Abstract
Objective
Evidence of the brain network involved in cognitive dysfunction has been inconsistent for major depressive disorder (MDD), especially during early stage of MDD. This study seeks to examine abnormal cognition connectivity network (CCN) in MDD within the whole brain.
Methods
Sixteen patients with MDD and 16 health controls were scanned during resting-state using 3.0 T functional magnetic resonance imaging (fMRI). All patients were first episode without any history of antidepressant treatment. Both the left and right dorsolateral prefrontal cortex (DLPFC) were used as individual seeds to identify CCN by the seed-target correlation analysis. Two sample t test was used to calculate between-group differences in CCN using fisher z-transformed correlation maps.
Results
The CCN was constructed by bilateral seed DLPFC in two groups separately. Depressed subjects exhibited significantly increased functional connectivity (FC) by left DLPFC in one cluster, overlapping middle frontal gyrus, BA7, BA43, precuneus, BA6, BA40, superior temporal gyrus, BA22, inferior parietal lobule, precentral gyrus, BA4 and cingulate gyrus in left cerebrum. Health controls did not show any cluster with significantly greater FC compared to depressed subjects in left DLPFC network. There was no significant difference of FC in right DLPFC network between depressed subjects and the health controls.
Conclusion
There are differences in CCN during early stage of MDD, as identified by increased FCs among part of frontal gyrus, parietal cortex, cingulate cortex, and BA43, BA22, BA4 with left DLPFC. These brain areas might be involved in the underlying mechanisms of cognitive dysfunction in MDD.
Keywords: Depression, First episode, Cognition connectivity network, Functional magnetic resonance imaging, Resting state
INTRODUCTION
Cognitive dysfunction is not rare among patients with major depressive disorder (MDD) which affects working memory, attention, psychomotor processing speed, and execution.1,2,3 There is an ongoing debate about whether cognitive dysfunction is a trait or a state in depression. Many studies have found long-term impairment in sustained attention, working memory and executive functioning after patients recover from depression.4,5,6 This evidence support that cognitive dysfunction is a trait, instead of a simple state marker in depression because it is consistent, and clinically significant in MDD.3 Thus, it is of interest to study the mechanisms of cognitive dysfunction in major depressive disorder.
Using functional magnetic resonance imaging (fMRI), some studies had found decreased activities in specific brain areas such as anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC) among patients with MDD.7,8 DLPFC plays a key role to regulate intellectual function and action by integration of sensory and mnemonic information. This brain area is also essential for the conflict-induced behavioral adjustment, which mediates encoding and maintenance of information about experienced conflict related with episode memory.9 There has been evidence that executive function improved after trans-cranial stimulation in the left DLPFC area among patients with MDD.10 Some studies found decreased activity in DLPFC among patients with MDD,7,8 and antidepressant treatment can increase activities in DLPFC during cognitive tasks.11 Thus, DLPFC may be an area that is important for the understanding of cognitive deficits in MDD.
Despite significant findings from studies of a specific brain area, an integrated view is necessary for investigations of complex cognitive dysfunction. Increasing efforts has been focused on functional connectivity (FC) between brain regions.12 Some evidence has been found from fMRI studies for very low frequency fluctuations (LFFs) (<0.08 Hz) in blood oxygen level dependence (BOLD) signals (LFBF) during the resting state.13,14 The fluctuations are synchronous and exhibit high temporal coherence in functionally related regions of the brain. The functional dependencies within whole brain by no neuropsychological task, defined as resting state functional connectivity (RSFC) based on the LFFs. Recent studies have reported increasing RSFC of depression.15,16 The cognition connectivity network (CCN) is an important RSFC network based on fMRI data which serves cognitive tasks involving hyperactivity in frontal and parietal regions,16,17 but there are still lack of research focused on CCN. Moreover, there has been little research on functional networks in early-stage MDD.
