over the past decade, and recent structural studies have revealed that the binding pocket of these positive modulators is located at the interface between the channel and calmodulin. SK/IK channel positive modulators can potentiate channel activity by enhancing the coupling between Ca2+ sensing via calmodulin and mechanical opening of the channel. Here, we review binding pocket studies that have provided structural insight into the mechanism of action for SK/IK channel positive modulators. These studies lay the foundation for structure-based drug discovery efforts that can identify novel SK/IK channel positive modulators.
Keywords: Small- and intermediate-conductance Ca2+-activated potassium channels, Small-molecule positive modulators, Structure-based drug discovery, Ataxia, Alcohol use disorders, Parkinson's disease
Introduction
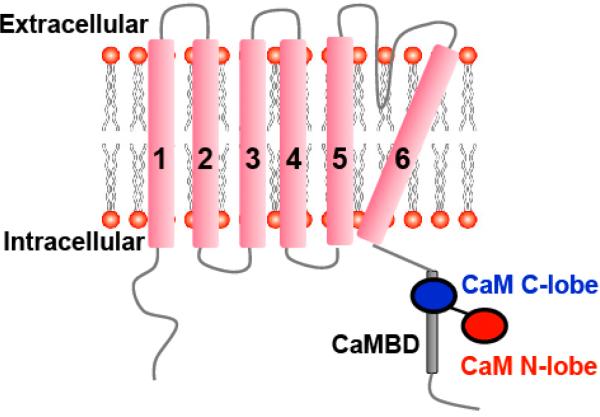
The small- and intermediate-conductance Ca2+-activated potassium (SK/IK) channels belong to a subfamily of Ca2+-activated potassium channels, which play important roles in the regulation of membrane excitability by the intracellular Ca2+ [1]. SK/IK channels share the same tetrameric architecture with the voltage-dependent potassium channels. Each channel subunit is composed of six transmembrane α-helical domains that are denoted S1--S6. A loop between the transmembrane S5 and S6 domains forms a potassium ion selectivity filter (fig. 1).
Fig. 1.
The architecture of one SK/IK channel subunit. Each subunit has six α-helical transmembrane domains (S1--S6, pink). The loop between the S5 and S6 domains forms the potassium ion filter. Both the N- and C-termini are located on the intracellular side of the membrane. The Ca2+-binding protein CaM is associated with the CaMBD that is located at the proximal C-terminus of the SK/IK channels. CaM serves as the Ca2+ sensor for the channels. In the absence of Ca2+, the CaM C-lobe (blue), but not the N-lobe (red), binds to the CaMBD.
Unlike the voltage-dependent potassium channels, SK/IK channel activation is not voltage dependent. Instead, elevated intracellular Ca2+ levels that occur in response to various physiological stimuli exclusively activate SK/IK channels. The Ca2+-binding protein calmodulin (CaM) is constitutively associated with SK/IK channels at the CaM-binding domain (CaMBD). The CaMBD is located in the proximal C-terminus of the channels (fig. 1). Ca2+ binding to CaM induces conformational changes in both CaM and the CaMBD that subsequently trigger an opening of the channel pore [2--4]. The mechanism by which these conformational changes in CaM and CaMBD translate into channel opening has only recently been elucidated.
In addition to intracellular elevated Ca2+ levels, the SK/IK channels are also subject to regulation by posttranslational modification (e.g., phosphorylation). The Ca2+ sensitivity of the SK/IK channels is reduced by phosphorylation of a specific CaM threonine residue (Thr79) by casein kinase 2. Importantly, the phosphorylation status of CaM Thr79, and thus the Ca2+ sensitivity of the channels, is regulated by casein kinase 2 and protein phosphatase 2A. Both of these are constitutively associated with the SK/IK channels [1].
Genes encoding SK/IK channels are highly conserved in many species including mammals, Drosophila melanogaster and Caenorhabditis elegans. There are four mammalian genes that encode the SK/IK channels: KCNN1 for SK1, KCNN2 for SK2, KCNN3 for SK3 and KCNN4 for IK (also known as SK4) channels [1]. SK1--3 channels have small single-channel conductance and are thus named ‘small-conductance’ channels. The protein encoded by KCNN4 has intermediate single-channel conductance and is accordingly denoted an ‘intermediate-conductance’ channel (table 1). SK1--3 channels are widely expressed in electrically excitable cells including the central nervous and cardiovascular systems. IK channel expression in the central nervous system is very low. However, in peripheral tissues, the IK channel is found in the vascular endothelium, secretory epithelia and T cells [5].
Table 1.
