I. Introduction
With the initial discovery of penicillin and the ensuing mass production of antibiotics in the 1940s, infectious bacteria quickly adapted and developed resistance to the deleterious molecules. In fact, a report published in 1947 found that of 100 staphylococcus infections tested, 38 were classified as highly resistant to penicillin (1). The initial resistance was primarily associated with individual enzymes inactivating specific antibiotics, such as β-lactamases on penicillin. As novel antibiotics were implemented to combat resistant pathogens, selective pressure led to fundamentally new methods of drug resistance. Currently, there are roughly three major mechanisms utilized by bacteria to evade the toxic effects of biocidal agents. These mechanisms include enzymes that modify the drug, alteration of the antibacterial target, and reduced drug uptake due to the presence of efflux pumps or a decrease in porin expression. Enzymatic modification involves two classes of enzymes, including those that degrade specific antibiotics (2) and enzymes that chemically modify the antibacterial compound (3), resulting in inhibition of drug function. The second mechanism employed by bacteria is alteration of the drug target. Nearly all relevant fluoroquinolone resistance has been attributed to target alteration, whereby specific mutations inhibit the drugs interaction to DNA gyrase and topoisomerase IV (4). Although highly effective, these mechanisms are limited by inhibiting only specific classes of antibiotics. A more critical issue is the broad spectrum of antibiotic resistance associated with multidrug efflux pumps. Ubiquitous in most living cells, multidrug efflux transporters have gained recognition as the major contributor to drug resistance observed in many pathogenic microorganisms (5, 6). These transporters are capable of capturing and exporting toxic compounds before they reach their cellular target, thereby decreasing the effectiveness of the drug (6–8). The first drug transporter TetA, identified in 1980 (9), specifically conferred tetracycline resistance on its host. Following this breakthrough, ensuing discoveries identified protein families capable of eliminating various structurally unrelated toxic compounds.
Based on sequence and functional similarities, there are currently five families of multidrug-resistant (MDR) efflux pumps, including primary transporters of the ATP-binding cassette (ABC) family (10), and secondary transporters in the resistance-nodulation-division (RND) (11), multidrug and toxic compound extrusion (MATE) (12, 13), major facilitator (MF) (14–16), and small multidrug resistance (SMR) families (17). Of these protein families, RND transporters are considered the primary contributor to multidrug resistance associated with gram-negative bacteria (11, 18, 19). Transporters in the RND family are energized through the proton-motive force (PFM), with translocation of protons occurring in the transmembrane (TM) domain. These pumps generally function as a tripartite efflux complex in conjunction with a membrane fusion protein and an outer membrane channel to export substrates completely out of the bacterial cell (11). Strict regulation of these proteins is maintained at the transcription level through repressors and activators that respond to a similar library of compounds to initiate protein expression.
Recently, tremendous strides have been made toward understanding the mechanisms that govern the RND transporter function. These works have focused on both the transporters and the regulatory networks that control their expression. In this chapter we focus on the tripartite RND transporters that are the primary cause of biocidal resistance identified in the three gram-negative bacterial species. These tripartite systems include the AcrAB-TolC and CusABC efflux complexes of Escherichia coli, the CmeABC pump of Campylobacter jejuni, and the MtrCDE transporter of Neisseria gonorrhea. Further, we discuss the regulation of these efflux pumps, especially for the structural aspects of the E. coli AcrR and C. jejuni CmeR transcriptional regulators. With a detailed understanding of the nature of these protein machineries, it will be possible to generate novel therapeutics capable of inhibiting the function of these efflux transporters, thus making futile antibiotics viable once again.
II. Escherichia coli AcrAB-TolC system
Bacteria are highly adaptive microorganisms with an impressive ability to acquire resistance to each antibiotic they encounter. Broad-spectrum antibiotic resistance, a critical problem associated with the treatment of bacterial infections, is attributed primarily to multidrug-resistant (MDR) efflux pumps. Through sequence analysis, it was identified that E. coli harbor 37 putative multidrug efflux transporters (20, 21). Thus far, about 20 of these transporters have been identified as contributors to multidrug resistance (21). MDR proteins of the RND transporter family have been suggested to play the most prominent role in drug resistance, with the multidrug efflux pump AcrB providing a resistant phenotype to the widest range of substrates (22, 23). This inner membrane transporter recognizes and drives the extrusion of various structurally dissimilar antibacterial compounds, including commonly used antibiotics, bile salts, dyes, and detergents (22, 23). It functions as part of a tripartite efflux complex in conjunction with the membrane fusion protein AcrA (24, 25) and outer membrane channel TolC (26, 27). Together, the AcrAB-TolC complex spans both the inner and outer membranes of gram-negative bacteria to effectively lower the intracellular concentration of bactericidal compounds. Drug extrusion is catalyzed through the proton-motive force (PMF), whereby the RND transporter AcrB constitutes both the site of drug recognition and energy transduction for the entire protein complex (8, 28–30).
The AcrB transporter has been implicated in many clinically significant cases of drug-resistant bacterial infections (31–34). For example, fluoroquinolone resistance in Salmonella enterica serovar typhimurium DT104 has been associated with increased efflux through the AcrB pump (31). Further, in drug-resistant, clinical E. coli isolates, AcrB overexpression was identified as the main cause of resistance to ciprofloxacin (34). To combat these pathogens and design better treatments against drug-resistant diseases, it is important to delineate the mechanisms of multiple drug recognition and expulsion associated with these transporters. Recently, the crystal structures of individual components of both the E. coli AcrAB-TolC (27, 35–40) and Pseudomonas aeruginosa MexAB-OprM (41–45) tripartite efflux complexes have been made available to us. These structures have provided invaluable insights into the efflux mechanisms of these RND transport systems.
The structures of E. coli AcrB (35–39) and P. aeruginosa MexB (41) suggested that these two proteins span the entire width of the inner membrane and protrude approximately 70 Å into the periplasm. The crystal structures of the outer membrane channels, E. coli TolC (27) and P. aeruginosa OprM (42), have also been determined. TolC is highlighted by a 100-Å-long α-helical tunnel that extends from the outer membrane anchored β-sheet domain and protrudes into the α-helical periplasmic domain. The P. aeruginosa OprM channel possesses a similar elongated α-helical tunnel that projects into the periplasmic space. Recently, the structures of the two periplasmic membrane fusion proteins, E. coli AcrA (40) and P. aeruginosa MexA (43–45), associated with these RND transporters have been solved. The structures suggest that these two periplasmic proteins are folded into elongated secondary structures consisting of an ~47-Å-long α-hairpin domain that presumably interacts with the α-helical tunnels of their corresponding outer membrane channels. Furthermore, the N- and C-terminal ends of these membrane fusion proteins are thought to contact their respective inner membrane transporters, creating a functional complex that spans both membranes (46).
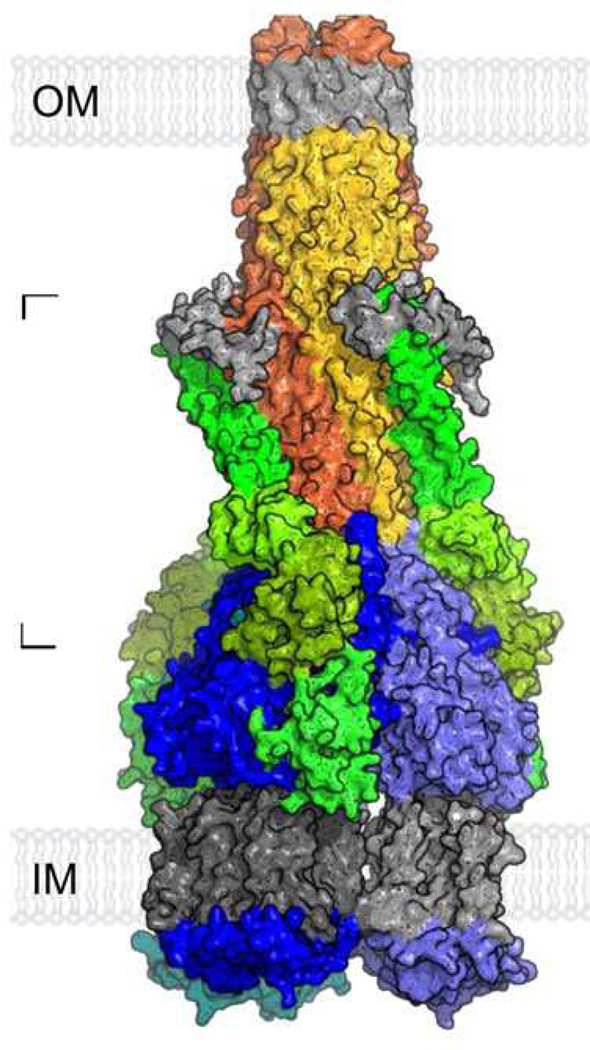
In 2009, the full-length MexA membrane fusion protein structure was made available (45). The structure revealed that this protein consists of four distinct regions, which include the α-hairpin, lipoyl, β-barrel and membrane proximal domains. These structural features are in good agreement with our recently determined crystal structure of the CusB membrane fusion protein, in which the full-length CusB protein also consists of four distinct domains (47). Based on the full-length structure of MexA and cross-linking experiments between AcrA, AcrB and TolC, the assembled structure of a complete tripartite efflux complex in the form TolC3-AcrA3-AcrB3, of size of ~600 kDa, has been generated (45). Figure 1 illustrates a proposed model of this assembled tripartite drug efflux pump. This model revealed that the α-hairpin domain of AcrA makes coiled-coil interactions with helices H3 and H4 of the TolC entrance coils. Presumably, the flexible nature of AcrA permits the TolC channel to shift from the closed state to open conformation, which allows substrates to exit the protein complex. In addition, the membrane proximal domain of AcrA, formed by both the N- and C-termini, directly contacts the PN2 and PC1 subdomains of AcrB in the periplasm. This structural model confirmed the earlier work that the C-terminal end of AcrA is important for AcrA and AcrB interaction (46). The model also indicated that AcrA does not contact the cleft region between the PC1 and PC2 subdomains of AcrB. Thus, the cleft is opened to the periplasm in the assembled structure, presumably allowing substrates to enter the pump. A possible pathway for ligand binding and extrusion in the AcrB pump has been suggested by the structure of the asymmetric trimer (37–39), in which the external cleft may serve as an entrance for AcrB substrates. Indeed, the structures of AcrB in complexes with various substrates have confirmed that these molecules are located inside the cleft (48, 49).
Figure 1.
Model of the assembled tripartite drug efflux pump of AcrAB-TolC in the form of TolC3-AcrA3-AcrB3. This possible model is generated based on the crystal structures of individual components of the complex in addition to cross-linked data between AcrA, AcrB, and TolC. The TolC trimer [orange, red, and yellow subunits with gray equatorial domains and outer membrane (OM) regions] was docked onto AcrA (green)-docked AcrB trimer (blue/light blue subunits with gray inner membrane (IM) regions). [From (45), with permission from the National Academy of Sciences and V. Koronakis.] (See insert for color representation.)
The asymmetric model of AcrB (37–39) suggests that the three protomers of the AcrB trimer may go through a cyclic conformational change, from an access form through a binding mode and finally, to the extrusion conformation. This mechanism is needed to couple with the process of proton translocation through the proton relay system in the transmembrane domain (29). The periplasmic domain of the binding protomer, containing an expanded binding pocket surrounded by several aromatic and hydrophobic amino acids, has also been found to bind drugs (37). A detailed description of the AcrAB-TolC system is provided in Chapter 1.
