Abstract
Prompt diagnosis and treatment of fungal meningitis are critical, but culture is insensitive. (1,3)-β-d-Glucan (BDG) testing is FDA approved for serological diagnosis of invasive fungal disease; however, BDG testing is not approved for cerebrospinal fluid (CSF), and the appropriate cutoff value is unknown. We aimed to validate the diagnostic accuracy of CSF BDG measurements for fungal meningitis among patients exposed to contaminated methylprednisolone acetate (MPA). A retrospective observational study was conducted at St. Joseph Mercy Hospital and Vanderbilt University from November 2013 to February 2014. Patients were included if they had received a contaminated MPA injection. Cases were classified as probable or proven meningitis according to Centers for Disease Control and Prevention guidelines. CSF BDG testing was performed according to the package insert instructions for serum samples, and results were validated using Clinical and Laboratory Standards Institute procedures (MiraVista Diagnostics). Of 233 patients, 45 had meningitis (28 proven cases), 53 had spinal/paraspinal infections (19 proven cases), and 135 did not develop disease. Using the manufacturer's cutoff value (≥80 pg/ml), the sensitivity and specificity were 96% and 95%, respectively, for proven meningitis and 84% and 95% for probable or proven meningitis. Receiver operating characteristic analysis identified the optimal cutoff value for proven meningitis to be 66 pg/ml (sensitivity, 100%; specificity, 94%) and that for probable or proven meningitis to be 66 pg/ml (sensitivity, 91%; specificity, 92%). Our results suggest that CSF BDG measurements are highly sensitive and specific for the diagnosis of fungal meningitis associated with contaminated MPA injections. Further study on the utility of CSF BDG testing for other types of fungal meningitis is needed.
INTRODUCTION
Prompt diagnosis and administration of appropriate therapy are critical to improving clinical outcomes for patients with invasive fungal disease (IFD) (1, 2); however, the diagnosis of IFD can be difficult (3). Definitive diagnoses are most often based on direct observation and/or isolation of a fungal organism from a sterile site (4). Fungal cultures have been shown to be insensitive (5), however, and the collection of adequate samples for culture may require invasive procedures (6). Sensitive and rapid serological assays have been approved for the diagnosis of IFD, but many have not been validated using nonserological samples (4).
In May 2004, the serum Fungitell assay (Associates of Cape Cod Inc., East Falmouth, MA) was approved by the Food and Drug Administration (FDA) for use in the United States. A recent meta-analysis comparing serum (1,3)-β-d-glucan (BDG) levels among patients with proven or probable IFD versus those without IFD revealed a pooled sensitivity of 76.8% (95% confidence interval [CI], 67.1% to 84.3%) and specificity of 85.3% (95% CI, 79.6% to 89.7%) (7). However, this assay has not been validated or FDA approved for testing of cerebrospinal fluid (CSF), and the appropriate cutoff value for positivity is not known.
In 2012, an unprecedented outbreak of fungal infections associated with the injection of contaminated methylprednisolone acetate (MPA) was identified (8, 9). By October 2013, 751 cases of fungal disease had been identified; central nervous system (CNS) disease developed in just over one-half of these cases, and the predominant fungal organism identified was the brown-black mold Exserohilum rostratum (http://www.cdc.gov/HAI/outbreaks/meningitis.html). A small case series, including specimens from 5 patients infected during this outbreak, suggested the possible utility of CSF BDG measurements in diagnosis and monitoring of responses to therapy (10). Another larger study included specimens from 41 confirmed cases of fungal meningitis and 66 controls. That study identified an optimal cutoff value of 138 pg/ml, which provided 100% sensitivity and 98% specificity for the diagnosis of proven fungal meningitis (11). We aim to further validate the diagnostic accuracy of CSF BDG measurements for both probable and proven fungal meningitis associated with contaminated MPA.
MATERIALS AND METHODS
Patients and setting.
This is a retrospective observational study of patients who received a contaminated methylprednisolone injection from one of two lots produced by the New England Compounding Center (NECC) (lot 06292012@26 or 08102012@51). All patients received their care at St. Joseph Mercy Hospital (SJMH) (Ann Arbor, MI) or Vanderbilt University Hospital (VUH) (Nashville, TN). Patients were included if a CSF specimen was available for analysis. The institutional review board at each study site approved the study protocol, with a waiver of informed consent requirements, and the study was conducted between November 2013 and February 2014.
