Abstract
Background
Three intrinsic connectivity networks in the brain, namely the central executive, salience, and default mode networks, have been identified as crucial to the understanding of higher cognitive functioning, and the functioning of these networks has been suggested to be impaired in psychopathology, including posttraumatic stress disorder (PTSD).
Objective
1) To describe three main large-scale networks of the human brain; 2) to discuss the functioning of these neural networks in PTSD and related symptoms; and 3) to offer hypotheses for neuroscientifically-informed interventions based on treating the abnormalities observed in these neural networks in PTSD and related disorders.
Method
Literature relevant to this commentary was reviewed.
Results
Increasing evidence for altered functioning of the central executive, salience, and default mode networks in PTSD has been demonstrated. We suggest that each network is associated with specific clinical symptoms observed in PTSD, including cognitive dysfunction (central executive network), increased and decreased arousal/interoception (salience network), and an altered sense of self (default mode network). Specific testable neuroscientifically-informed treatments aimed to restore each of these neural networks and related clinical dysfunction are proposed.
Conclusions
Neuroscientifically-informed treatment interventions will be essential to future research agendas aimed at targeting specific PTSD and related symptoms.
Keywords: Intrinsic networks, default mode network, salience network, central executive network, insula, PTSD, interoception, neurofeedback, mindfulness, dissociation
The objective of this commentary is threefold: 1) to describe three main large-scale networks of the human brain; 2) to discuss the functioning of these neural networks in posttraumatic stress disorder (PTSD); and 3) to offer hypotheses for neuroscientifically-informed interventions based on treating the abnormalities observed in these neural networks in PTSD and related disorders.
Three intrinsic connectivity networks (ICN) in the brain have been identified as crucial to the understanding of higher cognitive function (Menon, 2011), namely the central executive (CEN), salience (SN), and default mode (DMN) networks. Responses of these ICNs are thought to generally increase and decrease proportionally and antagonistically during performance of cognitive and emotional-processing tasks. The CEN is a network that is crucial to verbal learning and executive functioning (Habas et al., 2009; Koechlin & Hyafil, 2007; Koechlin & Summerfield, 2007; Miller & Cohen, 2001; Petrides, 2005; Seeley et al., 2007). The SN consists of the dorsal anterior cingulate cortex and the frontoinsular cortex and plays a key role in salience detection, in other words, directing behavior to the most pertinent actions (Dosenbach et al., 2007; Lovero, Simmons, Aron, & Paulus, 2009; Seeley et al., 2007; Sridharan, Levitin, & Menon, 2008). The DMN, consisting of anterior and posterior medial cortices and lateral parietal lobes, plays an important role in self-referential processing, autobiographical memory, and social cognition (Amodio & Frith, 2006; Buckner, Andrews-Hanna, & Schacter, 2008; Greicius, Krasnow, Reiss, & Menon, 2003; Qin & Northoff, 2011; Raichle et al., 2001; Spreng, Mar, & Kim, 2009). Crucially, the anterior insula of the SN is thought to mediate the engagement of the CEN and disengagement of the DMN, and thus the dynamic interplay between externally- and internally-focused attention and cognitive-affective processing (Menon & Uddin, 2010; Seeley et al., 2007; Sridharan et al., 2008).
Central executive network and PTSD
Several authors have examined CEN activity during cognitive processing in PTSD (Daniels et al., 2010; St. Jacques, Kragel, & Rubin, 2013). During a working memory task, whereas controls showed significantly stronger connectivity within areas implicated in the CEN, including the right inferior frontal gyrus and the right inferior parietal lobule, the PTSD group showed stronger connectivity with areas implicated in the DMN (namely enhanced connectivity between the medial prefrontal cortex and the left parahippocampal gyrus). Using an autobiographical memory retrieval paradigm, however, PTSD was associated, among other findings, with decreased recruitment of two networks identified as DMN-associated (medial temporal lobe and medial prefrontal cortex networks) when recalling autobiographical memories (St. Jacques et al., 2013). In addition, memories recalled in first-person perspective tended to recruit the medial temporal lobe network more so than those recalled in third-person. Finally, increased emotional intensity of the memory was associated with increased frontoparietal network activity for healthy controls but not for PTSD participants (St. Jacques et al., 2013).
Decreased resting-state connectivity within the CEN has also been found between PTSD participants and trauma-exposed controls during an emotional face viewing paradigm, with decreased connectivity between frontoparietal regions within a CEN component associated with trauma history and with PTSD symptoms (Cisler, Steele, Smitherman, Lenow, & Kilts, 2013). We also reported a positive correlation between frequency of dissociative experiences and DMN connectivity with the dorsolateral prefrontal cortex, a region involved in the CEN (Bluhm et al., 2009). This suggests that dissociative experiences may involve alterations in the relation between the DMN and CEN, which may relate to difficulties switching between DMN and CEN.
These differential patterns of connectivity are related to significant group differences with task-induced switches (i.e., engaging and disengaging the DMN and the CEN) (Daniels et al., 2010). Specifically, whereas controls engaged executive networks required for successful working memory performance, individuals with PTSD tended to engage brain regions involved in task-irrelevant processes (e.g., self-referential processing; connectivity between the posterior cingulate cortex (PCC) and the right superior frontal gyrus and between the medial prefrontal cortex and the left parahippocampal gyrus), signaling a potential key mechanism underlying the cognitive dysfunction observed in this population. By contrast, effective recruitment of brain regions associated with self-referential processing during autobiographical memory recall also seems to be impaired in PTSD. Taken together, these results point towards alterations in the ability to engage and switch between task-relevant (i.e., CEN) and task-irrelevant (i.e., DMN) brain networks during cognitive processing among individuals with PTSD, a function that is in turn known to be partly mediated by the anterior insula.
Restoring the CEN through cognitive remediation
In addition to its core affective components, PTSD is associated with poor cognitive functioning across multiple domains, including declarative memory (Moradi, Abdi, Fathi-Ashtiani, Dalgleish, & Jobson, 2012), short-term memory (Johnsen & Asbjornsen, 2008), attention, and executive functioning (Aupperle, Melrose, Stein, & Paulus, 2012; Polak, Witteveen, Reitsma, & Olff, 2012). The presence of cognitive impairments, in particular, deficits in executive functioning and memory, has also been associated with poor functional outcomes (e.g., return to work) among patients with affective disorders (Altshuler et al., 2007; Altshuler, Bearden, Green, Van Gorp, & Mintz, 2008; Bowie et al., 2010; Depp et al., 2009; Dickerson et al., 2004; Geuze, Vermetten, De Kloet, Hijman, & Westenberg, 2009; Gildengers et al., 2007). Critically, cognitive dysfunction impacts negatively on the outcome of pharmacological and non-pharmacological treatments for affective disorders, where the ability to engage in and successfully complete treatment relies heavily on higher-order cognitive processes (Dunkin et al., 2000; Jaeger & Vieta, 2007). Despite knowledge of the presence of cognitive dysfunction in PTSD, to the best of our knowledge, only one study has been conducted to examine the impact of a non-standardized intervention protocol aimed at improving cognitive functioning in this population. Here, clinically (but not statistically) significant improvements were noted in a small pilot on measures of cognitive functioning after using a bottom-up executive training intervention in conjunction with transcranial direct current sample of four patients (Saunders et al., 2015).