A. Crystal Structure of the AcrR Regulator
As a drug/proton antiporter with a wide substrate range, transcriptional regulation of AcrB is an important factor in maintaining cell viability. Unregulated overexpression of RND transporters can lead to deleterious side effects, including a loss of membrane integrity and potential export of important metabolites (50–52). Furthermore, expression of the multidrug efflux complex must be induced rapidly while the natural substrates are at subinhibitory concentrations.
The acrAB operon is globally regulated by the transcriptional activators MarA, SoxS, and Rob. These are well-characterized transcriptional activators that initiate expression of a group of 40 promoters (known as the marA/soxS/rob regulon), including acrAB (53, 54) and tolC (55), by interacting with the marbox operator sequence. Local regulation of acrAB is achieved through the transcriptional regulator AcrR, which is located 141 bp upstream of the acrAB operon and is transcribed divergently. AcrR is a prototypical member of the TetR family of transcriptional regulators and is responsible for fine-tuning the transcription of acrAB by repressing transcription initiation until the appropriate ligands are present (56).
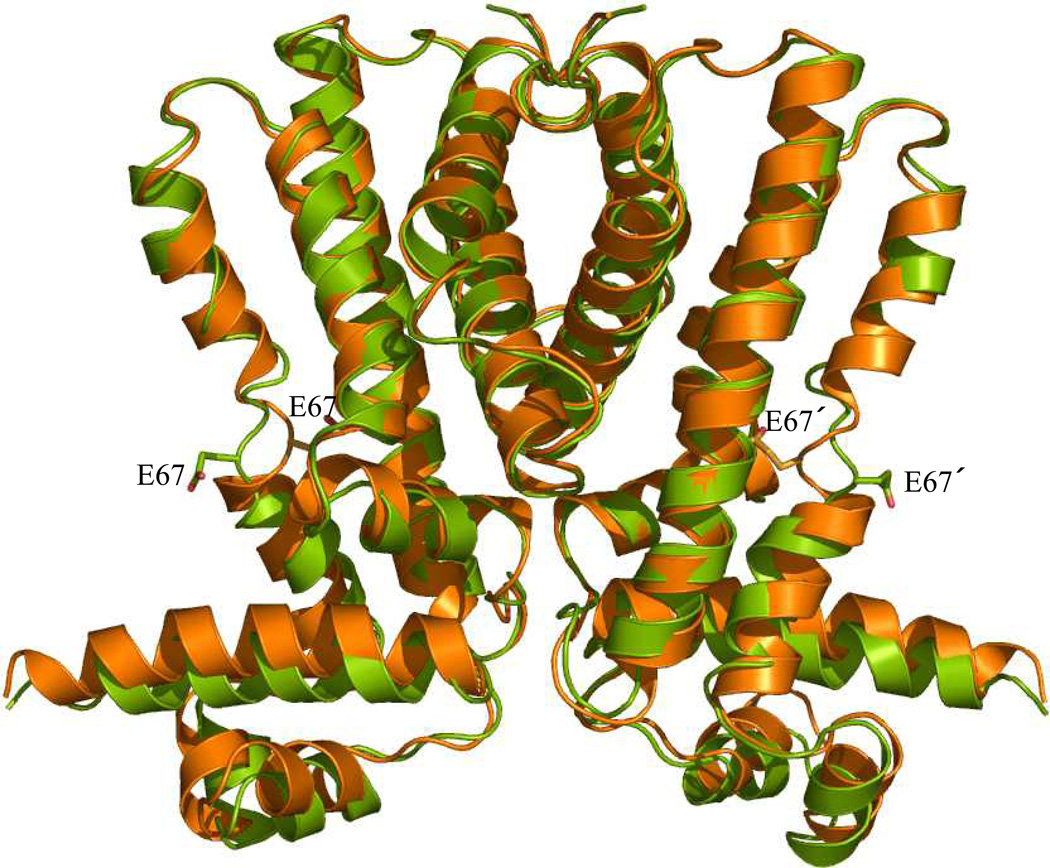
Transcription of acrR gives rise to a 215-amino acid regulatory protein (56). Similar to other members of the TetR family, it is characterized by a homologous N-terminal three-helix DNA binding domain composed of the hallmark helix-turn-helix (HTH) motif (57). This regulator presumably functions as a dimer of dimers to repress transcription of acrAB by interacting with the 24 base pair inverted repeat (IR) operator sequence, 5´-TACATACATTTGTGAATGTATGTA-3´. The IR sequence is positioned between the acrR and acrAB genes and overlapping with the acrAB promoter (56, 58, 59). In addition, the C-terminal domain, a region highly diverse among the TetR family, functions primarily in ligand recognition. As the regulator of AcrAB, AcrR responds to the same array of ligands as AcrB. In fact, recent studies indicate that both AcrB and AcrR bind these ligands with strikingly similar affinities. It appears that AcrR binds ethidium (Et), proflavin (Pf), and rhodamine 6G (R6G) with dissociation constants of 4.2, 10.1, and 10.7 µM (59), while AcrB interacts with these ligands with KD values of 8.7, 14.5, and 5.5 µM, respectively (60). Presumably, ligand interaction may initiate a conformational shift that results in the dissociation of AcrR from its cognate operator (57). Together, this suggests an effective mechanism to initiate AcrB expression and expulsion of substrates, while the toxic compounds are at subinhibitory levels. Recently, we have determined two distinct crystal structures of AcrR in space groups of P2221 (61) and P31 (62). Figure 2 illustrates a superimposition of these two AcrR structures. The structures have provided valuable insights into the mechanisms of ligand recognition and transcriptional regulation.
Figure 2.
Structural comparison of the P31 and P2221 structures of AcrR. Superimposition of the dimeric AcrR structures was performed using the program ESCET (green, P31 structure; orange, P2221 structure). The conformational differences highlighted in these two crystal structures provide a plausible model to describe transcriptional regulation by AcrR. Residue E67 in each subunit is shown as a stick model. (See insert for color representation.)
The crystal structures of AcrR reveal a dimeric protein with secondary structures composed entirely of α-helices and an overall architecture similar to other members of the TetR family, including TetR (63, 64), QacR (65, 66), CmeR (67), CprB (68), and EthR (69, 70). Each protomer of the AcrR dimer consists of helices (α1 to α9 and α1´ to α9´), with helices α1 to α3 making up the N-terminal DNA-binding domain and helices α4 to α9 forming the larger C-terminal ligand-binding region. Dimerization of AcrR occurs mostly through contacts made between helices α8 and α9, which creates a contact region of 2002 Å2 per monomer. The structures also demonstrate that a 350-Å3 internal hydrophobic pocket, surrounded by helices α4 through α8 of each monomer, forms a multifaceted ligand-binding pocket. Docking of known inducers, including Pf, Et and ciprofloxacin (Cip), into this ligand-binding pocket suggests that W63, I170, and F114 are supposed to perform hydrophobic contacts with the bound drugs. Additionally, residues E67 and Q130 are predicted to make important electrostatic interactions to the inducing compounds (61). Further confirmation of the binding site was identified with an E67A AcrR mutant that abolished the binding of Pf, Et, and rhodamine 6G (R6G) in the regulator (61). Evidence to support the multifaceted multidrug binding pocket was provided through fluorescence polarization experiments, which indicated that Pf and Et bind noncompetitively within the hydrophobic cavity (59).
Intriguing structural changes were observed when comparing the two conformations of AcrR (Figure 2), with the most significant changes occurring in the stretch of amino acids between helix α1 and the N-terminal half of helix α4 (residues 7–65) in the DNA-binding region [root-mean-square deviation (RMSD) of 2.8 Å]. Conformational changes in this region seem to be predominately rigid-body translation and rotation, resulting in a downward shift by 2.6 Å and a rotational movement of 10° of the N-terminal domain in the P31 structure with respective to that of P2221. As a result of these movements, the N-terminal domains of the dimer move closer together by about 3 Å (42 Å in the P2221 structure and 39 Å in the P31 conformation) (71). In both QacR (64) and TetR (66), similar changes have been observed in which the distances between N-terminal domains decrease by 11Å and 3Å, respectively, when converting from a drug-bound state to the DNA-bound conformation. Presumably, these changes allow the regulators to bind consecutive major grooves in B-form DNA. In addition to the overall movement of the N-terminal domain, the distance between two R45 residues in the dimeric AcrR shifts from 40 Å in the P2221 structure to 35 Å in the P31 structure (62). As AcrR and QacR share a similar DNA-binding mode, in which two dimeric regulators bind one double-stranded DNA, a speculative model of DNA-bound AcrR was generated by aligning individual domains of AcrR to those of QacR-DNA (61). The model suggests that two AcrR dimers interact with the DNA through amino acids, R45, G46, and W50. A study of fluoroquinolone-resistant E. coli strains highlights the critical role of R45. Six of the 36 isolated drug-resistant strains of E. coli examined had increased levels of AcrA and AcrB due to a mutation at R45 (arg→cys) that presumably inhibited AcrR-DNA binding (34).
When examining the changes in the C-terminal domain between the P2221 and P31 structures, the most striking distinction involves the shift of amino acid E67. In the P31 conformation, E67 is expelled from the ligand-binding pocket and faces the exterior of the protein, which contrasts the P2221 form, whereby E67 is completely buried within the binding cavity. These intriguing shifts of E67 suggest that this amino acid may act as a molecular switch that drives conformational change during ligand binding (71). The movement of E67 out of the binding pocket in the P31 structure initiates considerable changes in the C-terminal domain of AcrR, including helix α4a shifting toward the N-terminal domain by 2.3 Å and a local unwinding of N-terminus of α6, which shortens the helix by one turn. Moreover, an important hydrogen-bonded network between residues R105, Q14, and D18 identified in the P2221 structure is disrupted in the P31 crystal form (62).
With the evidence previously indicated, it is likely that the DNA-bound state of AcrR resembles the P31 crystal structure, whereby E67 is positioned outside the ligand-binding site. Upon ligand binding, E67 presumably flips into the cavity and makes appropriate electrostatic interactions with the ligand creating a conformation closely related to the P2221 structure. Thus, transmission of the signal from the C-terminal ligand-binding domain to the N-terminal DNA-binding region is thought to occur through the hydrogen-bonded network, R105, Q14, D18, between helices α1 and α4. The crystal structures of the DNA- and ligand-bound forms of AcrR are required to confirm the proposed model for transcriptional regulation of AcrR.
III. Escherichia coli CusABC Efflux System
E. coli contains seven different RND efflux transporters. These transporters can be categorized into two distinct subfamilies, the hydrophobic and amphiphilic efflux RND (HAE-RND) and heavy-metal efflux RND (HME-RND) families (11, 72). Six of these transporters—AcrB (35–39, 73), AcrD (74), AcrF (75), MdtB (76, 77), MdtC (76, 77) and YhiV (21, 78)–are multidrug efflux pumps, which belong to the HAE-RND protein family (11). In addition to these multidrug efflux pumps, E. coli consists of only one HME-RND transporter, CusA, which specifically recognizes and confers resistance to Ag(I) and Cu(I) ions (79, 80). These two metal ions are highly toxic to prokaryotes and have been widely used for centuries as effective antimicrobial agents to combat pathogens. The two sub-families of these RND transporters share relatively low protein sequence homology. For example, alignment of protein sequences suggests that CusA and AcrB possess only 19% identity. Because of this low protein sequence homology, the structural model of AcrB may not be precise enough to describe the conformation of the CusA transporter.