Biophysical properties and positive modulators of SK and IK channels adapted from Aldrich et al. [71]
| Channel | Human gene | Single-channel conductance, pS | Ca2+ sensitivity, pEC50 | Positive modulators |
|---|---|---|---|---|
| SK1 | KCNN1 | 9.2 | 6.2–6.5 | 1-EBIO, DCEBIO, NS309, riluzole, SKA-31, GW542573X |
| SK2 | KCNN2 | 9.5 | 6.2–6.5 | 1-EBIO, DCEBIO, NS309, riluzole, SKA-31, CyPPA, NS13001 |
| SK3 | KCNN3 | – | 6.0–6.5 | 1-EBIO, DCEBIO, NS309, riluzole, SKA-31, CyPPA, NS13001 |
| IK | KCNN4 | 11–40 | 6.1–7.0 | 1-EBIO, DCEBIO, NS309, riluzole, SKA-31, SKA-111 [60] |
Potassium efflux through SK/IK channels hyperpolarizes the cell. Accordingly, SK/IK channels modulate cell excitability. In addition, SK and IK channels have been implicated in multiple pathophysiological conditions such as ataxia, alcohol use disorders, Parkinson’s disease and hypertension. As a result, these channels have been recognized as potential therapeutic drug targets [6, 7].
In this review, we first summarize clinical conditions that have been linked with SK/IK channels in both the cardiovascular and central nervous systems. Then, we go on to review the recent development of SK/IK channel positive modulators that have shown promise as novel treatment options for ataxia, alcohol use disorders and hypertension. We next highlight the recently discovered binding pocket and mechanism whereby small molecules can positively modulate the SK/IK channels. This mechanism was elucidated through combined approaches including crystallography, molecular dynamic simulations, mutagenesis and electrophysiology. Finally, the state of the art of structure-based discovery of novel drugs that target the SK/IK channels will also be discussed.
SK/IK Channels in the Central Nervous System
Three subtypes of SK channels (SK1--3) are expressed in many areas of the central nervous system. Their activation is specifically involved in afterhyperpolarization, which regulates the firing frequency for many types of neurons [1]. In neurological diseases that involve abnormal neuronal firing patterns, such as ataxia, Parkinson's disease and alcohol use disorders, the pharmacological manipulation that leads to SK channel activation can serve neuroprotective roles.
The Cerebellum and Ataxia
Ataxia is a movement disorder characterized by a lack of coordination of muscle movements. Dysfunction of cerebellar Purkinje cells has been observed in many types of ataxia [8]. More specifically, the loss of firing precision by Purkinje cells is thought to underlie the symptoms of episodic ataxia and spinal cerebellar ataxia [9, 10]. Moreover, aberrant Ca2+ influx during action potential generation (either a shortage or an overload) can lead to dysfunction of Purkinje cell firing [11]. Pharmacological manipulations that restore the regular firing pattern of Purkinje cells have been suggested as a means of alleviating ataxia symptoms. The predominant subtype of SK channel expressed by Purkinje cells is SK2, and SK2 has emerged as a major ion channel involved in Purkinje cell pacemaking [12]. SK channels play important roles in the regulation of Purkinje cell excitability by modulating the afterhyperpolarization that occurs after action potential generation. Additionally, a positive modulation of SK channels can counteract genetic mutations that cause either reduced [13] or elevated [14] Ca2+ signaling, and SK channel positive modulators can alleviate some behavioral and neuropathological symptoms of ataxia in animal models [10, 13, 15, 16]. Lastly, riluzole, an SK/IK channel positive modulator, yielded promising results in a phase II clinical study of a mixed population of ataxia patients [17].
The Substantia Nigra and Parkinson's Disease
Parkinson's disease is a neurodegenerative disorder without curative treatment. The degeneration of midbrain substantia nigra dopaminergic neurons is thought to underlie the pathogenesis of Parkinson's disease. SK channels are also implicated in the physiology of dopaminergic neurons [18]. For example, an SK3 mutation (hSK3Δ) that has been associated with human disease (schizophrenia) generates a dominant negative for SK channels that suppresses endogenous SK currents. The introduction of this hSK3Δ mutation into mouse midbrain dopamine neurons reduced coupling between NMDA receptors and SK channels. Consequently, the normally occurring balance between tonic and phasic dopaminergic neuron activity was disrupted by the introduction of hSK3Δ [19]. In addition, a deletion of the mouse KCNN2 gene that encodes SK2 led to mouse behavioral phenotypes that are thought to model specific aspects of human Parkinson's disease [20]. SK channels expressed on the neuronal plasma membrane may exert neuroprotective effects through modulating the firing pattern of dopaminergic neurons [21]. SK channels were also identified on the membrane of mitochondria in dopaminergic neurons. The activation of mitochondrial SK channels could prevent mitochondrial dysfunction [22]. As such, SK channels have been suggested as a potential drug target for dopamine-neuron-related diseases, even though it is not clear whether SK channel activity is altered in Parkinson's disease patients [23]. Accordingly, further evaluation of this hypothesis is needed.
