Abstract
This study aimed to evaluate the serodiagnostic potential of immunoglobulin G (IgG) responses to Mycobacterium tuberculosis antigens in pulmonary tuberculosis (TB) patients, recent TB contacts with latent TB infection (LTBI), and healthy subjects. Infections were assessed using tuberculin skin tests, QuantiFERON-TB Gold In-Tube tests, drug susceptibility testing, and molecular genotyping of clinical isolates. Serum IgG responses to selective M. tuberculosis antigens, including the 38-kDa and 16-kDa antigens, lipoarabinomannan (LAM), and recombinant early secreted antigen target 6 kDa (ESAT-6) and culture filtrate protein 10 kDa (CFP-10), were determined. We found that the serum IgG responses to all antigens might differentiate between active TB and LTBI, with LAM having the highest diagnostic value (area under the curve [AUC] of 0.7756, P < 0.001). Recurrent TB cases showed significantly higher IgG responses to 38 kDa, CFP-10 (P < 0.01), and LAM (P < 0.05) than new cases, and male patients had higher levels of antigen-specific IgG than females (P < 0.05). Conversely, drug resistance and patient body mass index did not affect IgG responses (P > 0.05). LAM-specific IgG responses differentiated between acid-fast bacillus (AFB) smear-positive and -negative patients (P < 0.01), whereas antigen-specific IgG responses did not vary with the M. tuberculosis genotype (P > 0.05). Significantly higher IgG responses to 38 kDa and 16 kDa were observed in AFB smear-negative patients than in controls. These results suggest that assessment of serum IgG responses to selective purified M. tuberculosis antigens may help improve the diagnosis of active TB, particularly for sputum smear-negative patients or recurrent cases, and these may also help to differentiate between active TB and LTBI.
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, remains a major global health problem and is a significant cause of morbidity and mortality worldwide (1). It is estimated that nearly one-third of the world's population is latently infected with M. tuberculosis (2), and around 8.6 million new cases were reported to the World Health Organization (WHO) globally in 2012 (1). About 3.6% of new cases are caused by multidrug-resistant (MDR) strains, and the levels of MDR-TB were found to be higher (∼20%) in patients previously treated for TB (3). Notably, a high risk of multidrug resistance and epidemic spread of TB in Asia are associated with the M. tuberculosis Beijing strain (4, 5).
Current TB diagnostics rely mostly on identification of M. tuberculosis clinical isolates by acid-fast bacillus (AFB) staining or culture (6). Although AFB smear staining allows rapid detection of mycobacteria in clinical specimens, it has relatively low sensitivity, with a greater failure rate in children and immunocompromised groups, such as the elderly and patients with AIDS (6). The culture method is more sensitive than AFB staining, but it takes several weeks to obtain results and requires laboratory facilities that may be unavailable in resource-limited settings (6). Immunological approaches, such as the tuberculin skin test (TST) and gamma interferon (IFN-γ) release assay (IGRA), have also been developed for detecting latent TB infection (LTBI). The IGRA has higher specificity than the TST due to the use of M. tuberculosis-specific peptide antigens, such as early secreted antigen target 6 kDa (ESAT-6), culture filtrate protein 10 kDa (CFP-10), and TB7.7 (7, 8). However, both the TST and IGRA failed to discriminate between LTBI and active TB in areas with a high burden of TB (9, 10), indicating that there is no gold standard for the differential diagnosis between active TB and LTBI.
Development of a serodiagnostic method for TB diagnosis has garnered considerable interest, as these assays are both simple and cost-effective; however, efforts have been hampered by inconsistent results (11). Notably, while serodiagnosis of TB by combining immunoglobulin classes was suggested as a useful point-of-care test in South Africa (12), poor performance of the commercial serological tests for diagnosing pulmonary TB was reported in India (13), and to date, no commercial serodiagnostic tests have demonstrated acceptable sensitivity and specificity for routine laboratory use. In previously developed tests, these have varied, depending on the recombinant antigens tested (14), and changes in antibody levels following successful anti-TB treatment also varied with different antigens (15–17). Inconsistent M. tuberculosis antigen-specific antibody titers were also found in populations with various levels of M. tuberculosis exposure (18). Moreover, antibody responses were much stronger in sputum smear-positive TB than in sputum smear-negative TB (19, 20). These reports indicate that when TB serodiagnostics are being developed, factors such as the antigens utilized, population variation, stage of infection, and bacillary load should be considered.
