Abstract
CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (NCT01243424) is an ongoing, randomized trial in subjects with early type 2 diabetes and increased cardiovascular risk or established complications that will determine the long-term cardiovascular impact of linagliptin versus the sulphonylurea glimepiride. Eligible patients were sulphonylurea-naïve with HbA1c 6.5%–8.5% or previously exposed to sulphonylurea (in monotherapy or in a combination regimen <5 years) with HbA1c 6.5%–7.5%. Primary outcome is time to first occurrence of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for unstable angina. A total of 631 patients with primary outcome events will be required to provide 91% power to demonstrate non-inferiority in cardiovascular safety by comparing the upper limit of the two-sided 95% confidence interval as being below 1.3 for a given hazard ratio. Hierarchical testing for superiority will follow, and the trial has 80% power to demonstrate a 20% relative cardiovascular risk reduction. A total of 6041 patients were treated with median type 2 diabetes duration 6.2 years, 40.0% female, mean HbA1c 7.2%, 66% on 1 and 24% on 2 glucose-lowering agents and 34.5% had previous cardiovascular complications. The results of CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes may influence the decision-making process for selecting a second glucose-lowering agent after metformin in type 2 diabetes.
Keywords: Type 2 diabetes, cardiovascular complications, macrovascular, dipeptidyl-peptidase-4 inhibitor, sulphonylurea
Introduction
It remains unknown how specific agents compare with respect to long-term cardiovascular (CV) effect as only few,1 long-term head-to-head trials have compared the effects of different diabetes drugs on CV outcomes or CV surrogates, and most have been of relatively short duration with insufficient statistical power. Furthermore, although, since 2007, new glucose-lowering agents are required by the Food and Drug Administration (FDA) to demonstrate CV safety before or after regulatory approval,2 most of these CV outcome trials are conducted in a placebo-controlled setting with no active comparators. Hence, they do not allow an assessment of comparative effectiveness. Particularly debated in this respect are sulphonylureas (SUs) – frequently recommended as the preferred second-line therapy – where some studies, first suggested by the highly controversial University Group Diabetes Program (UGDP) conducted in the 1960s,3 led to uncertainty about their long-term CV safety, an effect perhaps related to binding on the potassium adenosine triphosphate (KATP) channel in cardiac myocytes and other cells,4 but the results of long-term randomized controlled trials (RCTs) have not supported a deleterious CV effect of SUs.5,6
Dipeptidyl-peptidase-4 (DPP-4) inhibitors are oral glucose-lowering agents for second-line therapy when glycaemic control cannot be achieved with metformin or as first-line therapy where metformin is contraindicated or not tolerated.7 DPP-4 inhibitors are associated with the benefits of significantly lowering blood glucose level without the side effects of hypoglycaemia or weight gain, two adverse effects that occur with SU.7,8 Linagliptin is a once-daily, DPP-4 inhibitor with a xanthine-based structure that is characterized by a pharmacological profile distinct from other drugs in this class,8 largely due to its non-renal route of elimination (80% hepatic vs 5% renal).9 Of interest, a 2-year randomized, double-blind phase III trial comparing linagliptin versus glimepiride in 1519 patients with type 2 diabetes (T2D) (HbA1c 6.5%–10.0%) on metformin background therapy – designed to assess the effects on glucose control – suggested a reduced relative CV risk with linagliptin [0.46; 95% confidence interval (CI) 0.23–0.91] for the composite CV outcome of CV death, non-fatal myocardial infarction (MI), non-fatal stroke or hospitalization for unstable angina [4-point major adverse CV events (4P-MACE)].10 Even though all CV events were prospectively adjudicated, the study was a registration trial neither designed nor powered to assess CV outcomes, and the overall number of CV events was low (n = 38). Nevertheless, these findings are suggestive and provide the basis for the hypothesis of a potential CV benefit of linagliptin over glimepiride, which is currently being tested in the ongoing CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®).
Methods
CAROLINA (clinicaltrials.gov identifier: NCT01243424) is an ongoing, multicentre, randomized, double-blind, active-controlled trial. It is designed to assess the effect of linagliptin compared with glimepiride, in addition to standard of care, on CV events in adults with relatively early T2D at increased CV risk or established CV complications and with less than optimized glycaemic control. The study protocol was approved by the respective Institutional Review Boards, Independent Ethics Committees and Competent Authorities according to national and international regulations.
Trial population
Patients with T2D who were naïve to therapy or on a non-insulin secretagogue (SU or glinide) with HbA1c ⩾ 6.5% and ⩽8.5%, or currently being treated with an insulin secretagogue (in monotherapy or in a dual combination regimen < 5 years) with HbA1c ⩾ 6.5% and ⩽7.5%, and at elevated risk of CV events according to specific criteria (Table 1) were eligible for inclusion. Upon randomization, if a patient was on a SU or glinide, this secretagogue therapy was discontinued and replaced with study medication. For patients not on a secretagogue, study medication was added to the existing regimen. At screening, any glucose-lowering background therapy should have been stable for at least 8 weeks. No patients requiring insulin therapy for glucose control were allowed in the trial. Previous exposure to DPP-4 inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists or thiazolidindiones (TZDs) was exclusionary. Subjects were required at baseline to have a body mass index (BMI) ⩽ 45 kg/m2 and age 40–85 years. Detailed inclusion and exclusion criteria are listed in online Appendix 1.