The aim of this study was to investigate the functional networks underlying cognitive deficits of MDD. We hypothesized that there are the abnormal functional networks in MDD. In order to test the hypothesis, a RSFC analysis was performed to construct the CCN based on seed DLPFC.16 The CCN measures correlations of spontaneous LFBF signals between the seed region (DLPFC) and all brain voxels. Then, CCN were compared between individuals with first-episode MDD who had no history of antidepressant treatment and non-MDD health controls.
METHODS
Participants
Patients with MDD were recruited from the outpatient clinic at Huashan Hospital and Shanghai Mental Health Center, Shanghai, China. Non-MDD health individuals matched on age, sex, and level of education with MDD patients, who were recruited via advertisement. Sixteen patients with MDD and 16 controls participated in the study after signing informed consents. The study was approved by the institutional review board of Shanghai Mental Health Center. All participants were right-handed and the right right-eye-dominant. All participants were assessed using the Structured Clinical Interview for DSM-IV (SCID), 24-item Hamilton Depression Scale (HAMD),18 and 14-item Hamilton Anxiety Scale (HAMA). Clinical laboratory tests included blood counts, metabolic panel, thyroid hormone tests, urinary analysis and pregnancy tests for female.
Patients. Inclusion criteria: All patients were 25-50 years of age, met DSM-IV diagnostic criteria of MDD, had first episode of MDD, were naïve to antidepressant, had a score greater than 20 in HAMD and a score less than 7 in HAMA, and were receiving outpatient treatment. Exclusion criteria included a history of other DSM-IV Axis I disorder (e.g., schizophrenia, schizoaffective disorder, bipolar disorder or an anxiety disorder) as the primary diagnosis, having taken any prescription or psychotropic medications in the prior 4 weeks, current suicidal ideation or attempt, undergoing inpatient treatment, substance dependence during the past year (except for dependence of caffeine or nicotine), positive urinary toxicology at baseline, alcohol drinking in the prior week, any serious medical or neurological illness, current pregnancy or breastfeeding, and metallic implants or other contraindications to MRI.
Controls. Inclusion criteria: health control were 25-50 years of age, had no history of psychiatric illnesses or substance use disorders, had no family history of major psychiatric or neurological illnesses in first degree relatives, were not currently taking any prescription or psychotropic medications, did not drink alcohol in the prior week, and did not have serious medical or neurological illnesses. Exclusion criteria: those who were pregnant or breastfeeding, or had metallic implants or other MRI contraindications were excluded.
Functional MRI procedure
A 3.0-T General Electric Signa scanner with a standard whole-head coil was used for MRI in this study. T1 structural images with a horizontal axis were acquired using the SE sequence with the following parameters: repeat time (TR)=500 ms, echo time (TE)=14 ms, flip angle=15°, 5.0 mm thickness, no interval, and NEX=1. The 3D reconstruction used rapid interference phase gradient echo flip recovery (FSPGRIR) and pulse sequence (T1 weighed) scanning with the following parameters: TR=5.9 ms, TE=1.4 ms, flip angle=15°, 1.0 mm thickness, no interval, field of vision (FOV)=240×240 mm, bandwidth=31.25, and resolution=1.0×1.0×1.0 mm. Multislice echo planar imaging (EPI) sequence was used to obtain whole-brain functional images with the following parameters: TR=3000 ms, TE=30 ms, flip angle=90°, 5.0 mm thickness, no interval, FOV=240×240 mm, matrix size=64×64, and spatial resolution=3.75×3.75×5.0 mm. Each brain volume was comprised of 22 axial slices and each functional run contained 100 volumes. The fMRI instruction: to lie and remain motionless, to keep your eyes closed and relax as possibly. The scanning time of resting state: 5 minutes and 12 seconds.