The Nucleus Accumbens and Alcohol Use Disorders
Alcohol abuse and misuse continues to exhaust significant socioeconomic resources. This is in part due to an increase in the motivation to seek alcohol that develops over time and that persists during abstinence. It has been demonstrated that SK channels may play an important role in the neurobiology of alcohol seeking [24]. For example, the expression and function of SK3 channels is decreased in the rat nucleus accumbens core after abstinence from ethanol self-administration [24] and in the ventral tegmental area after repeated ethanol injections [25]. A similar reduction was seen ex vivo after the application of ethanol to hippocampal slices [26]. This decrease in SK channel activity led to increased action potential generation by both nucleus accumbens core and ventral tegmental area neurons. Importantly, positive modulation of nucleus accumbens core neuron SK channels with 1-EBIO normalized neuronal firing and decreased the motivation of rats to self-administer ethanol [24]. 1-EBIO can also reduce ethanol-induced hyperexcitability, neurotoxicity and handling-induced convulsions in mice [26]. Similarly, the FDA-approved SK channel positive modulator chlorzoxazone decreased ethanol consumption in a rat model of high ethanol intake [27], and CyPPA has been shown to reduce both ethanol consumption and the preference for ethanol over water in mice [28]. Unfortunately, chlorzoxazone failed to reduce the number of drinks consumed per week in a clinical trial of 20 moderate-to-heavy social alcohol users compared to placebo (NCT01342341). However, because chlorzoxazone has off-target effects that confound an unequivocal interpretation of these studies, it remains to be determined whether other more potent and selective SK channel positive modulators would be clinically effective.
SK/IK Channels in the Cardiovascular System
In the normal heart, three SK channel subtypes are expressed (SK1, SK2 and SK3), and these subtypes play important roles in regulating the activity of atrial myocytes [29--32]. Moreover, in vascular endothelial cells [33], the predominant subtypes are IK and SK3. SK2 channels are also expressed in endothelial cells, but to a lesser extent, whereas SK1 channels are not expressed.
The Heart and Arrhythmias
The SK channels have emerged as major ion channels regulating cardiac action potentials. In the normal heart, SK channels are mostly expressed in the atria, while very little SK channel expression is seen in the normal ventricles [29]. However, ventricular SK channel activity is upregulated in the failing heart [34]. These data implicate a role of SK channels in cardiac pathophysiology or perhaps a homeostatic role in the diseased ventricle. An increase in both Ca2+ sensitivity and channel protein expression is thought to contribute to SK channel upregulation [35]. It remains unknown whether casein kinase 2 and/or protein phosphatase 2A regulate the Ca2+ sensitivity of cardiomyocyte SK channels, and this regulatory mechanism of phosphorylation/dephosphorylation needs further investigation.
Genetic studies also support a role of SK channels in arrhythmia. Genetic ablation of SK2 channels in mice resulted in atrial action potential prolongation and atrial arrhythmias [36]. Moreover, single nucleotide polymorphisms in the SK/IK genes have been implicated in cardiovascular abnormalities [37, 38]. Lastly, single nucleotide polymorphisms in the SK3 gene (KCNN3) have been associated with lone atrial fibrillation [38].
Vessels and Hypertension
SK/IK channels also play an equally important role in vascular system function [39--43]. SK/IK channels are especially important in vasodilation that is mediated by endothelium-derived hyperpolarizing factor (EDHF) [44--46]. Activation of SK/IK channels in endothelial cells can induce hyperpolarization that is transduced to smooth muscle cells through the myoendothelial gap junctions. Using transgenic mice with suppressed expression of SK3 channels, it was found that SK3 channel expression can modulate both arterial tone and blood pressure [39]. Further, a genetic deficiency of both SK3 and IK channels in mice severely impaired both endothelial hyperpolarization and EDHF-mediated vasodilation, which caused hypertension-like symptoms [47]. Additionally, SK3 expression is reduced by more than 40% in mesenteric arteries from spontaneously hypertensive rats [48]. Similarly, decreased SK3 mRNA and protein expression was observed in mesenteric arteries collected from rats with angiotensin II-induced hypertension [49]. Accordingly, it has been suggested that compromised SK channel activity may be a contributing factor to hypertension [41].
Genome-wide association studies of single nucleotide polymorphisms occurring within the IK channel locus revealed an association with myocardial infarction [50]. IK channels are also implicated in atherogenesis in both mice and humans [51]. Additionally, a positive modulation of endothelial SK/IK channels can lower blood pressure in hypertension animal models. These data, in part, led to the development of SKA-31, an SK/IK channel positive modulator based on riluzole. SKA-31 can lower blood pressure by enhancing EDHF-mediated responses [52]. Thus, SKA-31 illustrates the potential of SK/IK channels as potent therapeutic targets for the treatment of hypertension.