The few studies conducted thus far with PTSD patients contrast sharply with the extant literature in schizophrenia where, to date, more than 20 randomized controlled trials in patients with psychotic-spectrum disorders have been conducted (Barlati, Deste, De Peri, Ariu, & Vita, 2013; Kurtz, 2012; Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). Indeed, the results of a recent meta-analytic review point towards a moderate (effect size = 0.43) and sustained improvement in overall cognitive functioning (including for frontal-temporally mediated domains including processing speed, working memory, episodic memory, and executive functioning) among patients with schizophrenia after participating in cognitive remediation (Wykes et al., 2011). Critically, there was also a medium effect (effect size=0.37) of cognitive remediation on improving functional outcome when measured immediately post-treatment and at follow-up.
In our own laboratory, we have successfully applied cognitive remediation in patients with major depressive disorder, resulting in improvements in performance on working memory tasks in conjunction with increased functional activity in lateral prefrontal and parietal areas (Elgamel, McKinnon, Ramakrishan, Joffe, & MacQueen, 2007; Meusel, Hall, Fougere, McKinnon, & MacQueen, 2013) implicated in the CEN (Aupperle et al., 2012; Dunkin et al., 2000; Greenberg et al., 1999; Jaeger & Vieta, 2007; Johnsen & Asbjornsen, 2008; Kessler, 2000; Moradi et al., 2012; Olatunji, Cisler, & Tolin, 2007; Polak et al., 2012; Van Ameringen, Mancini, Patterson, & Boyle, 2008).
The majority of cognitive remediation interventions applied in psychiatric populations rely heavily upon a bottom-up restitution-based approach to training that focuses on the repair and recovery of function (Robertson & Murre, 1999). Here, participants engage in extensive, repetitive practice of tasks thought to be related to specific, cognitive domains based on the assumption that exercising memory, for example, on one task, will strengthen it for use on other memory tasks. By contrast, we are keen to investigate the efficacy of top-down remediation strategies that focus on the “stimulation” of higher-order systems (e.g., frontal executive systems; control of attention) to modify and regulate impaired processing in other systems (e.g., training in meta-cognitive and self-monitoring strategies). It is these interventions that we believe will have the greatest potential to restore CEN functioning. Notably, these approaches have been applied in other clinical and non-clinical populations, including older adults (Levine et al., 2007; Van Hooren et al., 2007; Winocur et al., 2007), individuals who have suffered a traumatic brain injury (Krasny-Pacini, Chevignard, & Evans, 2014; Krasny-Pacini, Limond, et al., 2014; Levine et al., 2000, 2011; Schweizer et al., 2008), have attention deficit hyperactivity disorder (ADHD) (In de Braek, Dijkstra, Ponds, & Jolles, 2012), polysubstance abuse disorder (Alfonso, Caracuel, Delgado-Pastor, & Verdejo-Garcia, 2011), or spina bifida (Stubberud, Langenbahn, Levine, Stanghelle, & Schanke, 2013), and that experience deficits in executive functioning, attention, and/or memory. Here, participants show improvement in completing everyday tasks (as measured by self-report), as well as improvements in executive functions such as decision making, working memory, and selective attention. Critically, these results are maintained at follow-up (when assessed) (Alfonso et al., 2011; In de Braek et al., 2012; Krasny-Pacini, Chevignard, et al., 2014; Krasny-Pacini, Limond, et al., 2014; Levine et al., 2000, 2007, 2011; Stubberud et al., 2013; Van Hooren et al., 2007).
Given the previous success of top-down intervention strategies in remediating frontal-, temporal- and parietally-mediated brain dysfunction across clinical populations, we hypothesize that these interventions may also have the ability to target core cognitive difficulties experienced by those suffering from PTSD. Moreover, by targeting core cognitive processes (e.g., executive functioning, decision making, and attention) mediated by frontoparietal networks involved in the CEN, we would expect to see functional reorganization of the CEN as a result of treatment (e.g., Green & Benzeval, 2013; HealthCanada, 2002; Parikh, Lam, & Group, 2001; Ratnasingham, Cairney, Rehm, Manson, & Kyndyak, 2012; World Health Organization, 2008).
Salience network and PTSD
In addition to CEN abnormalities in PTSD, a number of studies have demonstrated connectivity alterations among brain areas related to the SN, such as between the anterior insula and other SN regions, including the amygdala (Birn, Patriat, Phillips, Germain, & Herringa, 2014; Cisler et al., 2013, 2014; Fonzo et al., 2010; Peterson, Thome, Frewen, & Lanius, 2014; Rabinak et al., 2011; Shang et al., 2014; Simmons, Norman, Spadoni, & Strigo, 2013; Simmons et al., 2008, 2011; Sripada, King, Garfinkel, et al., 2012; Sripada, King, Welsh, et al., 2012; Stevens et al., 2013; Tursich, Ros, Frewen, Calhoun, & Lanius, 2015). Fonzo et al. (Fonzo et al., 2013) also have shown specific effects of childhood maltreatment on SN functioning in PTSD during emotional face processing, where childhood maltreatment was negatively correlated with connectivity between the insula and amygdala when viewing fearful and angry faces, but positively correlated with prefrontal-limbic connectivity when viewing fearful faces. In addition, Simmons et al. (Simmons et al., 2013) found that individuals in remission from PTSD showed an increase in functional connectivity between the left ventral anterior insula and the right anterior cingulate and middle frontal gyrus, as well as with left cerebellum. Finally, Cisler et al. (Cisler et al., 2014) demonstrated that repeated exposure to a traumatic memory during the experimental paradigm increased connectivity between the right anterior insula and both the right hippocampus and right amygdala, as well as between the right posterior insula and medial prefrontal cortex, whereas patients with higher PTSD symptoms showed this effect less strongly. On balance, the results of these studies suggest that altered connectivity within the SN may result in a change in threat-sensitivity circuits, contributing to the hypervigilance and hyperarousal symptoms in PTSD. Indeed, these findings are consistent with previous models that have associated increased anterior insula activation with heightened levels of interoception and awareness of bodily arousal during states of emotional undermodulation, including reexperiencing and hyperarousal symptoms (Hopper, Frewen, Van der Kolk, & Lanius, 2007; Lanius, Bluhm, & Frewen, 2011; Lanius, Vermetten, et al., 2010; Paulus & Stein, 2006; Shin & Liberzon, 2010).
However, it is worth noting here that decreased insula activation has been associated with emotional detachment or overmodulation of emotion, hypoarousal, and attenuated interoceptive awareness of bodily arousal, such as may be involved during states of depersonalization and derealization (Hopper et al., 2007), which are accompanied by increased prefrontal inhibition of limbic regions (Lanius, Vermetten, et al., 2010). With regard to interoceptive awareness, emotional overmodulation is frequently associated with alexithymia, the inability to know what one is feeling or not having words for one's feelings, and symptoms of alexithymia have been shown to be negatively correlated with anterior insula functioning in PTSD (Frewen et al., 2008). Hence, it is possible that differential activation of the insula underlies both emotional over- and undermodulation and associated alterations in interoceptive awareness in PTSD.