Based on the reports showing significantly higher sensitivities of the IgG test compared with the IgM, IgA, or IgG/IgM tests in response to mycobacterial antigens (21, 22), we aimed to evaluate the IgG responses to five different M. tuberculosis antigens, 38-kDa and 16-kDa antigens, ESAT-6, CFP-10, and lipoarabinomannan (LAM), in the sera of active TB patients, TB contacts with LTBI, and controls. We correlated antigen-specific IgG responses with infection state, TB recurrence, drug resistance, bacterial burden, M. tuberculosis genotype, and patient body mass index (BMI) and gender, in order to identify candidate antigens with clinical value for TB diagnosis and elucidate factors that may influence M. tuberculosis-specific IgG responses in TB patients.
MATERIALS AND METHODS
Enrollment of study subjects.
In total, 159 pulmonary TB patients, 51 recent TB contacts, and 133 healthy subjects were recruited at two hospitals in South Korea. From February 2011 to December 2012, 73 TB patients (mean age, 46 years) were enrolled at Mokpo National Hospital, and from November 2010 to December 2013, 86 TB patients (mean age, 32 years), 51 asymptomatic TB contacts (mean age, 44 years), and 133 healthy subjects (mean age, 31 years) were enrolled at Severance Hospital (Fig. 1). Informed written consent was obtained from all participants for interviews and for diagnostic and immunological tests. Active pulmonary TB was diagnosed by confirmation of M. tuberculosis strains from sputum smear/culture and chest X-ray (7), and the genotypes of infecting M. tuberculosis strains were confirmed by molecular genotyping (23) in 94 patients. The LTBI and control groups were defined based on results of TSTs and QuantiFERON-TB Gold In-Tube (QFT-IT) tests (7, 8). Among the 51 TB contacts, 26 showed positive IFN-γ responses in QFT-IT tests; double-positive responses for TSTs (≥10 mm) and QFT-IT tests were observed in 19 of the 26 TB contacts, while 7 of the 26 TB contacts had negative TST results (<10 mm). The control group consisted of 54 individuals with double-negative responses for TSTs (<10 mm) and QFT-IT tests among the 133 healthy individuals who had never had contact with TB patients. Subjects taking immunosuppressants or those with cancer, diabetes, or renal disease were excluded. Our final study population consisted of 94 TB patients, 26 contacts with LTBI, and 54 healthy controls (Fig. 1).
FIG 1.
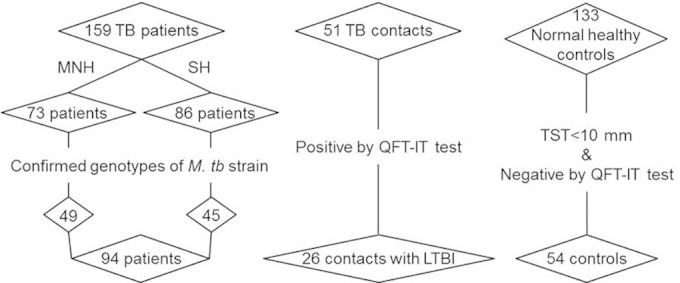
Enrollment of study participants. In total, 159 active TB patients, 51 TB contacts, and 133 healthy subjects were recruited from Mokpo National Hospital (MNH) and from Severance Hospital (SH). Of these, the genotypes of the infecting M. tuberculosis strains were confirmed in 94 TB patients. Based on QFT-IT tests and TSTs, 26 TB contacts were defined as individuals with LTBI and 54 normal healthy controls were identified.
Ethical approval was granted by the Mokpo National Hospital Ethics Review Committee (approval number 2010-001) and the Severance Hospital Ethics Review Committee (approval number 4-2010-0213).
TSTs.
An intradermal injection of tuberculin purified protein derivative (0.1 ml of RT-23; Statens Serum Institute, Copenhagen, Denmark) was given to all participants. Induration sizes were determined at 48 and 72 h, and measurements ≥10 mm were classified as positive.
