Abstract
Bartonella spp. are fastidious bacteria that cause blood culture-negative endocarditis and have been increasingly reported. In this study, we included all patients retrospectively and prospectively diagnosed with Bartonella endocarditis in our French reference center between 2005 and 2013. Our diagnosis was based on the modified Duke criteria and microbiological findings, including serological and PCR results. To review the published literature, we searched all human Bartonella endocarditis cases published in the PubMed database between January 2005 and October 2013. We report here a large series of 106 cases, which include 59 cases that had not previously been reported or mentioned. Indirect immunofluorescence assays, Western blotting, and real-time PCR from total blood, serum, and valve tissue exhibited sensitivities of 58%, 100%, 33%, 36%, and 91%, respectively. The number of cases reported in the literature between 2005 and 2013 increased to reach a cumulative number of 196 cases. The number of cases reported in the literature by other centers is increasing more rapidly than that reported by our French reference center (P < 10−2). Currently, there is a lack of criteria for the diagnosis of Bartonella endocarditis. We suggest that a positive PCR result from a cardiac valve or blood specimen, an IgG titer of ≥800 using an immunofluorescence assay, or a positive Western blot assay be considered major Duke criteria for Bartonella endocarditis. There is no real increase in the incidence of these infections but rather a better understanding and interest in the disease resulting from the improvement of diagnostic tools.
INTRODUCTION
Bartonella species are small Gram-negative hemotropic bacilli classified within the class Alphaproteobacteria. These facultative intracellular bacteria, which are generally transmitted by arthropod vectors (1), cause various clinical syndromes in immunocompetent and immunocompromised patients. To date, 23 Bartonella species have been described, 11 of which are proven or likely human pathogens (2). Bartonella species were first recognized as endocarditis agents in 1993 when 3 such cases were reported (3–5). After the seminal work of Drancourt et al. (6), 120 cases were reported in the literature until 2006 (7). Most reported cases were attributed to Bartonella quintana, the agent of trench fever that may also cause endocarditis, chronic bacteremia, and bacillary angiomatosis, and Bartonella henselae, which mainly causes cat scratch disease, endocarditis, bacillary angiomatosis, peliosis hepatis, and osteomyelitis (8). B. quintana primarily infects homeless people infested with body lice, whereas B. henselae is associated with patients with a previous valvulopathy and contact with cats (2, 7, 8). Single cases of endocarditis caused by other Bartonella species, including Bartonella elizabethae (4), Bartonella vinsonii subsp. berkhoffii (9, 10), B. vinsonii subsp. arupensis (11), Bartonella koehlerae (12), Bartonella alsatica (13, 14), and “Candidatus Bartonella mayotimonensis” (15), have occasionally been reported in the literature. Epidemiological data suggest a European-African gradient distribution in the prevalence of Bartonella endocarditis, the highest prevalence being reported in southern countries (7). In Europe, the prevalence of Bartonella endocarditis among those with infective endocarditis is 0% in Sweden, 1.1% in the United Kingdom, and 3% in France and Germany (7), and it reaches 15.6% and 9.8% in Algeria (16) and Tunisia (17), respectively.
Blood culture-negative endocarditis (BCNE) represents 2.5% to 31% of cases of endocarditis, depending on the series (18). In France, approximately 20% to 30% of all documented cases of BCNE are Bartonella endocarditis, which represents the second most common cause of endocarditis following Coxiella burnetii (19, 20). Over the past few years, in our national reference center for Q fever and Bartonella infection, we have developed diagnostic tools for the diagnosis of BCNE, including Bartonella endocarditis (19). Because Bartonella spp. are fastidious, serological analysis using an indirect immunofluorescence assay (IFA) remains the most common method for diagnosing endocarditis caused by these bacteria. However, these infections can also be successfully diagnosed by Western blotting or specific real-time PCR (RT-PCR), allowing for the rapid detection of Bartonella DNA in blood or resected valve samples. The bacteria can also be isolated by inoculation onto agar plates or in tissue culture (2) or visualized in valvular tissues using Warthin-Starry staining and immunohistochemical examination (2).