Normalizing arousal and interoceptive awareness through regulation of the SN
As described above, traumatized individuals often cycle between states of emotional undermodulation associated with hyperarousal, and emotional overmodulation associated with emotional detachment and hypoarousal, symptoms that have been associated with insula over- and underactivity, respectively (Frewen & Lanius, 2006; Hopper et al., 2007; Lanius, Vermetten et al., 2010). Traumatized individuals who exhibit emotional overmodulation frequently suffer from a profound detachment from their emotional states and a lack of interoceptive awareness as evidenced by dissociative experiences such as depersonalization and derealization (Lanius et al., 2011; Lanius, Frewen, Vermetten, & Yehuda, 2010), emotional numbing (Krystal, 1968; Krystal & Krystal, 1988; Van der Kolk & McFarlane, 1996), and alexithymia (Badura, 2003; Frewen et al., 2008; Yehuda et al., 1997), with accompanying insula underactivity. Detachment from one's emotional states often occurs in response to repeated traumatization during which the traumatized individual is frequently unable to initiate defensive actions due to overwhelming feelings and emotions. Emotional shutdown, numbness, and detachment instead ensue to the point where the traumatized person can become devoid of any positive (Etter, Gauthier, McDade-Montez, Cloitre, & Carlson, 2013; Frewen, Dean, & Lanius, 2012; Frewen, Dozois, & Lanius, 2012) and negative (Nawijn et al., 2015) emotional experience. Critically, without emotional experience, the capacity for salience detection becomes significantly impaired as emotions are crucial to directing behavior and physiological states to the most important actions, with the goal of maintaining homeostasis. The capacity to pursue the most salient endeavors in the present is therefore often lost in the aftermath of trauma. How can we help traumatized clients to awaken from this shutdown state and restore their interoceptive awareness and salience detection, which we hypothesize would have the concomitant effect of normalizing functioning of the insula and restoring the integrity of the SN?
Body scan meditations are a core part of the mindfulness-based stress reduction program developed by Jon Kabat-Zinn (Kabat-Zinn, 1990) and are thought to enhance interoceptive awareness and assist in overcoming emotional detachment in traumatized populations (e.g., Follette, Briere, Rozelle, Hopper, & Rome, 2014; Frewen & Lanius, 2015). Body scans encourage individuals to become aware of and to monitor bodily sensations experienced throughout the body. Given that an individual's subjective conscious emotional experience is thought to be based on the perception of physical sensations/bodily states arising from musculoskeletal, autonomic nervous, and endocrine system influences (Barrett, Mesquita, Ochsner, & Gross, 2007; Damasio, 1996; James, 1884) mediated by the anterior insula (Craig, 2010; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004), mapping what physical sensations are associated with specific emotions can then be used to help individuals identify what emotional feelings they experience. In support of this, a recent large cross-cultural study showed that specific maps of bodily sensations are associated with different emotions (Nummenmaa, Glerean, Hari, & Hietanen, 2014). Increasing awareness of bodily sensations and encouraging individuals to map what sensations are associated with specific emotions may therefore be an important strategy in overcoming emotional detachment and thus restore salience detection and insular and SN functioning in traumatized individuals. Interestingly, meditators have also been shown to exhibit greater gray matter thickness of the insula as compared to non-meditators (Holzel et al., 2008; Lazar et al., 2005), and a significant increase in right insula cortical thickness that correlated with decreased levels of alexithymia was reported in meditation naïve subjects following mindfulness-based stress reduction (Santarnecchi et al., 2014). It is also interesting to note that emerging evidence points to the efficacy of mindfulness-based interventions in the treatment of PTSD and related disorders (Bormann, Oman, Walter, & Johnson, 2014; Kearney, McDermott, Malte, Martinez, & Simpson, 2012; Kimbrough, Magyari, Langenberg, Chesney, & Berman, 2010; King et al., 2013; Niles et al., 2012). We predict that mindfulness-based treatments, specifically body scans coupled with helping individuals to identify which bodily sensations are associated with what specific emotions, would help to overcome emotional detachment and reestablish emotional awareness, salience detection, normalize insula functioning, and restore the integrity of the SN. Such interventions will therefore need to be the focus of future investigations (Koch et al., 2014) for medication (oxytocin)-enhanced psychotherapy to normalize salience processing.
In contrast to emotional overmodulation and associated symptoms of emotional detachment, emotional undermodulation and related hyperarousal symptoms are frequently associated with hypervigilance, increased levels of interoceptive awareness salience detection, and heightened insula activation. Here, we predict that increased insula activation will alter the functioning of the SN, thereby contributing to the hypervigilance and hyperarousal symptoms in PTSD. Such symptoms have been shown to be managed by anxiety management skills that are part of standard cognitive behavioral treatments, including relaxation training, breathing exercises, fostering mindful awareness, decreasing ruminations, and thought stopping to decrease distressing thoughts and associated worries. These treatments would therefore be expected to normalize interoceptive awareness and restore salience detection through normalization of insula and SN functioning. Future studies examining individuals with PTSD will need to examine the capacity for plastic modulation of the insula and the SN through specific treatments targeting hyperarousal symptoms.
Default mode network and PTSD
As described above, the DMN has been shown to play a key role in self-referential processing, autobiographical memory, and social cognition. Resting-state and task-related studies of PTSD participants show altered connectivity within brain structures associated with the DMN (Birn et al., 2014; Bluhm et al., 2009; Chen & Etkin, 2013; Cisler et al., 2013, 2014; Fonzo et al., 2013; Jin et al., 2014; Kennis, Rademaker, Van Rooij, Kahn, & Geuze, 2015; Lanius, Bluhm, et al., 2010; Lanius et al., 2005; Peterson et al., 2014; Qin et al., 2012; Shang et al., 2014; Sripada, King, Welsh, et al., 2012; St. Jacques et al., 2013; Tursich et al., 2015; Yin et al., 2011; Zhou et al., 2012). Moreover, the strength of connectivity within the DMN appears to correlate with PTSD symptom severity in patients with PTSD or acute posttraumatic stress symptoms (Birn et al., 2014; Cisler et al., 2013; Kennis, Rademaker, Van Rooij, Kahn, & Geuze, 2013; Lanius, Bluhm, et al., 2010; Sripada, King, Welsh, et al., 2012).
In addition, there is emerging evidence that altered resting-state connectivity between key regions of the DMN and regions associated with the SN, including the amygdala and anterior insula, may be a prognostic indicator of PTSD symptomatology (Lanius, Bluhm, et al., 2010; Zhou et al., 2012). Both Lanius et al. (Lanius, Bluhm, et al., 2010) and Zhou et al. (Zhou et al., 2012) found that resting connectivity between the PCC and left and/or right amygdala shortly after a traumatic event predicted PTSD symptom severity at 12 weeks posttrauma; the directionality of this effect, however, has not been clearly identified. Interestingly, Qin et al. (Qin et al., 2012) also found that connectivity of PCC with anterior insula distinguished between people who would later develop PTSD and those who would not, although they did not find associations between amygdala connectivity and PTSD symptoms. Studies have also reported alterations in DMN and SN regions in PTSD, suggesting an alteration within the interaction between large-scale brain networks (Bluhm et al., 2009; Jin et al., 2014; Kennis et al., 2013; Simmons et al., 2011, 2013; St. Jacques et al., 2013).