Preparation of blood samples.
Serum samples were separated by centrifugation from 4 ml of blood (Vacuette serum tube; Greiner Bio-One GmbH, Frickenhausen, Germany). For QFT-IT tests, a total of 3 ml of blood was collected directly into each of three QFT-IT tubes: nil, M. tuberculosis antigen (Ag) tube (ESAT-6, CFP-10, and TB7.7 peptide antigens), and mitogen (phytohemagglutinin [PHA] (Cellestis, CA, USA). Plasma was harvested after incubation at 37°C for 24 h, and IFN-γ enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer's protocol (QuantiFERON-TB Gold, Cellestis).
Molecular genotyping of M. tuberculosis.
M. tuberculosis genotypes were determined by M. tuberculosis Beijing genotyping (Optipham-M&D, Wonju, South Korea) based on large-sequence polymorphism analysis (23). Briefly, genomic DNA was extracted by boiling a mycobacterial suspension scraped from Lowenstein-Jensen media slants (Becton Dickinson and Company, MD, USA) in 400 μl buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). DNA served as a template for gene amplification using a Veriti 96-well fast thermal cycler (Applied Biosystems, Life Technologies, CA, USA), and products were analyzed using agarose gel electrophoresis.
Purification of recombinant protein antigens.
The 38-kDa and 16-kDa antigens were provided by Standard Diagnostics, Inc. (Suwon, South Korea), and LAM was obtained from the Mycobacteria Research Laboratory, Colorado State University (Fort Collins, CO, USA). Vector construction for expression of recombinant ESAT-6 and CFP-10 was performed using the NdeI and BamHI (New England BioLabs, MA, USA) sites of pET11a_KB, and genes were amplified from M. tuberculosis H37Rv genomic DNA. Plasmids encoding ESAT-6 and CFP-10 were transformed into Escherichia coli BL21(DE3) for protein overexpression, and recombinant proteins were purified using nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen, CA, USA) and fast protein liquid chromatography (ÄKTAFPLC; GE Healthcare Life Sciences, PA, USA) with a Mono Q anion-exchange column (GE Healthcare Life Sciences).
Measurement of IgG responses to M. tuberculosis antigens.
M. tuberculosis antigens were coated in 96-well microplates (Corning Inc., NY, USA) at 2 μg/ml (38 kDa, 16 kDa, ESAT-6, and CFP-10) or 0.1 μg/ml (LAM) in 0.5 M carbonate-bicarbonate buffer overnight at 4°C. Plates were washed three times in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 20 (PBST) and blocked with PBST containing 5% normal goat serum (PBST-NGS) at 37°C for 1 h. Blocked plates were incubated with human serum samples (1:300 dilution in PBST-NGS) at 37°C for 2 h, washed again with TBST, and incubated with peroxidase-conjugated anti-human IgG antibody (1:10,000 dilution in PBST-NGS; Calbiochem, CA, USA) at 37°C for 1 h. Plates were washed again with TBST, and tetramethylbenzidine (KPL, MD, USA) was added. The reaction was stopped with 2.5 N H2SO4, and the optical density (OD) was measured at 450 nm using the ELISA microplate reader (Molecular Devices, CA, USA).
Statistical analysis.
Differential M. tuberculosis antigen-specific IgG responses in TB patients, contacts with LTBI, and healthy controls were analyzed by Kruskal-Wallis and Dunn's multiple comparison tests. The IgG responses between groups based on TB recurrence, drug resistance, AFB staining, M. tuberculosis genotype, and patient BMI and gender were analyzed by Mann-Whitney tests. Diagnostic values of IgG responses to M. tuberculosis antigens were examined by analysis of receiver operating characteristic (ROC) curves.