Definitions.
Using the CDC case definitions, probable fungal meningitis was defined as signs or symptoms of meningitis (with white blood cell counts in CSF of ≥5 cells/μl, accounting for the presence of red blood cells) of unknown etiology or posterior circulation stroke without a cardioembolic source following epidural or paraspinal injection of contaminated methylprednisolone. Probable spinal or paraspinal infections were defined on the basis of magnetic resonance imaging (MRI) evidence of osteomyelitis, abscess, or other infection (e.g., soft tissue infection) of unknown etiology in the spinal or paraspinal structures, at or near the site of epidural or paraspinal injection of contaminated methylprednisolone. Proven cases were defined as cases with the aforementioned findings plus microbiological, molecular, or histopathological evidence of a fungal pathogen (http://www.cdc.gov/hai/outbreaks/clinicians). Control subjects were persons who had received an injection of contaminated methylprednisolone and did not meet the CDC case definitions related to the fungal outbreak.
Specimens.
When available, CSF specimens from patients who were exposed to contaminated MPA and evaluated with lumbar puncture were stored in −70°C freezers at the study sites. For most patients, the available CSF samples were from the initial lumbar puncture. A total of 229 deidentified specimens were thawed, placed in vials, refrozen, and shipped on dry ice to MiraVista Diagnostics (Indianapolis, IN). Four CSF specimens from VUH that had been previously evaluated at MiraVista Diagnostics were available for BDG testing.
CSF (1,3)-β-d-glucan testing.
CSF BDG testing was performed at MiraVista Diagnostics using the Fungitell assay (Associates of Cape Cod Inc., East Falmouth, MA), in accordance with the manufacturer's instructions for testing of serum. Specimens were tested in duplicate; the results were considered valid if the duplicates were both positive and the coefficient of variation (CV) for the positive duplicates was less than 30% or both samples tested negative or intermediate. Specimens not meeting these criteria were retested in the next assay, and the results were considered valid if they met the acceptance criteria. Results with CVs above 30% with repeat testing were judged to be invalid and were reported as “unable to report, invalid result.”
The reportable range for this assay using serum specimens is 31 pg/ml to 500 pg/ml, and the manufacturer recommends that <60 pg/ml be interpreted as negative, 60 pg/ml to 79 pg/ml as intermediate, and ≥80 pg/ml as positive (12). The Fungitell assay has been validated at MiraVista Diagnostics for testing CSF specimens according to the Clinical and Laboratory Standards Institute (CLSI) procedures. For the purposes of our study, CSF BDG levels higher than 500 pg/ml were reported numerically.
Precision.
Intra-assay repeatability was examined by comparing the results for duplicate aliquots, to determine the mean concentration values. Interassay reproducibility was evaluated by testing two aliquots of specimens from 22 randomly selected patients on 2 occasions, 1 day apart, in duplicate. Categorical reproducibility as positive or negative at the cutoff value of 30 pg/ml was determined by comparing initial and repeat results for the 22 patients. Quantitative reproducibility was evaluated by linear regression analysis.
Data analysis.
Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Carey, NC) and SigmaPlot (Systat Software, San Jose, CA). Sensitivity and specificity values, with 95% confidence intervals, were calculated for BDG cutoff values suggested by the manufacturers of the assay (≥80 pg/ml). Sensitivity was calculated in two ways, namely, (i) the proportion of patients with positive BDG results among all patients with proven fungal meningitis and (ii) the proportion of patients with positive BDG results among all patients with probable or proven fungal meningitis. Specificity was calculated as the proportion of patients with negative BDG results among all exposed patients without probable or proven fungal disease. For the purposes of this analysis, indeterminate BDG values were considered negative.
Receiver operating characteristic (ROC) curves were created by plotting the sensitivity of the BDG assay at various numerical cutoff values versus 1 − specificity at those values. The ability of the assay to distinguish cases from controls was quantified using the area under the curve (AUC), with 95% confidence intervals. The optimal cutoff value was determined by the Youden method (13), as implemented using the statistical software package Optimal Cutpoints 1.1 with R version 3.0.1 (www.r-project.org).