Restoring self-related processes and the sense of self through normalization of the DMN
Trauma can have lasting effects on the sense of self (Schore, 2003) manifested both cognitively and somatically. This is evidenced by altered core beliefs, disrupted self-referential processing (including poor emotional awareness, alexithymia), and dissociative symptoms including depersonalization and related identity disturbance. It is not unusual for traumatized individuals to experience altered beliefs about themselves, for example: “I have permanently changed for the worse,” “I will never be able to feel normal emotions again,” or “I don't know myself anymore” [see Posttraumatic Cognition Inventory (Foa, Ehlers, Clark, Tolin, & Orsillo, 1999) and Cox, Resnick, & Kilpatrick (2014)]. In addition to these cognitive alterations in self-referential processing, individuals with PTSD may also exhibit somatically-based alterations in self-referential processing, such as depersonalization and related identity disturbance, manifested by feelings like “I feel as if I am outside my body,” “I feel dead inside,” or “I feel like my body does not belong to me” (Bernstein & Putnam, 1986; Briere, 2002; Dell, 2006; Foa et al., 1999).
It has been suggested that the DMN is a key brain network underlying the continuous experience of the sense of self across time and into the future, given its role in autobiographical memory retrieval, envisioning the future, and conceiving the perspectives of others; these are all processes that have been shown to be disrupted in PTSD (Brewin, 2014; Brown et al., 2013, 2014; Freeman, Hart, Kimbrell, & Ross, 2009; Mazza et al., 2012; Moore & Zoellner, 2007; Nazarov, Frewen, Oremus, et al., 2014; Nazarov, Frewen, Parlar, et al., 2014; St. Jacques et al., 2013). Moreover, dissociative experiences, including depersonalization, have repeatedly also been shown to involve brain regions associated with the DMN, including the medial prefrontal cortex, medial parietal lobe, and the temporoparietal junction (reviewed in (Frewen & Lanius, 2015)), which suggests that alterations in the DMN are also a potential mechanism underlying depersonalization and related identity disturbances that follow psychological trauma. We would therefore expect that current PTSD treatments geared towards alleviating both cognitive and somatically-based disturbances in self-referential processing (including, but not limited to, cognitive processing therapy, exposure therapy, eye movement desensitization and reprocessing, mentalization based therapy, dialectical behavior therapy, trauma affect regulation: guide for education and therapy, emotion focused therapy, skills training in affective and interpersonal regulation, psychodynamic approaches, and sensorimotor) may aid in the restoration of DMN function and in reestablishing sense of self. As such, it is critical that future research explicitly measures changes in the sense of self as a treatment outcome. This effort will necessitate the development of appropriate measures to capture this complex concept. In addition, investigations examining whether a combination of cognitive and body-focused treatments, rather than each of these treatments alone, can more optimally relieve self-dysfunction and restore DMN integrity, will be necessary. In theory, combination treatment may simultaneously target both cognitive and somatically-based self-dysfunction through top-down and bottom-up processing, respectively.
Neurofeedback as a tool to aid in the restoration of large-scale brain network functioning
Neurofeedback is a form of biofeedback that uses a brain computer interface to provide feedback about brain functioning in the form of an electroencephalogram (EEG) or blood oxygenation level dependent response, thereby enabling self-regulation of brain activity. EEG neurofeedback training has been shown to aid in the regulation of major brain networks such as the SN and the DMN. For example, a recent functional magnetic resonance imaging (fMRI) study examined the effects of a single session of neurofeedback training versus SHAM (placebo neurofeedback) aimed at voluntarily reducing the alpha rhythm amplitude to gain insight into potential mechanisms underlying this form of neurofeedback. Activity in the alpha band (8–12 Hz) has previously been shown to be successfully modulated by naïve participants through neurofeedback (NFB) (Ros, Munneke, Ruge, Gruzelier, & Rothwell, 2010), and evidence suggests that alpha rhythm NFB may be beneficial in treating anxiety and attention problems (Hardt & Kamiya, 1978; Rasey, Lubar, McIntyre, Zoffuto, & Abbott, 1995), symptoms relevant for the treatment of PTSD and related disorders. In healthy individuals, alpha neurofeedback training led to plastic modulation of both SN and DMN functional connectivity, effects which were not observed in the SHAM condition (Ros et al., 2013).
A similar investigation was subsequently conducted in PTSD patients who had suffered repeated childhood trauma. The results of this study indicated that voluntarily reducing alpha rhythm amplitude was associated with decreased alpha amplitude during training, followed by a significant increase (“rebound”) in resting-state alpha rhythm amplitude. This rebound was related to increased calmness, greater default mode network connectivity, and enhanced SN connectivity (Kluetsch et al., 2014). The alpha “rebound,” which was only observed in the PTSD patients and not in healthy controls, may represent a form of metaplasticity (Muller-Dahlhaus & Ziemann, 2014). Metaplasticity is thought to allow the brain to maintain homeostasis, thus keeping network activity within a physiological range. Hence, the alpha rebound could be seen as a reaction of the brain towards restoring alpha activity to an optimal level for cognitive function (Ros, Baars, Lanius, & Vuilleumier, 2014). Although studies examining mechanisms underlying alpha neurofeedback training in healthy individuals and persons suffering from PTSD need to be repeated following multiple neurofeedback training sessions, they nevertheless point to promising effects this intervention may have for regulating key brain networks, including the SN and DMN.
Although, to our knowledge, no studies have examined the use of real-time fMRI in the regulation of large-scale networks in PTSD, studies in both healthy controls (Lawrence et al., 2013; Paret et al., 2014; Zhang, Yao, Zhang, Long, & Zhao, 2013; Zotev, Phillips, Young, Drevets, & Bodurka, 2013), meditators (Garrison, Santoyo, et al., 2013; Garrison, Scheinost, et al., 2013), and certain psychiatric disorders, including schizophrenia (Ruiz et al., 2013) and depression (Linden et al., 2012; Young et al., 2014; Yuan et al., 2014; Zotev, Phillips, Yuan, Misaki, & Bodurka, 2014), reveal promising results for the plastic modulation of brain regions such as the insula, amygdala, and PCC involved in the SN and DMN as a function of real-time fMRI neurofeedback. Future studies will need to examine this type of intervention for PTSD and related symptoms.
Another neural treatment, repetitive transmagnetic stimulation (rTMS), involves non-invasive, superficial stimulation of the brain and has shown encouraging outcomes for the treatment of PTSD when applied to right or left dorsolateral prefrontal cortex (for meta-analysis see (Berlim & Van den Eynde, 2014)). However, no studies have yet examined the effects of rTMS on the integrity of large-scale brain networks in PTSD, and future investigations of this type may also provide insight into the brain mechanisms underlying successful treatment. Finally, novel pharmacotherapeutic treatments (Watts et al., 2013) developed specifically to target intrinsic network functioning in PTSD would provide important research opportunities.