As an RND transporter, CusA works in conjunction with a periplasmic component, belonging to the membrane fusion protein (MFP) family, and an outer membrane channel to form a functional protein complex. CusA is a large PMF-dependent inner membrane efflux pump that contains 1047 amino acid residues (79, 80). CusC, however, is a 457-amino acid polypeptide that forms an outer membrane channel (79, 80). The membrane fusion protein CusB consists of 379 amino acids and contacts both the inner membrane CusA and outer membrane CusC proteins (79, 80). Presumably, the three components of this HME-RND system form a tripartite efflux complex that resembles the AcrAB-TolC complex, whereby heavy-metal efflux in CusABC is driven by proton import and catalyzed through CusA.
Between the cusC and cusB genes, there is a small chromosomal gene that produces a periplasmic protein CusF (79, 80). This small periplasmic protein is also involved in Cu+ and Ag+ resistance. The crystal structure of CusF suggests that this protein forms a five-stranded β-barrel, and its conserved residues H36, W44, M47, and M49 form a Cu+ or Ag+ binding site (81–83). CusF probably functions as a chaperone that carries Cu+ or Ag+ to the CusABC heavy-metal efflux pump. In fact, it has been recently shown that CusF is able to directly transfer bound Cu+/Ag+ ions to the membrane fusion CusB (84). The entire CusAB(F)C system is controlled by a two-component sensory circuit, which includes the histidine kinase CusS and the response regulator CusR (85).
Currently, little structural information is available for any components of the HME-RND tripartite efflux complexes. Different from the HAE-RND family, members of the HME-RND family are highly substrate specific, with the ability to differentiate between monovalent and divalent ions. Thus, there is a strong rationale to understand the structural aspect of this efflux system. As an initial step to examine the mechanisms used by the CusABC efflux system to facilitate recognition and extrusion of Ag(I) and Cu(I) ions, we recently determined the crystal structure of the periplasmic membrane fusion protein CusB (47).
A. Crystal Structure of the Membrane Fusion Protein CusB
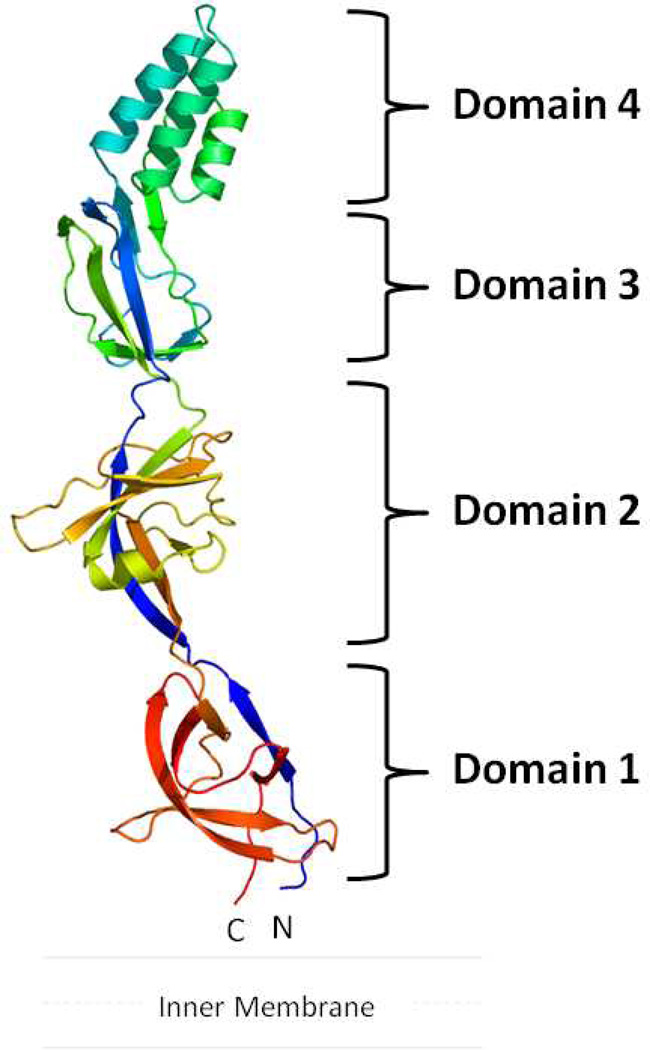
The crystal structure of CusB (47) suggests that this protein is folded into an elongated molecule with each protomer being divided into four different domains (Figure 3). The first three domains of the protein are mostly β-strands. However, the fourth domain forms an entirely α-helical domain featuring a three-helix bundle secondary structure.
Figure 3.
Crystal structure of the CusB membrane fusion protein. The structure can be divided into four distinct domains. Domain 1 is formed by the N- and C-termini and is located above the inner membrane. The loops between domains 2 and 3 appear to form an effective hinge to allow the molecule to shift from an open conformation to a more compact structure. Domain 4 is folded into an antiparallel, three-helix bundle, which is thought to be located near the outer membrane. (See insert for color representation.)
The first β-domain (domain 1) is formed by the N- and C-terminal ends of the polypeptide (residues 89–102 and 324–385). Presumably, this domain is located directly above the outer leaflet of the inner membrane and interacts with the CusA efflux pump. Overall, domain 1 is a β-barrel domain. It is composed of six β-strands, with the N-terminal end forming one of the β-strands while the C-terminus of the protein constitutes the remaining five strands (Figure 3).
The second β-domain (domain 2) of CusB is formed by residues 105–115 and 243–320. This domain consists of six β-strands and one short α-helix. Again, the N-terminal residues form one of the β-strands that is incorporated into this domain. The C-terminal residues contribute a β-strand, an α-helix, and four anti-parallel β-sheets.
Domain 3 is another globular β-domain adjacent to the second domain of CusB. This domain consists of residues 121–154 and 207–239, with a majority of these residues folding into eight β-strands.
Perhaps the most interesting motif appears to be in the fourth domain (domain 4) of CusB. This region forms an all-helical domain, which comprises residues 156–205. Surprisingly, this α-domain is folded into an antiparallel, three-helix bundle. This structural feature, not found in other known protein structures in the MFP family, highlights the uniqueness of the CusB protein. The helix bundle creates an ~27-Å-long helix-turn-helix-turn-helix secondary structure, making it at least 20 Å shorter than the two-helical hairpin domains of MexA (43–45) and AcrA (40). To date, CusB is the only periplasmic protein in the MFP family that possesses this three-helical domain instead of a two-helical hairpin motif. The overall structure of CusB is quite distinct from the known structures of other membrane fusion proteins.
B. The CusB-Cu(I) and CusB-Ag(I) Complexes
To identify the metal binding sites of CusB, we prepared the CusB-Cu(I) and CusB-Ag(I) crystal complexes by soaking these metal ions into the apo-CusB crystals. The overall structures of these complexes are very similar to that of apo-CusB. For example, the structures of CusB-Cu(I) and apo-CusB can easily be superimposed, giving an overall RMSD of 0.8 Å. It appears that we have found two Cu+ (designated sites C1 and C2) and one Ag+ (designated site A1) binding sites in this protein (47). To our knowledge, these are the first structures of any membrane fusion proteins that have been determined with their ligands. The structures suggest an unusual metal binding mode, in which each metal-binding site consists of only one methionine residue to facilitate metal binding.
Cu+ in site C1 is located in domain 1, which is formed by the N- and C-termini of the protein. Coordinating with the bound Cu+ ion at this site are M324, F358, and R368. Site C1 is located near the bottom of the elongated CusB molecule. Presumably, this region may interact directly with the periplasmic domain of the CusA efflux pump. The binding of Cu+ in site C2 is located close to the center of the three-helix bundle in Domain 4. This α-helical domain may make a direct contact with the outer membrane channel CusC. Cu+ in this location is bound by M190, W158, and Q162.
For the Ag+ binding, site A1 is found right next to M324 of CusB. It appears that the location of this Ag+-binding site is the same as that of site C1 for Cu+ binding. Thus, the bound Ag+ at site A1 is coordinated with M324, F358, and R368.
There is evidence that members of the MFP family play a functional role in the efflux of substrates. It has been found that the MFP EmrA is able to directly bind different transported drugs (86). Recently, the CusB protein has also been shown to interact with Ag(I) (87). The crystal structures of the CusB-Cu(I) and CusB-Ag(I) complexes provide direct evidence that this protein specifically interacts with and contacts Cu(I) and Ag(I). Thus, in addition to their role as adaptors to bridge the inner and outer membrane efflux components, these membrane fusion proteins may participate in recognizing and extruding their substrates.
C. Interaction Between CusA and CusB
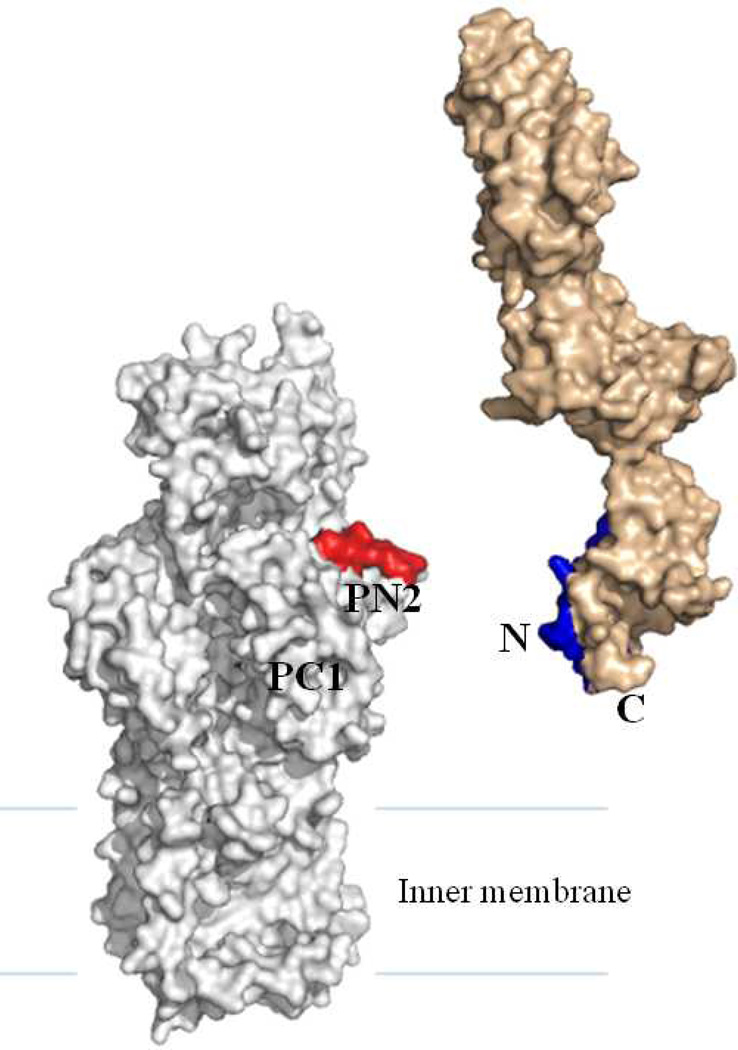
To determine how CusB interacts with the CusA efflux pump and the relative orientation of CusB in the efflux complex, we cross-linked the purified CusA and CusB proteins in vitro using the lysine-lysine cross-linker disuccinimidyl suberate (DSS) (47). The resulting product was digested with trypsin and examined using LC-MS/MS. Analysis of the mass spectral data suggests that the lysine residue of the polypeptide β (IDPTQTQNLGVKTATVTR), originating from the N-terminal residues (84–101) of CusB, interacts directly with the lysine residue of peptide α (SGKHDLADLR), which belongs to the periplasmic domain (residues 148–157) of the CusA efflux pump. Although the CusA and AcrB efflux pumps share only 19% protein sequence identity, we generated a structural model of the CusA transporter based on the crystal structure of AcrB and alignment of protein sequences of these two transporters (Figure 4). The model indicates that polypeptide α (residues 148–157 of CusA) is located directly above the vestibule region of CusA, facing the periplasm. This location should correspond to the PN2 region of AcrB. If this is the case, the C-terminus of CusB should interact with CusA at a position corresponding to the PC1 region in AcrB (Figure 4). According to the most recently determined MexA structure, it suggested that both the N- and C-terminal ends of MexA are located close to the MexB transporter (45). In addition, in vivo cross-linking studies also demonstrated that the N- and C-termini of AcrA interact directly with PN2 and PC1 of the periplasmic domain of AcrB, respectively (45). Together with the crystal structure of CusB and the mass spectrometric data, it has been suggested that domain 1, formed by the N- and C-terminal ends, of CusB should interact with the periplasmic domain of the CusA transporter (47).