RESULTS
M. tuberculosis antigen-specific IgG responses in active TB, LTBI, and controls.
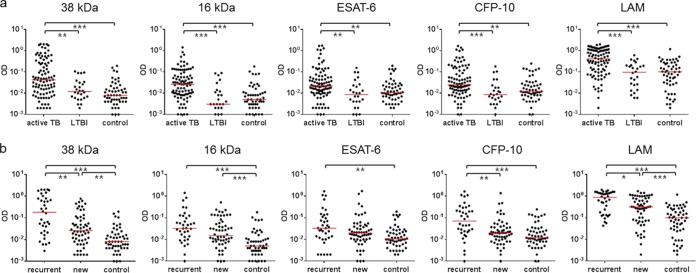
In order to determine M. tuberculosis antigen-specific IgG responses according to the infection status, IgG responses were evaluated in active TB, LTBI, and control groups. We found that active TB patients showed significantly higher IgG responses than controls to all antigens tested (P < 0.001 or P < 0.01) (Fig. 2a). The area under the ROC curve (AUC) for 38 kDa-specific IgG responses was the highest among the five antigens (AUC of 0.7695, P < 0.001), followed by those for LAM and 16 kDa (Table 1). The sensitivity (73.4%) and specificity (70.4%) were also highest for 38 kDa-specific IgG responses (Table 1). Although ESAT-6- and CFP-10-specific IgG responses differed between the active TB and control groups (Fig. 2a), the low AUCs (<0.7) and sensitivities (55.3% and 51.1%, respectively) of those responses did not provide diagnostic value (Table 1). Active TB and LTBI were differentiated by IgG responses to all antigens tested (P < 0.01) (Fig. 2a), with the highest AUC (0.7756, P < 0.001), sensitivity (67.0%), and specificity (80.8%) observed for LAM-specific responses (Table 1).
FIG 2.
Antigen-specific IgG responses according to infection state and TB recurrence. (a) TB patients (n = 94) had significantly higher IgG responses to all antigens tested than individuals with LTBI (n = 26) and controls (n = 54), whereas there were no significant differences in IgG responses between individuals with LTBI and controls. (b) M. tuberculosis antigen-specific IgG responses were compared between recurrent TB (n = 36) and new (n = 58) cases. In response to 38 kDa, CFP-10, and LAM, recurrent cases showed significantly higher IgG responses, as compared to new cases. The IgG responses to 38 kDa and LAM also differed between new cases and controls, whereas ESAT-6 and CFP-10 did not differentiate between these groups. The median levels of IgG responses are indicated with horizontal bars in red (*P < 0.05; **P < 0.01; ***P < 0.001).
TABLE 1.
Analysis of ROC curves for antigen-specific IgG responses in different patient groupsa
| Groups and antigens | AUC (P value) | % sensitivity | % specificity | Cutoff (OD) |
|---|---|---|---|---|
| Active TB vs control | ||||
| 38 kDa | 0.7695 (<0.001) | 73.4 | 70.4 | >0.015 |
| 16 kDa | 0.7535 (<0.001) | 71.3 | 70.4 | >0.010 |
| ESAT-6 | 0.6544 (<0.01) | 55.3 | 70.4 | >0.021 |
| CFP-10 | 0.6643 (<0.001) | 51.1 | 70.4 | >0.024 |
| LAM | 0.7655 (<0.001) | 68.1 | 70.4 | >0.189 |
| Active TB vs LTBI | ||||
| 38 kDa | 0.7064 (<0.01) | 58.5 | 73.1 | >0.027 |
| 16 kDa | 0.7535 (<0.001) | 64.9 | 73.1 | >0.012 |
| ESAT-6 | 0.6966 (<0.01) | 55.3 | 73.1 | >0.021 |
| CFP-10 | 0.7398 (<0.001) | 64.9 | 76.9 | >0.019 |
| LAM | 0.7756 (<0.001) | 67.0 | 80.8 | >0.210 |
| Recurrent case vs control | ||||
| 38 kDa | 0.8593 (<0.001) | 80.6 | 77.8 | >0.019 |
| 16 kDa | 0.8027 (<0.001) | 75.0 | 77.8 | >0.012 |
| ESAT-6 | 0.6965 (<0.01) | 63.9 | 74.1 | >0.024 |
| CFP-10 | 0.7744 (<0.001) | 72.2 | 72.2 | >0.025 |
| LAM | 0.8498 (<0.001) | 72.2 | 74.1 | >0.237 |
| New case vs control | ||||
| 38 kDa | 0.7138 (<0.001) | 67.2 | 70.4 | >0.015 |
| 16 kDa | 0.7230 (<0.001) | 67.2 | 70.4 | >0.010 |
| ESAT-6 | 0.6282 (<0.05) | 50.0 | 70.4 | >0.021 |
| CFP-10 | 0.5959 (>0.05) | 36.2 | 70.4 | >0.024 |
| LAM | 0.7131 (<0.001) | 65.5 | 70.4 | >0.189 |
AUC, sensitivity, specificity, and cutoff values were calculated by analysis of the ROC curves between the different groups tested. The serum IgG responses to 38 kDa, 16 kDa, and LAM showed higher sensitivities and specificities to diagnose active TB or LTBI than ESAT-6- and CFP-10-specific IgG responses. The AUC, sensitivity, and specificity were higher in recurrent cases than in new cases.
