Abstract
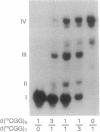
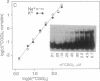
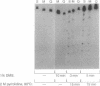
The fragile X mental retardation syndrome is associated with the expansion of trinucleotide 5'-d(CGG)-3' repeats within the FMR1 gene and with hypermethylation of the cytosine residues of these repeats. The expansion and hypermethylation may account for the suppression of the transcription of the FMR1 gene and for the delay of its replication during the cell cycle. Here we show that d(CGG)n oligomers can form a stable Hoogsteen-bonded structure that exhibits properties consistent with those of tetraplex DNA. Oligomers, d(mCGG)n, (n = 4, 5, or 7), at pH 8.0 and in the presence of an alkali metal ion form stable species exhibiting a reduced electrophoretic mobility in nondenaturing polyacrylamide gels. These species are denatured by heating at 90 degrees C for 10 min. With a short d(mCGG)5 oligomer, the slowly migrating species is formed only when the cytosine residue is 5-methylated, whereas with the longer d(CGG)7 it is generated whether or not cytosine is 5-methylated. By contrast, complementary cytosine-rich oligomers do not form analogous complexes. The second-order association kinetics of the formation of the slowly migrating species of d(mCGG)5 suggests that it is an interstrand complex. Formation of intermediate-size complexes between d(mCGG)5 and d(mCGG)7 indicates that the stoichiometry of the slowly migrating structures is tetramolecular. Protection of the complex from methylation by dimethyl sulfate indicates the involvement of the N-7 positions of the guanine residues in Hoogsteen hydrogen bonding, a characteristic of quadruplex structures. If formed in vivo along the expanded and hypermethylated d(mCGG)n stretch, this tetraplex structure could suppress transcription and replication of the FMR1 gene in the fragile X syndrome cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell M. V., Hirst M. C., Nakahori Y., MacKinnon R. N., Roche A., Flint T. J., Jacobs P. A., Tommerup N., Tranebjaerg L., Froster-Iskenius U. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell. 1991 Feb 22;64(4):861–866. doi: 10.1016/0092-8674(91)90514-y. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Kioschis P., Monaco A. P., Gross B., Korn B., Williams S. V., Sheer D., Heitz D., Oberle I., Toniolo D. Molecular cloning and analysis of the fragile X region in man. Nucleic Acids Res. 1991 May 25;19(10):2567–2572. doi: 10.1093/nar/19.10.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Cech T. R. The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell. 1993 Sep 10;74(5):875–885. doi: 10.1016/0092-8674(93)90467-5. [DOI] [PubMed] [Google Scholar]
- Gualberto A., Patrick R. M., Walsh K. Nucleic acid specificity of a vertebrate telomere-binding protein: evidence for G-G base pair recognition at the core-binding site. Genes Dev. 1992 May;6(5):815–824. doi: 10.1101/gad.6.5.815. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Canfield T. K., Lamb M. M., Gartler S. M., Laird C. D. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993 Jul 2;73(7):1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Gartler S. M., Scott C. R., Chen S. H., Laird C. D. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet. 1992 Nov;1(8):571–578. doi: 10.1093/hmg/1.8.571. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Corregan M., Brown B. A., 2nd, Frederick L. N. Cytosine-cytosine+ base pairing stabilizes DNA quadruplexes and cytosine methylation greatly enhances the effect. Biochemistry. 1993 Jun 8;32(22):5870–5880. doi: 10.1021/bi00073a021. [DOI] [PubMed] [Google Scholar]
- Jin R. Z., Breslauer K. J., Jones R. A., Gaffney B. L. Tetraplex formation of a guanine-containing nonameric DNA fragment. Science. 1990 Oct 26;250(4980):543–546. doi: 10.1126/science.2237404. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Lee J. S. The stability of polypurine tetraplexes in the presence of mono- and divalent cations. Nucleic Acids Res. 1990 Oct 25;18(20):6057–6060. doi: 10.1093/nar/18.20.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Frantz J. D., Gilbert W., Tye B. K. Identification and characterization of a nuclease activity specific for G4 tetrastranded DNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3157–3161. doi: 10.1073/pnas.90.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. L. Questions of expansion. Nat Genet. 1993 May;4(1):8–9. doi: 10.1038/ng0593-8. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Ledbetter D. H. Fragile X syndrome: a unique mutation in man. Annu Rev Genet. 1986;20:109–145. doi: 10.1146/annurev.ge.20.120186.000545. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boué J., Bertheas M. F., Mandel J. L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991 May 24;252(5009):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Cech T. R. Effect of monovalent cation-induced telomeric DNA structure on the binding of Oxytricha telomeric protein. Nucleic Acids Res. 1990 Aug 11;18(15):4543–4552. doi: 10.1093/nar/18.15.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Mandel J. L. The unstable and methylatable mutations causing the fragile X syndrome. Hum Mutat. 1992;1(2):91–96. doi: 10.1002/humu.1380010202. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Smith S. S. DNA methylation in eukaryotic chromosome stability. Mol Carcinog. 1991;4(2):91–92. doi: 10.1002/mc.2940040202. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Veselkov A. G., Malkov V. A., Frank-Kamenetskll M. D., Dobrynin V. N. Triplex model of chromosome ends. Nature. 1993 Aug 5;364(6437):496–496. doi: 10.1038/364496a0. [DOI] [PubMed] [Google Scholar]
- Walsh K., Gualberto A. MyoD binds to the guanine tetrad nucleic acid structure. J Biol Chem. 1992 Jul 5;267(19):13714–13718. [PubMed] [Google Scholar]
- Weisman-Shomer P., Fry M. QUAD, a protein from hepatocyte chromatin that binds selectively to guanine-rich quadruplex DNA. J Biol Chem. 1993 Feb 15;268(5):3306–3312. [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Yu S., Pritchard M., Kremer E., Lynch M., Nancarrow J., Baker E., Holman K., Mulley J. C., Warren S. T., Schlessinger D. Fragile X genotype characterized by an unstable region of DNA. Science. 1991 May 24;252(5009):1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]