RESULTS
Patients.
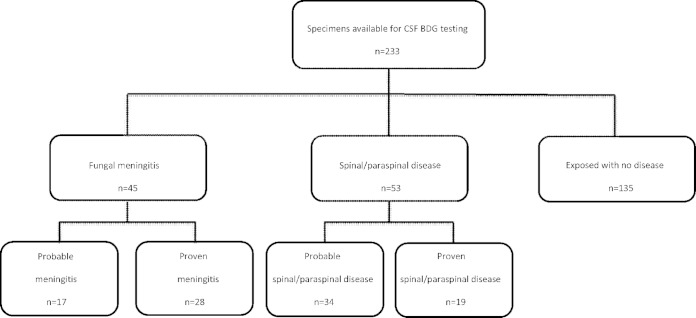
Among the 233 patients who were eligible for the study, there were 45 cases of fungal meningitis, in 32 of which concomitant spinal or paraspinal infections subsequently developed, and 53 spinal/paraspinal infections; 135 control patients had received injections of MPA but were not infected. Of the 45 cases of meningitis, 28 (62%) were proven; 19 patients (42%) had meningitis confirmed by either CSF culture or PCR, and 9 (20%) had fungal infections confirmed by tissue culture, PCR, or histopathological examination of specimens obtained during surgery for concomitant spinal or paraspinal infections. Of the 53 cases of probable spinal or paraspinal infections, 19 (36%) had proven evidence of fungal infections (Fig. 1). Among the proven cases of meningitis and spinal or paraspinal infections, Exserohilum rostratum was identified in 29 cases and Aspergillus fumigatus in one case. The age (mean ± standard deviation [SD]) of the patients was 58 ± 14 years, and 151 (65%) were women. Hypertension, hyperlipidemia, and diabetes were common, but few patients had immunosuppressive conditions.
FIG 1.
Flowchart of (1,3)-β-d-glucan testing of cerebrospinal fluid (CSF) samples from patients exposed to contaminated MPA injections. Probable meningitis was defined as signs or symptoms of meningitis with CSF white blood cell counts of ≥5 cells/μl, accounting for the presence of red blood cells. Probable spinal or paraspinal infections were defined on the basis of MRI evidence of osteomyelitis, abscess, or other infection (e.g., soft tissue infection) of unknown etiology in the spinal or paraspinal structures, at or near the site of epidural or paraspinal injection of contaminated methylprednisolone. Proven cases were defined as cases of probable disease with microbiological, molecular, or histopathological evidence of a fungal pathogen.
BDG results.
Of the 233 CSF specimens, 228 (98%) met the acceptance criteria for valid results, 208 (89%) in initial testing and 20 (9%) in repeat testing. Of the 20 samples that met the acceptance criteria in repeat testing, 19 tested negative and 1 intermediate on both tests. Five specimens (2%) (all controls) failed to yield valid results after repeat testing, given that the CV was greater than 30%.
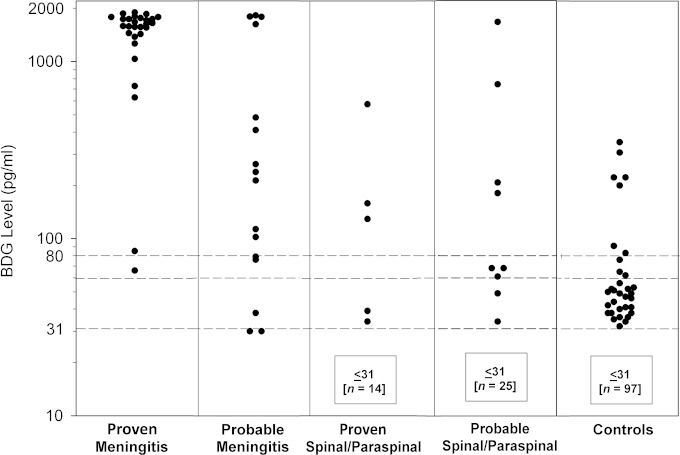
The distributions of CSF BDG levels in proven or probable cases of fungal meningitis, spinal or paraspinal infections, and controls are shown in Fig. 2. Using the manufacturer's recommended cutoff value of 80 pg/ml, results were positive in 27 (96%) of 28 proven cases of fungal meningitis, in 11 (65%) of 17 probable cases of fungal meningitis, and in 38 (84%) of 45 total cases of fungal meningitis. Accordingly, the sensitivity and specificity for diagnosis of proven fungal meningitis at the manufacturer's recommended cutoff value of 80 pg/ml were 96% (95% CI, 80% to 100%) and 95% (95% CI, 89% to 98%), respectively, and those for probable or proven meningitis were 84% (95% CI, 70% to 93%) and 95% (95% CI, 89% to 98%), respectively. BDG concentrations in the CSF of patients with proven spinal or paraspinal infections were above 80 pg/ml in 3 (16%) of 19 cases.