Figure 4.
Specific interaction between CusA and CusB. The model of CusA (gray) was created based on protein sequence alignment and the crystal structure of AcrB. Mass spectral data suggest that the periplasmic domain of CusA specifically interacts with the N-terminus of CusB (light brown). Polypeptides α, SGKHDLADLR (from CusA), and β, IDPTQTQNLGVKTATVTR (from CusB), are shown in red and blue, respectively. (See insert for color representation.)
As CusB is folded into a distinct secondary structure compared with the current crystal structures of other membrane fusion proteins, this may imply that its tripartite partners, the inner membrane transporter CusA and the outer membrane channel CusC, may also possess unique secondary structural features that distinguish them from the existing structures of their homologous proteins. Exactly how these individual heavy-metal efflux components assemble into a functional complex must await the elucidation of the CusA and CusC structures.
IV. Campylobacter jejuni CmeABC Efflux System
Campylobacter jejuni is a major causative agent of human enterocolitis and is responsible for more than 400 million cases of diarrhea each year worldwide (88). Campylobacter infection may also trigger an autoimmune response, which is associated with the development of Guillain-Barré syndrome, an acute flaccid paralysis caused by degeneration of the peripheral nervous system (89). C. jeuni is widely distributed in the intestinal tracts of animals and is transmitted to humans via contaminated food, water, or raw milk. For antibiotic treatment of human campylobacteriosis, fluoroquinolones and macrolides are frequently prescribed (90). Unfortunately, Campylobacter has developed resistance to both classes of antimicrobials, especially to fluoroquinolones (91–93). Resistance of Campylobacter to antibiotics is mediated by multiple mechanisms (94), including (1) synthesis of antibiotic-inactivating enzymes, (2) alteration or protection of antibiotic targets, and (3) active extrusion of drugs from Campylobacter cells through drug efflux transporters. Different from the first two mechanisms that are usually involved in the resistance to a specific class of drugs, antibiotic efflux pumps in Campylobacter contribute to both intrinsic and acquired resistance to a broad range of antimicrobials and toxic compounds.
C. jejuni harbors multiple drug efflux transporters of different families (94). Among them, CmeABC, an RND-type efflux pump, is the primary antibiotic efflux system and is the best functionally characterized transporter in Campylobacter (95, 96). CmeABC consists of three components including an outer membrane protein (CmeC), an inner membrane drug transporter (CmeB), and a periplasmic fusion protein (CmeA). CmeABC contributes significantly to the intrinsic and acquired resistance of Campylobacter to structurally diverse antimicrobials, including fluoroquinolones and macrolides, by reducing the accumulation of drugs in Campylobacter cells (95–100). It has been found that CmeABC functions synergistically with target mutations in conferring and maintaining high-level resistance to fluoroquinolones and macrolides (97, 98, 100–102). This efflux pump also plays an important role in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure (103). Inactivation of CmeABC reduced the frequency of emergence of fluoroquinolone-resistant mutants, while overexpression of CmeABC increased the frequency of emergence of the mutants (103). This contributing effect of CmeABC is due to the fact that many of the spontaneous gyrA mutants can not survive selection by ciprofloxacin in the absence of CmeABC.
In addition to conferring antibiotic resistance, CmeABC also has important physiological functions. It has been shown that CmeABC is functionally interactive with CmeDEF, another RND-type efflux pump, in maintaining optimal cell viability in Campylobacter, possibly by extruding endogenous toxic metabolites (104). Double mutations in CmeABC and CmeDEF appeared to be lethal to C. jejuni strain 11168 and significantly reduced the growth of strain 81–176 in conventional media. Another important function of CmeABC is bile resistance. As an enteric pathogen, C. jejuni must possess means to adapt in the animal intestinal tract, where bile acids are commonly present. Mutations of CmeB in C. jejuni resulted in a drastic increase in the susceptibility to various bile acids and a severe growth defect in bile-containing media or in chicken intestinal extracts (105). When inoculated into chickens, the CmeB mutant failed to colonize the inoculated birds. These findings provide compelling evidence that by mediating the resistance to bile acids, CmeABC is essential for Campylobacter adaptation to the intestinal environment. These findings also strongly suggest that bile resistance is a natural function of this RND-type efflux pump.
CmeABC is subject to regulation by a transcriptional factor named CmeR (106). The cmeR gene is located immediately upstream of the cmeABC operon and encodes a 210-amino acid protein that shares N-terminal sequence homology to the members of the TetR family of transcriptional repressors (57, 107). Similar to other members in the TetR family, the N-terminal region of CmeR contains a DNA-binding α-helix-turn-α-helix (HTH) motif, while the C–terminal region is involved in the interaction with inducing ligands (67). cmeR is transcribed in the same direction as cmeABC and the intergenic region between cmeR and cmeA contains the promoter (PcmeABC) for cmeABC. As a transcriptional repressor, CmeR binds directly to an inverted repeat in PcmeABC and inhibits the transcription of cmeABC (106). Deletion of cmeR or mutations in the inverted repeat of PcmeABC releases the repression and results in overexpression of CmeABC. Recently, it was shown by DNA microarray that CmeR functions as a pleiotropic regulator and modulates the expression of multiple genes in C. jejuni, occurring by direct or indirect methods (108). One of the newly identified CmeR-regulated genes is Cj0561c, which is predicted to be a periplasmic protein. The promoter of Cj0561c contains two CmeR-binding sites and is strongly repressed by CmeR. Although the exact function of Cj0561c was unknown, inactivation of this gene led to reduction of Campylobacter colonization in the intestinal tract of chicken (108), suggesting that Cj561c is important for Campylobacter physiology.
As a major mechanism in bile resistance, cmeABC is inducible by various bile conjugates (109). Thus, the expression level of cmeABC is influenced by bile. The induction of cmeABC is due to the inhibitory effect of bile on the binding of CmeR to PcmeABC, which promotes the release of CmeR from the promoter DNA (109). Since CmeR represses the expression of cmeABC, dissociation of CmeR from PcmeABC results in elevated expression of cmeABC. Not surprisingly, bile salts also strongly induce the expression of Cj0561c, which is also regulated by CmeR (108). These in vitro findings were consistent with the results from in vivo studies, in which DNA microarray and real-time RT-PCR revealed that expression of cmeABC and Cj0561c was greatly up-regulated in animal intestinal tracts (110). This suggests that bile-mediated induction of CmeR-regulated genes also occurs in animal hosts. Based on these findings, it is conceivable that CmeR senses the presence of bile compounds in the environment and accordingly, modulates the expression levels of its target genes.
The information discussed above indicates that the CmeR regulatory network plays an important role in Campylobacter physiology and in its resistance to various antimicrobials. To understand the structural basis of CmeR regulation and facilitate the development of anti-Campylobacter therapeutics, we have initiated our work to determine the three-dimensional structure of CmeR. Our protein crystallization studies confirmed the two-domain structure of CmeR and showed that CmeR functions as a homodimer (67).
A. Crystal Structure of the CmeR Regulator
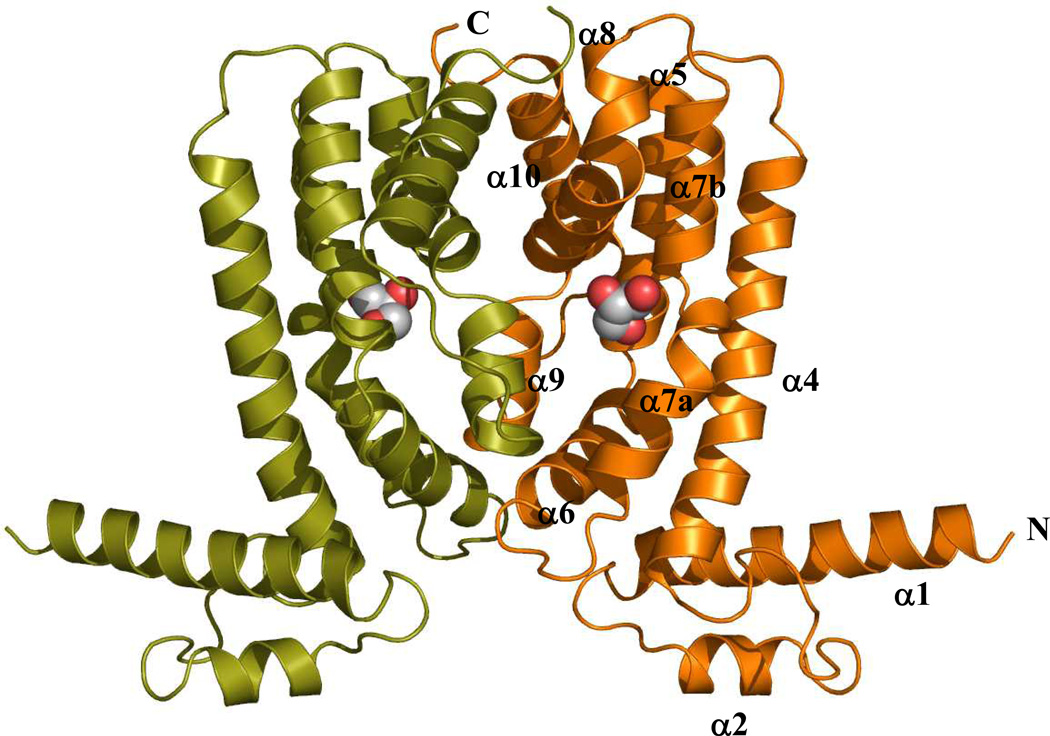
The crystal structure of dimeric CmeR, a member of the TetR family of regulators, is shown in Figure 5. This structure revealed that each subunit of CmeR is composed of nine α-helices, in which the characteristic short-recognition α3 helix, presumably formed by residues 47–53, is replaced by an intriguing random coil (67). This is, perhaps, the most striking feature that distinguishes CmeR from the other TetR family members. To date, the CmeR regulator is the only observed case of a random coil replacing helix α3 in a TetR family member. Presumably, the TetR regulators possess a HTH DNA-binding motif formed by helices α2 and α3. Owing to its important role in recognizing target DNA, helix α3 is named the recognition helix (57). Since CmeR is a pleiotropic regulator of a large set of genes and is predicted to bind multiple operator sites, with many of those not being of the consensus IR sequence located in the promoter region of cmeABC (108). It could be postulated that the flexibility of the DNA-binding domain, illustrated by the random coil in place of the helix α3, permits CmeR to recognize multiple cognate DNA sites. One other unique feature of the CmeR structure is its large center-to-center distance between the two N-termini of the dimer. This center-to-center distance (according to the separation between Cα atoms of Y51 and Y51' was measured to be 54 Å (67). The corresponding distances are 39 Å and 35 Å in the apo forms of QacR (65) and TetR (64). These center-to-center distances increase upon ligand binding. For the ligand-bound dimers of QacR (65), TetR (63), EthR (70), and YfiR (111), these distances become 41, 38, 52, and 54 Å, respectively. Thus, the relatively large center-to-center distance observed for CmeR reflected the fact that CmeR was liganded (67). Indeed, the crystal structure indicated that a fortuitous glycerol molecule was bound in each subunit of the CmeR dimer (Figure 5) (67). Although glycerol has not previously been identified as a natural inducer of cmeABC, it is plausible that it mimics the binding mode of other CmeR substrates.
