Abstract
Background
Several studies have focused on the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism; however, the results are conflicting. The effects of statins show significant variability between individuals. This meta-analysis aimed to investigate the effects of the SLCO1B1 c.521T>C polymorphism on the lipid-lowering effects of statins.
Methods
We systematically searched PubMed and Web of Science to screen relevant studies. Meta-analysis was performed to identify the association between SLCO1B1 c.521 polymorphisms and the lipid-lowering effects of statinson the basis of the standard mean difference (SMD) and 95% confidence intervals (CIs). Additionally, we checked for heterogeneity (I2) among studies and evidence of publication bias. We obtained eight studies including 2,012 wild genotype (T/T) and 526 variant genotype (T/C and C/C) cases.
Results
No significant difference was observed in the lipid-lowering efficacy of statins between the wildand variant genotypes of SLCO1B1, with a pooled SMD of 0.03 (95% CI: -0.07-0.13). Furthermore, there was no significant effect in the meta-analyses of the variant heterozygote, homozygote, and Chinese populations. Subgroup meta-analysis indicated that the timerequired for the statin to take effectdid notsignificantly affect the association between lipid-lowering efficacy of statins and SLCO1B1 c.521T>C polymorphism. However, thewild genotype improved the lipid-lowering efficacy of simvastatin with a pooled SMD of -0.26 (95% CI: -0.47- -0.05).
Conclusions
No significant association was detected between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism, with the exception of simvastatin.
Keywords: SLCO1B1 gene, Statins, Lipid-lowering effect, Meta-analysis
INTRODUCTION
Cardiovascular disease is the leading cause of death worldwide. Both high LDL-cholesterol and low HDL-cholesterol concentrations are important risk factors for cardiovascular disease. Lowering cholesterol is the most common method to prevent cardiovascular disease, especially during the primary and secondary levels of cardiovascular disease. Furthermore, statins are the most conventional and widely used cardiovascular disease prevention drugs for the treatment of hyperlipemia [1, 2].
By principally inhibiting hepatic β-hydroxy-β-methyl glutaryl CoA reductase (HMG-CoA reductase), statins limit the rate of cholesterol synthesis. This reduces plasma concentrations of both total and LDL-cholesterols [3]. There are various forms of statins, including pravastatin, fluvastatin, lovastatin, pitavastatin, rosuvastatin, cerivastatin, and simvastatin. However, the process of plasma reduction increases the risk of myopathy and rhabdomyolysis during statin therapy [4]. In spite of this, there is a great deal of variability between individuals with respect to the therapeutic reactions to these drugs; however, the origins of this variation are still only partially understood. Thus, to explain this variation better, recent studies have focused on hepatocytes, which are the primary sites of statin action. With a better understanding of hepatic influx and efflux transporters, researchers may be better able to explain the underlying genetic variations contributing to response to statin treatment [5].
The organic anion transporting polypeptide (OATP) 1B1 (also known as OATP-C, OATP2, and LST-1), which is encoded by the solute carrier organic anion transporter family member 1B1 gene (SLCO1B1), is located on the basolateral (sinusoidal) membrane of hepatocytes. OATP1B1 is a major determining factor for the transport (uptake) of several HMG-CoA reductase (statins) inhibitors from the portal circulation into hepatocytes. Previous single-dose studies have shown that plasma concentrations of pravastatin, rosuvastatin, and pitavastatin are considerably higher in subjects with certain SLCO1B1 single-nucleotide polymorphisms (SNPs), especially c.521T>C (Val174Ala, OATPC*5, rs4149056) [3]. Some research groups also reported that the SLCO1B1 c.521T>C SNP of SLCO1B1 increased the concentration of atorvastatin and simvastatin in human plasma [6, 7]. Therefore, whether or not the SLCO1B1 c.521T>C genetic variation can influence the lipid-lowering effect of statins is a crucial question. Zhang et al. [8] reported that the SLCO1B1 c.521T/C genotype (variant heterozygote) attenuated total cholesterol levels comparedwith the 521T/T genotype (wild genotype). Tachibana-Iimori et al. [9] performed a retrospective study on elderly Japanese patients who received treatment with atorvastatin (N=11), simvastatin (N=33), or pravastatin (N=22). They demonstrated that subjects with the SLCO1B1 c.521T>C genotype (N=20) showed a smaller mean percentage reduction in lipid-lowering effectsin patients with 521T/C genotype than in patients with the wild genotype 521T/T (N=44) after statin treatment. By contrast, some studies showed that SLCO1B1 c.521T>C polymorphisms may not be associated with the lipid-lowering effects of statins [5, 10]. Accordingly, the influence of the SLCO1B1 c.521T>C polymorphism on the lipid-lowering response to statins remains uncertain [5]. Thus, the objective of our meta-analysis was to determine the effect of SLCO1B1 c.521T>C genetic variation on the lipid-lowering efficacy of statins.