SK/IK Channel Positive Modulators
Given the many roles of SK/IK channels in central nervous and cardiovascular system functions, a great deal of effort has been devoted to developing small molecules that selectively target SK/IK channels [53]. The first positive modulator, 1-EBIO (1-ethyl-2-benzimidazolinone), was identified almost two decades ago [54]. 1-EBIO potentiates SK/IK channel activity, which enhances afterhyperpolarization and effectively modulates neuronal excitability [55]. The structural optimization of 1-EBIO led to DCEBIO. Compared to 1-EBIO, DCEBIO exhibits ~20-fold higher potency [56]. In the past decade, a Scandinavian biopharmaceutical company (NeuroSearch A/S) has developed additional compounds that target the SK/IK channels, including the potent SK/IK channel positive modulator NS309 [57].
Subtype-selective modulators have recently emerged for the SK/IK channels, and NeuroSearch A/S has developed several of these. For example, CyPPA is an SK2/SK3-selective positive modulator [58], and NS13001 is an orally effective SK2/SK3-selective compound that has shown promising beneficial effects in a mouse model of spinocerebellar ataxia type 2 [15]. Additionally, NeuroSearch A/S has also achieved a relatively selective activation of SK1 channels with GW542573X. This compound exhibited 10 times higher potency at SK1 channels over SK2 and SK3 channels [59]. Others have generated IK channel-selective compounds. Dr. Wulff's group from the University of California at Davis recently synthesized SKA-111, which displayed 160-fold selectivity for IK channels over SK3 channels [60]. The efforts of these investigators have made compounds that are selective for different subtypes of SK/IK channels available for the first time (table 1). There is no doubt that these compounds will be used as pharmacological tools to discriminate the individual roles of SK/IK channel subtypes in both physiological and pathophysiological conditions and may ultimately lead to clinically useful therapeutic agents.
It is important to note that GW542573X is unique among the compounds described above in that it has been described as a genuine SK1 channel opener [59]. In contrast, the selective SK2/SK3 positive modulators can only potentiate SK/IK channel activity, and this action requires the presence of Ca2+. This Ca2+-dependent mechanism is similar to that of the nonselective SK/IK channel positive modulators (e.g., 1-EBIO and NS309) that cannot activate the SK/IK channels without the presence of Ca2+. Accordingly, these compounds are termed positive modulators because they enhance the Ca2+ sensitivity of the SK/IK channels. It is this mechanism of action that led to the hypothesis that the binding site(s) for these compounds lie(s) within the cytoplasmic region where CaM is associated with the channel. However, the precise mechanism of action for these positive modulators has until recently been unclear.
The Binding Pocket for SK/IK Channel Positive Modulators
SK2-a is an RNA splice variant of the SK2 channel. We have recently identified a novel SK2 alternative RNA splice variant of the SK2 channel, which was named SK2-b [4]. In a crystal structure of the SK2-b channel fragment complexed with CaM (CaMBD2-b), we determined that the small molecule phenylurea (PHU, a component of the crystallization solution) was bound between CaM and the SK2-b channel fragment (fig. 2a). Electrophysiological measurements indicated that PHU can effectively potentiate the channel activity of both the SK2-a and SK2-b splice variants. Interestingly, PHU exhibits slightly lower potency than the prototypical positive modulator 1-EBIO [61].
Fig. 2.
Shared binding pocket of multiple positive modulators. Compounds such as PHU (a), 1-EBIO (b) and NS309 (c) bind at the interface of CaM (gray surface) and the SK2-a channel fragment (light blue ribbon). Atoms in the compounds are shown in the following colors: carbon in yellow, oxygen in red, nitrogen in blue and chloride in green. d Conformations of PHU, 1-EBIO and NS309 are overlaid (PDB codes: 4G27, 4G28 and 4J9Z). The carbon atoms in the compounds are shown in the following colors: PHU in gray, 1-EBIO in cyan and NS309 in magenta.
The similarity in the chemical structures between PHU and the prototypical compound 1-EBIO [61] led us to test whether this binding site at the CaM-channel interface is the common binding pocket for the positive modulators. This was done using a combination of approaches including structural biology, computational biology and a functional assay [61]. In the crystal structure (PDB code: 4G28), 1-EBIO bound in a similar manner as PHU at the interface of CaMBD2-a (from SK2-a) and the CaM N-lobe. 1-EBIO formed close contacts with both CaM and CaMBD2-a (fig. 2b). The most potent SK channel positive modulator, NS309 (fig. 2c), also binds into the same binding pocket as PHU and 1-EBIO (PDB code: 4J9Z). These data strongly indicate that this interface is a shared binding site for SK/IK channel positive modulators (fig. 2d).