From this study, we report a series of 106 patients with Bartonella endocarditis who were diagnosed in our laboratory during a 9-year period, and we compare our data to the increasing number of diagnosed cases reported in the literature during the same period.
MATERIALS AND METHODS
Patient recruitment.
The French Reference Center for Rickettsial Diseases is located in Marseille, southern France. We received samples from patients from all parts of France and other countries for Bartonella diagnosis. Between January 2005 and October 2013, we systematically searched for Bartonella species as the causative agents of BCNE using serology and/or RT-PCR, depending on the available samples. We tested 54,401 serum samples for antibodies to Bartonella spp., including 4,381 serum samples from patients suspected to have BCNE, and we received 750 cardiac valve samples that were tested by RT-PCR from patients with a BCNE diagnosis.
Serological assays.
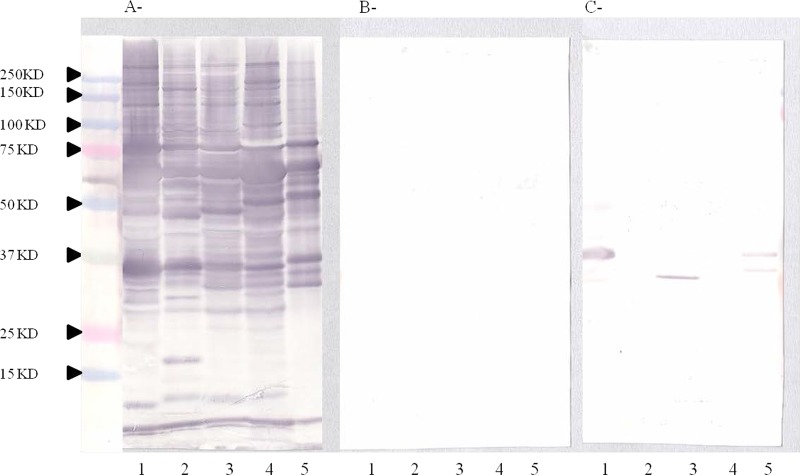
The IFA was performed as previously described to detect the titer of antibodies against B. henselae and B. quintana (21). An IFA was considered positive when the IgG antibody titer was ≥1:100, but for the diagnosis of endocarditis, we use a titer of 1:800 as the cutoff (22). To confirm positive serological results or when serology was negative in the context of BCNE, we performed Western blotting as previously described (23). This technique shows a very specific profile for Bartonella endocarditis (23). Six protein bands (Bq83, Bq65, Bq45, Bq30, Bq20, Bq10) react more frequently with antibodies from serum samples of patients presenting with B. quintana endocarditis and 4 protein bands (Bh65, Bh45, Bh35, Bh23) react more frequently with antibodies from serum samples of patients presenting with B. henselae endocarditis (23). Due to cross-reactions, current serological assays may not reliably distinguish antibody responses against different species of Bartonella. In such cases, Western blotting with cross-adsorption enables identification of the Bartonella species that generated the antibody response. We usually perform Western blotting using antigens from 5 Bartonella species, including B. quintana, B. henselae, B. vinsonii subsp. berkhoffii, B. elizabethae, and B. alsatica, which are proven endocarditis agents, and the serum of a patient diluted to 1:200 in Tris-buffered saline (TBS)–3% nonfat milk. Each serum sample was tested before and after cross-adsorption with B. quintana and B. henselae. When cross-adsorption is performed with the species involved in the disease, homologous and heterologous antibodies disappear, whereas only heterologous antibodies are removed when cross-adsorption is performed with a species that is not involved in the disease (Fig. 1).
FIG 1.
(A) Western immunoblotting of serum from a patient with B. quintana endocarditis presenting with negative serology and a positive PCR for the cardiac valve. The utilized antigens included B. quintana (lanes 1), B. henselae strain Houston (lanes 2), B. elizabethae (lanes 3), B. vinsonii subsp. berkhoffii (lanes 4), and B. alsatica (lanes 5). (B) B. quintana adsorbed sera. (C) B. henselae adsorbed sera. This Western blot presents a typical profile observed for Bartonella-induced endocarditis cases. Molecular masses (in kilodaltons) are given on the left.