Concluding remarks
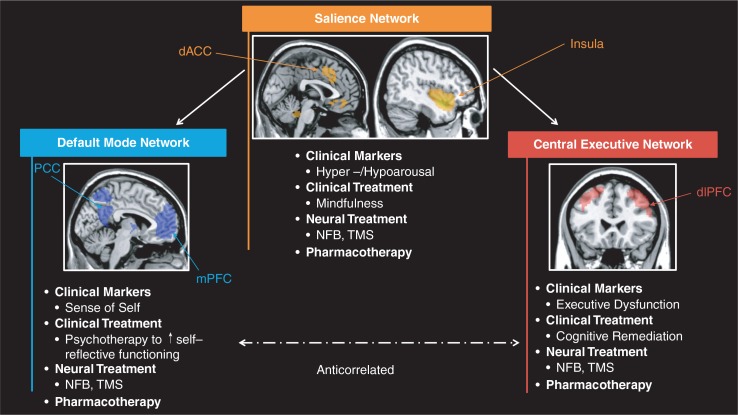
This commentary has demonstrated increasing evidence for altered functioning of three large-scale brain networks in PTSD, namely the CEN, SN, and DMN. We propose that each network is associated with specific clinical symptoms observed in PTSD, including cognitive dysfunction (CEN), hyper- and hypoarousal/interoception (SN), and an altered sense of self (DMN). Specific testable treatment interventions targeted to restore each of these neural networks and related clinical dysfunction were suggested (Fig. 1). Neuroscientifically-informed integrative treatment interventions will be central to research efforts aimed at targeting specific PTSD and related symptoms.
Fig. 1.
Neuroscientifically-informed treatment interventions in psychotraumatology: Three intrinsic networks, including the central executive network (CEN), salience network (SN), and default mode network (DMN) may be associated with specific clinical symptoms observed in PTSD, including cognitive dysfunction (CEN), hyper- and hypoarousal/interoception (SN), and an altered sense of self (DMN). Specific testable treatment interventions targeted to restore each one of these brain networks and related clinical dysfunction are suggested. Images were created using network templates available from http://findlab.stanford.edu/functional_ROIs.html (Shirer, Ryali, Rykhlevskaia, Menon, & Greicius, 2012).
Acknowledgements
This work was supported by funding from the Canadian Institute of Military and Veteran Health Research and the Canadian Institute of Health Research (#97914). We would like to thank Nancy Mazza for preparation of this manuscript for publication.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- Alfonso J. P, Caracuel A, Delgado-Pastor L. C, Verdejo-Garcia A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug and Alcohol Dependence. 2011;117(1):78–81. doi: 10.1016/j.drugalcdep.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bearden C. E, Green M. F, Van Gorp W, Mintz J. A relationship between neurocognitive impairment and functional impairment in bipolar disorder: A pilot study. Psychiatry Research. 2008;157(1–3):289–293. doi: 10.1016/j.psychres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Tekell J, Biswas K, Kilbourne A. M, Evans D, Tang D, et al. Executive function and employment status among veterans with bipolar disorder. Psychiatric Services. 2007;58(11):1441–1447. doi: 10.1176/appi.ps.58.11.1441. [DOI] [PubMed] [Google Scholar]
- Amodio D. M, Frith C. D. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aupperle R. L, Melrose A. J, Stein M. B, Paulus M. P. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura A. S. Theoretical and empirical exploration of the similarities between emotional numbing in posttraumatic stress disorder and alexithymia. Anxiety Disorders. 2003;17:349–360. doi: 10.1016/s0887-6185(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Barlati S, Deste G, De Peri L, Ariu C, Vita A. Cognitive remediation in schizophrenia: Current status and future perspectives. Schizophrenia Research and Treatment. 2013;2013:156084. doi: 10.1155/2013/156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. F, Mesquita B, Ochsner K. N, Gross J. J. The experience of emotion. Annual Reviews of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim M. T, Van den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: An exploratory meta-analysis of randomized, double-blind and sham-controlled trials, Canadian Journal of Psychiatry . Revue Canadienne de Psychiatrie. 2014;59(9):487–496. doi: 10.1177/070674371405900905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E. M, Putnam F. W. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease. 1986;174:727–734. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Birn R. M, Patriat R, Phillips M. L, Germain A, Herringa R. J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depression and Anxiety. 2014;31(10):880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R. L, Williamson P. C, Osuch E. A, Frewen P. A, Stevens T. K, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bormann J. E, Oman D, Walter K. H, Johnson B. D. Mindful attention increases and mediates psychological outcomes following mantram repetition practice in veterans with posttraumatic stress disorder. Medical Care. 2014;52(12 Suppl 5):S13–S18. doi: 10.1097/MLR.0000000000000200. [DOI] [PubMed] [Google Scholar]
- Bowie C. R, Depp C, McGrath J. A, Wolyniec P, Mausbach B. T, Thornquist M. H, et al. Prediction of real-world functional disability in chronic mental disorders: A comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167(9):1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C. R. Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychological Bulletin. 2014;140(1):69–97. doi: 10.1037/a0033722. [DOI] [PubMed] [Google Scholar]
- Briere J. Multiscale Dissociation Inventory. Odessa, FL: Psychological Assessment Resources; 2002. [Google Scholar]
- Brown A. D, Addis D. R, Romano T. A, Marmar C. R, Bryant R. A, Hirst W, et al. Episodic and semantic components of autobiographical memories and imagined future events in post-traumatic stress disorder. Memory. 2014;22(6):595–604. doi: 10.1080/09658211.2013.807842. [DOI] [PubMed] [Google Scholar]
- Brown A. D, Root J. C, Romano T. A, Chang L. J, Bryant R. A, Hirst W. Overgeneralized autobiographical memory and future thinking in combat veterans with posttraumatic stress disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44(1):129–134. doi: 10.1016/j.jbtep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chen A. C, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology. 2013;38(10):1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J. M, Steele J. S, Lenow J. K, Smitherman S, Everett B, Messias E, et al. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. Journal of Psychiatric Research. 2014;48(1):47–55. doi: 10.1016/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J. M, Steele J. S, Smitherman S, Lenow J. K, Kilts C. D. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Research. 2013;214:238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. S, Resnick H. S, Kilpatrick D. G. Prevalence and correlates of posttrauma distorted beliefs: Evaluating DSM-5 PTSD expanded cognitive symptoms in a national sample. Journal of Traumatic Stress. 2014;27(3):299–306. doi: 10.1002/jts.21925. [DOI] [PubMed] [Google Scholar]
- Craig A. D. The sentient self. Brain Structure & Function. 2010;214(5–6):563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Critchley H. D, Wiens S, Rotshtein P, Öhman A, Dolan R. J. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Daniels J. K, McFarlane A. C, Bluhm R. L, Moores K. A, Clark C. R, Shaw M. E, et al. Switching between executive and default mode networks in posttraumatic stress disorder: Alterations in functional connectivity. Journal of Psychiatry and Neuroscience. 2010;35(4):258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell P. F. The multidimensional inventory of dissociation (MID): A comprehensive measure of pathological dissociation. Journal of Trauma and Dissociation. 2006;7(2):77–106. doi: 10.1300/J229v07n02_06. [DOI] [PubMed] [Google Scholar]
- Depp C. A, Mausbach B. T, Eyler L. T, Palmer B. W, Cain A. E, Lebowitz B. D, et al. Performance-based and subjective measures of functioning in middle-aged and older adults with bipolar disorder. Journal of Nervous and Mental Disease. 2009;197(7):471–475. doi: 10.1097/NMD.0b013e3181ab5c9b. [DOI] [PubMed] [Google Scholar]
- Dickerson F. B, Boronow J. J, Stallings C. R, Origoni A. E, Cole S, Yolken R. H. Association between cognitive functioning and employment status of persons with bipolar disorder. Psychiatric Services. 2004;55(1):54–58. doi: 10.1176/appi.ps.55.1.54. [DOI] [PubMed] [Google Scholar]