Table 1.
Key inclusion criteria in CAROLINA.
| Insufficient glycaemic control | Elevated risk of CV events defined as any 1 (or more) of the criteria (a, b, c or d) |
|---|---|
|
|
CAROLINA: CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes; IC: informed consent; T2D: type 2 diabetes; BP: blood pressure; SU: sulphonylurea; MI: myocardial infarction; MDRD: modified diet in renal disease; eGFR: estimated glomerular filtration rate; CV: cardiovascular.
Study design and follow-up
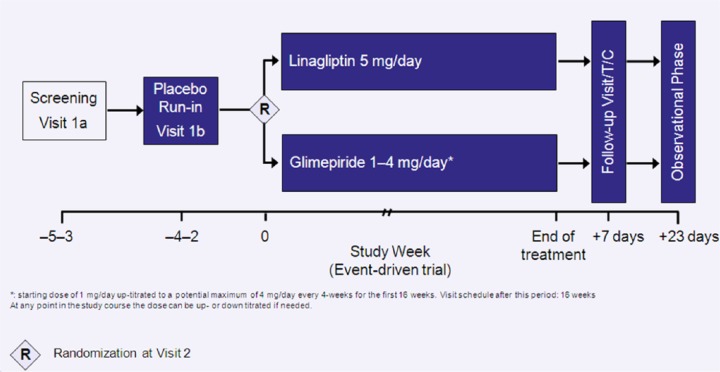
Eligible subjects underwent a 2- to 4-week, open-label, placebo run-in period (Figure 1) during which background glucose-lowering therapy was continued unchanged. Following the run-in, patients still meeting the inclusion or exclusion were randomly assigned 1:1 to receive linagliptin 5 mg, or glimepiride 1–4 mg, once daily in addition to their background therapy. After a starting dose of 1 mg/day, glimepiride was up-titrated at 4-week intervals during the first 16 weeks to a potential maximum dose of 4 mg/day. The dose of glimepiride was increased if the fasting self-monitored blood glucose (SMBG) values were >110 mg/dL (6.1 mmol/L), unless the investigator considered that it would place the patient at an increased risk of hypoglycaemia. The average of previous recent fasting SMBG measurements (from the patient’s diary) prior to the day of visit could also be used to guide up-titration at the discretion of the investigator. Of note, patients on previous glimepiride treatment were randomized to linagliptin or to continue on their current dose (i.e. if the glimepiride dose was ⩾4 mg/day, the masked starting dose would be 4 mg/day).
Figure 1.
CAROLINA study design.
If applicable, patients are to continue their metformin therapy (preferably >1500 mg daily) and other background therapy throughout the trial with an unchanged dose unless for medical emergencies or other patient safety reasons. To ensure an adequate level of glycaemic control for participants, investigators could institute glycaemic rescue medication provided specific protocol criteria were met (details in online Appendix 2). Investigators were also encouraged to treat all other CV risk factors [lipids, blood pressure (BP), albuminuria, unhealthy lifestyle and smoking) in the context of local or regional guidance for primary or secondary CV prevention. Changes to medication were ultimately left to the investigator’s clinical judgement.
Patients are instructed to attend the clinic at pre-specified times (e.g. every 16th week in the maintenance phase) over the duration of the study, including patients who prematurely discontinue study drug. Irrespective of whether on study drug or not, all patients are followed to capture CV events. Attempts are consistently made to avoid missing data and prevent withdrawal of informed consent or lost to follow-up that can compromise the integrity of the study. All subjects will undergo a final visit during the close-out period of the study and are to be followed-up for adverse events (AEs) for a period of 30 days after individual study completion (Figure 1).
Randomization and patient inclusion
Once inclusion criteria were confirmed, randomization was undertaken using a computer-generated random sequence and an interactive voice and web response system, without any stratification, as is recommended for large trials.11
Outcomes and adjudication
The primary outcome of the study is the time to first occurrence of CV death, non-fatal MI (excluding silent MI), non-fatal stroke or hospitalization for unstable angina, that is, 4P-MACE.
Three key secondary outcomes were defined: (1) time to the first occurrence of any of the classical 3P-MACE components (i.e. 4P-MACE excluding occurrence of hospitalization for unstable angina), (2) proportion of patients on-treatment and maintaining HbA1c ⩽ 7.0% at final visit without the need for rescue medication, without any moderate or severe hypoglycaemic episodes and without >2% weight gain between end of titration and final visit and (3) proportion of patients on-treatment and maintaining HbA1c ⩽ 7.0% at final visit without the need for rescue medication and without >2% weight gain between end of titration and final visit.
Further secondary CV outcomes include the occurrence and time to first event of a composite outcome of all independently confirmed adjudicated events (individually outlined below as tertiary CV outcomes but excluding silent MI), whereas tertiary CV outcomes are the occurrence of, and time to, each of the following adjudicated events: CV death (including fatal stroke and fatal MI), non-fatal MI, non-fatal stroke, hospitalization for unstable angina, transient ischaemic attack (TIA), hospitalization for congestive heart failure (CHF), composite of hospitalization for or death from CHF, hospitalization for coronary revascularization procedures and silent MI. All-cause mortality is a tertiary outcome.