M. tuberculosis antigen-specific IgG responses based on history of TB patients.
Of 94 active TB patients, 58 were newly diagnosed, and 36 previously had TB. These 36 patients, defined as recurrent cases, included individuals with relapsed TB after successful treatment and those whose initial treatment failed, either due to self-stop or drug resistance. The IgG responses to all antigens tested were significantly higher in recurrent cases than in controls (Fig. 2b), and AUC, sensitivity, and specificity values were higher for recurrent cases than for new cases versus controls (Table 1). In particular, almost a 2-fold difference was found in the sensitivity of the CFP-10-specific IgG responses between recurrent (72.2%) and new cases (36.2%) (Table 1). The IgG responses to 38 kDa, 16 kDa, and LAM also differentiated between new cases and controls (Fig. 2b).
Effects of drug susceptibility and BMI on IgG responses.
A total of 23 patients were classified with drug-resistant M. tuberculosis, including two extensively drug-resistant (XDR) and three MDR patients out of 88 patients tested for drug susceptibility. The IgG responses to all antigens were not significantly different in these patients than in those with drug-sensitive M. tuberculosis (P > 0.05, data not shown). The M. tuberculosis antigen-specific IgG responses also did not vary in the 24 patients with BMIs of <18.5 kg/m2 and the 64 patients with BMIs in the normal range (18.5 kg/m2 < BMI < 25 kg/m2) (data not shown).
Association between antigen-specific IgG responses and M. tuberculosis isolates.
M. tuberculosis antigen-specific IgG responses were analyzed based on the results of AFB smear staining and M. tuberculosis genotyping. In response to 38 kDa, 16 kDa, ESAT-6, and CFP-10, we observed no significant differences depending on AFB smear-staining results in 91 TB patients tested (Fig. 3a). However, AFB smear-positive patients showed significantly higher IgG responses to LAM than those with negative results (P < 0.01) (Fig. 3a); analysis of the ROC curve in AFB smear-positive (AUC of 0.8909, P < 0.001) and -negative patients (AUC of 0.6857, P < 0.001) confirmed these results. Conversely, 38 kDa- and 16 kDa-specific IgG responses showed good diagnostic values (AUC of >0.7, P < 0.001) in sputum smear-negative patients. Genotyping revealed that Beijing strains accounted for about 81% (76/94) of the isolates in our study; however, the antigen-specific IgG responses did not differ according to strain genotype (P > 0.05) (Fig. 3b).
FIG 3.
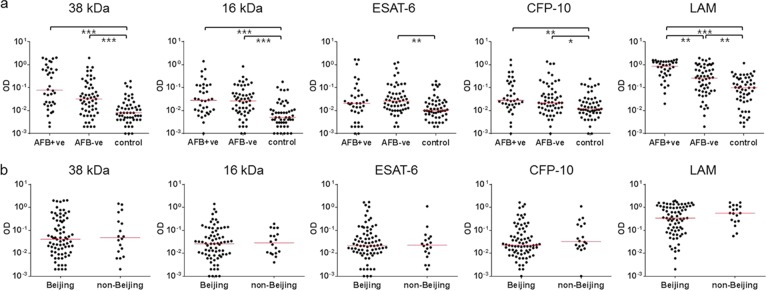
Antigen-specific IgG responses depending on identification of M. tuberculosis strains by AFB smear tests and M. tuberculosis genotypes. (a) The median IgG response to LAM was significantly higher in patients who showed positive AFB smear test results (AFB+ve, n = 36) than those with negative results (AFB-ve, n = 55). However, all AFB smear-negative patients showed significantly higher IgG responses than controls in response to all antigens tested. (b) The majority of patients (81%) were infected with the M. tuberculosis Beijing strain, but genotype did not affect antigen-specific IgG responses (n = 76 for Beijing strain, n = 18 for non-Beijing strain). The median responses are indicated with horizontal bars in red (*P < 0.05; **P < 0.01; ***P < 0.001).
