FAST-TRACK COMMUNICATION
In the late summer and fall of 2014, the province of Alberta, Canada (4.1 million people), was in the midst of peak entero-rhinovirus (ERV) activity, as descriptions of enterovirus D68 (EV-D68) activity were being publicized (1, 2). This analysis was undertaken with several goals: (i) to understand the burden of respiratory illness in emergency department (ED) visits on <18-year-old Albertans in the periods from 14 August to 10 September in both 2013 and 2014; (ii) to understand the impact of enteroviruses, including EV-D68, on pediatric hospital patients (from 14 August to 10 September 2014, when rates of ERV activity were increasing); and (iii) to determine whether those infected with EV-D68 are more likely to have asthma or other respiratory illness than those with EV of a strain other than D68. This analysis was initiated as a public health investigation and did not require ethics approval, and all clinical specimens and data were collected for the purposes of routine ongoing diagnostics and surveillance.
To compare the burdens of respiratory illness in Alberta, we analyzed the ED data for <18-year-old Albertans visiting ED facilities in Edmonton and Calgary, Canada. Data for ED visitors who screened positive for influenza-like illness (ILI) and whose chief complaints were shortness of breath, cough/congestion, and wheezing were extracted from the Alberta Real Time Syndromic Surveillance Net (ARTSSN) (3) for the periods from 14 August to 10 September in both 2013 and 2014. The numbers of ED visits per day were analyzed using Poisson regression to assess if the differences were statistically significant. All analyses were performed using SAS version 14. Case definitions for ERV are not standardized; however, within this investigation, they were defined as an acute symptom(s) of respiratory tract infection and laboratory confirmation of EV-D68 from the first clinical specimen.
For the time period from 14 August to 10 September 2014, as per routine testing approaches, all specimens received at the Provincial Laboratory for Public Health (ProvLab) were routinely tested for influenza A and influenza B viruses and then excluded from further testing if they were readily identifiable as collected from community settings. This time period was chosen for further EV-D68 analysis, as this was the period in our jurisdiction when public health services noticed increased ERV activity. Respiratory testing was dependent on clinician-directed ordering and was focused primarily on hospitalized patients. All specimens were tested for influenza A and influenza B viruses; if specimens were found to be negative, they were tested by the xTAG respiratory viral panel (RVP; Luminex, Austin, TX, USA) (4). All ERV-positive specimens were then tested by a real-time enterovirus/parechovirus (EPV) assay that does not cross-detect rhinoviruses; when a specimen was enterovirus positive, a sequencing assay was used to type EV-D68 (2, 5).
Prior to this analysis, we had tested the EV-D68 specimens stored in our laboratory with both the Luminex RVP and enterovirus reverse transcription (RT)-PCR, and in both assays, specimens were reactive. We were also part of a collaborative project across Canada using the Fermon strain of EV-D68 (ATCC VR1076) and found that the analytical sensitivities of both methods using Probit regression are roughly equivalent to each other (real-time enterovirus assay, 3.45 log10 copies/ml; RVP classic assay, 3.43 log10 copies/ml). Both assays also performed well in comparison to other technologies (unpublished data).
Cases were grouped into three categories: (i) ERV positive but enterovirus negative, (ii) enterovirus positive but not for strain D68, and (iii) EV-D68 positive. Hospitalizations, stays in an intensive care unit (ICU), asthma, and respiratory conditions were assessed via linkage to the Supplemental Enhanced Service Event (SESE) system (physician claims) and the Morbidity and Ambulatory Care Abstract Reporting (MACAR) databases held by the Alberta Ministry of Health. Asthma was identified using a validated case definition for asthma that has been modified to ensure that two or more physician claims are used to identify a case and that the claims are at least 30 days apart (6). Respiratory conditions were defined as an inpatient visit with an International Statistical Classification of Diseases and Related Health Problems of ICD-9 (diagnostic codes 460 to 519 [diseases of the respiratory system]) in the previous year but prior to 30 days before the patient was identified as having ERV, enterovirus of a strain other than D68, or EV-D68 and excludes any asthma diagnoses (ICD-9 diagnostic code 460).