- Dosenbach N. U, Fair D. A, Miezin F. M, Cohen A. L, Wenger K. K, Dosenbach R. A, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkin J. J, Leuchter A. F, Cook I. A, Kasl-Godley J. E, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of Affective Disorders. 2000;60(1):13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Elgamel S, McKinnon M. C, Ramakrishan K, Joffe R. T, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: A proof of principle study. Psychological Medicine. 2007;37:1229–1238. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- Etter D. W, Gauthier J. R, McDade-Montez E, Cloitre M, Carlson E. B. Positive affect, childhood adversity, and psychopathology in psychiatric inpatients. European Journal of Psychotraumatology. 2013;4:20771. doi: 10.3402/ejpt.v4i0.20771. doi: http://dx.doi.org/10.3402/ejpt.v4i0.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E. B, Ehlers A, Clark D. M, Tolin D. F, Orsillo S. M. The posttraumatic cognitions inventory (PTCI): Development and validation. Psychological Assessment. 1999;11:303–314. [Google Scholar]
- Follette V. M, Briere J, Rozelle D, Hopper J. W, Rome D. I, editors. Mindfulness-oriented interventions for trauma: Integrating contemplative practices. New York: Guilford Press; 2014. [Google Scholar]
- Fonzo G. A, Flagan T. M, Sullivan S, Allard C. B, Grimes E. M, Simmons A. N, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research. 2013;211(2):93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G. A, Simmons A. N, Thorp S. R, Norman S. B, Paulus M. P, Stein M. B. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T. W, Hart J, Kimbrell T, Ross E. D. Comprehension of affective prosody in veterans with chronic posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21(1):52–58. doi: 10.1176/appi.neuropsych.21.1.52. [DOI] [PubMed] [Google Scholar]
- Frewen P. A, Dean J. A, Lanius R. A. Assessment of anhedonia in psychological trauma: Development of the Hedonic Deficit and Interference Scale. European Journal of Psychotraumatology. 2012;3:8585. doi: 10.3402/ejpt.v3i0.8585. doi: http://dx.doi.org/10.3402/ejpt.v3i0.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P. A, Dozois D. J, Lanius R. A. Assessment of anhedonia in psychological trauma: Psychometric and neuroimaging perspectives. European Journal of Psychotraumatology. 2012;3:8587. doi: 10.3402/ejpt.v3i0.8587. doi: http://dx.doi.org/10.3402/ejpt.v3i0.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P. A, Lanius R. A. Toward a psychobiology of posttraumatic self-dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Frewen P. A, Lanius R. A. Healing the traumatized self: Consciousnes, neuroscience, and treatment. New York: W.W. Norton; 2015. [Google Scholar]
- Frewen P. A, Lanius R. A, Dozois D. J, Neufeld R. W, Pain C, Hopper J. W, Stevens T. K. Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Journal of Abnormal Psychology. 2008;117(1):171–181. doi: 10.1037/0021-843X.117.1.171. [DOI] [PubMed] [Google Scholar]
- Garrison K. A, Santoyo J. F, Davis J. H, Thornhill T. A, 4th, Kerr C. E, Brewer J. A. Effortless awareness: Using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Frontiers in Human Neuroscience. 2013;7:440. doi: 10.3389/fnhum.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K. A, Scheinost D, Worhunsky P. D, Elwafi H. M, Thornhill T. A, 4th., Thompson E, et al. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage. 2013;81:110–118. doi: 10.1016/j.neuroimage.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, De Kloet C. S, Hijman R, Westenberg H. G. Neuropsychological performance is related to current social and occupational functioning in veterans with posttraumatic stress disorder. Depression and Anxiety. 2009;26:7–15. doi: 10.1002/da.20476. [DOI] [PubMed] [Google Scholar]
- Gildengers A. G, Butters M. A, Chisholm D, Rogers J. C, Holm M. B, Bhalla R. K, et al. Cognitive functioning and instrumental activities of daily living in late-life bipolar disorder. American Journal of Geriatric Psychiatry. 2007;15(2):174–179. doi: 10.1097/JGP.0b013e31802dd367. [DOI] [PubMed] [Google Scholar]
- Green M. J, Benzeval M. The development of socioeconomic inequalities in anxiety and depression symptoms over the lifecourse. Social Psychiatry and Psychiatric Epidemiology. 2013;48(12):1951–1961. doi: 10.1007/s00127-013-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. E, Sisitsky T, Kessler R. C, Finkelstein S. N, Berndt E. R, Davidson J. R, et al. The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry. 1999;60(7):427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Greicius M. D, Krasnow B, Reiss A. L, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.01350581000135058100. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann C. F, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J. V, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science. 1978;201(4350):79–81. doi: 10.1126/science.663641. [DOI] [PubMed] [Google Scholar]
- Health Canada. A report on mental illness. Ottawa, Canada: Health Canada; 2002. [Google Scholar]
- Holzel B. K, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. W, Frewen P. A, Van der Kolk B. A, Lanius R. A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress. 2007;20(5):713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- In de Braek D. M, Dijkstra J. B, Ponds R. W, Jolles J. Goal management training in adults with ADHD: An intervention study. Journal of Attention Disorders. 2012;27(6):433–42. doi: 10.1177/1087054712468052. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Vieta E. Functional outcome and disability in bipolar disorders: Ongoing research and future directions. Bipolar Disorders. 2007;9(1–2):1–2. doi: 10.1111/j.1399-5618.2007.00441.x. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, et al. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychological Medicine. 2014;44(9):1927–1936. doi: 10.1017/S0003329171300250X. [DOI] [PubMed] [Google Scholar]
- Johnsen G. E, Asbjornsen A. E. Consistent impaired verbal memory in PTSD: A meta-analysis. Journal of Affective Disorders. 2008;111(1):74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrohic living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Dell; 1990. [Google Scholar]
- Kearney D. L, McDermott K, Malte C, Martinez M, Simpson T. L. Effects of participation in a mindfulness program for Veterans with posttraumatic stress disorder: A randomized controlled pilot study. Journal of Clinical Psychology. 2012;69:14–27. doi: 10.1002/jclp.21911. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker A. R, Van Rooij S. J, Kahn R. S, Geuze E. Altered functional connectivity in posttraumatic stress disorder with versus without comorbid major depressive disorder: A resting state fMRI study. F1000Res. 2013;2:289. doi: 10.12688/f1000research.2-289.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, Rademaker A. R, Van Rooij S. J, Kahn R. S, Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Human Brain Mapping. 2015;36(1):99–109. doi: 10.1002/hbm.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C. Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61(Suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- Kimbrough E, Magyari T, Langenberg P, Chesney M, Berman B. Minfulness intervention for child abuse survivors. Journal of Clinical Psychology. 2010;66:17–33. doi: 10.1002/jclp.20624. [DOI] [PubMed] [Google Scholar]
- King A. P, Erickson T. M, Giardino N. D, Favorite T, Rauch S. A, Robinson E, et al. A pilot study of group mindfulness-based cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD) Depression and Anxiety. 2013;30(7):638–645. doi: 10.1002/da.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R. C, Ros T, Theberge J, Frewen P. A, Calhoun V. D, Schmahl C, et al. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatrica Scandinavica. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S. B, Van Zuiden M, Nawijn L, Frijling J. L, Veltman D. J, Olff M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: Salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Krasny-Pacini A, Chevignard M, Evans J. Goal Management Training for rehabilitation of executive functions: A systematic review of effectiveness in patients with acquired brain injury. Disability and Rehabilitation. 2014;36(2):105–116. doi: 10.3109/09638288.2013.777807. [DOI] [PubMed] [Google Scholar]