PCR assays.
Extraction of bacterial DNA was performed from surgically excised valves or EDTA blood samples using a QIAamp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RT-PCR amplification was performed using a LightCycler thermal cycler (Roche Diagnostics, Meylan, France) between 2005 and 2010 and a CFX thermal cycler (Bio-Rad, Marne la Coquette, France) between 2011 and 2013. All the samples were screened using primers and TaqMan probes targeting a portion of the Bartonella 16S-23S RNA intergenic region, as previously described (19). All positive reactions were confirmed using primers and TaqMan probes targeting the pap31 gene for B. henselae and the yopP gene for B. quintana, as previously described (2, 24). A case was considered definite when 2 positive PCR results in assays targeting 2 different Bartonella sp. genes with a threshold cycle (CT) value of ≤35 were obtained. Identification of other Bartonella species was obtained by sequencing the amplified 16S-23S RNA intergenic region (25). DNAs from B. henselae and B. quintana strains were used as positive controls, and sterile water was used as a negative control. We verified the quality of the extracted DNA by detecting the human actin gene by RT-PCR.
All the valvular specimens were also tested using a broad-range 16S rRNA PCR assay, followed by sequencing when positive, as previously described (26).
Immunohistochemical analysis and Warthin-Starry staining.
Warthin-Starry staining and immunohistochemical analysis were performed on paraffin-embedded valve sections. Anti-B. henselae or anti-B. quintana polyclonal rabbit antibodies were used for immunohistochemistry as previously described (27).
Case definition.
Between January 2005 and October 2013, Bartonella endocarditis was diagnosed according to the modified Duke criteria, which includes positive blood culture, positive histologic examination of valve tissue, and vegetations on echocardiography as major criteria (28). We considered Bartonella to be the etiological agent of endocarditis and a major diagnosis criterion when we found either an elevated serological titer of anti-Bartonella antibodies with an IgG titer of ≥1:800 using IFA (22), a Western blot analysis with a typical endocarditis profile (23), a positive PCR from valve or blood specimens using two different target genes (25), or a positive immunohistochemical analysis (26). The causative Bartonella species was identified by PCR and sequencing (25) or Western blotting following cross-adsorption (23). A standardized questionnaire, including criteria used in the diagnostic score of the modified Duke criteria, was completed for each patient with documented Bartonella endocarditis.
Literature review.
To review the published literature, we searched the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) using the keywords “Bartonella” and “endocarditis” and included all human Bartonella endocarditis case reports published between January 2005 and October 2013. We compared the number of published and unpublished cases diagnosed in our laboratory between 2005 and 2013 to those reported in the literature during the same period. Redundant cases were excluded. All references used for this analysis are listed in the supplemental material. To establish a cumulative frequency curve, we used the year of publication for the published cases or the year of diagnosis for the unpublished cases.
Statistical analysis.
The statistical analysis was performed using Epi Info 3.5.1 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Differences were considered statistically significant when the P value was <0.05.
RESULTS
During the study period, we diagnosed 106 cases of Bartonella endocarditis, 47 of which have been mentioned or reported (11, 13–15, 19). Serum, EDTA blood, and valvular specimens were available for 102, 60, and 52 of these patients, respectively. The IFA was positive in 93 of the 102 tested patients (91%) and negative in 9 cases, 8 of which had a positive Western blot result. Western blotting was not performed for 1 patient. Among the 93 patients with positive IFA titers, 34 (37%) had an IgG titer of <1:800, and 59 (63%) patients had an IgG titer of ≥1:800. In the 34 patients with an IgG titer of <1:800, the diagnosis of Bartonella endocarditis was confirmed by Western blotting for 25 patients, by RT-PCR from valve samples of 7 patients, and by RT-PCR from the blood of 2 patients. Among the 59 patients with an IgG titer of ≥1:800, Western blotting was performed and positive for 40 patients (Table 1).
TABLE 1.