FIG 2.
Cerebrospinal (1,3)-β-d-glucan (BDG) levels in proven fungal meningitis cases, probable meningitis cases, proven spinal or paraspinal infection cases, probable spinal or paraspinal infection cases, and controls. Manufacturer recommendations for serum BDG specimens are indicated by the dashed lines (values of <60 are interpreted as negative, and values of 80 or higher are interpreted as positive).
Levels were greater than 500 pg/ml in 26 (93%) of the 28 proven meningitis cases, 4 (24%) of the 17 probable meningitis cases, and 3 (6%) of the 53 spinal/paraspinal infection cases. The mean CSF BDG concentrations were highest in proven meningitis (1,448 pg/ml), followed by probable meningitis (537 pg/ml), spinal/paraspinal infections (98 pg/ml), and controls (43 pg/ml).
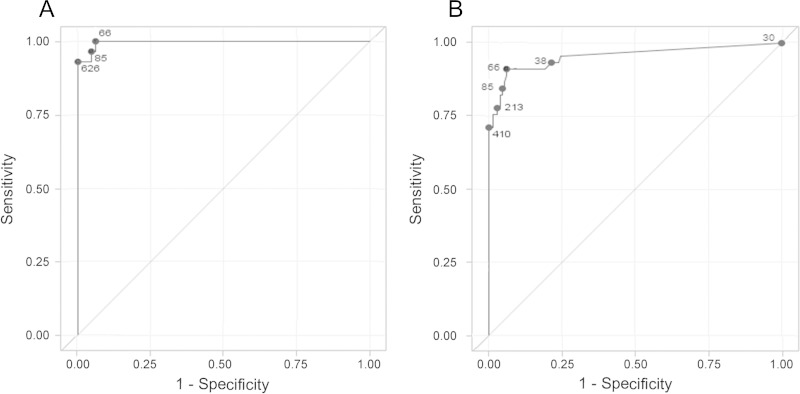
The ROC curve for the diagnosis of proven meningitis versus no disease is shown in Fig. 3A. The AUC was 1.00 (95% CI, 0.99 to 1.00). The optimal cutoff value was 66 pg/ml, with a sensitivity of 100% (95% CI, 87% to 100%) and a specificity of 94% (95% CI, 88% to 97%). The results were positive in all 28 cases of proven meningitis and in 8 (6%) of 130 controls. The ROC curve for the diagnosis of probable or proven meningitis versus no disease is shown in Fig. 3B. The AUC was 0.95 (95% CI, 0.91 to 0.99). The optimal cutoff value also was 66 pg/ml, resulting in a sensitivity of 91% (95% CI, 79% to 98%) and a specificity of 92% (95% CI, 87% to 96%). The results were positive in 41 (84%) of 45 cases of probable or proven meningitis and 8 (6%) of 130 controls.
FIG 3.
(A) Receiver operating characteristic (ROC) curve of cerebrospinal fluid (1,3)-β-d-glucan cutoff values to distinguish proven meningitis cases from noncases. The area under the ROC curve was 1.00 (95% CI, 0.99 to 1.00). (B) ROC curve of cerebrospinal fluid (1,3)-β-d-glucan cutoff values to distinguish probable or proven meningitis cases from noncases. The area under the ROC curve was 0.95 (95% CI, 0.91 to 0.99). Numbers represent different (1,3)-B-d-glucan cutoff values.