All CV outcome events and deaths are being prospectively adjudicated by a Clinical Events Committee, as recommended in FDA guidelines.2 The study also has defined several secondary diabetes-related outcomes [e.g. change from baseline to final visit in HbA1c, urine albumin-to-creatinine ratio (UACR) or transition in albuminuria categories], several tertiary diabetes-related outcomes as well as other outcomes. In addition, a number of analyses from dedicated sub-studies involving a subset of the study cohort (Table 2) are defined. Definitions of the major clinical outcomes are presented in online Appendix 3, and a broader list of efficacy and safety outcomes is presented in online Appendix 4.
Table 2.
Outline of CAROLINA sub-studies.
| Rationale for sub-studies | |
|---|---|
| Cognition sub-study | Cognitive dysfunction is increased in T2DM |
| Effects of glucose-lowering therapies on cognitive decline remain unknown | |
| DPP-4 inhibition has a theoretical basis for potential benefits12 | |
| Glycaemic variability sub-study | Improving glucose diurnal patterns may have an impact on vascular complications and β-cell dysfunction |
| DPP-4 inhibitors may mimic normal glucose diurnal patterns to a greater degree than SUs and may have salutary effects on these outcomes13 | |
| β-cell function sub-study | The inevitably progressive decline in β-cell function in T2D is a major challenge to its effective management |
| Long-term β-cell function studies in T2D with different therapies are required14 | |
| Latent autoimmune diabetes in adults (LADA) sub-study | There are currently no gold standard treatments for LADA |
| Role of SUs in the natural disease progression of LADA are debated | |
| Linagliptin prevented accelerated C-peptide decline in LADA in an exploratory clinical study15 |
CAROLINA: CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes; T2DM: type 2 diabetes; DPP-4: dipeptidyl-peptidase-4; SU: sulphonylurea.
Safety will be assessed based on AEs reported throughout the study, clinical laboratory tests, vital signs, 12-lead electrocardiogram, physical examination and the use of rescue medication. Pre-specified AEs of special interest include hypersensitivity reactions, skin lesions, renal AEs, pancreatitis, pancreatic cancer and hepatic events. Pancreatitis or pancreatic cancers are being adjudicated by a group of independent external experts. For qualifying events, relevant source documentation will be requested including laboratory values, histological analysis, results of imaging tests, hospital discharge letters and medical reports from other physicians. All evaluations will be performed in a blinded fashion.
Study oversight and organization
The trial was jointly designed by employees of Boehringer Ingelheim (BI) and the academic investigators who are members of the steering committee. The steering committee, which is led by the academic investigators and included members who are employees of the sponsor, supervises the trial design and operation. An independent data monitoring committee (DMC) reviews interim safety data approximately every 90 days or on an ad hoc basis on request. The DMC will also perform two interim analyses (IAs) as discussed below. The data will be analysed by the sponsor and also independently analysed and validated by independent biostatisticians at L-Biostat at KU Leuven Research and Development, Belgium. Interpretation and reporting of the data and the decisions for publications will be conducted by the steering committee. A list of committees involved in the trial conduct is presented in online Appendix 5.
Statistical considerations
Sample size and power calculations
The primary hypothesis is that linagliptin is not inferior to glimepiride for the incidence of 4P-MACE. This is by means of comparing the upper limit of the two-sided repeated 95% CI at a non-inferiority margin of 1.3, which is mandated by the FDA for CV trials evaluating new therapies for T2D.2 This test is the first in a five-step hierarchical testing strategy, where a subsequent test is only performed in case of a significant prior result: (1) non-inferiority test of the primary outcome (4P-MACE), (2) superiority test of the primary outcome (4P-MACE), (3) superiority test of key secondary CV outcome (3P-MACE), (4) superiority test of the first key secondary efficacy outcome (HbA1c ⩽ 7.0% without rescue medication, moderate or severe hypoglycaemic episodes and >2% weight gain at final visit) and (5) superiority test of the second key secondary efficacy outcome (HbA1c ⩽ 7.0% without rescue medication and without >2% weight gain at final visit).
N = 631 confirmed primary outcome events provides 91% power to show CV non-inferiority, if the underlying hazard ratio (HR) for the incidence of the primary outcome between the linagliptin and glimepiride groups is 1.0 for the specified one-sided alpha of 2.5%. If 1-year event rates are 2% in each treatment group, accrual time is 2 years, follow-up time is 4.8 years and the 1-year loss to follow-up rate is 1.5%, enrolment of 6000 patients is projected to yield the necessary number of events.
Furthermore, 631 patients with 4P-MACE provides 80% power to demonstrate superiority assuming a HR of 0.80, corresponding to a 20% risk reduction in 4P-MACE for linagliptin as compared to glimepiride.
IAs
The DMC will perform two formal IAs of the primary outcome after, respectively, 190 and 411, 4P-MACE events have occurred. At each of these IAs, the trial could be terminated if superiority for linagliptin is demonstrated with respect to 4P-MACE and overall CV safety, with particular emphasis on CV mortality. Of note, at the second formal IA, the trial may also be stopped for futility, if superiority of glimepiride with respect to the primary outcome can be shown prematurely or if a futility assessment shows that the 1.3 non-inferiority margin likely will not be met, based on a conditional power of less than 20%. The DMC is the sole group with access to unblinded results.