- Krasny-Pacini A, Limond J, Evans J, Hiebel J, Bendjelida K, Chevignard M. Context-sensitive goal management training for everyday executive dysfunction in children after severe traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2014;29(5):E49–64. doi: 10.1097/htr.0000000000000015. [DOI] [PubMed] [Google Scholar]
- Krystal H. Massive psychic trauma. New York: International University Press; 1968. [Google Scholar]
- Krystal H, Krystal J. H. Integration and self-healing: Affect, trauma, alexithymia. Hillsdale, NJ: Analytic Press; 1988. [Google Scholar]
- Kurtz M. M. Cognitive remediation for schizophrenia: Current status, biological correlates and predictors of response. Expert Review of Neurotherapeutics. 2012;12(7):813–821. doi: 10.1586/ern.12.71. [DOI] [PubMed] [Google Scholar]
- Lanius R. A, Bluhm R. L, Coupland N. J, Hegadoren K. M, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius R. A, Bluhm R. L, Frewen P. A. How understanding the neurobiology of complex posttraumatic stress disorder can inform clinical practice: A social cognitive and affective neuroscience approach. Acta Psychiatrica Scandinavica. 2011;124(5):331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- Lanius R. A, Frewen P. A, Vermetten E, Yehuda R. Fear conditioning and early life vulnerabilities: Two distinct pathways of emotional dysregulation and brain dysfunction in PTSD. European Journal of Psychotraumatology. 2010;1:5467–5477. doi: 10.3402/ejpt.v1i0.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R. A, Vermetten E, Loewenstein R. J, Brand B, Schmahl C, Bremner J. D, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R. A, Williamson P. C, Bluhm R. L, Densmore M, Boksman K, Neufeld R. W, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: A functional magnetic resonance imaging investigation. Biological Psychiatry. 2005;57(8):873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Lawrence E. J, Su L, Barker G. J, Medford N, Dalton J, Williams S. C, et al. Self-regulation of the anterior insula: Reinforcement learning using real-time fMRI neurofeedback. Neuroimage. 2013;88C:113–124. doi: 10.1016/j.neuroimage.2013.10.069. [DOI] [PubMed] [Google Scholar]
- Lazar S. W, Kerr C. E, Wasserman R. H, Gray J. R, Greve D. N, Treadway M. T, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Robertson I. H, Clare L, Carter G, Hong J, Wilson B. A, et al. Rehabilitation of executive functioning: An experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society. 2000;6(3):299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- Levine B, Schweizer T. A, O'Connor C, Turner G, Gillingham S, Stuss D. T, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Frontiers in Human Neuroscience. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Stuss D. T, Winocur G, Binns M. A, Fahy L, Mandic M, et al. Cognitive rehabilitation in the elderly: Effects on strategic behavior in relation to goal management. Journal of the International Neuropsychological Society. 2007;13(1):143–152. doi: 10.1017/s1355617707070178. [DOI] [PubMed] [Google Scholar]
- Linden D. E, Habes I, Johnston S. J, Linden S, Tatineni R, Subramanian L, et al. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7(6):e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovero K. L, Simmons A. N, Aron J. L, Paulus M. P. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45(3):976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Giusti L, Albanese A, Mariano M, Pino M. C, Roncone R. Social cognition disorders in military police officers affected by posttraumatic stress disorder after the attack of An-Nasiriyah in Iraq. Psychiatry Research. 2012;198:248–252. doi: 10.1016/j.psychres.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple netowrk model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin L. Q. Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel L. A, Hall G. B, Fougere P, McKinnon M. C, MacQueen G. M. Neural correlates of cognitive remediation in patients with mood disorders. Psychiatry Research. 2013;214(2):142–152. doi: 10.1016/j.pscychresns.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Miller E. K, Cohen J. D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore S. A, Zoellner L. A. Overgeneral autobiographical memory and traumatic events: An evaluative review. Psychological Bulletin. 2007;133(3):419–437. doi: 10.1037/0033-2909.133.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi A. R, Abdi A, Fathi-Ashtiani A, Dalgleish T, Jobson L. Overgeneral autobiographical memory recollection in Iranian combat veterans with posttraumatic stress disorder. Behaviour Research and Therapy. 2012;50(6):435–441. doi: 10.1016/j.brat.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Muller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. The Neuroscientist: A Review Journal Bridging Neurobiology, Neurology and Psychiatry. 2014 doi: 10.1177/1073858414526645. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Nawijn L, Van Zuiden M, Frijling J. L, Koch S. B, Veltman D. J, Olff M. Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neuroscience and Biobehavioral Reviews. 2015;51C:189–204. doi: 10.1016/j.neubiorev.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Nazarov A, Frewen P, Oremus C, Schellenberg E. G, McKinnon M. C, Lanius R. Comprehension of affective prosody in women with post-traumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica. 2014:1–8. doi: 10.1111/acps.12364. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Nazarov A, Frewen P, Parlar M, Oremus C, Macqueen G, McKinnon M, et al. Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica. 2014;129(3):193–201. doi: 10.1111/acps.12142. [DOI] [PubMed] [Google Scholar]
- Niles B. L, Klunk-Gillis J, Ryngala D. J, Silberbogen A. K, Paysnick A, Wolf E. J. Comparing mindfulness and psychoeducation treatments for combat-related PTSD using a telehealth approach. Psychological Trauma: Theory, Research, Practice, Policy. 2012;4:538–547. [Google Scholar]
- Nummenmaa L, Glerean E, Hari R, Hietanen J. K. Bodily maps of emotions. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):646–651. doi: 10.1073/pnas.1321664111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji B. O, Cisler J. M, Tolin D. F. Quality of life in the anxiety disorders: A meta-analytic review. Clinical Psychology Review. 2007;27(5):572–581. doi: 10.1016/j.cpr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Paret C, Kluetsch R, Ruf M, Demirakca T, Hoesterey S, Ende G, et al. Down-regulation of amygdala activation with real-time fMRI neurofeedback in a healthy female sample. Frontiers in Behavioral Neuroscience. 2014;8:299. doi: 10.3389/fnbeh.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S. V, Lam R. W, Group C. D. W. Clinical guidelines for the treatment of depressive disorders, I. Definitions, prevalence, and health burden. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2001;46(Suppl 1):13S–20S. [PubMed] [Google Scholar]
- Paulus M. P, Stein M. B. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peterson A, Thome J, Frewen P. A, Lanius R. A. Resting state neuroimaging studies: A new way of identifying differences and similarities among the anxiety disorders? Canadian Journal of Psychiatry. 2014;59:294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A. R, Witteveen A. B, Reitsma J. B, Olff M. The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Qin L. D, Wang Z, Sun Y. W, Wan J. Q, Su S. S, Zhou Y, et al. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Research. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabinak C. A, Angstadt M, Welsh R. C, Kenndy A. E, Lyubkin M, Martis B, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in psychiatry/Frontiers Research Foundation. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E, MacLeod A. M, Snyder A. Z, Powers W. J, Gusnard D. A, Shulman G. L. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasey H, Lubar J. F, McIntyre A, Zoffuto A, Abbott P. L. EEG biofeedback for the enhancement of attentional processing in normal college students. Journal of Neurotherapy. 1995;1:15–21. [Google Scholar]