Compared to daily ED visits in the 2013 period, daily ED visits during the study period in 2014 were significantly higher for ILI (in 2013, n = 922; in 2014, n = 1,078; relative risk [RR] = 1.17; 95% confidence intervals [95% CIs] = 1.07, 1.28), shortness of breath (in 2013, n = 519; in 2014, n = 645; RR = 1.24; 95% CIs = 1.11, 1.40), and cough/congestion (in 2013, n = 1,005; in 2014, n = 1,195; RR = 1.19; 95% CIs = 1.09, 1.14).
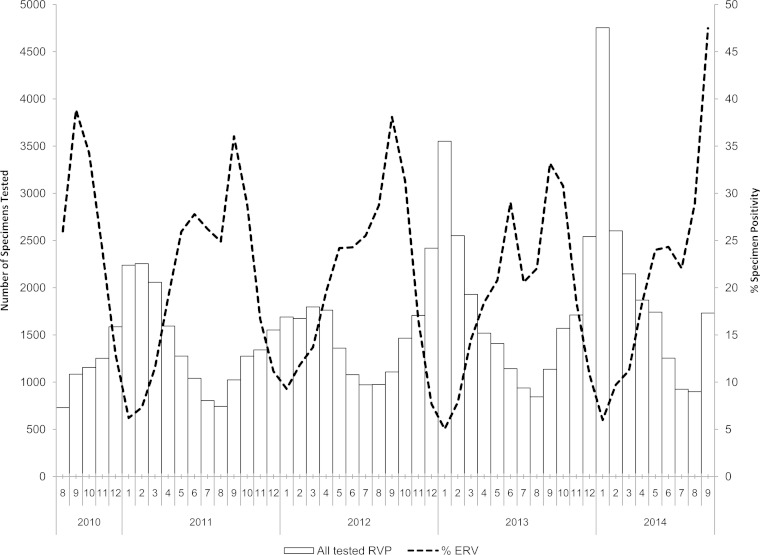
ERV-positive specimen counts and relative positivity rates are shown in Fig. 1. Following conversion of ERV specimen counts to case counts, ERV cases were further categorized in Table 1, and they exhibited a trend toward an increased risk for asthma in EV-D68 cases regardless of analysis approach.
FIG 1.
Distribution of non-influenza A/B respiratory viruses detected by the Luminex RVP from 2010 to 2014.
TABLE 1.
Characterization of laboratory-confirmed EPV-negative, untypeable non-D68 EV-positive, and EV-D68-positive casesa
| Group (n = 210) | Median age (mo) | No. of cases (% males) | No. (%) hospitalized | No. (%) admitted to ICU | No. (%) with asthma | No. (%) with respiratory disease |
|---|---|---|---|---|---|---|
| Enterovirus/parechovirus negative (n = 128) | 13 | 70 (55) | 78 (61) | 18 (14) | 10 (8) | 53 (41) |
| Non-D68 enterovirus (n = 33) | 12 | 18 (55) | 19 (58) | 2 (6) | 6 (18) | 0 |
| EV-D68 (n = 49) | 72 | 27 (55) | 38 (78) | 2 (4) | 15 (31) | 36 (73) |
Data are for cases tested for respiratory viral pathogens from 14 August to 10 September 2014 in Alberta, Canada. Specimens were not available for the same time period in 2013. Of the non-D68 enterovirus cases (n = 33), 12 could be typed as coxsackievirus A6 (n = 7), echovirus 18 (n = 1), echovirus 25 (n = 1), EV 104 (n = 1), EV 71 (n = 1), and a mixture of coxsackievirus A6 and rhinovirus A (n = 1). The remaining enterovirus-positive cases could not be typed because of the low viral load.