To prevent an inflation of the significance level, a group sequential design was chosen, where the O’Brien and Fleming α-spending function defines the allocation of the one-sided overall significance level of 2.5% to the IA and final analysis, and based on, respectively 190, 411 and 631 numbers of patients with 4P-MACE, the cumulative alpha spent is 0.000043997, 0.005469768 and 0.025.
Analysis plan
The primary analysis will be performed on the full analysis set (FAS). The FAS consist of all randomized patients who were treated with at least one dose of study drug. For the primary and secondary or tertiary CV outcomes, a classical intention-to-treat (ITT) analysis on the FAS will be done including all adjudicated and confirmed events which occur until study end. The time to (first) event will be derived from the date of randomization. Patients who do not experience an event during the trial period will be censored at their last documented study visit.
The analysis of primary and secondary or tertiary CV outcomes will be based on a Cox’s proportional hazards regression with a term for treatment assignment included in the model. The second and third composite key secondary outcomes will be analysed with a chi-square test.
For the primary outcome as well as the time to first 3P-MACE sensitivity analysis will be done based on events occurring within the time patients are on-treatment +30 days after permanent treatment discontinuation or date of last documented study visit, whichever comes first. Sensitivity analyses will be performed on the per protocol set (PPS), which consists of patients included in the FAS excluding those who have important protocol violations (i.e. if it can be expected to have a distorting influence on the assessment of the primary outcome and/or key secondary outcomes) as well as on the 30-day-treatment set, including all randomized patients with a minimum treatment duration of 30 days.
To examine the degree of consistency of the overall treatment effect, the primary and key secondary outcomes will be explored in certain subgroups, including, but not limited to, CV risk inclusion group (history of vascular disease, evidence of vascular-related end-organ, elevated age and CV risk factors), age, baseline BP, gender, prior therapy with an insulin secretagogue, background therapy of metformin, race and geographical region. These subgroup analyses are considered as being of exploratory nature, and analyses will not be adjusted for multiple comparisons. Further details on this are provided in online Appendix 6.
Results
Patient recruitment and baseline characteristics
Recruitment into CAROLINA began in December 2010 and was completed in December 2012. On 12 March 2012, the steering committee recommended to stop further recruitment of patients solely fulfilling CV risk category ‘d’ (i.e. the lowest CV risk). In total, 10,639 patients were screened, and 6051 were randomized at 606 clinical sites in 43 countries. The main reason for screen failure was an HbA1c value not meeting protocol specifications. Of those randomized, 6041 were treated with study drug. Most participants come from Europe (45.4%), 19.2% from North America, 16.7% from Asia, 15.0% from South America, 2.2% from South Africa and 1.4% from New Zealand or Australia. At baseline, the mean age of participants was 64 years with 14.0% aged ⩾75 years. Sixty percent are male, 73% are White and median T2D duration was 6.2 years (40.6% ⩽5 years). At baseline, mean HbA1c was 7.2%, with only 9.4% of participants having HbA1c ⩾ 8.0%. Mean ± standard deviation (SD) systolic BP was 136 ± 16 mmHg, diastolic BP 79 ± 10 mmHg and pulse rate 71 ± 11 bpm, indicating that the BP was reasonably well managed, with 88% of patients using any anti-hypertensive therapies. Lipids were also well controlled [total cholesterol 177 ± 44 mg/dL, low-density lipoprotein (LDL)-cholesterol 95 ± 36 mg/dL, high-density lipoprotein (HDL)-cholesterol 48 ± 13 mg/dL, median triglyceride 144 mg/dL (interquartile range: 105–198)] with 64% of the overall study cohort taking a statin (74% in those with existing CV complications). Acetylsalicylic acid was used by half of all patients and in 80% of those with existing CV complications.
At screening, 9.2% of participants were drug-naïve; 66% were receiving monotherapy (among whom 88.4% were taking metformin), and 24% were receiving dual therapy. In total, 82% of patients used metformin background medication at a mean ± SD daily baseline dose of 1.6 ± 0.6 g. CAROLINA included patients based on four CV risk categories [from low to high (Table 1)], and 34.5% of the cohort had previous CV complications (MI: 13.8%, coronary artery disease: 17.1%, stroke: 7.8% or peripheral arterial occlusive disease: 5.5%), 8.5% had evidence of vascular-related end-organ damage as defined by impaired renal function [estimated glomerular filtration rate 30–59 mL/min/1.73 m2 as defined by the Modified Diet of Renal Disease Formula (MDRD), albuminuria (urinary albumin:creatinine ⩾30 µg/mg in two out of three unrelated specimens] or proliferative retinopathy. An age ⩾70 years was the main inclusion criteria in 19.3% of subjects, and 37.3% had multiple CV risk factors (without having established CV complications). Further baseline characteristics and key laboratory data of treated participants (FAS) according to baseline CV risk is provided in Tables 3 and 4.
Table 3.