METHODS
1. Literature search
We searched the PubMed database from 1990 to April 2014 as well as the Web of Science database, with an index ranging from 1985 to April 2014. We ran searches based on the following terms: "SLCO1B1," "OATP1B1," "cardiovascular disease," "LDL-cholesterol," "lipid-lowering," "polymorphism," and "statins," including all possible combinations therein. We also conducted manual searches following up on all of the studies' references. Lastly, we inspected several related articles from reviews and other pertinent sources such as research bibliographies.
2. Inclusion and exclusion criteria
The criteria that we used to determine whether a study was suitable for our meta-analysis included five factors: (1) the research must involve cases; (2) the relationships between SLCO1B1 c.521T>C polymorphisms and the lipid-lowering efficacy of statins must be assessable; (3) the concentration change of LDL-cholesterol must be provided; (4) the article must be written in English; and (5) the research should provide sufficient information to estimate the standard mean difference (SMD) and corresponding 95% confidence intervals (CIs).
We excluded the following materials: (1) reviews, letters, conference abstracts, and case reports; (2) studies lacking information on the change in the LDL-cholesterol concentration; (3) articles that did not offer enough data to estimate the SMD related to the SLCO1B1 c.521T>C variant and statins' lipid-lowering efficacy; (4) non-English articles; and (5) overlapping articles. Accordingly, these articles were not applied into the scope of our meta-analysis.
3. Data extraction and assessment
After careful review, we extracted the following data from each of the eligible articles: the name of the first author, publication year, nationality, number of patients (T/T, T/C, and C/C genotype), drug type, the cycle of drugs, daily dosage, change ratio (%) in LDL-cholesterol (T/T, T/C, and C/C genotype), and the genotyping method. The quality of each study was evaluated according to the Newcastle-Ottawa quality assessment scale [11].
4. Description of studies
A total of 550 studies were initially identified from a search of the two data bases, according to the aforementioned inclusion and exclusion criteria (Fig. 1). After a thorough survey of these identified studies, we found and selected eight eligible studies for closer analysis [1, 3, 4, 5, 8, 10, 12, 13].
Fig. 1. Flow diagram of the study selection process.
Table 1 shows a summary of the extracted data of the eight included studies item by item. In total, these studies included 2,538 cases comprising 2,012 wild genotype (T/T) cases and 526 variant genotypes cases (T/C and C/C). These cases were compared to determine the lipid-lowering efficacy of statins between the wild and variant genotypes. Five studies included multi-parallel data, owing to the specific drug type and genotype in the respective experiment groups. Sample sizes ranged from five to 305 cases. Four studies recruited less than 100 cases and the other four studies involved more than 100 cases. Three of the eight studies were conducted in China, and the remaining five studies were conducted in Finland, Germany, France, the United Kingdom, and Brazil, respectively.
Table 1. Summary of the data used in the meta-analysis.