Data preprocessing
The data were preprocessed by standard procedures including slice timing, realignment, and spatial normalization to the standard Montreal Neurological Institute (MNI) space and re-sampled at 3 mm3. Then data were spatially smoothed with a 6-mm Full Wave at Half Maximum (FWHM) Gaussian kernel. A low-pass frequency filter (0.01<f<0.08 Hz) was applied by using AFNI (http://www.afni.nimh.nih.gov/).19 Linear regression was used to control for confounding factors including six motion parameters, mean time series of white matter, cerebrospinal fluid and global signal using SPM8 (www.fil.ion.ucl.ac.uk/spm).15,20
Seed region selection and functional connectivity analysis
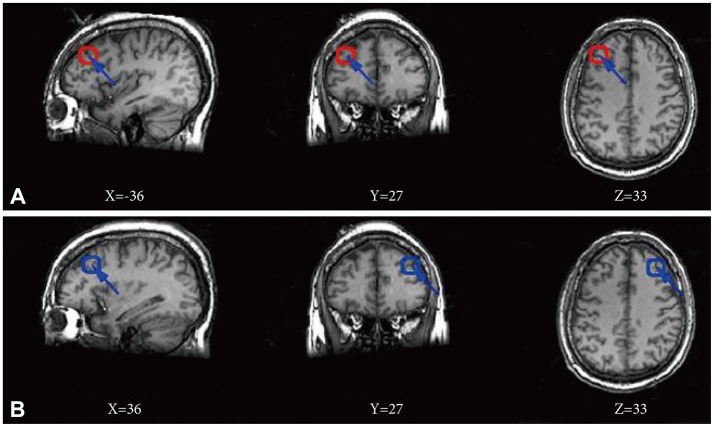
The left and right DLPFC region was chosen to serve as individual seeds (Figure 1) based on literature.16 We defined the seed region by using a 6 mm radius sphere respectively centering on the points (±36, 27, 33). The BOLD time series of the voxels within each seed were averaged to generate the reference time series for the specific seed region using SPM8 (www.fil.ion.ucl.ac.uk/spm). A correlation map was subsequently constructed by computing the Pearson's correlation coefficient between the reference time series and the time series of each voxel in the whole brain. Fisher's r-to-z transform {z=0.5 Ln [(1+r)/(1-r)]} was used to normalize data.21 Statistical significance was considered when p<0.05 using false discovery rate (FDR) for multiple comparisons correction with a cluster size ≥10 voxels (270 mm3). The significance maps were created by combining results across participants within a group, which were defined as the cognition connectivity network (CCN) maps. Based on CCN map obtained by the correlation coefficients, we calculated significant differences in mean correlation coefficients for both left and right DLPFCs between the MDD and control groups using two ample t tests, notably with age, gender distribution and education level as covariates. The t values were converted to equally probable z score and statistical significance was considered when p<0.05. A combined threshold for the contrast maps was set at p<0.05 (FDR correction) for a cluster size ≥10 voxels (270 mm3).
Figure 1.
Location of seed regions. Red circle in three maps of line A corresponds to a seed region in the left dorsolateral prefrontal cortex, and blue circle in three maps of line B corresponds to a seed region in the right dorsolateral prefrontal cortex. All maps using the Montreal Neurological Institute spatial array coordinate system (MNI). X: sagittal view, Y: coronal view, Z: axial view.
Association analysis between functional connectivity and clinical characteristics
First, the mean values of functional connectivity in the clusters showing significant differences of CCN between the two groups were extracted. Multivariate linear regressions were then performed to evaluate the relationships between the mean values of functional connectivity in MDD patients and total score of HAMD, while the age, gender, and educational level were controlled as the covariates.
RESULTS
Demographics and psychometrics
Sixteen individuals with MDD included: 7 females and 9 males between 26 to 45 years of age (mean age: mean±SD, 33.44±5.77 years) with a level of education between 12 to 21 years (mean level: 15.48±2.69 years). Sixteen health control subjects included 7 females and 9 males between 27 to 43 years of age (33.25±5.09 years) with a level of education between 12 to 22 years (15.72±3.03 years). All patients had first episode MDD and were naïve to antidepressants. Their duration of disorder varied from one to five months (mean duration: 3.08±1.30 months). Their age of onset varied from 26 to 44 years of age (33.13±5.74 years). There were no statistical significance in sex, age (t=0.09, p>0.05) and years of education (t=-0.27, p>0.05) between the two groups. HAMD scores varied from 26 to 36 (mean value: 31.19±2.95) in the MDD group, and 1 to 6 (2.63±1.96) in the control group (t=32.26, p<0.05). HAMA scores varied from 4 to 6 (mean value: 5.62±0.72) in the MDD group, and 3 to 6 (3.31±0.87) in the control group (t=8.18, p<0.05).