Previous studies showed that IK channels are more sensitive to 1-EBIO than SK channels [53]. An amino acid sequence alignment showed that IK channels contain unique residues at the equivalent positions of A477 and L480 in SK2-a (fig. 3a). These residues are implicated in the formation of the binding pocket for 1-EBIO (fig. 3b). In our recent report, a double SK2-a mutant (A477V/L480M) was generated that mimics the corresponding residues in IK channels. This SK2-a double mutant was more sensitive to potentiation by 1-EBIO mimicking IK channels [61]. These data suggest that the binding pocket identified by crystallography is indeed the functional binding site through which the compounds exert the positive modulation of channel activity.
Fig. 3.
Molecular basis for the higher sensitivity of IK channels to 1-EBIO compared with SK2-a channels. a Amino acid sequences of rat SK2-a and human IK channels at the region of the 1-EBIO binding pocket were aligned. The major SK2-a channel residues that are required for 1-EBIO coordination (Ala477 and Leu480) are highlighted in magenta. The equivalent IK channel residues (Val365 and Met368) are highlighted in blue. b The 1-EBIO binding pocket of SK2-a with the A477V/L480M double mutations. The slightly bulkier residues in the double mutant channel can potentially form better contacts with 1-EBIO. Green spheres: calcium ions.
The Mechanism of Action for SK/IK Channel Positive Modulators
A simplified chemomechanical gating scheme [2] of Ca2+-dependent SK channel activation might include two steps: (1) binding of Ca2+ to CaM associated with the SK/IK channel and (2) mechanical coupling between Ca2+ binding to CaM and subsequent channel opening (fig. 4). Positive modulators could therefore theoretically enhance either one of these two steps in order to potentiate channel activity. To test this hypothesized mechanism, a biochemical binding assay was performed with NS309 [62]. In the presence of a saturating NS309 concentration, no change was observed in the apparent Ca2+ affinity during formation of the CaM/CaMBD complex. As a result, NS309 did not influence the first step of the hypothesized gating scheme; i.e., Ca2+ binding to the CaM-channel complex.
Fig. 4.
A simplified chemomechanical gating scheme of SK/IK channels that was adapted from a previous report [2]. The first step in channel activation is Ca2+ binding to CaM. Next, PIP2 mediates mechanical coupling into the opening of the channel pore. For simplicity, only the S6 transmembrane domains from two channel subunits are shown. The N-lobe of CaM is in red, while the C-lobe of CaM is in blue. PIP2 is shown in cyan. Ca2+ ions are shown as gray dots. Positive SK/IK channel modulators (e.g., NS309 or 1-EBIO) can enhance the mechanical coupling of Ca2+ binding into the channel opening. Conversely, phosphorylation of CaM by casein kinase 2 can negatively modulate the PIP2-mediated mechanical coupling.
We have recently identified a flexible region (R396-M412) in the SK2 channels that is responsible for the second phase of the hypothesized step; i.e., mechanical coupling between Ca2+ binding to CaM and subsequent channel opening. A previous report showed that the SK2-a channel fragment located immediately after the transmembrane S6 domain, R396-M412 (fig. 5a), was not visible in the CaM/CaMBD2-a crystal structure [2]. Our structure determination, from multiple sets of X-ray diffraction data of the same complex, consistently showed the interaction between the CaM linker and part of the missing fragment (E399-K402, cuff; fig. 5b) instead of an interaction between the CaM linker and the poly-His tag used to purify CaMBD2-a, as previously reported [2]. Most proteins form well-organized three-dimensional structures to carry out their specific functions. On the other hand, intrinsically disordered fragments (IDF) in proteins are equally important for protein function [63--65]. The channel fragment E404-M412, which connects the cuff and CaMBD, is visible neither in our determined crystal structure (fig. 5b) nor in the previous report [2]; suggesting this fragment is an IDF.
Fig. 5.
An IDF connects the CaMBD to the SK/IK channel transmembrane S6 domain. The IDF is critical for the coupling of Ca2+ binding and mechanical opening of the channel. a Amino acid sequence alignment of SK/IK channels at the region that connects the CaMBD and the S6 transmembrane domain. The amino acid residues of the IDF are shown in salmon. b The IDF is invisible in the absence of NS309 (shown as a dashed line). c The IDF (in salmon) becomes well structured in the presence of NS309 (PDB code: 4J9Z).