| First author | Year | Country | Drug | N of patient | Duration of treatment | Medication dose | Percentage change (%) of LDL-cholesterol level | Genotyping method | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TT | TC | CC | TT | TC | CC | |||||||
| Igel M | 2006 | Germany | Pravastatin | 8 | 8 | 3 weeks | 40 mg/day | -19.10±8.30 | -13.10±9.10 | PHASE program | ||
| Hedman M | 2006 | Finland | Pravastatin | 14 | 6 | - | 2 months | 10 mg/day | -20.10±10.20 | -23.20±11.60 | - | TaqMan |
| Zhang W | 2007 | China | Pravastatin | 36 | 9 | - | 30 days | 20 mg/day | -22.40±10.30 | -14.50±6.60 | - | PCR |
| Couvert P | 2008 | France | Fluvastatin | 305 | 110 | 5 | 2 months | 80 mg/day | -34.00±15.90 | -30.70±17.40 | -31.30±5.20 | TaqMan |
| Bailey KM | 2010 | United Kingdom | Simvastatin | 200 | 82 | 9 | 3 months | - | -79.52±25.36 | -77.77±25.25 | TaqMan | |
| Rosuvastatin | 231 | 72 | 7 | -73.24±23.41 | -76.34±20.85 | |||||||
| Yang GP | 2010 | China | Pitavastatin | 64 | 21 | 4 weeks | 2 mg/day | -31.00±21.00 | -30.00±26.00 | PCR-RFLP | ||
| ARMS-PCR | ||||||||||||
| Yang GP | 2010 | China | Pitavastatin | 64 | 21 | 8 weeks | 2 mg/day | -29.00±26.00 | -27.00±29.00 | PCR-RFLP | ||
| ARMS-PCR | ||||||||||||
| Sortica VA | 2012 | Brazil | Simvastatin | 152 | 59 | 5 | 6 months | 20 mg/day | -38.60±8.00 | -39.90±8.60 | -42.10±15.80 | TaqMan |
| Fu Q | 2013 | China | Atorvastatin | 133 | 49 | 7 | 4 weeks | 20 mg/day | -27.80±5.30 | -26.50±6.00 | -26.80±3.50 | AS-PCR |
| Simvastatin | 123 | 46 | 5 | -27.20±5.40 | -28.90±5.90 | -30.80±5.40 | RFLP | |||||
Please delete this line. These footnote belong to Fig. 2.
Abbreviations: ARMS, amplification refractory mutation system; AS, allele-specific; RFLP, restriction fragment length polymorphism.
5. Statistical analysis
The SMDs and corresponding 95% CIs were combined to determine the overall effect size of the continuous variables. We then used this result to assess the association between the SLCO1B1 c.521T>C variant and the statins' lipid-lowering efficacy (based on LDL-cholesterol) in relation to several variables, including the type of statin, the medication dose, the length of time taken for the medicine to be effective, and the genotype (only SLCO1B1 c.521T>C). For the pooled analysis of the difference in the effect of the SLCO1B1 c.521T>C variant on the lipid-lowering (LDL-cholesterol) ability of statins, the SMD and 95% CI served as the summary statistics for our meta-analysis using the fixed-effects (FE) model. In some cases, we were able to pool statistical variables directly; this occurred whenever the statistical information was adequately described in the literature. In other cases, however, these statistical variables were calculated from available numerical data provided in the articles using the Parmar methods [14]. According to the SLCO1B1 c.521T>C genotype, the participants were divided into two different groups: (1) wild type genotype and (2) variant genotype. In the variant genotype group, patients were further divided into two subgroups: (1) variant heterozygote or (2) homozygote. While these subgroups were treated as two different studies in the literature, they were considered as parts of the same study during our meta-analysis.
Whenever observed, SMD=0 implied unfavorable parameters for the group, as it indicated a lack of association between the SLCO1B1 c.521T>C polymorphism and the lipid-lowering efficacy of statins. We identified the differential impact of the SLCO1B1 c.521T>C variant on the lipid-lowering effect of statins as statistically significant if the 95% CI did not overlap with 0. We assessed the heterogeneity of all studies by using the chi-square statistic, which is based on the Q statistical test. The quantification of the proportion of total heterogeneity across studies was based on the I2 statistic, which is measured from 0% to 100%. Absence of heterogeneity was confirmed if I2<50% or P>0.10 among the studies. The pooled SMD estimate of each study was then calculated by using the FE model (specifically, the inverse-variance method). The probability of publication bias was evaluated by using the funnel plot method. We carried out the statistical analyses using Review Manager 5.2 software (The Nordic Cochrane Center, The Cochrane Collaboration,Copenhagen, Denmark). All P values were for a two-sided test, and differences were considered statistically significant whenever P<0.05.