BDG assay precision.
The intra-assay repeatability of duplicate results above 80 pg/ml was assessed by regression analysis and determination of CVs between duplicates. The mean CV for the duplicate results was 5.8% (range, 0 to 26.0%), and the R2 value determined by linear regression analysis was 0.985. Interassay reproducibility was determined by testing, on consecutive days, aliquots of specimens from 22 randomly selected patients, with initial results between 16 and 1,896 pg/ml. The results were reproducibly positive for 17 of 17 specimens and reproducibly negative for five of five specimens, and the R2 comparing day 1 and day 2 results was 0.989 by linear regression analysis. Of the 8 specimens with results above 500 pg/ml (range, 839 to 1,749 pg/ml), the mean CV for the day 1 and day 2 results was 1.28% (range, 0.2% to 3.0%).
DISCUSSION
Our results demonstrate that measurement of CSF BDG levels is a highly sensitive and specific test for the diagnosis of fungal meningitis associated with contaminated MPA injections. Using the manufacturer's cutoff value of 80 pg/ml for serum testing, the sensitivity and specificity for CSF were 96% and 95%, respectively, for proven disease and 84% and 95% for proven or probable disease. We found that the accuracy of CSF BDG measurements for the diagnosis of fungal meningitis in our study was higher than that reported previously from studies validating serum BDG measurements for the diagnosis of IFD (7, 12). It is expected that false-negative results would occur in the setting of zygomycosis, as these fungal species produce little or no BDG (14–16). Therefore, it is possible that the spectrum of fungal species implicated in this outbreak had an effect on the sensitivity of the assay. False-positive serum BDG results have been reported with a variety of factors, including bacteremia (17), intravenous β-lactam antibiotic therapy (18, 19), hemodialysis membranes (20, 21), gauze packing (22), and intravenous immunoglobulin administration (23). It is possible that these factors may not cause false-positive results with CSF samples due to the blood-brain barrier, leading to increased specificity with CSF samples.
Interestingly, the Youden method (13) identified identical optimal cutoff values of 66 pg/ml for both proven disease and proven or probable disease. In the Youden method, the CSF BDG level that maximizes (sensitivity + specificity − 1) is chosen as the optimal cutoff value. Because of a relatively small sample size (2 proven and 3 probable cases of meningitis) with values between 31 and 100 pg/ml, it is not surprising that the point represented by the level of 66 pg/ml was chosen both times. This cutoff value is lower than that currently used for the serum BDG assay (80 pg/ml), which may be related to factors decreasing the proportions of false-negative and false-positive results, as discussed above. These findings suggest that CSF BDG levels between 66 and 80 pg/ml should be taken into consideration for diagnosis of Exserohilum meningitis and not dismissed as clinically insignificant. Whether these findings apply to other forms of fungal meningitis is unknown.
A previous study by Litvintseva et al. that included only proven fungal meningitis cases identified an optimal cutoff value for CSF BDG levels of 138 pg/ml (11). Using this cutoff value, the authors reported a sensitivity of 100% (95% CI, 95% to 100%) and a specificity of 98% (95% CI, 83% to 99%) for proven cases. Using the proposed cutoff value of 138 pg/ml with our data, the sensitivity and specificity for the diagnosis of proven fungal meningitis were 93% (95% CI, 75% to 99%) and 96% (95% CI, 91% to 99%), respectively, and those for proven or probable meningitis were 78% (95% CI, 63% to 88%) and 96% (95% CI, 91% to 99%).
The reason that Litvintseva et al. (11) identified a different optimal cutoff value than our analysis (138 pg/ml versus 66 pg/ml) may be related to sample size. First, since both samples were relatively small, each cutoff value was estimated with some uncertainty and was dependent on the levels obtained. In addition, our control group was composed of exposed patients with no disease, while 39 (59%) of 66 specimens in their control group came from patients not exposed to contaminated MPA. Patients exposed to contaminated MPA but not identified as having CNS disease may have detectable BDG levels in the CSF. It is possible that some of the exposed patients cleared the fungal infection before meningitis developed.