From the time period of 14 August to 10 September 2014, 210 pediatric cases of ERV were identified and are characterized in Tables 1 and 2. In Table 1, of the 210 cases, 61% (n = 128) were ERV negative, 16% (n = 33) were enterovirus positive but not for strain D68, and 23% (n = 49) were EV-D68 positive. Of those positive for enterovirus but not for D68 (n = 33), 11 specimens could be typed as coxsackievirus A6 (n = 7), echovirus 18 (n = 1), echovirus 25 (n = 1), EV 104 (n = 1), and EV 71 (n = 1). Twelve specimens were enterovirus positive but could not be typed because of the low viral load. A further 10 specimens contained enteroviruses, with 1 specimen containing a mixture of coxsackievirus A6 and rhinovirus A and the remaining 9 specimens containing an enterovirus that could not be typed, as well as rhinovirus C (n = 6), rhinovirus A12 (n = 1), rhinovirus A19 (n = 1), and rhinovirus A44 (n = 1).
TABLE 2.
Characterization of laboratory-confirmed EPV-negative, typeable non-D68 enterovirus-positive, and EV-D68-positive casesa
| Group (n = 189) | Median age (mo) | No. of cases (% males) | No. (%) hospitalized | No. (%) admitted to ICU | No. (%) with asthma | No. (%) with respiratory disease |
|---|---|---|---|---|---|---|
| Enterovirus/parechovirus negative (n = 128) | 13 | 70 (55) | 78 (61) | 18 (14) | 10 (8) | 53 (41) |
| Non-D68 enterovirus (n = 12) | 12 | 4 (33) | 7 (58) | 0* | 0* | 0* |
| EV-D68 (n = 49) | 72 | 27 (55) | 38 (78) | 2 (4) | 15 (31) | 36 (73) |
Data are for cases tested for respiratory viral pathogens from 14 August to 10 September 2014 in Alberta, Canada. Specimens were not available for the same time period in 2013. Untypeable enteroviruses were removed from the analysis to avoid the potential of missing a low-titer D68 enterovirus. *, numbers are too small for statistical analysis.
When the 33 non-D68 enterovirus cases from Table 1 were analyzed as a group and compared to EV-D68 cases, the odds of presenting with asthma for EV-D68 was greater than for non-D68 enterovirus (odds ratio [OR], 2.0; 95% CIs, 0.7, 5.8). Due to a selection bias for RVP testing in hospitalized patients, they were oversampled in this analysis. However, not all cases were hospitalized; 38/49 (78%) EV-D68 cases and 19/33 (58%) non-D68 enterovirus cases were hospitalized. EV-D68 cases were no more at risk for ICU admission (ICU admission, 2/49 [4%]) than patients infected with non-D68 enterovirus (ICU admission, 2/36 [6%]) (Fisher's exact test, P = 0.53). None of these children were placed on a ventilator or died.
Due to concerns that some of the untyped enteroviruses included in Table 1 might in fact be low titers of enterovirus D-68, the untypeable enteroviruses were removed from the non-D68 enterovirus group and are presented in Table 2. When the nontypeable enterovirus cases were removed from the analysis, 189 cases remained in the study for analysis (Table 2). This step removed all specimens that were non-D68 enterovirus from the ICU, asthma, and respiratory disease categories; consequently, the OR comparison between this group and the EV-D68 group for these categories could not be done. In this analysis, we also stress that the “untypeable” EVs could not be typed solely because of a low viral load. However, Table 2 clearly shows that patients infected with EV-D68 were more likely to have asthma or an underlying respiratory illness than patients infected with non-D68 EV.