RESULTS
1. Meta-analysis
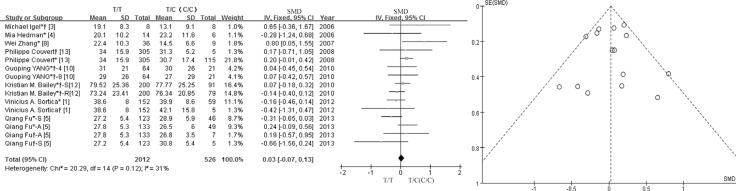
We observed no obvious heterogeneity (I2=31%) among the eight studies that evaluated the lipid-lowering efficacy of statins between the wild and variant genotypes, based on a change inLDL-cholesterol concentration (Fig. 2). Accordingly, the FE model was used to calculate the pooled SMDs with corresponding 95% CIs. Overall, the meta-analysis results indicated that there was nostatistically significant association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism. The overall SMD was 0.03 (95% CI: -0.07-0.13; P=0.59). When stratifying among the Chinese population specifically, we obtained an SMD of 0.03 (95% CI: -0.15-0.21; P=0.74) and inconsistent coefficients, indicating moderate heterogeneity (I2=49%; Fig. 3A). We found no significant association in the stratified analyses according to the Chinese population sample size.
Fig. 2. Forest plot of SMD for the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism, and funnel plots for evaluating publication bias. *TC genotype; †CC genotype; *†TC and CC genotype.
Abbreviations: SMD, standard mean difference; CI, confidence interval; df, degrees of freedom; S, simvastatin; A, atorvastatin; R, rosuvastatin; 4, 4 weeks; 8, 8 weeks.
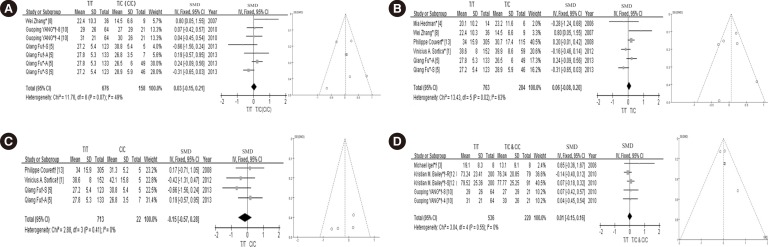
Fig. 3. Forest plots and funnel plots of each subgroup. (A) Forest plots of SMD for the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism of Chinese populations, and funnel plot for evaluating publication bias; (B) Forest plot of SMD for the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C heterozygous genotype, and funnel plot for evaluating publication bias; (C) Forest plot of SMD for the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C homozygote genotype, and funnel plotfor evaluating publication bias; (D) Forrest plot of SMD for the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C variant genotypes (T/C and C/C genotype), and funnel plot for evaluating publication bias. *TC genotype; †CC genotype; *†TC and CC genotype; S: simvastatin; A: atorvastatin; R: rosuvastatin; 4: 4 weeks; 8: 8 weeks.
Abbreviations: SMD, standard mean difference; CI, confidence interval; df, degrees of freedom.
Subsequently, we assessed the effect of thevariant heterozygote (T/C) and homozygote (C/C) genotypes of the SLCO1B1 c.521T>C polymorphism on the lipid-lowering efficacy of statins, both independently and conjointly. As shown in Fig. 3B and C, the overall SMD of thevariant heterozygote (T/C) and the homozygote genotype (C/C) was 0.06 (95% CI: -0.08-0.20; P=0.38) and -0.15 (95% CI: -0.57-0.28; P=0.50), respectively. When assessed conjointly, the SMD of the variant genotype (T/C and C/C) was 0.01 (95% CI: -0.15-0.16; P=0.94) (Fig. 3D). The above results were summarized in the Table 2.
Table 2. Meta-analysis results of all studies and each subgroup studies.
| Cases | Heterogeneity analysis | Pooled SMD | 95% CI | ||
|---|---|---|---|---|---|
| I2 (%) | P value | ||||
| Variant genotype* | 526 | 31 | 0.12 | 0.03 | -0.07-0.13 |
| China populations† | 158 | 49 | 0.07 | 0.03 | -0.15-0.21 |
| Variant genotype (T/C)‡ | 284 | 63 | 0.02 | 0.06 | -0.08-0.20 |
| Variant genotype (C/C)§ | 22 | 0 | 0.41 | -0.15 | -0.57-0.28 |
| Variant genotype (T/C & C/C)∥ | 220 | 0 | 0.55 | 0.01 | -0.15-0.16 |
*Analysis of the association between the lipid-lowering efficacy of statins and SLCO1B1 c.521T>C polymorphism; †Analysis of the association between the lipid-lowering efficacy of statins and SLCO1B1 c.521T>C polymorphism in Chinese populations; ‡Analysis of the association between the lipid-lowering efficacy of statins and SLCO1B1 variant heterozygotes (T/C); §Analysis of the association between the lipid-lowering efficacy of statins and SLCO1B1 variant homozygotes (C/C); ∥These studies did not provide the cases of single heterozygote (T/C) or homozygote (C/C).