The inherent flexibility of IDF makes it a perfect motif for the regulation of protein function, which, in this case, is SK/IK channel activity. Unfortunately, there was not enough electron density to model the IDF in the crystal structures that were obtained in the presence of the weak positive modulator 1-EBIO [61]. However, with the most potent modulator NS309 [53], the IDF became readily identifiable (fig. 5c). The appearance of the IDF structure in the presence of NS309 suggested that binding of NS309 to the protein complex might stabilize IDF and consequently facilitate the transfer of conformational changes that occur during Ca2+ binding to CaM to the mechanical opening of the channel (fig. 4). This mechanism of action for NS309 was further confirmed with site-directed mutagenesis and electrophysiology [62].
The Possible Involvement of PIP2 in the Action of SK/IK Channel Positive Modulators
In the past decade, it has been established that plasma membrane phosphoinositides such as phosphatidylinositol 4,5-biphosphate (PIP2) can regulate the activities of many different types of ion channels [66]. We recently demonstrated that SK channels are PIP2 sensitive [67]. The putative PIP2 binding site in the SK2/CaM complex involves the central linker of CaM as well as the cuff and the IDF, which connects the transmembrane S6 domain to the CaMBD. This region is implicated in the coupling of Ca2+ binding to CaM with the opening of the SK channel pore [62]. A threonine residue (Thr79) in the CaM central linker that is known to be phosphorylated by casein kinase 2 lies near the putative PIP2 binding site of the SK2/CaM complex.
In general, interactions between channels and PIP2 are electrostatic in nature. The PIP2 binding sites consist of positively charged amino acid residues that are clustered close to the bottom of the pore-forming S6 segments and the immediate C-linker region of the channel that connects the S6 transmembrane domain to the cytosolic domain of the channel proteins. The introduction of negative charges by phosphorylation at this site can effectively reduce apparent affinity of PIP2 for the channel complex and negatively modulate channel activity, indicating that channel-PIP2 interactions are a critical element in channel modulation.
The binding pocket for positive modulators (e.g., NS309) is adjacent to the PIP2 binding site. However, no direct interaction between NS309 and PIP2 was indicated in the structural model. Interestingly, the IDF residues Glu404 and Lys405 bridge the binding pockets for NS309 and PIP2. This raises the exciting possibility that NS309 could allosterically affect PIP2 binding to the CaM-SK channel complex. This hypothesis needs to be tested in the future.
The Future Discovery of Drugs That Target SK/IK Channels
Riluzole is an FDA-approved drug that is used to prolong the survival of patients with amyotrophic lateral sclerosis. Riluzole could exert its neuroprotective effects at least partly through the positive modulation of SK channels [68]. Chlorzoxazone [69], a centrally acting drug used to relieve muscle pain and stiffness, has also been found to positively modulate SK channels. Both drugs are being evaluated for ataxia [17] and alcohol use disorders [27] in clinical trials targeting SK channels for new clinical applications. However, neither riluzole nor chlorzoxazone exhibit satisfactory potency [53]; on the one hand, this reflects the need for SK channel modulators as therapeutic agents and, on the other hand, the lack of new modulators as drug candidates.
Despite the recent progress in our understanding of the structural biology of ion channels, the structure of SK/IK channel transmembrane domains is not yet available. This deficiency could make the structure-based discovery of new drugs that target the channel pore region a difficult task. However, the Ca2+ gating domains (CaM and CaMBD) from two alternative splice variants of SK2 channels (SK2-a and SK2-b) have been crystallized [2--4]. This opens the possibility of an accelerated development of SK channel positive modulators. Importantly, the SK2-b variant differs from SK2-a by only an insertion of three amino acid residues in the CaMBD. Nonetheless, this difference is enough to force CaM to adopt a completely different conformation when complexed with either variant. Because the two variants exhibit a significantly different Ca2+ sensitivity for activation [4], these data suggest a critical role of the Ca2+ gating domain (CaM/CaMBD complex) in regulating SK/IK channel activity. Accordingly, these observations raise the question of whether it is possible to develop novel SK/IK channel modulators by targeting the CaM/CaMBD region. Importantly, positive modulators have been shown to bind at the CaM/CaMBD interface. This drug-binding pocket for SK/IK channel positive modulators is shared by multiple compounds with different potency, including PHU, 1-EBIO and NS309 (fig. 2). A systematic analysis of the CaM/CaMBD structure complexed with various compounds could reveal the structural determinants for drug potency. These studies are crucial in the structure-based discovery of more potent modulators. For example, a recent structure-activity relationship study conducted by Dr. Wulff's group led to the potent IK channel-selective modulator SKA-111 [60]. That effort was a very exciting example of rational drug discovery targeting SK/IK channels that was aided by structural information. As such, structural insights may not only help improve the potency of positive modulators, but this information may also be useful in the identification of modulators that exhibit more subtype selectivity.