The period from 14 August to 10 September 2014 was selected as a time frame for analysis, as this was a time period prior to extensive press and public health communication on EV-D68 activity. Our study indicates an increase in daily ED visits for <18-year-old Albertans for ILI, shortness of breath, and cough/congestion that was significantly higher (RR, 1.24; 95% CIs, 1.11, 1.40) in 2014 than during the same period of time in 2013. Previous studies have identified increases in asthma admissions in Canada and other countries during the period of July to October (7, 8). Seasonal upswings in ERV activity, while other respiratory viral pathogens are routinely at negligible levels, in time frames matching increases in ERV activity have been identified in our province, as seen in Fig. 1 and in other locations (9). Much of the originally released information on emergency department or hospital visits as a result of ILI during August and September 2014 was based on reports from individual hospitals or groups of hospitals identifying increases in ILI or asthma exacerbation visits to emergency departments (1). Although it may be that ILI increases in our region may not have been as dramatic as in other regions (10), we did see significant increases that also occurred at the same time that physicians were individually notifying public health officials that there was a perceived increase as well. We feel that our approach is warranted, as simple fold increases compared across jurisdictions may not take into account differences in baseline health care delivery, where that health care is delivered, and whether universal health care systems are utilized.
In our study, we note that although the study numbers were too low to determine a difference in illness between patients with EV-D68 and non-D68 enteroviruses for many of the categories, there seemed to be a trend toward asthma that was greater in patients with EV-D68 than in patients with non-D68 enterovirus. This is comparable to other trends identified in the U.S. Midwest (1) and in other locations prior to 2014 (11). Unlike with other reports in the United States (1), we saw relatively few ICU admissions for EV-D68 patients and no deaths or clusters of severe illness (12).
Limitations in the study include the selection bias for a high proportion of hospitalized cases. This is due to the testing approach in our jurisdiction, which utilizes RVP testing primarily for hospitalized cases while undertesting community patients. Despite this, there were cases of EV-D68 found in the community, indicating that mild cases exist. From this analysis, it is evident that we must clearly define how these viral infections are diagnosed, characterized, and categorized. In some specimens, enteroviruses were detected due to the high sensitivity of the assay used but could not be typed, chiefly because of the low viral load. Since it is unclear what fraction of these isolates are EV-D68 and since EV-D68 has been shown to cocirculate with other enteroviruses, we have used two different approaches for analysis. Groups undertaking these types of analyses should be aware that different diagnostic approaches may impact our ability to clearly identify EV-D68 and rule out its presence in specimens from comparator groups containing enterovirus. Although numbers were low and it was difficult to make strong statistical conclusions, similar trends were shown for the increased presence of EV-D68 in asthma patients regardless of the categorization approach for non-D68 enterovirus patients.
This analysis described the presence of EV-D68 in our province during an increase in ERV activity as well as an increase in respiratory disease visits to EDs. As in other locations, there is a spectrum of illness, with cases ranging from those found in community settings to ED visits due to respiratory disease and severe illness, with some patients being admitted to the ICU (1, 13). Although the ILI increases were not as large as in other regions in 2014, we also noted an increase in ERV positivity in September 2014 (45 to 50% increases in September 2014 versus 35 to 40% increases in the same period in other years). Specimen loads in September also increased approximately 50% compared to loads in other years. In contrast, the number of August 2014 specimens tested and ERV positivity were similar to those in other years (Fig. 1). We cannot confidently determine whether this is a unique presentation of this virus in Alberta, Canada. Due to our different testing approaches over time (EV-D68 testing was done only in 2014) it is unclear in what proportions EPVs and EV-D68 have existed within our province historically. Additionally, a lack of nonbiased historical specimens in pediatric patients from our province makes retrospective analysis difficult. We note that EV-D68, non-D68 enterovirus, and ERV are not routinely reportable to public health agencies on a case-by-case basis, although public health officials do track general trends of ERV in Alberta on a routine basis. In our province, the chief medical officer of health has the authority to track emerging pathogens of public health concern, and we believe that this investigation will act as a framework for future public health investigations linking the various arms of public health, including diagnostic virology laboratories
ACKNOWLEDGMENTS
We thank all members of the Molecular Diagnostics and Virology Laboratories at the Edmonton and Calgary ProvLab sites. The typing was done with the assistance of Kara Gill and Danielle Zarra, who work as technologists at the ProvLab, Alberta, Calgary, Canada, site.