Functional connectivity construction of CCN
Seed location was shown in Figure 1. Results of ROI connectivity analysis for the left and right seed DLPFC in each group were shown in Table 1 and Figure 2 for the two groups separately. Specific regions included the frontal lobe (BA 9), parietal lobe (BA 7), and limbic lobe especially near the posterior anterior cingulate cortex (ACC). Additionally, connectivity in both left and right DLPFCs with the temporal lobe was also shown in Figure 2.
Table 1.
Significant clusters of CCN by both left and right DLPFC positive correlation
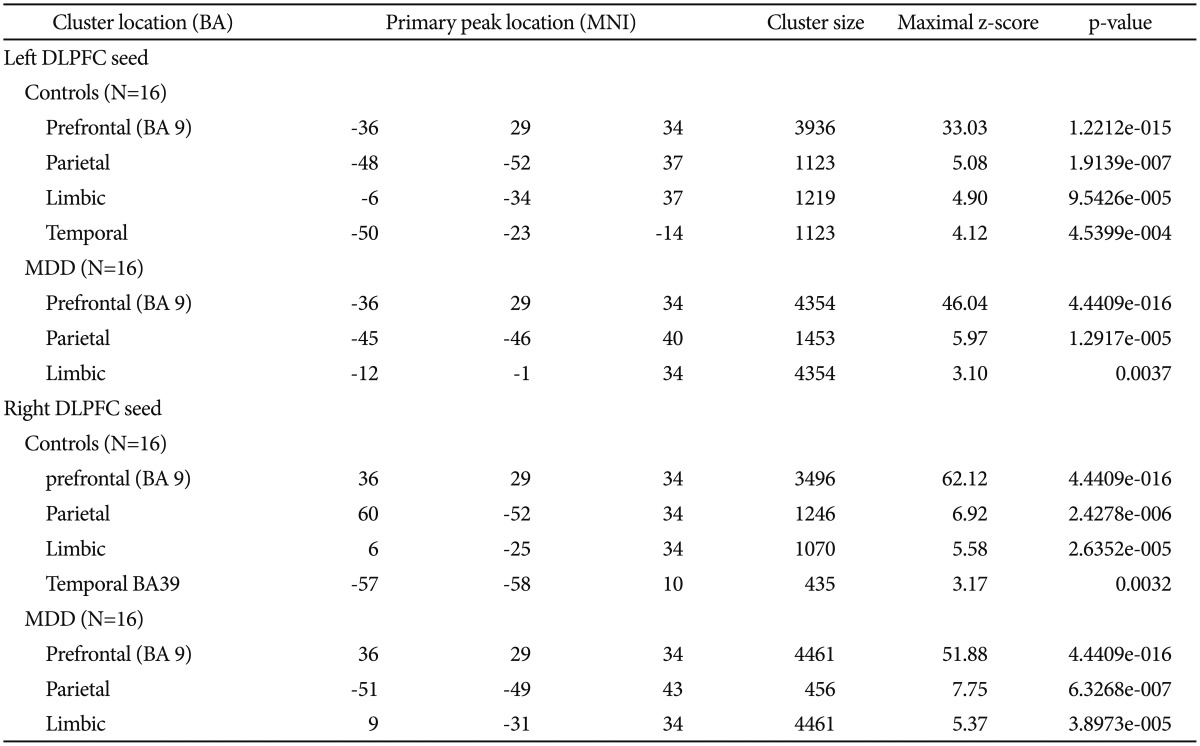
Significance criteria of p<0.001, random effects analysis. Listed primary peak location is Montreal Neurological Institute spatial array coordinate system (MNI), cluster size (mm3). BA: Broadmann's Area, DLPFC: dorsolateral prefrontal cortex, MDD: major depressive disorder, CCN: cognition connectivity network
Figure 2.