Influence of gender on IgG responses.
We found that the antigen-specific IgG responses were significantly higher in male (n = 64) than in female (n = 30) patients in response to all antigens, with the exception of ESAT-6 (Fig. 4). The differences were particularly significant for 38 kDa- and LAM-specific IgG responses (P < 0.001). It should be noted that the percentages of recurrent cases, drug resistance, and positive AFB smear test results were all more than 2-fold higher in male patients than in female patients, while mean age, BMI, and proportions of M. tuberculosis genotypes were similar between the two groups (Table 2).
FIG 4.

Effect of gender on antigen-specific IgG responses in TB patients. M. tuberculosis antigen-specific IgG responses were compared between male (n = 64) and female (n = 30) TB patients. In response to all antigens, with the exception of ESAT-6, male patients showed significantly higher IgG responses. The median responses are indicated with horizontal bars in red (*P < 0.05; **P < 0.01; ***P < 0.001).
TABLE 2.
Characteristics of male and female TB patientsa
| Characteristic | Male (n = 64) | Female (n = 30) | Fold differenceb |
|---|---|---|---|
| Mean age (yr) | 43 | 32 | 1.3 |
| Mean BMI (kg/m2) | 20 | 19 | 1.0 |
| % recurrence in TB | 47 | 20 | 2.4 |
| % drug resistance | 32 | 14 | 2.3 |
| % AFB+ve | 54 | 10 | 5.4 |
| % Beijing strain | 81 | 80 | 1.0 |
Male patients had higher percentages of TB recurrence, drug resistance, and positive AFB smear test results (% AFB+ve) results than female patients. However, age and BMI were not significantly different between the groups.
Fold difference was calculated by dividing the values of male patients by those of female patients.
DISCUSSION
In this study, we found that M. tuberculosis antigen-specific IgG responses are affected by infection state (active TB versus LTBI), TB recurrence, bacterial burden, and gender, whereas age, BMI, and M. tuberculosis genotype had no effect. While considerable effort has been focused on identifying biomarkers for the differential diagnosis of LTBI and active TB (9, 24), we observed that LAM-specific IgG responses reliably differentiated these, suggesting that this might be an informative marker for clinical diagnosis. LAM is a major cell wall component that contributes to M. tuberculosis pathogenicity by arresting host phagosomal maturation and blocking T cell proliferation (25). We found that only LAM-specific IgG responses differed between AFB smear-positive and -negative TB patients (P < 0.01), suggesting that these are more associated with bacillary load than the other antigens tested.
Many studies have focused on identifying novel antigens to improve the sensitivities and specificities of current TB diagnostics, particularly for sputum smear-negative patients or those incapable of producing sputum. In our study, approximately 60.4% of 94 patients with culture-confirmed TB failed to identify M. tuberculosis by the AFB smear test. We observed that IgG responses to the 38-kDa antigen did not vary based on sputum smear results; however, both smear-positive and -negative patients showed significantly higher levels of IgG than controls. The 38-kDa antigen is a surface-exposed lipoprotein, which has previously been exploited for TB serodiagnosis (26). The sensitivities and specificities of serodiagnostic tests based on this antigen have been reported to range from 40 to 89% and from 44 to 100%, respectively, depending on the study population and test laboratory (26). We observed approximately 70% sensitivity and specificity, with a good diagnostic value (AUC of >0.7, P < 0.001), for the 38-kDa antigen in both AFB smear-positive and -negative patients. These data indicate that measuring the IgG response to the 38-kDa antigen may facilitate early diagnosis of TB, particularly in sputum smear-negative patients or those unable to produce sputum. Recurrent disease with a poor prognosis has also been associated with high levels of anti-38-kDa antibody (19), which corroborates our findings showing a high diagnostic value (AUC of 0.8593, P < 0.001) and enhanced sensitivity and specificity (81% and 78%, respectively) of the 38-kDa antigen in recurrent cases. The proportion of patients infected with the Beijing strain was about 4 times higher than that of those infected with non-Beijing strains (81% versus 19.1%); however, this did not correlate with the antigen-specific IgG responses, perhaps due to the limited number of XDR/MDR patients in our drug-resistant cohort.