Figure 5.
Crystal structure of the CmeR regulator. The dimeric structure of CmeR indicates that CmeR is an all-helical protein (α1-α10 and α1'-α10', respectively) which can easily be divided into two domains (the N-terminal DNA-binding and C-terminal ligand-binding domains). The bound glycerol molecule in each subunit of CmeR is represented as a hard-sphere model. (See insert for color representation.)
The C-terminal domain of CmeR consists of helices α4 through α10, with helices α4, α5, α7, α8, and α10 forming an antiparallel five-helix bundle. In view of the crystal structure, helices α6, α8, α9, and α10 are involved in the formation of the dimer. Dimerization occurs mainly by couplings between pairs of helices (α6 and α9', α8 and α10', and their identical counter pairs). A surface area of 1950 Å2 per monomer is buried in the contact region of the dimer (67). This interaction surface is mostly hydrophobic in character.
The C-terminal domain also forms a large tunnel-like cavity in each subunit of CmeR. This tunnel, surrounded by mostly hydrophobic residues of helices α4 to α9, opens horizontally from the front to the back of each protomer. The length of this tunnel is approximately 20 Å. Helices α7 and α8 from one subunit, and α9' from the other subunit of the regulator make the entrance of the tunnel. Helices α4-α6, however, contribute to form the end of this hydrophobic tunnel. Each hydrophobic tunnel, occupying a volume of about 1000 Å3, spans horizontally across the C-terminal domain and can be seen through from the front to the back of the dimer without obstruction. This unique feature, not found in other structures of the TetR family of regulators, highlights the flexibility of the CmeR regulator (67). As indicated above, the crystal structure of CmeR revealed the presence of a glycerol molecule inside this large ligand-binding tunnel (Figure 5). Glycerol binds identically in each subunit, as indicated by the crystallographic two-fold symmetry of the CmeR dimer (67). This ligand-binding mode is different from that of QacR, in which one dimer of QacR binds one drug (65), but similar to that of TetR, which interacts with tetracycline in a manner of a 1:1 monomer-to-drug molar ratio (63). The volume of the ligand-binding tunnel of CmeR is large enough to accommodate a few of the ligand molecules. Additional water molecules fill the portion of the large tunnel that is unoccupied by ligand. The structure suggests that CmeR might be able to bind more than one drug molecule at a time, or possibly accommodate significantly larger ligands, which spans across the entire binding tunnel. Indeed, a docking study showed that the hydrophobic tunnel of CmeR should be able to accommodate large, negatively charged bile acid molecules, such as taurocholate and cholate (67). The bound bile acids are predicted to anchor to several hydrophobic, polar, and positively charged residues, including H72, F99, F103, F137, S138, Y139, V163, C166, T167, K170, and H174. These anionic ligands were predicted to span almost the entire length of the ligand-binding tunnel of the regulator, respectively. The large tunnel, possibly consisting of multiple minipockets that may be employed to interact with different ligands, is rich in aromatic residues and contains three positively charged amino acids (two histidines and one lysine). It is very likely that these positively charged residues are crucial for CmeR to recognize negatively charged ligands. Site-directed mutagenesis is needed for an understanding of the detailed function of these charged residues.
V. Efflux Pumps of Neisseria gonorrhoeae : Repertoire and Contributions to Antimicrobial Resistance
The strict human pathogen N. gonorrhoeae expresses four drug efflux pumps (112), which belong to the resistance-nodulation-division (RND) family (MtrCDE), the major facilitator (MF) family (FarAB-MtrE), the ABC transporter family (MacAB), and the multidrug toxic compound extrusion (MATE) family (NorM). These pumps are also possessed by N. meningitides (112), but in this chapter we concentrate on their structure, function, and regulation in gonococci. In addition to these four efflux pumps, some clinical isolates have acquired the mef gene, which encodes a pump that recognizes macrolides (113).
There is evidence that gonococcal efflux pumps can contribute to levels of bacterial resistance to classical antibiotics since inactivation of efflux pump-encoding genes can enhance susceptibility to pump substrates (114–116). Moreover, mutations that increase efflux pump gene expression can also increase antimicrobial resistance of N. gonorrhoeae. From a clinical perspective, the important question is whether efflux pumps can influence the efficacy of antibiotic treatment. In this respect, work on the MtrCDE efflux pump in clinical isolates indicates that this is indeed the case. As an example, overexpression of the mtrCDE operon due to mutations in the mtrR-coding sequence, which encodes a repressor of mtrCDE expression (see below), or its promoter can provide gonococci with a twofold increase in resistance to penicillin (116). However, when strains have co-resident mutations in other chromosomal genes that influence the affinity of penicillin for penicillin-binding proteins (PBPs) or drug influx, resistance can become clinically significant (≥ 2.0 µg/ml). The outbreak of penicillin-resistant gonorrhea that occurred in Durham, North Carolina in the 1980s (117), due to a strain (termed FA6140) that had mtrR mutations as well as other mutations that both decreased the binding of penicillin to PBP-1 and PBP-2 and the influx of penicillin (118), is an example of the impact that efflux can have on gonococcal resistance to antibiotics. Thus, while introduction of the mtrR mutations from penicillin-resistant strain FA6140 by transformation into highly penicillin-sensitive strain FA19, resulted in only a twofold increase in resistance, inactivation of the mtrD gene, which encodes the inner membrane RND transporter protein, in resistant strain FA6140 decreased resistance from 4 to 0.25 µg/ml. This decrease in resistance, due to the loss of efflux activity, was intriguing as it represented a transition from clinical resistance to sensitivity and provides support for the notion that inhibitors of efflux pumps could reverse antibiotic resistance exhibited in pathogenic organisms. In addition to penicillin, gonococcal clinical isolates bearing mtrR mutations can express decreased susceptibility to macrolides and tetracycline (119). In fact, a cluster of azithromycin-resistant gonococci identified in a cohort of patients in Kansas City, Missouri were found to have a Correia insertion within the DNA sequence that intervenes the divergently transcribed mtrR and mtrCDE genes (120).
It has been suggested (112) that efflux pumps endow bacteria with the ability to resist natural or manufactured antimicrobial agents in their local environment and that such resistance is important for their survival in ecosystems. For strict human pathogens, such as gonococci that do not naturally exist for long periods of time outside the human body, these antimicrobial agents would be compounds (e.g., antimicrobial peptides, long-chain fatty acids, bile salts, certain hormones) that are at the frontline of the innate host defense system. In this respect, the MtrCDE efflux pump appears to recognize certain antimicrobial peptides (121), progesterone (115) and bile salts (115), while the FarAB-MtrE pump recognizes long-chain fatty acids (122). In support of the hypothesis that efflux pumps can promote bacterial survival during infection, Jerse et al. (123) found that the MtrCDE efflux pump is required for survival of gonococci in the lower genital tract of experimentally infected female mice (115). More recently, this group reported (124, 125) that the degree of in vivo fitness expressed by gonococci is related to the presence of the MtrR repressor or MtrA activator, which modulate levels of mtrCDE expression (see below). This is a unique example of how a mechanism of antibiotic resistance can actually increase in vivo fitness and is probably due to the ability of the MtrC-MtrD-MtrE pump to recognize both classical antibiotics (e.g., penicillin) and host-derived antimicrobials.
A. Structure of the MtrCDE Efflux System
The mtr (multiple transferable resistance) system was first identified by Maness and Sparling (126) when they isolated a spontaneous mutant that exhibited increased resistance to multiple structurally diverse antimicrobial hydrophobic compounds. It was originally thought that mtr modified outer membrane permeability (127). However, subsequent cloning and sequencing experiments (114, 115, 128, 129) showed that the mutation was located within a gene encoding a transcriptional repressor (MtrR) of a downstream but transcriptionally divergent operon (mtrCDE) encoding the tripartite MtrCDE efflux pump. Similar to other RND-type pumps of gram-negative bacteria, the three proteins are a cytoplasmic membrane transporter (MtrD), a membrane fusion protein (MtrC), and an outer membrane channel protein (MtrE). Directly or indirectly, other proteins also participate in efflux mediated by the pump. Veal and Shafer (130) identified an accessory protein (MtrF) which, for reasons that are not yet clear, is required for efflux activity when the host strain is expressing high levels of the pump during stressful conditions. Energy supplied by the TonB-ExbB-ExbD system is also needed for inducible antimicrobial resistance mediated by MtrC-MtrD-MtrE (131).
B. Regulation of the mtrCDE System
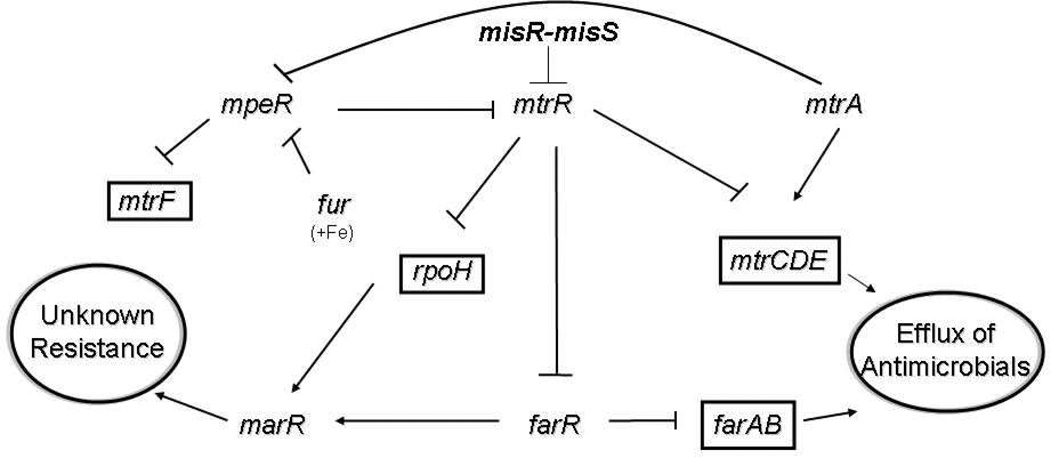
Expression of the mtrCDE operon is controlled by both cis- and trans-acting regulatory elements that negatively or positively control production of the pump proteins at the level of transcription. The DNA-binding proteins that are involved in regulation of this system and their biological importance are described in Figure 6. The degree of mtrCDE gene expression that is controlled by these elements corresponds to constitutive or inducible levels of gonococcal resistance to antimicrobials recognized by the pump. The important trans-acting factors that control mtrCDE expression directly or indirectly in gonococci are MtrR, MpeR, and MtrA (Figure 6). Recent studies indicate (132) that the two-component regulatory system termed MisR-MisS (133) also controls mtrR expression and, as a consequence, mtrCDE. In meningococci, regulation of mtrCDE appears to be controlled by a Correia element (CE) within the mtrR and mtrCDE intervening region that contains a binding site for integration host factor (134). This element was found to be similarly positioned in azithromycin-resistant gonococcal clinical isolates in Kansas City, Missouri (120), but the presence of the CE at this site is apparently unique to these strains.