While positive modulators have been hypothesized to potentiate SK/IK channels through enhancing Ca2+ binding to CaM since the early years of their discovery, recent research has indicated that NS309 does not influence the Ca2+-dependent formation of the CaM/CaMBD complex [62]. Instead, the binding of NS309 stabilizes an IDF that connects the CaMBD to the transmembrane domains. In this model, NS309 acts as a positive modulator via enhancing the second hypothesized step (coupling) rather than the first step (Ca2+ binding) of the gating scheme (fig. 4). This mechanism of action might also apply to other SK/IK channel positive modulators such as 1-EBIO. It is also a fair question to ask whether negative modulators could be developed by differentially targeting the same binding pocket. In other words, a negative modulator could change the IDF into an unfavorable conformation, which would reduce the efficiency of coupling to channel opening.
Lastly, CaM has been implicated in the modulation of many ion channels including voltage-gated Na+ and Ca2+ channels [70]. Thus, it would be interesting to investigate whether the interface of CaM and these ion channels could also accommodate the pharmacological modulation by small molecules. If true, this could lead to novel strategies for targeting voltage-gated Na+ and Ca2+ channels.
Acknowledgements
This work was supported by the American Heart Association (13SDG16150007, M.Z.), the National Institutes of Health (S10RR027411, M.C.; P50AA022537, M.S.B.), the Alcohol Beverage Medical Research Foundation (M.S.B.), the National High Technology Research and Development Program of China (863 Program, No. 2012AA020301 and 2012AA01A305, K.Y.) and the Chinese Academy of Sciences Project (KSZD-EW-L09-4, K.Y.).
References
- 1.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher MA, et al. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 3.Li W, et al. EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J Gen Physiol. 2009;134:281–293. doi: 10.1085/jgp.200910295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, et al. Structural basis for calmodulin as a dynamic calcium sensor. Structure. 2012;20:911–923. doi: 10.1016/j.str.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen BS, et al. The Ca2+-activated K+ channel of intermediate conductance: a possible target for immune suppression. Expert Opin Ther Targets. 2002;6:623–636. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- 6.Lam J, et al. The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin Ther Targets. 2013;17:1203–1220. doi: 10.1517/14728222.2013.823161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wulff H, Kohler R. Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets. J Cardiovasc Pharmacol. 2013;61:102–112. doi: 10.1097/FJC.0b013e318279ba20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol. 2012;197:167–177. doi: 10.1083/jcb.201105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasumu AW, et al. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar Purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32:12786–12796. doi: 10.1523/JNEUROSCI.1643-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakkottai VG, et al. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31:13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum. 2012;11:630–639. doi: 10.1007/s12311-010-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci. 2003;23:2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter JT, et al. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- 14.Gao Z, et al. Cerebellar ataxia by enhanced CaV2.1 currents is alleviated by Ca2+-dependent K+-channel activators in Cacna1aS218L mutant mice. J Neurosci. 2012;32:15533–15546. doi: 10.1523/JNEUROSCI.2454-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasumu AW, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol. 2012;19:1340–1353. doi: 10.1016/j.chembiol.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakkottai VG, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113:582–590. doi: 10.1172/JCI20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ristori G, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74:839–845. doi: 10.1212/WNL.0b013e3181d31e23. [DOI] [PubMed] [Google Scholar]
- 18.Aumann TD, et al. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp Neurol. 2008;213:419–430. doi: 10.1016/j.expneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Soden ME, et al. Disruption of dopamine neuron activity pattern regulation through selective expression of a human KCNN3 mutation. Neuron. 2013;80:997–1009. doi: 10.1016/j.neuron.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szatanik M, et al. Behavioral effects of a deletion in Kcnn2, the gene encoding the SK2 subunit of small-conductance Ca2+-activated K+ channels. Neurogenetics. 2008;9:237–248. doi: 10.1007/s10048-008-0136-2. [DOI] [PubMed] [Google Scholar]
- 21.Herrik KF, Christophersen P, Shepard PD. Pharmacological modulation of the gating properties of small conductance Ca2+-activated K+ channels alters the firing pattern of dopamine neurons in vivo. J Neurophysiol. 2010;104:1726–1735. doi: 10.1152/jn.01126.2009. [DOI] [PubMed] [Google Scholar]