Baseline characteristics, overall and according to CV risk category in CAROLINA.
| Total (n = 6041)a | Group A: previous CV events (n = 2084, 34.5% of total) | Group B: retinopathy/albuminuria (n = 513, 8.5% of total) | Group C: age >70 years (n = 1163, 19.3% of total) | Group D: ⩾2 CV risk factors (n = 2252, 37.3% of total) | |
|---|---|---|---|---|---|
| Age, years (mean ± SD) ⩾ 75 years of age (n, %) | 64.0 ± 9.5 (14.0%) | 64.6 ± 8.9 (14.3%) | 65.6 ± 9.7 (21.2%) | 74.0 ± 3.4 (37.9%) | 58.0 ± 7.2 (0%) |
| Male (n, %) | 3622 (60.0%) | 1511 (72.5%) | 284 (55.4%) | 593 (51.0%) | 1219 (54.1%) |
| Race (n, %) | |||||
| White | 4408 (73.0%) | 1489 (71.4%) | 367 (71.5%) | 911 (78.3%) | 1634 (72.6%) |
| Asian | 1061 (17.6%) | 427 (20.5) | 85 (16.6%) | 145 (12.5%) | 402 (17.9%) |
| Black/African American | 331 (5.5%) | 101 (4.8%) | 32 (6.2%) | 40 (3.4%) | 157 (7.0%) |
| Otherb | 241 (4.0%) | 67 (3.2%) | 29 (5.7%) | 67 (5.8%) | 59 (2.6%) |
| Ethnicity (n, %) | |||||
| Hispanic or Latino | 1033 (17.1%) | 309 (14.8%) | 96 (18.7%) | 260 (22.4%) | 368 (16.3%) |
| Smoking: current/ex-smoker (%) | 19.5%/33.7% | 16.3%/46.2% | 14.4%/30.2% | 6.6%/32.8% | 30.7%/23.6% |
| Time since T2D diagnosis, years (median, IQR) | 6.2 (2.9–11.0) | 5.8 (2.7–10.4) | 6.6 (3.6–11.3) | 7.7 (4.3–12.0) | 5.8 (2.6–10.7) |
| Time since T2D diagnosis (%) | |||||
| ⩽5 years | 40.6% | 43.7% | 36.1% | 30.5% | 44.2% |
| >5–10 years | 28.2% | 29.1% | 30.4% | 30.5% | 25.9% |
| >10 years | 30.9% | 27.2% | 33.5% | 39.0% | 29.9% |
| Region (%) | |||||
| Europe | 45.4% | 46.0% | 44.4% | 48.2% | 44.2% |
| North America (including New Zealand and Australia) | 20.7% | 18.8% | 18.9% | 17.5% | 23.6% |
| South America | 15.0% | 13.5% | 17.7% | 19.3% | 13.8% |
| Africa | 2.2% | 1.8% | 2.7% | 2.8% | 2.1% |
| Asia | 16.7% | 20.0% | 16.2% | 12.1% | 16.3% |
| Glucose-lowering therapy at screening (n, %) | |||||
| None | 557 (9.2%) | 184 (8.8%) | 50 (9.7%) | 118 (10.1%) | 201 (8.9%) |
| 1 glucose lowering | 3988 (66.0%) | 1345 (64.5%) | 318 (62.0%) | 793 (68.2%) | 1530 (67.9%) |
| 2 glucose lowering | 1439 (23.8%) | 546 (26.2%) | 139 (27.1%) | 245 (21.1%) | 505 (22.4%) |
| 3 or no reliable data | 57 (0.9%) | 9 (0.4%) | 6 (1.2%) | 7 (0.6%) | 16 (0.7%) |
| Any metformin | 4982 (82.5%) | 1721 (82.6%) | 391 (76.2%) | 927 (79.7%) | 1937 (86.0%) |
| Any SU | 1728 (28.6%) | 651 (31.2%) | 193 (37.6%) | 315 (27.1%) | 565 (25.1%) |
| Any glinide | 65 (1.1%) | 22 (1.1%) | 8 (1.6%) | 16 (1.4%) | 19 (0.8%) |
| Any α-GI inhibitor | 188 (3.1%) | 63 (3.0%) | 19 (3.7%) | 40 (3.4%) | 66 (2.9%) |
| Monotherapy (n, % of monotherapy) | 3988 (100.0%) | 1345 (100.0%) | 318 (100.0%) | 793 (100.0%) | 1530 (100.0%) |
| Metformin (% of monotherapy) | 3526 (88.4%) | 1171 (87.1%) | 249 (78.3%) | 681 (85.9%) | 1423 (93.0%) |
| SU (% of monotherapy) | 391 (9.8%) | 148 (11.0%) | 61 (19.2%) | 92 (11.6%) | 90 (5.9%) |
| Dual therapy (n, % of dual therapy) | 1439 (100.0%) | 546 (100%) | 139 (100%) | 245 (100%) | 505 (100%) |
| Metformin + SU (% of dual therapy) | 1280 (89.0%) | 489 (89.6%) | 124 (89.2%) | 211 (86.1%) | 452 (89.5%) |
| Metformin + glinide | 44 (3.1%) | 16 (2.9%) | 2 (1.4%) | 12 (4.9%) | 14 (2.8%) |
| Metformin + α-GI inhibitor | 82 (5.7%) | 31 (5.7%) | 8 (5.8%) | 12 (4.9%) | 31 (6.1%) |
| Other therapies (%) | |||||
| Acetylsalicylic acid | 50.1% | 79.8% | 39.4% | 40.0% | 30.8% |
| Statins | 64.1% | 74.0% | 51.9% | 53.4% | 63.9% |
| Fibrates | 5.9% | 6.0% | 6.8% | 4.4% | 6.5% |
| Any anti-hypertensive therapy (%) | 87.7% | 92.9% | 88.1% | 81.9% | 86.4% |
| Blockers of the renin–angiotensin system (ACEi/ARBs) | 44.1%/31.1% | 48.0%/29.1% | 45.0%/36.3% | 38.7%/31.0% | 43.4%/32.1% |
| Beta-blockers | 38.8% | 61.8% | 30.0% | 27.1% | 26.0% |
| Calcium channel blockers | 29.3% | 32.3% | 33.9% | 29.5% | 25.7% |
CV: cardiovascular; CAROLINA: CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes; T2DM: type 2 diabetes; DPP-4: dipeptidyl-peptidase-4; SU: sulphonylurea; GI: glucosidase inhibitor; IQR: interquartile range; SD: standard deviation; ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blocker.