Figure 6.
Proposed model of the regulatory system in N. gonorrhoeae. Shown is a proposed regulatory system in N. gonorrhoeae strain FA19 that impacts levels of antimicrobial resistance via drug efflux pumps. The lines with bars indicate transcriptional repression; the lines with arrows indicate transcriptional activation.
The MtrR repressor, which is similar to the TetR family of repressors (128), binds as two homodimers to a DNA sequence that lies just upstream of the mtrCDE operon (135). The 15-bp MtrR-binding site is within the mtrCDE promoter and is characterized by two pseudo-direct 7-bp repeats. Mutations in the mtrR helix-turn-helix coding region that reduce or abrogate DNA binding, as well as missense mutations in the C-terminal-encoding region that probably impact homodimer formation, can enhance transcription of mtrCDE and elevate gonococcal resistance to antimicrobials (114, 136, 137). This regulation has important biological consequences. First, by dampening mtrCDE expression levels of gonococcal, resistance to hydrophobic antimicrobials is decreased. Second, and probably linked to the first point, production of an active MtrR decreases gonococcal fitness in vivo (but not in vitro), as assessed by the use of an experimental murine model of lower genital tract infection (124, 125). Expression of mtrR seems to be regulated by both direct and indirect processes involving DNA-binding proteins (Figure 6) and a cis-acting 13-bp inverted repeat within the mtrR promoter (see below). We reported on an AraC-like transcriptional regulator that represses mtrF (Figure 6) expression and termed the protein MpeR (138). In the same year, Dyer’s group reported that mpeR (NGO0025) is maximally expressed under iron-limiting conditions (139), and recent studies showed that iron-replete conditions dampen mtrR expression by an MpeR-dependent mechanism during late-log (140), a phase of growth likely to be iron limiting. It may be that mpeR expression is negatively controlled by the ferric uptake regulator (Fur) in the presence of iron (141), which provides a connection between iron limitation and the regulation of genes encoding transcriptional modulators of efflux pump genes. mtrR expression is also negatively regulated by a two-component regulatory system termed MisR-MisS (142) that is functionally similar (133), but not identical, to the PhoP-PhoQ system in Salmonella enterica serovar typhimurium, which is known to modulate levels of bacterial susceptibility to cationic APs (143).
In addition to regulating mtrCDE, we found (144) through microarray analysis that MtrR can positively or negatively regulate the expression of nearly 70 genes, including genes known or presumed to be involved in the regulation of antimicrobial resistance (marR and farR), response to stress conditions (rpoH), polyamine uptake (potF), glutamine biosynthesis (glnA and glnE) and transport (glnM and glnQ), peroxide detoxification (ccp), and sodium-glutamate symporter activity (gltS). FarR is a transcriptional repressor of the farAB efflux pump system (139), which exports antimicrobial fatty acids (122), but can also activate glnA and marR (Figure 6). Thus, we propose that a regulatory circuit exists in gonococci as demonstrated in Figure 6, which controls not only levels of bacterial resistance to host antimicrobials and antibiotics, but also expression levels of important metabolic processes (e.g., glutamine biosynthesis).
Thus, apart from its ability to control levels of MtrCDE, MtrR may control other processes important for basic metabolism and pathogenesis. Given this hypothesis, it is fair to ask: Why did evolution not select against mtrR if its loss increases fitness during infection? We believe that the answer is, in part, because MtrR can directly or indirectly activate certain genes important for metabolism and other cellular processes. For example, MtrR appears to up-regulate expression of glnE, which encodes the enzymatic regulator of glutamine synthetase (GlnA). Thus, the initial advantage afforded by loss of MtrR (resistance to host antimicrobials) might be negated later during infection if biosynthetic processes are negatively impacted due to loss of MtrR. This, in part, may explain why natural mtrR mutants represent <25% of all clinical isolates (112) and why mtrR expression is subject to transcriptional regulatory processes.
While mtrR-coding mutations can enhance transcription of mtrCDE and elevate HA resistance in gonococci, high-level resistance requires a cis-acting mutation within the overlapping mtrR and mtrCDE promoters (114, 133, 146). The mtrR promoter contains a 13-bp inverted repeat sequence between the −10 and −35 hexamers (114,146). A single bp deletion (114,146) or a dinucleotide insertion within this inverted repeat is sufficient to reduce transcription of mtrR dramatically. These cis-acting promoter mutations are observed in clinical isolates expressing high levels of HA resistance. We proposed (146) that because the mtrR and mtrCDE promoters overlap on opposite strands at their −35 hexamer region, the single bp deletion or the dinucleotide insertion enhance mtrCDE expression by both reducing mtrR expression and making the mtrCDE promoter more available for interacting with RNA polymerase. This model provides a mechanism to explain why mtrR promoter mutants express higher levels of antimicrobial resistance and mtrCDE than those of strains with mtrR point mutations that cause radical amino acid changes, inactivating MtrR function (114, 125, 137). High-level antimicrobial resistance mediated by overexpression of the mtrCDE operon can also be afforded by a point mutation 120 nucleotides upstream of the mtrC translational start (125). This mutation, found in strain MS11, when transferred to strain FA19 could result in levels of antimicrobial resistance and in vivo fitness similar to that endowed by the single bp deletion in the mtrR promoter. Warner et al. (125) found that this point mutation could enhance the half-life of the mtrCDE transcript, and recent evidence (147) indicates that it generates a new promoter element that is used preferentially for mtrCDE transcription.
Transcriptional activation of efflux pump genes is common in bacteria and can lead to inducible resistance to antibiotics and other antimicrobials recognized by the specific pump (112). For example, environmental and antimicrobial stimuli have been shown to modulate expression of the acrAB efflux pump system in E. coli through the action of several DNA-binding proteins (MarR, Rob and SoxS) (148). This inducible resistance process is typically transient and allows bacteria to quickly respond to antimicrobials in their local environment. Expression of the mtrCDE efflux pump locus can be up-regulated when gonococci are grown in the presence of a sublethal level of an antimicrobial agent recognized by the pump (149). This induction requires the presence of an AraC/XylS-like protein termed MtrA (149) and energy supplied by the TonB-ExbB-ExbD system (131). MtrA can activate expression of mtrCDE even in the presence of an active MtrR and we (150) found that it can bind to a DNA sequence upstream of mtrCDE . Moreover, MtrA can bind certain substrates (e.g., TX-100) that induce the expression of the mtrCDE operon and these interactions enhance its binding to target DNA sequences. Like MtrR, MtrA can regulate directly or indirectly (positively or negatively) several genes in gonococci, including mpeR (Figure 6). The capacity of MtrA to regulate gonococcal genes is probably of importance during infection because an mtrA null mutant of strain FA19 was less fit than its mtrA+ parent strain in the lower genital tract of experimentally infected female mice.
VI. Concluding Remarks
It has been well established that overexpression of RND multidrug efflux pumps led to a resistant phenotype in pathogenic organisms. This problem is exacerbated by the ease with which many of these resistant genes can pass from one microorganism to another through plasmid transfer (151). The availability of the three-dimensional structures of these efflux transporters potentially allows us rationally to design agents that block the function of these pumps. However, there is still quite a mountain to climb in the development toward achieving this goal. To date, no efflux pump inhibitor has been licensed for use in the clinical treatment of bacterial infections. As RND pumps assemble as tripartite complexes, one method to inhibit the drug-resistant phenotype is to prevent the assembly of complex formation by blocking the different subunits from forming a functional complex. The possibility of this approach has been demonstrated by the crystal structure of AcrB in complex with the designed ankyrin repeat protein inhibitor (DARPin), in which the inhibitor is bound in such a way that AcrB is not able to form a functional complex with TolC (33). Another potential approach is to target the transcriptional regulators that modulate the expression of these RND pumps. For example, the global regulator CmeR controls the expression of several genes, including cmeABC (108). By inhibiting the interactions between the regulator and its substrates, expression of these transporter genes could potentially be blocked. Recently, more examples of RND transporters regulated by two-component systems have been identified, including E. coli CusRS, which controls the expression of CusABC (85). The structures of different components of these regulatory systems may potentially allow us to rationally design inhibitors that reduce the level of efflux pump expression.