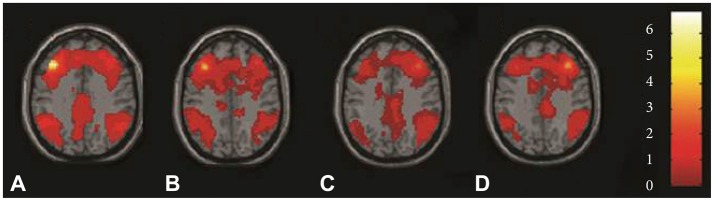
Functional connectivity maps of individual groups by using Z axial views for whole brain. A controls and B major depressive disorder (MDD) subjects using seed left dorsolateral prefrontal cortex. C controls and D MDD subjects using seed right dorsolateral prefrontal cortex. All maps using the Montreal Neurological Institute spatial array coordinate system (MNI).
Differences in CCN between the two groups
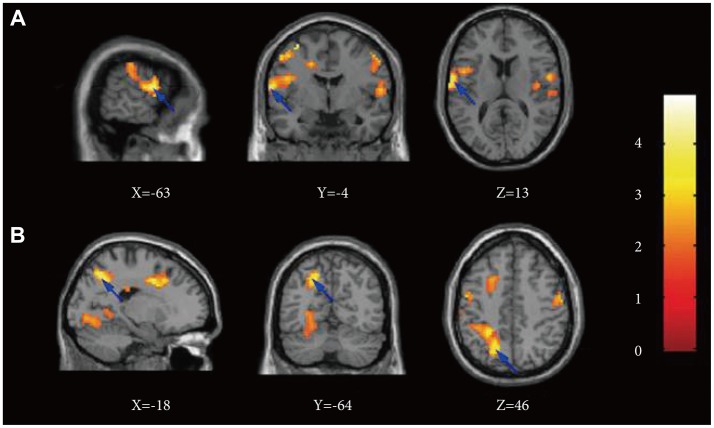
The MDD group exhibited increased FC in the left DLPFC in one cluster, overlapping the middle frontal gyrus, BA7, BA43, precuneus, BA6, BA40, superior temporal gyrus, BA22, inferior parietal lobule, precentral gyrus, BA4 and cingulate gyrus in the left cerebrum (Table 2, Figure 3). Health controls did not show any cluster with significantly greater FC compared to depressed subjects in left DLPFC network. There was not significantly different of FC in right DLPFC network between the two groups (Table 2).
Table 2.
Significant clusters by comparing FC of left and right DLPFC in MDD subjects versus controls
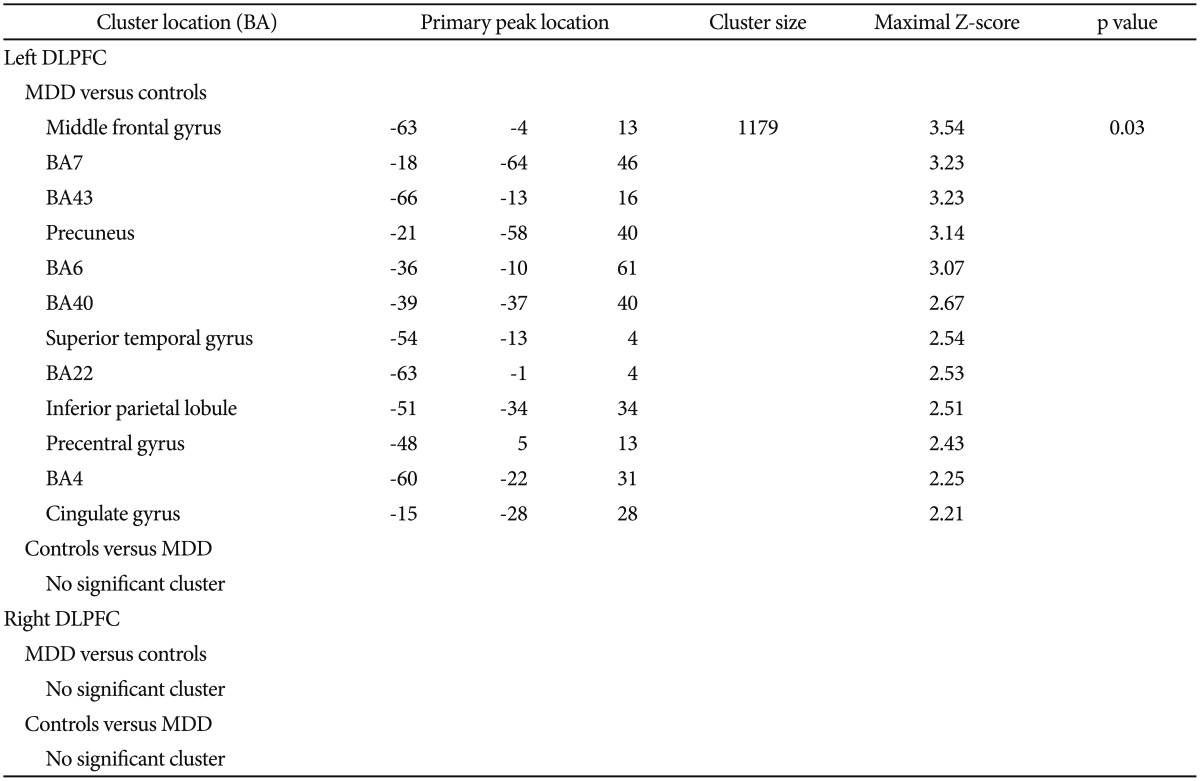
Listed primary peak location is Montreal Neurological Institute spatial array coordinate system (MNI), cluster size (mm3), and significance level for each region. Height and extent thresholds of p<0.05 were used to determine significant clusters between groups. FCs: functional connectivity, MDD: major depressive disorder, BA: Broadmann's Area, DLPFC: dorsolateral prefrontal cortex
Figure 3.
Comparison of functional connectivity maps for depressed and control subjects across the network of the left dorsolateral prefrontal cortex. The color bar indicates that images were thresholded at p<0.05. Major depressive disorder subjects>controls, between-group comparison with X, Y, and Z coordinates shown at one cluster including two primary voxels in frontal (line A) and parietal lobe (line B). All maps using the Montreal Neurological Institute spatial array coordinate system (MNI). X: sagittal view, Y: coronal view, Z: axial view.
Association between CCN and clinical characteristics
No significant correlation was found between the functional connectivity of CNN and symptom severity assessed by the HAMD in MDD group (p>0.05).
DISCUSSION