A few patients had no reliable CV risk categorization.
American Indian/Native, Alaskan/Native, Hawaiian/Pacific Islander.
Table 4.
Key laboratory data, overall and according to CV risk category in CAROLINA.
| Total (n = 6041)a | Group A: previous CV events (n = 2084) | Group B: retinopathy/albuminuria (n = 513) | Group C: age >70 years (n = 1163) | Group D: ⩾2 CV risk factors (n = 2252) | |
|---|---|---|---|---|---|
| HbA1c, % (mean ± SD) | 7.2 ± 0.6 | 7.2 ± 0.6 | 7.1 ± 0.6 | 7.1 ± 0.5 | 7.2 ± 0.6 |
| HbA1c ⩾ 8.0% (%) | 9.4% | 9.3% | 9.7% | 6.8% | 10.7% |
| Fasting plasma glucose, mg/dL (mean ± SD) | 140 ± 31 | 140 ± 31 | 139 ± 32 | 138 ± 29 | 141 ± 30 |
| BMI, kg/m2 (mean ± SD); ⩾35 kg/m2 (%) | 30.1 ± 5.1; 17.1% | 29.7 ± 5.0; 14.9% | 30.1 ± 5.3; 17.7% | 28.8 ± 4.6; 10.6% | 31.1 ± 5.3; 22.5% |
| Weight, kg (mean ± SD) | 84.0 ± 18.0 | 84.1 ± 17.7 | 82.4 ± 17.7 | 78.2 ± 15.8 | 87.2 ± 18.6 |
| Waist circumference, cm (mean ± SD) | 103 ± 13 | 103 ± 13 | 103 ± 14 | 101 ± 12 | 104 ± 13 |
| Systolic/diastolic BP, mmHg (mean ± SD) | 136 ± 16/79 ± 10 | 136 ± 17/78 ± 10 | 138 ± 18/79 ± 10 | 138 ± 16/77 ± 10 | 135 ± 15/81 ± 9 |
| Heart rate (mean ± SD) | 71 ± 11 | 69 ± 11 | 72 ± 11 | 70 ± 10 | 73 ± 10 |
| Lipids, mg/dL (mean ± SD) | |||||
| Total cholesterol | 177 ± 44 | 169 ± 43 | 182 ± 48 | 180 ± 40 | 181 ± 45 |
| LDL-cholesterol | 95 ± 36 | 90 ± 36 | 100 ± 38 | 98 ± 34 | 98 ± 35 |
| HDL-cholesterol | 48 ± 13 | 46 ± 12 | 49 ± 13 | 52 ± 14 | 48 ± 13 |
| Triglycerides (median, IQR) | 144 (105, 198) | 145 (105, 199) | 145 (109, 199) | 130 (99, 178) | 147 (109, 206) |
| eGFR according to MDRD, mL/min/1.73m2 (mean ± SD) | 77 ± 20 | 75 ± 19 | 63 ± 23 | 73 ± 17 | 84 ± 18 |
| eGFR according to MDRD (%) | |||||
| ⩾90 mL/min/1.73m2 | 23.4% | 19.9% | 13.8% | 15.0% | 33.3% |
| 60–<90 mL/min/1.73m2 | 57.5% | 58.3% | 30.8% | 64.7% | 59.6% |
| 30–<60 mL/min/1.73m2 | 18.2% | 21.4% | 52.8% | 19.7% | 6.8% |
| UACR, µg/mg; median (Q1, Q3) | 9.7 (5.3–30.9) | 10.6 (5.3–38.0) | 24.8 (7.1–108.3) | 9.7 (5.3–30.9) | 8.0 (4.4–20.3) |
| UACR (%) | |||||
| ⩽30 µg/mg | 74.0% | 71.0% | 53.0% | 74.2% | 82.1% |
| >30–300 µg/mg | 21.2% | 23.8% | 32.6% | 22.2% | 15.9% |
| >300 µg/mg | 4.3% | 5.1% | 14.2% | 3.5% | 1.8% |
IC: informed consent; T2D: type 2 diabetes; BP: blood pressure; SU: sulphonylurea; MI: myocardial infarction; MDRD: modified diet in renal disease; eGFR: estimated glomerular filtration rate; CV: cardiovascular; IQR: interquartile range; SD: standard deviation; UACR: urine albumin-to-creatinine ratio; LDL: low-density lipoprotein; HDL: high-density lipoprotein; BMI: body mass index.
n = 6019 for HbA1c, n = 6007 for fasting plasma glucose, n = 6016 for BMI, n = 6020 for weight and vital signs, n = 6015 for waist circumference, n = 6017 for eGFR, n = 6014 for UACR.