References
- 1.Barber M. Staphylococcal infection due to penicillin-resistant strains. Br. Med. J. 1947;29:863–865. doi: 10.1136/bmj.2.4534.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson KS, Smith ME. Version 2000: the new β-lactamases of gram-negative bacteria at the dawn of the new millennium. Microbes Infect. 2000;2:1225–1235. doi: 10.1016/s1286-4579(00)01276-4. [DOI] [PubMed] [Google Scholar]
- 3.Wright GD. Aminoglycoside-modifying enzymes. Curr. Opin. Microbiol. 1999;2:499–503. doi: 10.1016/s1369-5274(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 4.Hooper DC. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000;31:S24–S28. doi: 10.1086/314056. [DOI] [PubMed] [Google Scholar]
- 5.Piddock LJV. Clinically relevant bacterial chromosomally encoded multi-drug resistance efflux pumps. Clin. Microbiol. Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy S. Active efflux mechanisms for antibiotic resistance. Antimicrob. Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhuis H, van Veen HW, Poolman B, Driessen AJ, Konings WN. Mechanisms of multidrug transporters. FEMS Microbiol. Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 9.McMurry L, Petrucci RE, Jr, Levy SB. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli . Proc. Natl. Acad. Sci. USA. 1980;77:3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins CF. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 11.Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, Saier MH., Jr The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999;1:107–125. [PubMed] [Google Scholar]
- 12.Brown MH, Paulsen LT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 1999;31:394–395. doi: 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 13.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli . Antimicrob. Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith JK, Baker ME, Rouch DA, Page MG, Skurray RA, Paulsen IT, Chater KF, Baldwin SA, Henderson PJ. Membrane transport proteins: implications of sequence comparisons. Curr. Opin. Cell Biol. 1992;4:684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 15.Marger M, Saier MH., Jr A major superfamily of transmembrane facilitators that can catalyze uniport, symport and antiport. Trends Biochem. Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 16.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen IT, Skurry RA, Tam R, Saier MH, Jr, Turner RJ, Weiner JH, Goldberg EB, Grinius LL. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol. Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 18.Schweizer HP. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet. Mol. Res. 2003;31:48–62. [PubMed] [Google Scholar]
- 19.Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 1998;27:S32–S41. doi: 10.1086/514920. [DOI] [PubMed] [Google Scholar]
- 20.Paulsen IT, Sliwinski MK, Saier MH., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 21.Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli . J. Bacteriol. 2001;183:5803–5812. doi: 10.1128/JB.183.20.5803-5812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 2000;37:219–225. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- 24.Zgurskaya HI, Nikaido H. Cross-linked complex between oligomeric periplasmic lipoprotein AcrA and the inner-membrane-associated multidrug efflux pump AcrB from Escherichia coli . J. Bacteriol. 2000;182:4264–4267. doi: 10.1128/jb.182.15.4264-4267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure. 2006;14:577–587. doi: 10.1016/j.str.2005.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fralick JA. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli . J. Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 28.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli . Mol. Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 29.a Takatsuka Y, Nikaido H. Threonine-978 in the transmembrane segment of the multidrug efflux pump AcrB of Escherichia coli is crucial for drug transporter as a probable component of the proton relay network. J. Bacteriol. 2006;188:7284–7289. doi: 10.1128/JB.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Su C-C, Li M, Gu R, Takatsuka Y, McDermott G, Nikaido H, Yu EW. Conformation of the AcrB multidrug efflux pump in mutants of the putative proton relay pathway. J. Bacteriol. 2006;188:7290–7296. doi: 10.1128/JB.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zgurskaya HI, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli . Proc. Natl. Acad. Sci. USA. 1999;96:7190–7196. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baucheron S, Tyler S, Boyd D, Mulvey MR, Chaslus-Dancla E, Cloeckaert A. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar typhimurium DT104. Antimicrob. Agents Chemother. 2004;48:3729–35. doi: 10.1128/AAC.48.10.3729-3735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bratu S, Landman D, George A, Salvani J, Quale J. Correlation of the expression of acrB and the regulatory genes marA soxS and ramA with antimicrobial resistance in clinical isolates of Klebsiella pneumoniae endemic to New York City. J. Antimicrob. Chemother. 2009 May 21; doi: 10.1093/jac/dkp186. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber MA, Talukder A, Piddock LJ. Contribution of mutation at amino acid 45 of AcrR to acrB expression and ciprofloxacin resistance in clinical and veterinary Escherichia coli isolates. Antimicrob Agents Chemother. 2005;49:4390–4392. doi: 10.1128/AAC.49.10.4390-4392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 36.Yu EW, McDermott G, Zgurskaya HI, Nikaido H, Koshland DE., Jr Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science. 2003;300:976–980. doi: 10.1126/science.1083137. [DOI] [PubMed] [Google Scholar]
- 37.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 38.Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 39.Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grütter MG. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol. 2007;5:e7. doi: 10.1371/journal.pbio.0050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure. 2006;14:577–587. doi: 10.1016/j.str.2005.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sennhauser G, Bukowska MA, Briand C, Grütter MG. Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa . J. Mol. Biol. 2009;389:134–45. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, Yoneyama H, Narita S, Nakagawa A, Nakae T. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J. Biol. Chem. 2004;279:52816–52819. doi: 10.1074/jbc.C400445200. [DOI] [PubMed] [Google Scholar]
- 43.Higgins MK, Bokma E, Koronakis E, Hughes C, Koronakis V. Structure of the periplasmic component of a bacterial drug efflux pump. Proc. Natl. Acad. Sci. USA. 2004;101:9994–9999. doi: 10.1073/pnas.0400375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akama H, Matsuura T, Kashiwagi S, Yoneyama H, Narita S, Tsukihara T, Nakagawa A, Nakae T. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa . J. Biol. Chem. 2004;279:25939–25942. doi: 10.1074/jbc.C400164200. [DOI] [PubMed] [Google Scholar]
- 45.Symmons MF, Bokma E, Koronakis E, Hughes C, Koronakis V. The assembled structure of a complete tripartite bacterial multidrug efflux pump. Proc. Natl. Acad. Sci. USA. 2009;106:7173–7178. doi: 10.1073/pnas.0900693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkins CA, Nikaido H. Chimeric analysis of AcrA function reveals the importance of its C-terminal domain in its interaction with the AcrB multidrug efflux pump. J. Bacteriol. 2003;185:5349–5356. doi: 10.1128/JB.185.18.5349-5356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su C-C, Yang F, Long F, Reyon D, Routh MD, Kuo DW, Mokhtari AK, Van Ornam JD, Rabe KL, Hoy JA, et al. Crystal structure of the membrane fusion protein CusB from Escherichia coli . J. Mol. Biol. 2009;393:342–355. doi: 10.1016/j.jmb.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu EW, Aires JR, McDermott G, Nikaido H. A periplasmic drug-binding site of the AcrB multidrug efflux pump: a crystallographic and site-directed mutagenesis study. J. Bacteriol. 2005;187:6804–6815. doi: 10.1128/JB.187.19.6804-6815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klepsch MM, Newstead S, Flaig R, De Gier JW, Iwata S, Beis K. The structure of the efflux pump AcrB in complex with bile acid. Mol Membr. Biol. 2008;25:677–682. doi: 10.1080/09687680802552257. [DOI] [PubMed] [Google Scholar]
- 50.Eckert B, Beck CF. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J. Bacteriol. 1989;171:3557–3559. doi: 10.1128/jb.171.6.3557-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurland CG, Dong H. Bacterial growth inhibition by overproduction of protein. Mol. Microbiol. 1996;21:1–4. doi: 10.1046/j.1365-2958.1996.5901313.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee SW, Edlin G. Expression of tetracycline resistance in pBR322 derivatives reduces the reproductive fitness of plasmid-containing Escherichia coli . Gene. 1985;39:173–180. doi: 10.1016/0378-1119(85)90311-7. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 2003;48:1609–1619. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 54.Martin RG, Rosner JL. Analysis of microarray data for the marA soxS, and rob regulons of Escherichia coli . Methods Enzymol. 2003;370:278–80. doi: 10.1016/S0076-6879(03)70024-X. [DOI] [PubMed] [Google Scholar]
- 55.Zhang A, Rosner JL, Martin RG. Transcriptional activation by MarA, SoxS and Rob of two tolC promoters using one binding site: a complex promoter configuration for tolC in Escherichia coli . Mol Microbiol. 2008;69:1450–1455. doi: 10.1111/j.1365-2958.2008.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. The local repressor AcrR plays a moderating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang XD, Gallegos MT, Brennan R, Tobes R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodionov DA, Gelfand MS, Mironov AA, Rakhmaninova AB. Comparative approach to analysis of regulation in complete genomes: multidrug resistance systems in gramma-proteobacteria. J. Mol. Microbiol. Biotechnol. 2001;3:319–324. [PubMed] [Google Scholar]
- 59.Su C-C, Rutherford DJ, Yu EW. Characterization of the multidrug efflux regulator AcrR from Escherichia coli . Biochem. Biophys. Res. Commun. 2007;361:85–90. doi: 10.1016/j.bbrc.2007.06.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su C-C, Yu EW. Ligand-transporter interaction in the AcrB multidrug pump determined by fluorescence polarization assay. FEBS Lett. 2007;581:4972–4976. doi: 10.1016/j.febslet.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Gu R, Su C-C, Routh MD, Harris KC, Jewell ES, McDermott G, Yu EW. Crystal structure of the transcriptional regulator AcrR from Escherichia coli . J. Mol. Biol. 2007;374:591–603. doi: 10.1016/j.jmb.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu R, Li M, Su C-C, Long F, Routh MD, Yang F, McDermott G, Yu EW. Conformational change of the AcrR regulator reveals a possible mechanism of induction. Acta Crystallogr. 2008;F64:584–588. doi: 10.1107/S1744309108016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinrichs W, Kisker C, Duvel M, Muller A, Tovar K, Hillen W, Saenger W. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science. 1994;264:418–420. doi: 10.1126/science.8153629. [DOI] [PubMed] [Google Scholar]
- 64.Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat. Struct. Biol. 2000;7:215–219. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- 65.Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. Structural mechanisms of QacR induction and multidrug recognition. Science. 2001;294:2158–2163. doi: 10.1126/science.1066020. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002;21:1210–1218. doi: 10.1093/emboj/21.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu R, Su C-C, Shi F, Li M, McDermott G, Zhang Q, Yu EW. Crystal structure of the transcriptional regulator CmeR from Campylobacter jejun. J. Mol. Biol. 2007;372:583–593. doi: 10.1016/j.jmb.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natsume R, Ohnishi Y, Senda T, Horinouchi S. Crystal structure of a γ-butyrolactone autoregulator receptor protein in Streptomyces coelicolor A3(2) J. Mol. Biol. 2003;336:409–419. doi: 10.1016/j.jmb.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 69.Dover LG, Corsino PE, Daniels IR, Cocklin SL, Tatituri V, Besra GS, Futterer K. Crystal structure of the TetR/CamR family repressor Mycobacterium tuberculosis EthR implicated in ethionamide resistance. J. Mol. Biol. 2004;340:1095–1105. doi: 10.1016/j.jmb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Frenois F, Engohang-Ndong J, Locht C, Baulard AR, Villeret V. Structure of EthR in a ligand bound conformation reveals therapeutic perspectives against tuberculosis. Mol. Cell. 2004;16:301–307. doi: 10.1016/j.molcel.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Routh MD, Su C-C, Zhang Q, Yu EW. Structures of AcrR and CmeR: insight into the mechanisms of transcriptional repression and multi-drug recognition in the TetR family of regulators. Biochim. Biophys. Acta. 2009;1794:844–851. doi: 10.1016/j.bbapap.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 73.Zgurskaya H, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli . Proc. Natl. Acad. Sci. U.S.A. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aires JR, Nikaido H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli . J. Bacteriol. 2005;187:1923–1929. doi: 10.1128/JB.187.6.1923-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lau SY, Zgurskaya HI. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. J. Bacteriol. 2005;187:7815–7825. doi: 10.1128/JB.187.22.7815-7825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baranova N, Nikaido H. The BaeSR two-component regulatory system activates transcription of yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 2002;184:4168–4176. doi: 10.1128/JB.184.15.4168-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 2002;184:4161–4167. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elkins CA, Mullis LB. Mammalian steroid hormones are substrates for the major RND- and MFS-type tripartite multidrug efflux pumps of Escherichia coli . J. Bacteriol. 2006;188:1191–1195. doi: 10.1128/JB.188.3.1191-1195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franke S, Grass G, Nies DH. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology. 2001;147:965–972. doi: 10.1099/00221287-147-4-965. [DOI] [PubMed] [Google Scholar]