- 22.Dolga AM, et al. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014;5:e999. doi: 10.1038/cddis.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XK, Wang G, Chen SD. Modulation of the activity of dopaminergic neurons by SK channels: a potential target for the treatment of Parkinson's disease? Neurosci Bull. 2010;26:265–271. doi: 10.1007/s12264-010-1217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopf FW, et al. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65:682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopf FW, et al. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 26.Mulholland PJ, et al. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69:625–632. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopf FW, et al. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2011;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padula A, et al. Novel anticonvulsants for reducing alcohol consumption: a review of evidence from preclinical rodent drinking models. OA Alcohol. 2013;1:2. doi: 10.13172/2053-0285-1-1-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 30.Tuteja D, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, et al. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via α-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnham MP, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua SK, et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang PC, et al. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J Am Heart Assoc. 2013;2:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Köhler R. Single-nucleotide polymorphisms in vascular Ca2+-activated K+-channel genes and cardiovascular disease. Pflugers Arch. 2010;460:343–351. doi: 10.1007/s00424-009-0768-6. [DOI] [PubMed] [Google Scholar]
- 38.Ellinor PT, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor MS, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 40.Feng J, et al. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008;118(suppl 14):S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garland CJ. Compromised vascular endothelial cell SKCa activity: a fundamental aspect of hypertension? Br J Pharmacol. 2010;160:833–835. doi: 10.1111/j.1476-5381.2010.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy N, et al. Role of Ca2+-sensitive K+ currents in controlling ventricular repolarization: possible implications for future antiarrhythmic drug therapy. Curr Med Chem. 2011;18:3622–3639. doi: 10.2174/092986711796642463. [DOI] [PubMed] [Google Scholar]
- 43.Sonkusare SK, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 45.McNeish AJ, Dora KA, Garland CJ. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke. 2005;36:1526–1532. doi: 10.1161/01.STR.0000169929.66497.73. [DOI] [PubMed] [Google Scholar]
- 46.McNeish AJ, et al. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 47.Brahler S, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 48.Weston AH, et al. Impairment of endothelial SKCa channels and of downstream hyperpolarizing pathways in mesenteric arteries from spontaneously hypertensive rats. Br J Pharmacol. 2010;160:836–843. doi: 10.1111/j.1476-5381.2010.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2275–H2284. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi M, et al. Relationship between haplotypes of KCNN4 gene and susceptibility to human vascular diseases in Japanese. Med Sci Monit. 2009;15:CR389–CR397. [PubMed] [Google Scholar]
- 51.Toyama K, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankaranarayanan A, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devor DC, et al. Modulation of Cl– secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- 55.Pedarzani P, et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I (AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- 56.Singh S, et al. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- 57.Strøbaek D, et al. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Hougaard C, et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151:655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hougaard C, et al. Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol Pharmacol. 2009;76:569–578. doi: 10.1124/mol.109.056663. [DOI] [PubMed] [Google Scholar]
- 60.Coleman N, et al. New positive KCa channel gating modulators with selectivity for KCa3.1. Mol Pharmacol. 2014;86:342–357. doi: 10.1124/mol.114.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang M, et al. Identification of the functional binding pocket for compounds targeting small-conductance Ca2+-activated potassium channels. Nat Commun. 2012;3:1021. doi: 10.1038/ncomms2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Pascal JM, Zhang JF. Unstructured to structured transition of an intrinsically disordered protein peptide in coupling Ca2+-sensing and SK channel activation. Proc Natl Acad Sci USA. 2013;110:4828–4833. doi: 10.1073/pnas.1220253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babu MM, et al. Intrinsically disordered proteins: regulation and disease. Curr Opin Struct Biol. 2011;21:432–440. doi: 10.1016/j.sbi.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature. 2007;447:1021–1025. doi: 10.1038/nature05858. [DOI] [PubMed] [Google Scholar]
- 65.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 66.Logothetis DE, et al. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 2010;460:321–341. doi: 10.1007/s00424-010-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, et al. Modulation of the PIP2 sensitivity of the CaM-SK channel complex through selective phosphorylation. Nat Chem Biol. 2014;10:753–759. doi: 10.1038/nchembio.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao YJ, et al. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur J Pharmacol. 2002;449:47–54. doi: 10.1016/s0014-2999(02)01987-8. [DOI] [PubMed] [Google Scholar]
- 69.Cao Y, et al. Modulation of recombinant small-conductance Ca2+-activated K+ channels by the muscle relaxant chlorzoxazone and structurally related compounds. J Pharmacol Exp Ther. 2001;296:683–689. [PubMed] [Google Scholar]
- 70.Kovalevskaya NV, et al. Structural analysis of calmodulin binding to ion channels demonstrates the role of its plasticity in regulation. Pflugers Arch. 2013;465:1507–1519. doi: 10.1007/s00424-013-1278-0. [DOI] [PubMed] [Google Scholar]
- 71.Aldrich RW, et al. Ca2+ activated potassium channels. [16/12/2013];IUPHAR Database. http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=69.