In contrast to Litvintseva et al. (11), we included an analysis with probable meningitis. Litvintseva et al. (11) included only specimens from patients with proven disease in their calculations of diagnostic accuracy. Given the low sensitivity of culture (14%) and PCR (29%) in the outbreak, we thought that laboratory confirmation missed patients who clinically had the disease (http://www.cdc.gov/hai/outbreaks/laboratory/index.html).
Litvintseva et al. (11) provided important data on testing of a small number of serial samples (n = 20), which suggested that BDG levels that decline with therapy may predict therapeutic responses. In this situation, it would be useful to know whether serial values over 500 pg/ml were changing; in their study, however, results were reported only as >500 pg/ml. In our study, the reproducibility of the assay for specimens with BDG values of >500 pg/ml was high (mean CV, 1.28% [range, 0.2% to 3.0%]). This suggests that numerical values of >500 pg/ml could be reported to clinicians for better assessment of serial changes, an important issue requiring further study of a larger number of cases.
There are several limitations to this study. First, the sample size was relatively small, as reflected in the width of the confidence intervals around the sensitivity and specificity values. Second, the gold standard method used in our study was imperfect, probably missing true cases, so we included an analysis based on clinical diagnosis (probable meningitis). Third, given that only the specimens that were available and stored in the laboratory freezers were tested for CSF BDG levels, the proportion of diagnosed cases in our study may not reflect the prevalence of disease in the population at risk. Therefore, we did not report positive and negative predictive values, because those metrics are highly dependent on prevalence or the pretest probability of disease. Additionally, BDG levels are not helpful in determining the species of fungus responsible for infection. However, given the high specificity of the assay, clinicians can be confident that further diagnostic evaluation for fungal meningitis, including species identification, is warranted in the setting of positive test results. Finally, we were unable to test serial samples to assess whether CSF BDG levels decline with therapy and predict therapeutic response.
In conclusion, CSF BDG measurement is a highly sensitive and specific test for the diagnosis of fungal meningitis associated with contaminated MPA injections. Future studies are needed to determine the accuracy of CSF BDG measurements for the diagnosis of fungal meningitis not due to contaminated MPA injections.
ACKNOWLEDGMENTS
We thank Janet Obear for helping with coordination of the study.
A.N.M., B.S., O.A.S., and A.C.P. all report no conflicts. L.J.W., T.A.S., and M.M.D are employees of MiraVista Diagnostics, the laboratory that performed the testing for this study.
There was no financial support given for this study.
REFERENCES
- 1.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 2.von Eiff M, Roos N, Schulten R, Hesse M, Zuhlsdorf M, van de Loo J. 1995. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 62:341–347. doi: 10.1159/000196477. [DOI] [PubMed] [Google Scholar]
- 3.Perfect JR. 2013. Fungal diagnosis: how do we do it and can we do better? Curr Med Res Opin 29(Suppl 4):S3–S11. doi: 10.1185/03007995.2012.761134. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal and National Institute of Allergy and Infectious Diseases Mycoses Study Group . 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer J, Buck M, Witebsky F, Stock F, Pizzo PA, Walsh TJ. 1993. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis: disseminated versus single-organ infection. Diagn Microbiol Infect Dis 17:103–109. doi: 10.1016/0732-8893(93)90020-8. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar P. 2010. Diagnostic challenges and recent advances in the early management of invasive fungal infections. Eur J Haematol 84:281–290. doi: 10.1111/j.1600-0609.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 7.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. 2011. β-d-Glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 8.Chiller TM, Roy M, Nguyen D, Guh A, Malani AN, Latham R, Peglow S, Kerkering T, Kaufman D, McFadden J, Collins J, Kainer M, Duwve J, Trump D, Blackmore C, Tan C, Cleveland AA, MacCannell T, Muehlenbachs A, Zaki SR, Brandt ME, Jernigan JA, Multistate Fungal Infection Clinical Investigation Team . 2013. Clinical findings for fungal infections caused by methylprednisolone injections. N Engl J Med 369:1610–1619. doi: 10.1056/NEJMoa1304879. [DOI] [PubMed] [Google Scholar]