Abbreviations: SMD, standard mean difference; CI, confidence interval.
The association of the lipid-lowering efficacy of statins was also evaluated with respect to drug types and the length of time that the medicine required to be effective. The results (Table 3) showed that the statistically insignificant association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism was not influenced by the length of time thatthe medicine required to take effect (patients were treated with statins for >1 or ≤1 month), nor was it influenced when considering patients treated with pravastatin. Surprisingly, however, the lipid-lowering efficacy of simvastatin did in fact show an improvement in patients with the SLCO1B1 c.521T>C wild genotype relative to patients with the variant genotype. This became apparent when the data from Bailey et al. [12] were removed owing to a difference in evaluation standards.
Table 3. Main results of meta-analysis for the drug type and treatment length subgroups.
| N of cases | Heterogeneity analysis | Pooled SMD | 95% CI | ||
|---|---|---|---|---|---|
| I2 (%) | P value | ||||
| Pravastatin | 23 | 37 | 0.20 | 0.46 | -0.05-0.97 |
| Simvastatin | 206 | 24 | 0.26 | -0.12 | -0.28-0.04 |
| Simvastatin* | 115 | 0 | 0.71 | -0.26 | -0.47- -0.05 |
| Length of time required for medicine to be effective (≤1 month) | 145 | 54 | 0.04 | 0.05 | -0.14-0.24 |
| Length of time required for medicine to be effective (>1 month) | 381 | 1 | 0.42 | 0.02 | -0.10-0.14 |
*The data by Bailey et al. [12] were excluded. If P>1, there is no heterogeneity in simvastin subgroup.
Abbreviations: SMD, standard mean difference; CI, confidence interval.
Except in the case of simvastatin, the above meta-analysis results consistently indicate that there is no statistically significantassociation between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism, regardless of whether the genotype is heterozygote (T/C) or homozygote (C/C). These results included studies of a variety of statins, including pravastatin, fluvastatin, simvastatin, rosuvastatin, pitavastatin, and atorvastatin.
2. Publication bias assessment
The data of the eight studies were included in a funnel plot, which was used to analyze the publication bias of the literature included in this meta-analysis. The funnel plot results did not suggest any evidence of publication bias (Figs. 2 and 3).
DISCUSSION
This is the most comprehensive meta-analysis conducted to date with respect to the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism. The main advantage of this study was due to the accumulation of published data on the PubMed and Web of Science databases. These resources providea great deal of information that proved useful in generating enough statistical power to detect significant differences between studies. Our meta-analysis involved eight studies including 2,012 wild genotype (T/T) cases and 526 variant genotype (T/C and C/C) cases. These cases were all used to analyze the effect of the SLCO1B1 c.521T>C polymorphism. The main results of our meta-analysis are displayed in Table 1, and demonstrate that no significant association was detected between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism, which includes patients with both variant heterozygote (T/C) and homozygote (C/C) genotypes. Our results also show that there may be a difference between heterozygotes (T/C) and homozygotes (C/C) with respect to thelipid-lowering efficacy of statins, because their SMD swere 0.06 [95% CI: -0.08-0.20] and -0.15 [95% CI: -0.57-0.28], respectively.
In the subgroup analysis that focused on drug type and the length of time required for the medicine to take effect, we found that the latter did not alter the association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism. However, the SLCO1B1 c.521T>C wild genotype could, in fact, improve the lipid-lowering efficacy of simvastatin, with an SMD of-0.26 (95% CI: -0.47- -0.05).
Previous research found that the SLCO1B1 c.521T>C polymorphism altered the pharmacokinetics of statins [6, 7]. For example, Tachibana-Iimori et al. [9] demonstrated that statins had stronger lipid-lowering efficacy in patients with wild genotypes (T/T) compared with variant heterozygotes (T/C). By contrast, some other studies showed that the SLCO1B1 c.521T>C polymorphism may not be associated with the lipid-lowering effects of statins [5, 10]. Considering the non-parallel effects of SLCO1B1 c.521T>C polymorphisms on pharmacokinetics and the drugs' lipid-lowering efficacies, it appears that other elements such as dosage, the duration of the treatment, the timing of cholesterol level measurement, and ethnic differences between samples from various studies may play a significant role. As a result, the lipid-lowering efficacies of statins vary considerably across different studies. Our meta-analysis results suggest that there is no significant association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism. Specifically, there appears to bea slight difference between heterozygotes (T/C) and homozygotes (C/C) with respect to the lipid-lowering efficacy of statins.