A few patients had no reliable CV risk categorization.
Discussion
The CAROLINA is an ongoing RCT designed to assess whether linagliptin 5 mg once daily is non-inferior, and if so, superior compared with glimepiride 1–4 mg once daily with respect to CV events in adults with relatively early T2D at increased risk of CV events and with less than optimized glycaemic control. Given that medications of both classes are currently advocated as second-line therapy after metformin,7 and since SUs have been associated with concerns regarding their CV safety2 while DPP-4 inhibitors have been suggested to exhibit CV benefits in preclinical and mechanistic trials,16 this study will provide answers to several clinically relevant questions.
The CAROLINA study having selected a cohort with early T2D (median duration 6.2 years) and a mean baseline HbA1c of 7.2% has the potential to adequately answer the study question as two-thirds were on a monotherapy regimen predominantly with metformin and only 34.5% of subjects have established CV complication at study entry with just 13.8% having a previous MI. Recently, the first two placebo-controlled CV outcome trials (SAVOR-TIMI 5317 and EXAMINE18), involving the DPP-4 inhibitors saxagliptin and alogliptin, respectively, reported a neutral effect on a composite of 3P-MACE. Of note, both of these studies were relatively short in duration and drug exposure (median follow-up, respectively, 2.1 and 1.5 years) and included patients predominantly, or exclusively, with manifest CV complications. Whether a longer follow-up would have led to a different result is not known; further an hypothesis generating findings supporting the concept of early intervention was seen in a subgroup analysis of the EXAMINE trial,18 suggesting that patients with a shorter duration of T2D seemed to have benefited from alogliptin therapy as compared to those with a longer duration.
An unexpected finding in the SAVOR-TIMI 53 trial was a statistically significant increased risk for hospitalization for CHF, associated with saxagliptin therapy, and a recent analysis of data in EXAMINE for alogliptin suggesting an increase in risk which was, however, not statistically significant.19 CAROLINA will also address through independent adjudication whether CHF hospitalizations are increased with linagliptin or glimepiride and whether death due to CHF occurs more frequently. This study will provide the greatest possible insight into whether DPP-4 inhibitors hold advantages over SUs in terms of CV outcomes, and despite other ongoing comparative effectiveness studies, like GRADE,20 CAROLINA is the only active-controlled comparator trial sufficiently powered to address CV outcomes. One interesting aspect relates to the potential that hypoglycaemia, in particular severe hypoglycaemia, may modulate and increase CV risk.21 In a former study, it was demonstrated that hypoglycaemia occurred at lower incidence with linagliptin as compared to glimepiride.10 However, the relative deleterious contribution of hypoglycaemia on MACE remains to be fully elucidated, and CAROLINA will also be able to address this.
Of note, in a recent pooled CV meta-analysis analysing the comparative impact on CV events of SUs versus other agents, an overall neutral effect on CV events versus total comparators was reported [odds ratio (OR) 1.08 (95% CI: 0.86–1.36), p = 0.52].22 However, when compared with individual compounds (i.e. metformin, GLP-1 receptor analogues, DPP-4 inhibitors and TZDs), a neutral effect was reported for all classes of therapies except versus DPP-4 inhibitors where the analysis suggested a benefit of the latter [OR 0.54 (95% CI: 0.35–0.83), p = 0.005 (93 events)]. It should be noted, however, that CAROLINA also has its limitations, in particular since we do not include a placebo arm to assess the independent effects of linagliptin and glimepiride.
With 6041 patients randomized, CAROLINA will also provide insights beyond CV outcomes from the sub-studies, where recent data indicate a potential role for DPP-4 inhibition, including impact on microvascular,23 renal outcomes,24 cognitive function,12 long-term beta-cell function14 and ambulatory glucose profiles13 and may demonstrate further evidence on a recent observation of potential beta-cell protection with linagliptin in latent autoimmune diabetes in adults (LADA).15
In summary, the CAROLINA trial will help clarify the CV safety and potential CV protection of long-term linagliptin compared to glimepiride in early T2D predominantly on background metformin therapy. The outcome of the CAROLINA trial may generate the most robust evidence in the decision-making process for selecting a second glucose-lowering agent after metformin in T2D.
Acknowledgments
The authors would like to thank the patients and staff who participated in this clinical trial.