Gender appeared to affect the antigen-specific IgG responses, although this result is likely due, at least somewhat, to the differential frequency and severity of TB in male and female patients. TB rates are approximately twice as high in men as in women, suggesting that men are more susceptible to TB (1), although the biological basis for this discrepancy remains unclear. A higher frequency of exposure to TB in men and other gender-specific factors might also influence antigen-specific antibody responses. Sex steroid hormones have been suggested to play a protective role against mycobacterial infection (27, 28), but their role remains to be elucidated.
In conclusion, we found that a serodiagnostic test for TB using 38-kDa and LAM antigens may be developed as a rapid and economic point-of-care test in resource-limited settings, particularly for early diagnosis in sputum smear-negative cases and recurrent cases and for the differential diagnosis between active TB and LTBI. However, the sensitivities and specificities of the responses should be enhanced for clinical use in diagnosis between active TB and LTBI. Further studies should focus on improving the sensitivities and specificities of serodiagnostic tests by selecting epitopes to enhance antibody responses and by assessing combinations of antigens for possible synergistic effects.
ACKNOWLEDGMENTS
We thank all of the study participants and staff at Mokpo National Hospital and Severance Hospital in South Korea for their assistance.
This study was funded by the Ministry for Health, Welfare, and Family Affairs, Republic of Korea (Korean Health Technology R&D Project HI 10C1708). The funding sources had no role in the study process, including study design, sample collection, analysis, and interpretation of the results.
We declare no conflicts of interest.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1.
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677–686. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2014. Multidrug-resistant tuberculosis (MDR-TB): 2014 update. http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf.
- 4.Glynn JR, Whiteley J, Bifani PJ, Raviglione MC, van Soolingen D. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis 8:843–849. doi: 10.3201/eid0808.020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis 12:736–743. doi: 10.3201/eid1205.050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuberculosis Division International Union Against Tuberculosis and Lung Disease. 2005. Tuberculosis bacteriology—priorities and indications in high prevalence countries: position of the technical staff of the Tuberculosis Division of the International Union Against Tuberculosis and Lung Disease. Int J Tuberc Lung Dis 9:355–361. [PubMed] [Google Scholar]
- 7.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 8.Pai M, Riley LW, Colford JM Jr. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 9.Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G. 2009. Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med 9:21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ra SW, Lyu J, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Shim TS. 2011. Distinguishing tuberculosis from Mycobacterium avium complex disease using an interferon-gamma release assay. Int J Tuberc Lung Dis 15:635–640. doi: 10.5588/ijtld.10.0485. [DOI] [PubMed] [Google Scholar]
- 11.Steingart KR, Flores LL, Dendukuri N, Schiller I, Laal S, Ramsay A, Hopewell PC, Pai M. 2011. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med 8:e1001062. doi: 10.1371/journal.pmed.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann R, Kaempfer S, Chegou NN, Oehlmann W, Loxton AG, Kaufmann SH, van Helden PD, Black GF, Singh M, Walzl G. 2014. Serologic diagnosis of tuberculosis by combining Ig classes against selected mycobacterial targets. J Infect 69:581−589. doi: 10.1016/j.jinf.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Singh J, Kumar S, Gopinath K, Balooni V, Singh N, Mani K. 2012. Poor performance of serological tests in the diagnosis of pulmonary tuberculosis: evidence from a contact tracing field study. PLoS One 7:e40213. doi: 10.1371/journal.pone.0040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Su Z, Zhang X, Hu C, Yu J, Gao Q, Wang H. 2013. Generation of Mycobacterium tuberculosis-specific recombinant antigens and evaluation of the clinical value of antibody detection for serological diagnosis of pulmonary tuberculosis. Int J Mol Med 31:751–757. doi: 10.3892/ijmm.2013.1254. [DOI] [PubMed] [Google Scholar]