In summary, the results from our meta-analysis show that there is no significant association between the lipid-lowering efficacy of statins and the SLCO1B1 c.521T>C polymorphism, with an exception of simvastatin, which showed a significant effect in the drug type subgroup meta-analysis. Future studies should be carried out to analyze why the SLCO1B1 c.521T>C polymorphism might alter the pharmacokinetics of statins but does not appear to influence the lipid-lowering efficacy of statins.
Acknowledgments
The Shangdong Province Young and Middle-Aged Scientists Research Awards Fund (BS2013SF031), the Natural Scientific Foundation of Chinese Shandong Province (ZR2010CQ031 & ZR2014CM046), the Key Laboratory of Dryland Technology of Shandong Province, and the National Science and Technology Plan "Twelfth Five-Year" in Rural Areas (2011BAD16B09-03) provided grants for this work.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Sortica VA, Fiegenbaum M, Lima LO, Van der Sand CR, Van der Sand LC, Ferreira ME, et al. SLCO1B1 gene variability influences lipid-lowering efficacy on simvastatin therapy in Southern Brazilians. Clin Chem Lab Med. 2012;50:441–448. doi: 10.1515/cclm.2011.804. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Wang M, Zhang H, Wang Q. Effects of statins on rat liver microsomal aspirin esterase activities in vitro. Lat Am J Pharm. 2013;32:1501–1507. [Google Scholar]
- 3.Igel M, Arnold KA, Niemi M, Hofmann U, Schwab M, Lütjohann D, et al. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther. 2006;79:419–426. doi: 10.1016/j.clpt.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Hedman M, Antikainen M, Holmberg C, Neuvonen M, Eichelbaum M, Kivistö KT, et al. Pharmacokinetics and response to pravastatin in paediatric patients with familial hypercholesterolaemia and in paediatric cardiac transplant recipients in relation to polymorphisms of the SLCO1B1 and ABCB1 genes. Br J Clin Pharmacol. 2006;61:706–715. doi: 10.1111/j.1365-2125.2006.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Q, Li YP, Gao Y, Yang SH, Lu PQ, Jia M, et al. Lack of association between SLCO1B1 polymorphism and the lipid-lowering effects of atorvastatin and simvastatin in Chinese individuals. Eur J Clin Pharmacol. 2013;69:1269–1274. doi: 10.1007/s00228-012-1453-9. [DOI] [PubMed] [Google Scholar]
- 6.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 7.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Chen BL, Ozdemir V, He YJ, Zhou G, Peng DD, et al. SLCO1B1 521T-->C functional genetic polymorphism and lipid-lowering efficacy of multiple-dose pravastatin in Chinese coronary heart disease patients. Br J Clin Pharmacol. 2007;64:346–352. doi: 10.1111/j.1365-2125.2007.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, et al. Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet. 2004;19:375–380. doi: 10.2133/dmpk.19.375. [DOI] [PubMed] [Google Scholar]
- 10.Yang GP, Yuan H, Tang B, Zhang W, Wang LS, Huang ZJ, et al. Lack of effect of genetic polymorphisms of SLCO1B1 on the lipid-lowering response to pitavastatin in Chinese patients. Acta Pharmacol Sin. 2010;31:382–386. doi: 10.1038/aps.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Bailey KM, Romaine SP, Jackson BM, Farrin AJ, Efthymiou M, Barth JH, et al. Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: the GEOSTAT-1 Study. Circ Cardiovasc Genet. 2010;3:276–285. doi: 10.1161/CIRCGENETICS.109.898502. [DOI] [PubMed] [Google Scholar]
- 13.Couvert P, Giral P, Dejager S, Gu J, Huby T, Chapman MJ, et al. Association between a frequent allele of the gene encoding OATP1B1 and enhanced LDL-lowering response to fluvastatin therapy. Pharmacogenomics. 2008;9:1217–1227. doi: 10.2217/14622416.9.9.1217. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]