There are two main findings from this study. First, the cognition connectivity network (CCN) was constructed separately by both left and right DLPFC seed-region analysis in each group. It included the frontal lobe (BA 9), parietal lobe (BA 7), and limbic lobe especially near posterior ACC in both groups. The result of CCN was consistent with prior literatures on resting state fMRI.16,22 All the brain areas were involved in attention, episodic memory, and execution of cognition.1,7,23 Second, depressed subjects exhibited significantly increased FCs in the gray matter via the left seed DLPFC including middle frontal gyrus, BA7, BA43, precuneus, BA6, BA40, superior temporal gyrus, BA22, inferior parietal lobule, precentral gyrus, BA4 and cingulate gyrus in the left cerebrum. However, health controls did not show any cluster with significantly greater FC compared to depressed subjects in left DLPFC network. No significant difference was found in FC in the right DLPFC network between the MDD and non-MDD groups.
To the best of authors' knowledge, three has been few studies that focused on functional connectivity network of cognition control in MDD. Differences in methodology (e.g., analytical methods and sampling approaches), duration of illnesses, and medication status precluded direct comparison of findings from the current study with previous studies. Findings from the current study are in line with a previous study, which found increased functional connectivity in the left DLPFC and the cerebellum in MDD patients compared to healthy controls using task fMRI.24 Findings from the current study contradict with that from a previous study where a decreased resting FC within the CCN was found in the MDD group compared to the control group. Moreover, this previous study found that lower resting FC within the CCN predicted low remission and persistence of depressive symptoms, apathy, and dysexecutive behavior after treatment with escitalopram.25 These discrepancies in results may be attributed to differences in study samples between the current study and the previous study. In specific, the sample of the previous study consisted of elderly adults who were 60 years of age or older. In contrast, participants of the current study were individuals who had their first episodes of MDD and were 45 years of age or younger. Thus, it is arguable that the current study is less biased by the persistence of MDD, for which the underlying mechanism can be quite different from the occurrence of MDD. In the early stage, the increasing of functional connectivity might contribute to the underlying mechanism for developing MDD.