Microbiologic diagnosis of 106 patients with Bartonella endocarditis
| Test type and criteria | No. of positive samples/no. of samples tested (%) |
|---|---|
| IFA | |
| IFA with IgG titer ≥ 100 | 93/102 (91) |
| IFA with IgG titer ≥ 800 | 59/93 (63) |
| IFA with IgG titer from 1:100 to 1:800 | 34/93 (37) |
| Negative IFA | 9/102 (9) |
| Western blotting | |
| Total | 73/73 (100) |
| Patient with IgG titer ≥ 1:800 | 40/40 (100) |
| Patient with IgG titer from 1:100 to 1:800 | 25/25 (100) |
| Patient with negative IFA | 8/8 (100) |
| Specific RT-PCR for Bartonella spp. | |
| Cardiac valves | 48/52 (92) |
| Blood | 20/60 (33) |
| Serum | 25/70 (36) |
| 16S RNA amplification | |
| Cardiac valves | 21/35 (60) |
| Blood | 0/15 (0) |
RT-PCR was performed on 182 samples from 103 patients and was positive for 48/52 (92%) valvular specimens, 20/60 (33%) blood samples, and 25/70 (36%) tested serum samples. Sera and blood were tested by PCR for 40. The PCR was negative for the sera and blood from 16 patients and positive on the 2 samples from 8 patients. However, a dissonance was observed between the 2 samples for 15 patients; PCR was positive for serum and negative for blood from 9 patients and positive for blood and negative for sera from 6 patients. In addition, 16S RNA gene amplification was positive for 21/35 (60%) cardiac specimens and 0/15 blood samples from the tested patients. 16S rRNA PCR on valvular biopsy specimens was significantly less sensitive than RT-PCR (P = 0.0003 according to the chi-square test). Immunohistochemistry and Warthin-Starry staining were performed on 28 paraffin-embedded valve sections and were positive for 8 (29%) and 13 (46%) of them, respectively.
Overall, the IFA and Western blotting exhibited sensitivities of 58% (59/102) and 100% (72/72), respectively, and PCR from valvular, blood, and serum samples exhibited sensitivities of 92% (48/52), 33% (20/60), and 36% (25/70), respectively. As 33% of our cases presented Bartonella endocarditis with low antibody titers (IgG < 1:800), we reevaluated the positive predictive value (PPV) of the Bartonella IgG titer for an endocarditis diagnosis. We included 461 patients with positive serology for Bartonella spp. from our laboratory between 2005 and 2013, and we defined cases of endocarditis according to serological or PCR findings, as described above. The PPVs for the diagnosis of endocarditis according to the IgG titers were 20%, 32%, 54%, 94%, 91%, and 100% for IgG titers ranging between 1:100 and 1:6,400, 1:200 and 1:6,400, 1:400 and 1:6,400, 1:800 and 1:6,400, 1:1600 and 1:6,400, and 1:3200 and 1:6,400 (Table 2), respectively.
TABLE 2.
Positive predictive value (PPV) of Bartonella IgG titers for patients with endocarditis, France, 2005–2013
| Patients (n = 461) | Result for patients with IgG titer range of: |
|||||
|---|---|---|---|---|---|---|
| 1:100–1:6,400 | 1:200–1:6,400 | 1:400–1:6,400 | 1:800–1:6,400 | 1:1,600–1:6,400 | 1:3,200–1:6,400 | |
| No. of patients with definite endocarditis | 93 | 87 | 72 | 59 | 32 | 9 |
| Total no. of patients | 461 | 267 | 133 | 63 | 35 | 9 |
| PPV, definite endocarditis (%) | 20 | 32 | 54 | 94 | 91 | 100 |
The species causing endocarditis was identified in 91 patients (Table 3). Four Bartonella species were identified as causative agents of endocarditis in our study. The two main etiologic agents were B. quintana, which was detected in 48 cases (53%), and B. henselae, which was detected in 39 cases (43%). We also diagnosed 3 cases (3%) of B. alsatica and 1 case (1%) of B. vinsonii subsp. berkhoffii infections.
TABLE 3.