- 9.Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J, Duwve J, Fontaine E, Chu A, Carothers B, Reilly A, Fiedler J, Wiese AD, Feaster C, Gibson L, Griese S, Purfield A, Cleveland AA, Benedict K, Harris JR, Brandt ME, Blau D, Jernigan J, Weber JT, Park BJ, Multistate Fungal Infection Outbreak Response Team . 2013. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med 369:1598–1609. doi: 10.1056/NEJMoa1213978. [DOI] [PubMed] [Google Scholar]
- 10.Lyons JL, Roos KL, Marr KA, Neumann H, Trivedi JB, Kimbrough DJ, Steiner L, Thakur KT, Harrison DM, Zhang SX. 2013. Cerebrospinal fluid (1,3)-β-d-glucan detection as an aid for diagnosis of iatrogenic fungal meningitis. J Clin Microbiol 51:1285–1287. doi: 10.1128/JCM.00061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvintseva AP, Lindsley MD, Gade L, Smith R, Chiller T, Lyons JL, Thakur KT, Zhang SX, Grgurich DE, Kerkering TM, Brandt ME, Park BJ. 2014. Utility of (1–3)-β-d-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin Infect Dis 58:622–630. doi: 10.1093/cid/cit808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Associates of Cape Cod Inc. 2011. Fungitell package insert. Associates of Cape Cod Inc., Falmouth, MA: http://www.acciusa.com/pdfs/accProduct/Fungitell_multilang_pisheets/Fungitell%20Insert%20EN.pdf. [Google Scholar]
- 13.Fluss R, Faraggi D, Reiser B. 2005. Estimation of the Youden Index and its associated cutoff point. Biom J 47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 14.Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, McGinnis MR, Ostrosky-Zeichner L. 2006. Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med Mycol 44:267–272. doi: 10.1080/13693780500474327. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K. 1995. Plasma (1→3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol 33:3115–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girouard G, Lachance C, Pelletier R. 2007. Observations on (1–3)-β-d-glucan detection as a diagnostic tool in endemic mycosis caused by Histoplasma or Blastomyces. J Med Microbiol 56:1001–1002. doi: 10.1099/jmm.0.47162-0. [DOI] [PubMed] [Google Scholar]
- 17.Mennink-Kersten MA, Ruegebrink D, Verweij PE. 2008. Pseudomonas aeruginosa as a cause of 1,3-β-d-glucan assay reactivity. Clin Infect Dis 46:1930–1931. doi: 10.1086/588563. [DOI] [PubMed] [Google Scholar]
- 18.Mennink-Kersten MA, Warris A, Verweij PE. 2006. 1,3-β-D-Glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med 354:2834–2835. doi: 10.1056/NEJMc053340. [DOI] [PubMed] [Google Scholar]
- 19.Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR. 2006. Reactivity of (1→3)-β-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother 50:3450–3453. doi: 10.1128/AAC.00658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda H, Kubo K, Hamasaki K, Kanda Y, Nakao A, Kitamura T, Fujita T, Yamamoto K, Mimura T. 2001. Influence of various hemodialysis membranes on the plasma (1→3)-β-d-glucan level. Kidney Int 60:319–323. doi: 10.1046/j.1523-1755.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagasawa K, Yano T, Kitabayashi G, Morimoto H, Yamada Y, Ohata A, Usami M, Horiuchi T. 2003. Experimental proof of contamination of blood components by (1→3)-β-d-glucan caused by filtration with cellulose filters in the manufacturing process. J Artif Organs 6:49–54. doi: 10.1007/s100470300008. [DOI] [PubMed] [Google Scholar]
- 22.Kanamori H, Kanemitsu K, Miyasaka T, Ameku K, Endo S, Aoyagi T, Inden K, Hatta M, Yamamoto N, Kunishima H, Yano H, Kaku K, Hirakata Y, Kaku M. 2009. Measurement of (1–3)-β-d-glucan derived from different gauze types. Tohoku J Exp Med 217:117–121. doi: 10.1620/tjem.217.117. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa M, Hori H, Niiguchi S, Azuma E, Komada Y. 2004. False-positive plasma (1→3)-β-d-glucan test following immunoglobulin product replacement in an adult bone marrow recipient. Int J Hematol 80:97–98. doi: 10.1532/IJH97.04030. [DOI] [PubMed] [Google Scholar]