Our work was supported by operational funding from Alberta Health Services and Alberta Health.
We declare no conflict of interests.
REFERENCES
- 1.Midgley CM, Jackson MA, Selvarangan R, Turabelidze G, Obringer E, Johnson D, Giles BL, Patel A, Echols F, Oberste MS, Nix WA, Watson JT, Gerber SI. 2014. Severe respiratory illness associated with enterovirus d68—Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 2.Fonseca K, Kellner JD, Talbot J, Simmonds K, Anselmo M, Kuhn S, Drews S, Fathima S, Pabbaraju K, Tipples G, Tellier R, Wong S. 19 September 2014. Human enterovirus 68—Canada: (Alberta). ProMED-mail archive no. 20140919.2788704. [Google Scholar]
- 3.Fan S, Blair C, Brown A, Gabos S, Honish L, Hughes T, Jaipaul J, Johnson M, Lo E, Lubchenko A, Mashinter L, Meurer DP, Nardelli V, Predy G, Shewchuk L, Sosin D, Wicentowich B, Talbot J. 2010. A multi-function public health surveillance system and the lessons learned in its development: the Alberta Real Time Syndromic Surveillance Net. Can J Public Health 101:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaramillo-Gutierrez G, Benschop KS, Claas EC, de Jong AS, van Loon AM, Pas SD, Pontesilli O, Rossen JW, Swanink CM, Thijsen S, van der Zanden AG, van der Avoort HG, Koopmans MP, Meijer A. 2013. September through October 2010 multi-centre study in the Netherlands examining laboratory ability to detect enterovirus 68, an emerging respiratory pathogen. J Virol Methods 190:53–62. doi: 10.1016/j.jviromet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Pabbaraju K, Wong S, Chan EN, Tellier R. 2013. Genetic characterization of a Coxsackie A9 virus associated with aseptic meningitis in Alberta, Canada in 2010. Virol J 10:93. doi: 10.1186/1743-422X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muggah E, Graves E, Bennett C, Manuel DG. 2013. Ascertainment of chronic diseases using population health data: a comparison of health administrative data and patient self-report. BMC Public Health 13:16. doi: 10.1186/1471-2458-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dales RE, Schweitzer I, Toogood JH, Drouin M, Yang W, Dolovich J, Boulet J. 1996. Respiratory infections and the autumn increase in asthma morbidity. Eur Respir J 9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 8.Scheuerman O, Meyerovitch J, Marcus N, Hoffer V, Batt EE, Garty BZ. 2009. The September epidemic of asthma in Israel. J Asthma 46:652–655. doi: 10.1080/02770900902963102. [DOI] [PubMed] [Google Scholar]
- 9.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. 2006. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ 55:1–20. [PubMed] [Google Scholar]
- 10.Brooks D. 2014. Influx of patients with asthma-like symptoms strains resources in many pediatric EDs. ED Manag 26:121–124. [PubMed] [Google Scholar]
- 11.Hasegawa S, Hirano R, Okamoto-Nakagawa R, Ichiyama T, Shirabe K. 2011. Enterovirus 68 infection in children with asthma attacks: virus-induced asthma in Japanese children. Allergy 66:1618–1620. doi: 10.1111/j.1398-9995.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson LM, Redd JT, Schneider E, Lu X, Chern SW, Oberste MS, Erdman DD, Fischer GE, Armstrong GL, Kodani M, Montoya J, Magri JM, Cheek JE. 2012. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J 31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- 13.Linsuwanon P, Puenpa J, Suwannakarn K, Auksornkitti VV, Vichiwattana P, Korkong S, Theamboonlers A, Poovorawan Y. 2012. Molecular epidemiology and evolution of human enterovirus serotype 68 in Thailand, 2006–2011. PLoS One 7:e35190. doi: 10.1371/journal.pone.0035190. [DOI] [PMC free article] [PubMed] [Google Scholar]