Epidemiologic features and biological data of the 91 patients with endocarditis induced by Bartonella spp. identified to the species level
| Species | No. of cases | Mean age (yr) | Sex ratio (no. male/no. female) | No. of samples positive in the indicated test/total no. of samples tested |
||||
|---|---|---|---|---|---|---|---|---|
| IFA with IgG ≥ 1:800 | Western blotting | PCR on valve sample | PCR on blood sample | PCR on serum sample | ||||
| B. quintana | 48 | 54 | 43/5 | 28/47 | 35/35 | 26/27 | 13/28 | 13/32 |
| B. henselae | 39 | 49 | 26/13 | 19/36 | 28/28 | 19/20 | 6/24 | 12/24 |
| B. alsatica | 3 | 64 | 2/1 | 0/3 | 3/3 | 1/1 | 0/3 | 0/1 |
| B. vinsonii | 1 | 19 | 1/0 | 1/1 | 1/1 | ND | 1/1 | 0/1 |
The mean age (± the standard deviation [SD]) of the 48 patients with B. quintana endocarditis was 54 ± 14 years, and 43 (90%) were male. Epidemiologic information was not available for all 48 patients, but we identified 18 homeless individuals, 8 alcoholic men, 4 intravenous drug users, and 3 patients who had contact with body lice. Seventeen (35%) patients originated from Marseille, 24 (50%) from other parts of France, and 7 (15%) from other countries (2 cases from Ireland and 1 case each from Belgium, Chile, Great Britain, Italy, and the United States). IFA titers of ≥1:800 were observed in 28/47 (60%) B. quintana cases, and Western blotting was positive in 100% of the cases. PCR of valve samples was positive in 26/27 (96%) tested patients. PCR of the blood samples was positive in 13/28 (46%) cases, and PCR of the serum samples was positive in 13/32 (41%) cases.
The mean age (±SD) of the 39 patients with B. henselae endocarditis was 49 ± 21 years, and 26 patients (67%) were male. Twenty-two patients (56%) reported contact with cats. Six patients (15%) originated from Marseille, 30 (77%) from other cities in France, and 3 (8%) from other countries (Belgium, Thailand, and the United States). IFA titers of ≥800 were obtained in 19/36 (53%) B. henselae cases, PCR of valve samples was positive in 19/20 (95%) cases, PCR of blood was positive in 6/24 (25%) tested patients, and PCR of serum samples was positive in 12/24 (50%) cases.
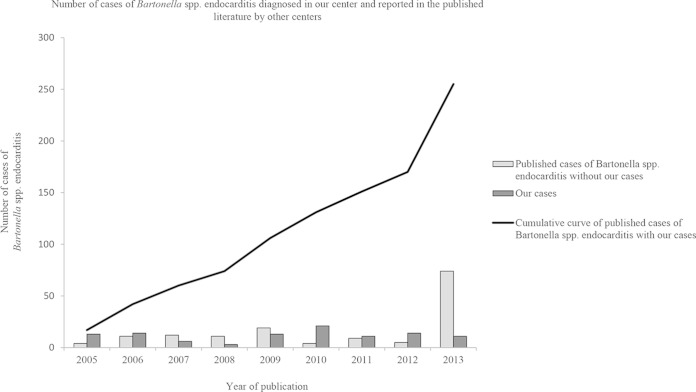
The annual number of cases diagnosed in our laboratory did not increase during the 9-year study period. We diagnosed a mean (±SD) of 12 ± 6 Bartonella endocarditis cases per year, with a minimum of 3 in 2008 and a maximum of 21 in 2010. Following a literature review from January 2005 to October 2013 (see supplemental material), we observed that the number of cases reported in the literature doubled every 1.5 years, reaching a cumulative number of 196 cases in 2013 (Fig. 2). In this study, we added 59 unpublished cases. Between 2005 and 2006, we reported more than half of the published cases; however, since 2007, we observed a slight increase in the number of cases reported by other centers, with a more important accentuation in 2013. Using a chi-square test to examine the trend, we found that the number of cases reported in the literature by other centers (excluding ours) is increasing significantly more rapidly than the number of cases diagnosed at our French reference center (P < 10−2) (Fig. 2).
FIG 2.
Number of cases of endocarditis induced by Bartonella spp. between January 2005 and October 2013 diagnosed in our center and number of cases reported in the published literature by other centers. A cumulative frequency curve of the total cases of endocarditis induced by Bartonella spp. was generated. Chi-square test for trends: P < 10−2.