- Ratnasingham S, Cairney J, Rehm J, Manson H, Kyndyak P. A. Opening eyes, opening minds: The Ontario burden of mental illness and addictions report. Toronto, Ontario: An ICES/PHO Report; 2012. [Google Scholar]
- Robertson I. H, Murre J. M. Rehabilitation of brain damage: Brain plasticity and principles of guided recovery. Psychological Bulletin. 1999;125(5):544–575. doi: 10.1037/0033-2909.125.5.544. [DOI] [PubMed] [Google Scholar]
- Ros T, Baars B. J, Lanius R. A, Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Frontiers in Human Neuroscience. 2014;8:1008. doi: 10.3389/fnhum.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T, Munneke M. A, Ruge D, Gruzelier J. H, Rothwell J. C. Endogenous control of waking brain rhythms induces neuroplasticity in humans. European Journal of Neuroscience. 2010;31(4):770–778. doi: 10.1111/j.1460-9568.2010.07100.x.. [DOI] [PubMed] [Google Scholar]
- Ros T, Theberge J, Frewen P. A, Kluetsch R, Densmore M, Calhoun V. D, et al. Mind over chatter: Plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Neuroimage. 2013;65:324–335. doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekadar S. R, Caria A, Veit R, Kircher T, et al. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Human Brain Mapping. 2013;34(1):200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, D'Arista S, Egiziano E, Gardi C, Petrosino R, Vatti G, et al. Interaction between neuroanatomical and psychological changes after mindfulness-based training. PLoS One. 2014;9(10):e108359. doi: 10.1371/journal.pone.0108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N, Downham R, Turman B, Kropotov J, Clark R, Yumash R, et al. Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase. 2015;21:271–278. doi: 10.1080/13554794.2014.890727. [DOI] [PubMed] [Google Scholar]
- Schore A. N. Affect dysregulation and disorders of the self. New York: W.W. Norton; 2003. [Google Scholar]
- Schweizer T. A, Levine B, Rewilak D, O'Connor C, Turner G, Alexander M. P, et al. Rehabilitation of executive functioning after focal damage to the cerebellum. Neurorehabilitation and Neural Repair. 2008;22(1):72–77. doi: 10.1177/1545968307305303. [DOI] [PubMed] [Google Scholar]
- Seeley W. W, Menon V, Schatzberg A. F, Keller J, Glover G. H, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: A resting-state fMRI study. PLoS One. 2014;9(5):e96834. doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L. M, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W. R, Ryali S, Rykhlevskaia E, Menon V, Greicius M. D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A. N, Matthews S. C, Strigo I. A, Baker D. G, Donovan H. K, Motezadi A, et al. Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biology of Mood & Anxiety Disorders. 2011;1(1):6. doi: 10.1186/2045-5380-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A. N, Norman S. B, Spadoni A. D, Strigo I. A. Neurosubstrates of remission following prolonged exposure therapy in veterans with posttraumatic stress disorder. Psychotherapy and Psychosomatics. 2013;82(6):382–389. doi: 10.1159/000348867. [DOI] [PubMed] [Google Scholar]
- Simmons A. N, Paulus M. P, Thorp S. R, Matthews S. C, Norman S. B, Stein M. B. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64(8):681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N, Mar R. A, Kim A. S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin D. J, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R. K, King A. P, Garfinkel S. N, Wang X, Sripada C. S, Welsh R. C, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry and Neuroscience. 2012;37(2):110069. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R. K, King A. P, Welsh R. C, Garfinkel S. N, Wang X, Sripada C. S, et al. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques P. L, Kragel P. A, Rubin D. C. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cognitive, Affective & Behavioral Neuroscience. 2013;13(3):554–566. doi: 10.3758/s13415-013-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. S, Jovanovic T, Fani N, Ely T. D, Glover E. M, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubberud J, Langenbahn D, Levine B, Stanghelle J, Schanke A. K. Goal management training of executive functions in patients with spina bifida: A randomized controlled trial. Journal of the International Neuropsychological Society. 2013;19(6):672–685. doi: 10.1017/s1355617713000209. [DOI] [PubMed] [Google Scholar]
- Tursich M, Ros T, Frewen P. A, Calhoun V. D, Lanius R. A. Distinct intrinsic network connectivity patterns of Posttraumatic Stress Disorder symptom clusters. Acta Psychiatrica Scandinavica. 2015 doi: 10.1111/acps. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Boyle M. H. Post-traumatic stress disorder in Canada. CNS Neuroscience & Therapeutics. 2008;14(3):171–181. doi: 10.1111/j.1755-5949.2008.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kolk B. A, McFarlane A. C. The black hole of trauma. In: Van der Kolk B. A, McFarlane A. C, Weisaeth L, editors. Traumatic stress. New York, NY: Guilford Press; 1996. pp. 97–102. [Google Scholar]
- Van Hooren S. A, Valentijn S. A, Bosma H, Ponds R. W, Van Boxtel M. P, Levine B, et al. Effect of a structured course involving goal management training in older adults: A randomised controlled trial. Patient Education and Counseling. 2007;65(2):205–213. doi: 10.1016/j.pec.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts B. V, Schnurr P. P, Mayo L, Young-Xu Y, Weeks W. B, Friedman M. J. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2013;74(6):e541–550. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- Winocur G, Craik F. I, Levine B, Robertson I. H, Binns M. A, Alexander M, et al. Cognitive rehabilitation in the elderly: Overview and future directions. Journal of the International Neuropsychological Society. 2007;13(1):166–171. doi: 10.1017/s1355617707070191. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The global burden of disease 2004 update; Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk S. R, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. The American Journal of Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Steiner A, Kahana B, Binder-Brynes K, Southwick S. M, Zemelman S, Giller E. L. Alexithymia in Holocaust survivors with and without PTSD. Journal of Traumatic Stress. 1997;10(1):93–100. doi: 10.1023/a:1024860430725. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, et al. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: A functional magnetic resonance imaging study. Brain Research. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Young K. D, Zotev V, Phillips R, Misaki M, Yuan H, Drevets W. C, Bodurka J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9(2):e88785. doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Young K. D, Phillips R, Zotev V, Misaki M, Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connectivity. 2014;4(9):690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Yao L, Zhang H, Long Z, Zhao X. Improved working memory performance through self-regulation of dorsal lateral prefrontal cortex activation using real-time fMRI. PLoS One. 2013;8(8):e73735. doi: 10.1371/journal.pone.0073735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin L. D, Wan J. Q, Sun Y. W, Su S. S, et al. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS One. 2012;7(10):e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Phillips R, Young K. D, Drevets W. C, Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8(11):e79184. doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Phillips R, Yuan H, Misaki M, Bodurka J. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. Neuroimage. 2014;85(Pt 3):985–995. doi: 10.1016/j.neuroimage.2013.04.126. [DOI] [PubMed] [Google Scholar]