DLPFC is an essential brain area for attention, episode memory and executive functioning.9,10,11 Previous studies suggest that the left cerebral hemisphere is dominant for speech perception during verbal tasks, and the right hemisphere was dominant during visuospatial tasks.21,26 These findings were consistent with a functional trans-cranial Doppler study (fTCD), in which a stable flow was evident in the dominant hemisphere in successive cognitive tasks.27 In this study, increased FCs were found in the network from left DLPFC during verbal tasks. It provides supporting evidence for the role of left cerebrum in cognitive deficits related with speech perception.
The results of frontal gyrus in this study are consistent with one previous study, which found increased FC in the dorsal medial prefrontal cortex and junction to the DLPFC.16 In this study, both middle frontal gyrus and BA6 show significantly greater FCs within the left DLPFC. This large area of the frontal cortex is believed to play a role in the planning of complex and coordinated movements. Since the cognition network behaved especially in the precentral frontal cortex within the default mode network during resting-state,16 the present findings implicate that the precentral frontal cortex keeps on increasing FCs of the CCN in MDD.
In this study, the findings of increased FC in the parietal cortex are in line with one previous study, in which increased FCs were located in the DLPFC and the inferior parietal lobes in the task-positive networks (TPN).15 The inferior parietal lobule has been found to play a role in language, mathematical operations, body image, and interpretation of sensory information.28,29 In this study, increased FC overlapped with postcentral parietal cortex including BA7, BA40 and precuneus. BA7 has been shown to play a role in visuo-spatial coordination.30 It serves as a convergence point between vision and proprioception to determine the location of objects in relation to parts of the body. BA40 is involved in reading.31 Precuneus plays a major role in integrated metal processes, including visuo-spatial imagery, episodic memory and self-operation.32 Some studies had found that postcentral gyrus of parietal cortex showed decreased volume in voxel33,34 and decreased serotonin 1A receptor binding among individuals with depression.35 The structure deficit has been hypothesized as a marker of cognition dysfuntion.33,34 The change of serotonin 1A receptor implicated the histopathological pattern involved in depressed mood, which might also predict the treatment effectiveness related to cognition dysfunction on depression.35 Additionally, one earlier study showed postcentral parietal cortex was a brain area with increasing FCs in late-life depression.36 Thus, the altered FCs on postcentral parietal cortex found in this study may serve as a marker for cognitive deficits in MDD.
This study also found some novel brain areas with increased FCs via left DLPFC in depression. The BA 43 is a gustatory cortical area. The superior temporal gyrus serves as the primary auditory cortex. The BA22 is involved in language processing. The BA 4 is the primary motor cortex of the human brain. The cingulate cortex is involved in emotions, learning, and memory; it is also important for executive functioning and respiratory control.37 Increased FCs in these brain areas suggests that these areas are involved in integrated cognitive functioning, and are associated with cognitive deficits in MDD.
No significant correlation was found between FCs of CCN and clinical severity assessed by HAMD. The CCN is specifically used to evaluate the patterns of functional connectivity network which is constructed of brain areas responsible for the cognitive function.16 The HAMD reflects primarily the severity of depression or related clinical factors rather than the cognitive function. However, cognitive function is also associated with depressive symptoms, the negative result from current regression analysis may not be an evidence as the association of the altered connectivity of cognitive deficit in MDD.