- 80.Franke S, Grass G, Rensing C, Nies DH. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli . J. Bacteriol. 2003;185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loftin IR, Franke S, Roberts SA, Weichse A, Heroux A, Montfort WR, Rensing C, McEvoy MM. A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry. 2005;44:10533–10540. doi: 10.1021/bi050827b. [DOI] [PubMed] [Google Scholar]
- 82.Xue Y, Davis AV, Balakrishnan G, Stasser JP, Staehlin BM, Focia P, Spiro TG, Penner-Hahn JE, O'Halloran TV. Cu(I) recognition via cation-π and methionine interactions in CusF. Nat. Chem. Biol. 2008;4:107–109. doi: 10.1038/nchembio.2007.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loftin IR, Franke S, Blackburn NJ, McEvoy MM. Unusual Cu(I)/Ag(I) coordination of Escherichia coli CusF as revealed by atomic resolution crystallography and x-ray absorption spectroscopy. Protein Sci. 2007;16:2287–2293. doi: 10.1110/ps.073021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bagai I, Rensing C, Blackburn NJ, McEvoy MM. Direct metal transfer between periplasmic proteins identifies a bacterial copper chaperone. Biochemistry. 2008;47:11408–11414. doi: 10.1021/bi801638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munson GP, Lam DL, Outten FW, O'Halloran TV. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 2000;182:5864–5871. doi: 10.1128/jb.182.20.5864-5871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borges-Walmsley MI, Beauchamp J, Kelly SM, Jumel K, Candlish D, Harding SE, Price NC, Walmsley AR. Identification of oligomerization and drug-binding domains of the membrane fusion protein EmrA. J. Biol. Chem. 2003;278:12903–12912. doi: 10.1074/jbc.M209457200. [DOI] [PubMed] [Google Scholar]
- 87.Bagai I, Liu W, Rensing C, Blackburn NJ, McEvoy MM. Substrate-linked conformational change in the periplasmic component of a Cu(I)/Ag(I) efflux system. J. Biol. Chem. 2007;282:35695–35702. doi: 10.1074/jbc.M703937200. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Palacios GM. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin. Infect. Dis. 2007;44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 89.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 90.Blaser MJ, Engberg J. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd ed. Washington, DC: ASM Press; 2008. pp. 99–121. [Google Scholar]
- 91.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg.Infect.Dis. 2001;7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibreel A, Taylor DE. Macrolide resistance in Campylobacter jejuni and Campylobacter coli . J. Antimicrob. Chemother. 2006;58:243–255. doi: 10.1093/jac/dkl210. [DOI] [PubMed] [Google Scholar]
- 93.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future. Microbiol. 2009;4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q, Plummer P. Mechanisms of antibiotic resistance in Campylobacter . In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd ed. Washington, DC: ASM Press; 2008. pp. 263–276. [Google Scholar]
- 95.Lin J, Michel LO, Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni . Antimicrob. Agents. Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pumbwe L, Piddock LJ. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol. Lett. 2002;206:185–189. doi: 10.1111/j.1574-6968.2002.tb11007.x. [DOI] [PubMed] [Google Scholar]
- 97.Luo N, Sahin O, Lin J, Michel LO, Zhang Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 2003;47:390–394. doi: 10.1128/AAC.47.1.390-394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cagliero C, Mouline C, Payot S, Cloeckaert A. Involvement of the CmeABC efflux pump in the macrolide resistance of Campylobacter coli . Journal of Antimicrobial Chemotherapy. 2005;56:948–950. doi: 10.1093/jac/dki292. [DOI] [PubMed] [Google Scholar]
- 99.Mamelli L, Prouzet-Mauleon V, Pages JM, Megraud F, Bolla JM. Molecular basis of macrolide resistance in Campylobacter: role of efflux pumps and target mutations. J. Antimicrob. Chemother. 2005;56:491–497. doi: 10.1093/jac/dki253. [DOI] [PubMed] [Google Scholar]
- 100.Ge B, McDermott PF, White DG, Meng J. Role of efflux pumps and topoisomerase mutations in fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli . Antimicrob. Agents Chemother. 2005;49:3347–3354. doi: 10.1128/AAC.49.8.3347-3354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin J, Yan M, Sahin O, Pereira S, Chang YJ, Zhang Q. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob. Agents Chemother. 2007;51:1678–1686. doi: 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cagliero C, Mouline C, Cloeckaert A, Payot S. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli . Antimicrob. Agents Chemother. 2006;50:3893–3896. doi: 10.1128/AAC.00616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan M, Sahin O, Lin J, Zhang Q. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J. Antimicrob. Chemother. 2006;58:1154–1159. doi: 10.1093/jac/dkl412. [DOI] [PubMed] [Google Scholar]
- 104.Akiba M, Lin J, Barton YW, Zhang QJ. Interaction of CmeABC and CmeDEF in conferring antimicrobial resistance and maintaining cell viability in Campylobacter jejuni . J. Antimicrob.Chemother. 2006;57:52–60. doi: 10.1093/jac/dki419. [DOI] [PubMed] [Google Scholar]
- 105.Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni . Infect. Immun. 2003;71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin J, Akiba M, Sahin O, Zhang Q. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni . Antimicrob. Agents Chemother. 2005;49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grkovic S, Brown MH, Skurray RA. Regulation of Bacterial Drug Export Systems. Microbiol. Mol. Biol. Rev. 2002;66:671–701. doi: 10.1128/MMBR.66.4.671-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo B, Wang Y, Shi F, Barton YW, Plummer P, Reynolds DL, Nettleton D, Grinnage-Pulley T, Lin J, Zhang Q. CmeR functions as a pleiotropic regulator and is required for optimal colonization of Campylobacter jejuni in vivo. J. Bacteriol. 2008;190:1879–1890. doi: 10.1128/JB.01796-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin J, Cagliero C, Guo B, Barton YW, Maurel MC, Payot S, Zhang Q. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni . J. Bacteriol. 2005;187:7417–7424. doi: 10.1128/JB.187.21.7417-7424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stintzi A, Marlow D, Palyada K, Naikare H, Panciera R, Whitworth L, Clarke C. Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni . Infect. Immun. 2005;73:1797–1810. doi: 10.1128/IAI.73.3.1797-1810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rajan SS, Yang X, Shuvalova L, Collart F, Anderson WF. Crystal structure of YfiR, an unusual TetR/CamR-type putative transcriptional regulator from Bacillus subtilis . Proteins: Struct. Funct. Genet. 2006;65:255–257. doi: 10.1002/prot.21073. [DOI] [PubMed] [Google Scholar]
- 112.Rouquette-Loughlin C, Veal WL, Lee E-H, Zarantonelli L, Balthazar JT, Shafer WM. Antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis viewed as virulence factors. In: Paulsen I, Lewis K, editors. Microbial Drug Efflux. Wymonham, UK: Horizon Scientific Press; 2002. pp. 187–200. [Google Scholar]
- 113.Luna VA, Cousin S, Jr, Whittington WLH, Roberts MC. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 2000;44:2503–2506. doi: 10.1128/aac.44.9.2503-2506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrCDE efflux system. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 115.Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC, Shafer WM. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology. 1997;143:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 116.Veal WL, Nicholas RA, Shafer WM. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae . J. Bacteriol. 2002;184:5619–5624. doi: 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faruki H, Kohmescher RN, McKinney WP, Sparling PF. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally-mediated resistance) N. Engl. J. Med. 1985;313:607–611. doi: 10.1056/NEJM198509053131004. [DOI] [PubMed] [Google Scholar]
- 118.Olesky M, Hobbs M, Nicholas RA. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae . Antimicrob. Agents Chemother. 2002;46:2811–2820. doi: 10.1128/AAC.46.9.2811-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zarantonelli L, Borthagary G, Lee EH, Veal W, Shafer WM. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtrR promoter mutation in Neisseria gonorrhoeae . J. Antimicrob. Chemother. 2001;47:651–654. doi: 10.1093/jac/47.5.651. [DOI] [PubMed] [Google Scholar]
- 120.Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae . Int. J. Antimicrob. Agents. 2003;21:414–419. doi: 10.1016/s0924-8579(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 121.Shafer WM, Qu X-D, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee EH, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 1999;33:839–845. doi: 10.1046/j.1365-2958.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 123.Jerse AE, Sharma ND, Bodner ANB, Snyder LA, Shafer WM. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Warner DM, Folster JP, Shafer WM, Jerse AE. Regulation of the MtrC-MtrD-MtrE efflux pump system modulates the in vivo fitness of Neisseria gonorrhoeae . J. Infect. Dis. 2007;196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 125.Warner DM, Shafer WM, Jerse AE. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 2008;70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maness MJ, Sparling PF. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae . J. Infect. Dis. 1973;128:321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- 127.Guymon LF, Walstad DL, Sparling PF. Cell envelope alterations in antibiotic-sensitive and -resistant strains of Neisseria gonorrhoeae . J. Bacteriol. 1978;136:391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan W, Spratt BG. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 129.Delahay RM, Robertson BD, Balthazar JT, Shafer WM, Ison C. Involvement of the gonococcal MtrE in the resistance of Neisseria gonorrhoeae to toxic hydrophobic compounds. Microbiology. 1997;143:2127–2133. doi: 10.1099/00221287-143-7-2127. [DOI] [PubMed] [Google Scholar]
- 130.Veal WL, Shafer WM. Identification of a cell envelope protein (MtrF) involved in hydrophobic antimicrobial resistance in Neisseria gonorrhoeae . J. Antimicrob. Chemother. 2003;51:27–37. doi: 10.1093/jac/dkg031. [DOI] [PubMed] [Google Scholar]
- 131.Rouquette-Loughlin C, Stojiljkovic I, Hrobowski T, Balthazar JT, Shafer WM. Inducible, but not constitutive resistance of gonococci to hydrophobic agents due to the MtrC-MtrD-MtrE efflux pump requires the TonB-ExbB-ExbD proteins. Antimicrob. Agents Chemother. 2002;46:561–565. doi: 10.1128/AAC.46.2.561-565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shafer WM. Atlanta, GA: Emory University; 2009. Unpublished work. [Google Scholar]
- 133.Tzeng Y-L, Datta A, Ambrose KA, Davies JK, Carlson RW, Stephens DS, Kahler CM. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitides . J. Biol. Chem. 2004;279:3503–35062. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- 134.Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM. Modulation of the mtrCDE-encoded efflux pump gene complex due to a Correia Element insertion sequence. Mol. Microbiol. 2004;54:731–741. doi: 10.1111/j.1365-2958.2004.04299.x. [DOI] [PubMed] [Google Scholar]
- 135.Hoffman KM, Williams D, Shafer WM, Brennan RG. Characterization of the multiple transferrable repressor, MtrR, from Neisseria gonorrhoeae . J. Bacteriol. 2005;187:5008–5012. doi: 10.1128/JB.187.14.5008-5012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lucas CE, Balthazar JT, Hagman KE, Shafer WM. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae . J. Bacteriol. 1997;179:4123–4128. doi: 10.1128/jb.179.13.4123-4128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shafer WM, Balthazar JT, Hagman KE, Morse SA. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to fecal lipids. Microbiology. 1995;41:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 138.Folster JP, Shafer WM. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J. Bacteriol. 2005;187:3713–3720. doi: 10.1128/JB.187.11.3713-3720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ducey TF, Carson JM, Orvis B, Stinzi AP, Dyer DW. Identification of the iron-responsive genes of Neisseria gonorrhoeae by microarray analysis in defined media. J. Bacteriol. 2005;187:4865–4874. doi: 10.1128/JB.187.14.4865-4874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dyer DW, Jackson L, Mercante, Shafer WM. Emory University; 2009. Unpublished work. [Google Scholar]
- 141.Dyer D. Personal communication. Norman, OK: University of Oklahoma; 2009. [Google Scholar]
- 142.Lee E-H, Shafer WM. Atlanta, GA: Emory University; 2009. Unpublished work. [Google Scholar]
- 143.Soncini FC, Garcia E, Vescovi EG, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella: identification of PhoP-regulated genes. J. Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Folster JP, Johnson PJT, Jackson L, Dhulipali V, Dyer DW, Shafer WM. MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae . J. Bacteriol. 2009;191:287–297. doi: 10.1128/JB.01165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee E-H, Rouquette-Loughlin C, Folster JP, Shafer WM. FarR regulates the farAB-encoded efflux pump of Neisseria gonorrhoeae via an MtrR regulatory mechanism. J. Bacteriol. 2003;185:7145–7152. doi: 10.1128/JB.185.24.7145-7152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hagman KE, Shafer WM. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae . J. Bacteriol. 1995;171:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Warner DM, Shafer WM. Atlanta, GA: Emory University; 2009. Unpublished work. [Google Scholar]
- 148.Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. The local repressor AcrR plays a modulating role in regulation of the acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 149.Rouquette C, Harmon JB, Shafer WM. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 1999;33:651–658. doi: 10.1046/j.1365-2958.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 150.Shafer WM. Atlanta, GA: Emory University; 2009. Unpublished work. [Google Scholar]
- 151.Courvalin P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 1994;38:1447–1451. doi: 10.1128/aac.38.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]