DISCUSSION
Here, we report a large series of 106 patients diagnosed in our laboratory with Bartonella endocarditis. We employed a strategy of performing strict, validated protocols with specific RT-PCR, serology by IFA or Western blot analysis, and immunohistochemistry to avoid false-positive and false-negative results and to increase the sensitivity of diagnosis. The combination of these different techniques provided a specificity of 100%. In our study, the IFA contributed to the diagnosis of endocarditis induced by Bartonella spp. in 91% of the tested samples but was confirmatory with IgG titers of ≥800 in only 58% of cases. Western blotting provided the diagnosis in 100% of the tested samples, and RT-PCR and 16S rRNA PCR of valvular specimens were positive in 92% and 60% of the tested samples, respectively.
Serological testing is the easiest and most frequently used tool for the laboratory diagnosis of Bartonella endocarditis. IFA is the reference method, despite the cross-reactivity among Bartonella spp. and with Chlamydia spp. and C. burnetii (22, 29). All the samples in our study were also tested by serology and PCR for C. burnetii. Only 2 patients presented a cross-reaction with C. burnetii by IFA, but Bartonella endocarditis was determined through a positive Western blot analysis and a positive PCR on sera and cardiac valves, respectively. In our center, we have estimated that an IgG titer of ≥1:800 has a positive predictive value of 95% in patients with infective endocarditis (22). However, in this study, we observed that such a titer was found in fewer than 60% of the cases. Therefore, an IgG titer of <800 does not exclude the diagnosis of Bartonella endocarditis in patients with valvulopathy. Moreover, in our study, we found 8 cases of endocarditis caused by Bartonella spp. with a negative IFA for which the serological diagnosis was confirmed only by Western blotting. Western blotting exhibited a sensitivity of 100%, as it was positive for all patients from whom direct evidence of Bartonella endocarditis was obtained by PCR. In a previous study, we demonstrated the high PPV of Western blotting for Bartonella endocarditis (23). Our study suggests that any patient with a Bartonella IgG titer of <800 and a medical history evocative of endocarditis should be tested by Western blotting and RT-PCR following cardiac valve removal.
RT-PCR is a useful, sensitive, specific, and rapid tool for the diagnosis of Bartonella endocarditis. We observed a high sensitivity of RT-PCR for valvular biopsy specimens (92%), whereas this technique exhibited lower sensitivities when applied to blood and serum samples (33% and 36%, respectively). However, RT-PCR on blood may enable the diagnosis before cardiac surgery. Our results confirmed those from a previous study in which a positive PCR result was found in 97.8% of the available valvular biopsy specimens, despite the fact that 62.2% of the specimens were obtained from patients receiving antibiotic therapy (8). Similar results demonstrating the high value of RT-PCR on valves in the diagnosis of Bartonella endocarditis were recently reported by Chaloner et al. (30), with positive results in 13 of 14 patients. Furthermore, as previously reported (19, 31), RT-PCR is significantly more sensitive than 16S rRNA gene amplification for the diagnosis of Bartonella endocarditis in valvular tissues and blood.
Immunohistochemistry and Warthin-Starry staining were the less sensitive techniques. In our series, Warthin-Starry staining was the most sensitive of these 2 methods. Five samples were positive for Warthin-Starry staining and negative in the immunohistochemical analysis; however, this histologic stain is not specific for Bartonella spp. Because the infective process may be confined, a negative immunohistochemistry result does not definitively exclude the diagnosis of Bartonella endocarditis (27). Development of a technique based on fluorescence in situ hybridization would increase the sensitivity of detection of bacteria and in assessing their viability by bacterial RNA detection.
Bartonella endocarditis occurs in people with preexisting valvular abnormalities that promote the development of infective endocarditis and generates significant destruction of the valve; therefore, valvular surgery is required in more than 90% of cases, which is higher than that required for patients with endocarditis caused by other pathogens. In our study, we found a rate of valve replacement inferior to those described earlier, but information on this criterion is missing in our study, and this number is probably underestimated. However, we did not observe a significant difference in the rates of valvular surgery depending on the species of Bartonella (56% for B. quintana, 51% for B. henselae, and 33% for B. alsatica).