- 15.Gomez MP, Donkor S, Adetifa IM, Ota MO, Sutherland JS. 2012. Analysis of LAM and 38 kDa antibody levels for diagnosis of TB in a case-control study in West Africa. ISRN Immunol 2012:237823. doi: 10.5402/2012/237823. [DOI] [Google Scholar]
- 16.Feng X, Yang X, Xiu B, Qie S, Dai Z, Chen K, Zhao P, Zhang L, Nicholson RA, Wang G, Song X, Zhang H. 2014. IgG, IgM and IgA antibodies against the novel polyprotein in active tuberculosis. BMC Infect Dis 14:336–344. doi: 10.1186/1471-2334-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzurri A, Kanaujia GV, Sow OY, Bah B, Diallo A, Del Prete G, Gennaro ML. 2006. Serological markers of pulmonary tuberculosis and of response to anti-tuberculosis treatment in a patient population in Guinea. Int J Immunopathol Pharmacol 19:199–208. [PubMed] [Google Scholar]
- 18.Hoff ST, Abebe M, Ravn P, Range N, Malenganisho W, Rodriques DS, Kallas EG, Soborg C, Mark Doherty T, Andersen P, Weldingh K. 2007. Evaluation of Mycobacterium tuberculosis-specific antibody responses in populations with different levels of exposure from Tanzania, Ethiopia, Brazil, and Denmark. Clin Infect Dis 45:575–582. doi: 10.1086/520662. [DOI] [PubMed] [Google Scholar]
- 19.Bothamley GH, Rudd R, Festenstein F, Ivanyi J. 1992. Clinical value of the measurement of Mycobacterium tuberculosis-specific antibody in pulmonary tuberculosis. Thorax 47:270–275. doi: 10.1136/thx.47.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P, Hopewell PC, Ramsay A, Pai M, Laal S. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol 16:260–276. doi: 10.1128/CVI.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Selma W, Harizi H, Boukadida J. 2011. Immunochromatographic IgG/IgM test for rapid diagnosis of active tuberculosis. Clin Vaccine Immunol 18:2090–2094. doi: 10.1128/CVI.05166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demkow U, Filewska M, Bialas B, Szturmowicz M, Zielonka T, Wesolowski S, Kus J, Ziolkowski J, Augustynowicz-Kopec E, Zwolska Z, Skopiniska-Rabewska E, Rowinska-Zakrzewska E. 2004. Antimycobacterial antibody level in pleural, pericardial and cerebrospinal fluid of patients with tuberculosis. (In Polish.) Pneumonol Alergol Pol 72:105–110. [PubMed] [Google Scholar]
- 23.Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Gey van Pittius NC, van Helden PD. 2004. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 169:610–614. doi: 10.1164/rccm.200305-714OC. [DOI] [PubMed] [Google Scholar]
- 24.Hur YG, Gorak-Stolinska P, Ben-Smith A, Lalor MK, Chaguluka S, Dacombe R, Doherty TM, Ottenhoff TH, Dockrell HM, Crampin AC. 2013. Combination of cytokine responses indicative of latent TB and active TB in Malawian adults. PLoS One 8:e79742. doi: 10.1371/journal.pone.0079742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajni Rao N, Meena LS. 2011. Biosynthesis and virulent behavior of lipids produced by Mycobacterium tuberculosis: LAM and cord factor: an overview. Biotechnol Res Int 2011:274693. doi: 10.4061/2011/274693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abebe F, Holm-Hansen C, Wiker HG, Bjune G. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol 66:176–191. doi: 10.1111/j.1365-3083.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, Kuze F. 2001. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 123:428–434. doi: 10.1046/j.1365-2249.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Ying H, Demei J, Xie J. 2012. Tuberculosis and sexual inequality: the role of sex hormones in immunity. Crit Rev Eukaryot Gene Expr 22:233–241. doi: 10.1615/CritRevEukarGeneExpr.v22.i3.60. [DOI] [PubMed] [Google Scholar]