Footnotes
Declaration of conflicting interests: N.M. has served as a consultant to AstraZeneca, Amgen, BMS, Boehringer Ingelheim, Merck, Novo Nordisk, Roche and Sanofi-Aventis. He has received grant support from Merck and Boehringer Ingelheim. In addition, he has served as a speaker for AstraZeneca, Amgen, Bayer, BMS, Boehringer Ingelheim, Lilly, Merck, Mitsubishi Tanabe Pharma Corporation, Novartis, Novo Nordisk, Pfizer, Roche and Sanofi-Aventis. J.R. has served on scientific advisory boards and received honorarium or consulting fees from Sanofi, Novo Nordisk, Eli Lilly, GlaxoSmithKline, Takeda, Merck, Daiichi Sankyo, Janssen, Novartis, Boehringer Ingelheim, MannKind, Intarcia and Lexicon. In addition, he has received grants or research support from Merck, Pfizer, Sanofi, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, Janssen, Daiichi Sankyo, MannKind, Boehringer Ingelheim, Intarcia and Lexicon. S.E.K. has served as a consultant for Boehringer Ingelheim, Elcelyx, Eli Lilly, GlaxoSmithKline, Intarcia, Janssen, Merck, Novo Nordisk, Receptos and Takeda. B.Z. has served as a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Takeda and Sanofi. He has received grant support from Merck, Novo Nordisk and Boehringer Ingelheim. J.J.K. has acted as a consultant and received honouraria from the following companies: Aegerion, Amgen, AstraZeneca, Atheronova, Boehringer Ingelheim, Catabasis, Cerenis, CSL Behring, Dezima Pharmaceuticals, Eli Lilly, Esperion, Genzyme, Isis, Merck, Novartis, Omthera, Pronova, Regeneron, Sanofi, The Medicines Company, UniQure and Vascular Biogenics. J.M.L. has during the past year consulted with Boehringer Ingelheim, Gilead, Janssen, Lilly, Merck and Novartis. M.A.E. has been a consultant for Boehringer Ingelheim, Takeda Pharmaceuticals, Terumo Medical Corporation and Amgen. E.B., M.M., B.R., S.P., O.E.J. and H.-J.W. are employees of BI, the developer of linagliptin. All authors contributed to the development of the manuscript and read and approved the final manuscript.
Funding: The CAROLINA trial is sponsored by Boehringer Ingelheim and Eli Lilly.
References
- 1. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Food and Drug Administration (Center for Drug Evaluation and Research). Guidance for industry: diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf (2008, accessed 18 October 2014).
- 3. Meinert CL, Knatterud GL, Prout TE, et al. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 1970; 19(Suppl.): 789–830. [PubMed] [Google Scholar]
- 4. Ye Y, Perez-Polo JR, Aguilar D, et al. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res Cardiol 2011; 106: 925–952. [DOI] [PubMed] [Google Scholar]
- 5. Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA® trial. Diabetes Vasc Dis Re 2013; 10: 289–301. [DOI] [PubMed] [Google Scholar]
- 6. Hemmingsen B, Schroll JB, Lund SS, et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane DB Syst Rev 2013; 4: Article CD009008. DOI: 10.1002/14651858.CD009008.pub2. [DOI] [PubMed] [Google Scholar]
- 7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Inv Drug 2010; 19: 133–140. [DOI] [PubMed] [Google Scholar]
- 9. Blech S, Ludwig-Schwellinger E, Grafe-Mody EU, et al. The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos 2010; 38: 667–678. [DOI] [PubMed] [Google Scholar]
- 10. Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012; 380: 475–483. [DOI] [PubMed] [Google Scholar]
- 11. Grizzle JE. A note on stratifying versus complete random assignment in clinical trials. Control Clin Trials 1982; 3: 365–368. [DOI] [PubMed] [Google Scholar]
- 12. Shannon RP. DPP-4 inhibition and neuroprotection: do mechanisms matter? Diabetes 2013; 62: 1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013; 227: 349–354. [DOI] [PubMed] [Google Scholar]
- 14. van Genugten RE, van Raalte DH, Diamant M. Dipeptidyl peptidase-4 inhibitors and preservation of pancreatic islet-cell function: a critical appraisal of the evidence. Diabetes Obes Metab 2012; 14: 101–111. [DOI] [PubMed] [Google Scholar]
- 15. Johansen OE, Boehm BO, Grill V, et al. C-peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a two year double-blind, randomized, controlled study. Diabetes Care 2014; 37: e11–e12. [DOI] [PubMed] [Google Scholar]
- 16. Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vasc Pharmacol 2011; 55: 10–16. [DOI] [PubMed] [Google Scholar]
- 17. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. New Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 18. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. New Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 19. Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR–TIMI 53 randomized trial. Circulation. Epub ahead of print 4 September 2014. DOI: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 20. Nathan D, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013; 36: 2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Hong J, Chi J, et al. Head-to-head comparison of dipeptidyl peptidase-IV inhibitors and sulphonylureas – a meta-analysis from randomized clinical trials. Diabetes Metab Res 2014; 30: 241–256. [DOI] [PubMed] [Google Scholar]
- 23. Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care 2014; 37: 2884–2894. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka T, Higashijima Y, Wada T, et al. The potential for renoprotection with incretin-based drugs. Kidney Int 2014; 86: 701–711. [DOI] [PubMed] [Google Scholar]