Findings of the current study should be interpreted in light of the following limitations. First, longer TR (3000 ms) was used to measure spontaneous activities of the whole brain. The results may have been biased by heat beating. Future studies using shorter TR are needed to address this limitation and focus on cognition-mood brain areas if there are no hardware limitations such as limbic lobe near to ACC.15,16,36 Second, sample size is small and only out-patients were included in the study. Thus, results may not apply to individuals with MDD who are undergoing inpatient treatment. Counterbalancing strength is that all patients were first episode patients, and were naïve to any psychotropic medication. This sample of individuals is arguably superior to past-onset individuals who had a history of antidepressant treatment which changes FCs in the brain and thus masks intrinsic characteristics related to depression.11 Third, what we explored were only the patterns of CCN among the special patient sample by the similar approaches used in the Sheline et al's work.16 Using the seed correlation analysis, the presumed cognition functional connectivity might be assessed.16 It is just a primary study that focuses on the brain regions involved in the disease at global brain level. However, future study needs to measure the clinical characteristics including the intelligence quotient among larger sample of MDD. By further exploring the relationship between the CCN and clinical cognitive characteristics while regressing out all kinds of bias factors, the potential mechanisms of cognitive symptoms in MDD may be determined by the neuroimaging indices.
Despite limitations like these, the current study observed alterations in CCN of the left DLPFC during early stages of MDD. These brain areas might be involved in the cognitive dysfunction in MDD. Future longitudinal studies of both first-episode individuals and recurrent individuals are needed to provide evidence underlying the mechanisms of the cognitive symptoms about whether the increased FCs in the left DLPFC is a state or a trait.
Acknowledgments
The authors express appreciation to Hui Chen for the English language assistance.
References
- 1.Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: a review. Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- 2.Hammar A, Lund A, Hugdahl K. Long-lasting cognitive impairment in unipolar major depression: a 6-month follow-up study. Psychiatry Res. 2003;118:189–196. doi: 10.1016/s0165-1781(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30:515–527. doi: 10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- 4.Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, et al. Evidence for continuing neuropsychological impairments in depression. J Affect Disord. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. J Nerv Ment Dis. 2010;198:513–515. doi: 10.1097/NMD.0b013e3181e4c5ba. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 10.Boggio PS, Bermpohl F, Vergara AO, Muniz AL, Nahas FH, Leme PB, et al. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. J Affect Disord. 2007;101:91–98. doi: 10.1016/j.jad.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 14.Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol. 2000;21:1397–1401. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C. 2nd Edition. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- 22.Hunter MD, Lee KH, Tandon P, Parks RW, Wilkinson ID, Woodruff PW. Lateral response dynamics and hemispheric dominance for speech perception. Neuroreport. 2007;18:1295–1299. doi: 10.1097/WNR.0b013e32827420e4. [DOI] [PubMed] [Google Scholar]
- 23.Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim DW, Min BC, Kim HJ, Choi MH, Lee SJ, Jun JH, et al. Cerebral lateralization index based on intensity of bold signal of FMRI. Int J Neurosci. 2008;118:1628–1642. doi: 10.1080/00207450802330777. [DOI] [PubMed] [Google Scholar]
- 27.Vadikolias KM, Artemis ND, Mitsias PD, Heliopoulos JN, Tripsianis GA, Vadikolia CM, et al. Evaluation of the stability of blood flow over time in the dominant hemisphere: a functional transcranial Doppler study. J Cereb Blood Flow Metab. 2007;27:1870–1877. doi: 10.1038/sj.jcbfm.9600484. [DOI] [PubMed] [Google Scholar]
- 28.Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, et al. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radua J, Phillips ML, Russell T, Lawrence N, Marshall N, Kalidindi S, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49:939–946. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Coull JT, Walsh V, Frith CD, Nobre AC. Distinct neural substrates for visual search amongst spatial versus temporal distractors. Brain Res Cogn Brain Res. 2003;17:368–379. doi: 10.1016/s0926-6410(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 31.Stoeckel C, Gough PM, Watkins KE, Devlin JT. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45:1091–1096. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 33.Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Vasic N, Walter H, Hose A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109:107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 36.Kenny ER, O'Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, et al. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am J Geriatr Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- 37.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]