The number of reported cases of Bartonella endocarditis appears to have increased between 2005 and 2013 (Fig. 2), and in this study, we added 59 cases to the 196 previously reported to reach a cumulative number of 255 cases. The emergence of an infectious disease can usually be linked to one or more of the following factors: an increase in clinician interest in the infection, an improvement in diagnostic techniques, or a real increase in incidence (32). If we consider the distribution of sera tested for Bartonella spp. and the numbers of cases of Bartonella endocarditis in our laboratory from 2005 to 2013, an increase in the diagnosed cases was not observed. The observation of an increasing number of cases in the literature by our group and other centers can be explained by the fact that clinicians request more Bartonella tests for patients with endocarditis. Therefore, we believe that there is no real increase in the incidence of these infections but rather a better understanding of and interest in the disease resulting from the improvement of diagnostic tools.
The strategy for diagnosing Bartonella endocarditis is not clearly established, and specific diagnostic tools were available in only a few laboratories. We showed recently that expert centers play a key role in improving the diagnosis, management, and prevention of Q fever endocarditis (33, 34). In 2012, a new score based on evidence of endocardial involvement and microbiological results was proposed specifically for the diagnosis of Q fever endocarditis (35). Currently, there is a lack of criteria for the diagnosis of Bartonella endocarditis, and we think that the establishment of a new score-based diagnosis would be a valuable tool for clinicians in diagnosing Bartonella endocarditis. We have demonstrated that RT-PCR from valve samples can be a major element in the diagnosis of these infections, and we suggest that a positive PCR result from a valvular biopsy specimen can be considered a definite criterion (28). In addition, we suggest that a positive PCR from blood, an IgG titer of ≥800 using an IFA, and a positive Western blot analysis may be considered major criteria in patients with BCNE.
Supplementary Material
ACKNOWLEDGMENT
We declare no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02827-14.
REFERENCES
- 1.Chomel BB, Kasten RW, Williams C, Wey AC, Henn JB, Maggi R, Carrasco S, Mazet J, Boulouis HJ, Maillard R, Breitschwerdt EB. 2009. Bartonella endocarditis: a pathology shared by animal reservoirs and patients. Ann N Y Acad Sci 1166:120–126. doi: 10.1111/j.1749-6632.2009.04523.x. [DOI] [PubMed] [Google Scholar]
- 2.Edouard S, Raoult D. 2010. Bartonella henselae, an ubiquitous agent of proteiform zoonotic disease. Med Mal Infect 40:319–330. doi: 10.1016/j.medmal.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Spach DH, Callis KP, Paauw DS, Houze YB, Schoenknecht FD, Welch DF, Rosen H, Brenner DJ. 1993. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol 31:692–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O'Connor SP. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 31:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadfield TL, Warren R, Kass M, Brun E, Levy C. 1993. Endocarditis caused by Rochalimaea henselae. Hum Pathol 24:1140–1141. doi: 10.1016/0046-8177(93)90196-N. [DOI] [PubMed] [Google Scholar]
- 6.Drancourt M, Mainardi JL, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med 332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 7.Brouqui P, Raoult D. 2006. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol 47:1–13. doi: 10.1111/j.1574-695X.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Lelievre H, Eykyn SJ, Mainardi JL, Marrie TJ, Bruneel F, Roure C, Nash J, Clave D, James E, Benoit-Lemercier C, Deforges L, Tissot-Dupont H, Raoult D. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245–251. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Roux V, Eykyn SJ, Wyllie S, Raoult D. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol 38:1698–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olarte L, Ampofo K, Thorell EA, Sanderson S, Doby E, Pavia AT, Rosado H, Raoult D, Socolovschi C, Hersh AL. 2012. Bartonella vinsonii endocarditis in an adolescent with congenital heart disease. Pediatr Infect Dis J 31:531–534. doi: 10.1097/INF.0b013e31824ba95a. [DOI] [PubMed] [Google Scholar]
- 11.Fenollar F, Sire S, Raoult D. 2005. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol 43:945–947. doi: 10.1128/JCM.43.2.945-947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avidor B, Graidy M, Efrat G, Leibowitz C, Shapira G, Schattner A, Zimhony O, Giladi M. 2004. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol 42:3462–3468. doi: 10.1128/JCM.42.8.3462-3468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, Choutet P. 2006. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol 44:278–279. doi: 10.1128/JCM.44.1.278-279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeanclaude D, Godmer P, Leveiller D, Pouedras P, Fournier PE, Raoult D, Rolain JM. 2009. Bartonella alsatica endocarditis in a French patient in close contact with rabbits. Clin Microbiol Infect 15(Suppl):S110–S111. doi: 10.1111/j.1469-0691.2008.02187.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin EY, Tsigrelis C, Baddour LM, Lepidi H, Rolain JM, Patel R, Raoult D. 2010. Candidatus Bartonella mayotimonensis and endocarditis. Emerg Infect Dis 16:500–503. doi: 10.3201/eid1603.081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benslimani A, Fenollar F, Lepidi H, Raoult D. 2005. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis 11:216–224. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Znazen A, Rolain JM, Hammami N, Kammoun S, Hammami A, Raoult D. 2005. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg 72:503–507. [PubMed] [Google Scholar]
- 18.Brouqui P, Raoult D. 2001. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 14:177–207. doi: 10.1128/CMR.14.1.177-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, Maurin M, Célard M, Mainardi JL, Caus T, Collart F, Habib G, Raoult D. 2010. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 20.Houpikian P, Raoult D. 2005. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 21.Maurin M, Rolain JM, Raoult D. 2002. Comparison of in-house and commercial slides for detection by immunofluorescence of immunoglobulins G and M against Bartonella henselae and Bartonella quintana. Clin Diagn Lab Immunol 9:1004–1009. doi: 10.1128/CDLI.9.5.1004-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier PE, Mainardi JL, Raoult D. 2002. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol 9:795–801. doi: 10.1128/CDLI.9.4.795-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houpikian P, Raoult D. 2003. Western immunoblotting for Bartonella endocarditis. Clin Diagn Lab Immunol 10:95–102. doi: 10.1128/CDLI.10.1.95-102.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelakis E, Rolain JM, Raoult D, Brouqui P. 2011. Bartonella quintana in head louse nits. FEMS Immunol Med Microbiol 62:244–246. doi: 10.1111/j.1574-695X.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 25.Roux V, Raoult D. 1995. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J Clin Microbiol 33:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. 2000. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol 38:3623–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepidi H, Fournier PE, Raoult D. 2000. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol 114:880–889. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 28.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 29.La Scola B, Raoult D. 1996. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol 34:2270–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaloner GL, Harrison TG, Birtles RJ. 2013. Bartonella species as a cause of infective endocarditis in the UK. Epidemiol Infect 141:841–846. doi: 10.1017/S0950268812001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edouard S, Million M, Lepidi H, Rolain JM, Fournier PE, La Scola B, Grisoli D, Raoult D. 2013. Persistence of DNA in cured patient and positive culture in cases with low antibody levels questioned the diagnosis of Q fever endocarditis. J Clin Microbiol 51:3012–3017. doi: 10.1128/JCM.00812-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raoult D. 2009. Reemergence of Q fever after 11 September 2001. Clin Infect Dis 48:558–559. doi: 10.1086/596706. [DOI] [PubMed] [Google Scholar]
- 33.Edouard S, Million M, Royer G, Giorgi R, Grisoli D, Raoult D. 2014. Reduction in incidence of Q fever endocarditis: 27 years of experience of a national reference center. J Infect 68:141–148. doi: 10.1016/j.jinf.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Million M, Walter G, Thuny F, Habib G, Raoult D. 2013. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis 57:836–844. doi: 10.1093/cid/cit419. [DOI] [PubMed] [Google Scholar]
- 35.Raoult D. 2012. Chronic Q fever: expert opinion versus literature analysis and consensus. J Infect 65:102–108. doi: 10.1016/j.jinf.2012.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.