Abstract
Objective
Increasing evidence suggests that cancer-associated inflammation is associated with poor prognosis in patients with cancer. The role of the neutrophil–lymphocyte ratio (NLR) as a predictor in renal cell carcinoma (RCC) remains controversial. We conducted the meta-analysis to determine the association between NLR and clinical outcome of patients with RCC.
Methods and materials
Studies were identified from PubMed and EMBASE databases in March 2014. Meta-analysis was performed to generate combined HRs with 95% CIs for overall survival (OS) and recurrence-free/progress-free survival (RFS/PFS).
Results
15 cohorts containing 3357 patients were included. Our analysis results indicated that elevated NLR predicted poorer OS (HR=1.82, 95% CI 1.51 to 2.19) and RFS/PFS (HR=2.18, 95% CI 1.75 to 2.71) in patients with RCC. These findings were robust when stratified by study region, sample size, therapeutic intervention, types of RCC and study quality. However, it differed significantly by assessment of the cut-off value defining ‘elevated NLR’ in RFS/PFS (p=0.004). The heterogeneity in our meta-analysis was mild to moderate.
Conclusions
Elevated NLR indicates a poorer prognosis for patients with RCC. NLR should be monitored in patients with RCC for rational risk stratification and treatment individualisation.
Strengths and limitations of this study.
Our study is the first systematic meta-analysis evaluating the relationship between elevated NLR and prognosis in patients with RCC. Our analysis provides substantial evidence that elevated NLR is significantly associated with poorer outcomes of patients with RCC. However, there were some limitations in our study.
Firstly, the enrolled studies were retrospective cohort studies in which publication bias inevitably existed. We conducted a ‘trim and fill’ analysis to show that our conclusion was robust.
Secondly, there was some heterogeneity in the included patient populations, so we confirmed the prognostic role of NLR in patients at different disease stages through subgroup analysis stratified by therapeutic intervention and types of RCC.
Thirdly, we only searched limited databases (PubMed and EMBASE), which might weaken the estimating power of the pooled estimate.
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all malignant diseases in adults. It is the seventh most common cancer in men and the ninth in women worldwide.1 2 The incidence of this cancer varies geographically and has increased over past decades owing to changes in the lifestyle and environment.1 Despite a rapid development in surgical resection, immunotherapy and targeted therapy in RCC management, the long-term outcome is still not promising mainly due to common local recurrence, distal metastasis and limited drug response.3 Hence, it is important to identify significant biomarkers, which can help clinicians to stratify patients in terms of prognosis and possibility of metastatic recurrence together with the tumour staging system, that is, the TNM staging system and Robson's staging system, and then set the most appropriate therapeutic strategy.
It is well recognised that the heterogeneity in clinical outcomes is determined by the oncological characteristics of the tumour itself and the host's response to the progressing malignancy.4 Mechanisms involved in the interaction between cancer and inflammation were complicated. Inflammation impacts every single step of tumorigenesis, from tumour initiation to promotion and metastatic progression.5 Recently, several serum biomarkers and haematological indices representative of inflammatory response, notably C reactive protein (CRP), fibrinogen, lymphocyte–monocyte ratio, neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio , have been demonstrated to be closely related to poor prognosis of patients with RCC.6–9
Generally speaking, lymphopenia well reflects impaired cell-mediated immunity, while neutrophilia represents a response to systematic inflammation.5 So the NLR, defined as neutrophil counts divided by lymphocyte counts, is particularly noteworthy. Emerging evidences have shown that NLR gained its prognostic value in patients with colorectal cancer10 and hepatocellular carcinoma.11 Patients with RCC with elevated levels of pretreatment NLR may be more likely to gain a poorer clinical outcome.12 However, the exact role of NLR in patients with RCC is not consistent in different studies due to the variance in study design, sample size and other factors. Some concluded a significant relationship between higher NLR and poorer prognosis, while others did not. Therefore, it is necessary to perform a meta-analysis to systematically and comprehensively understand the prognostic value of NLR in patients with RCC.
In this study, we aimed to assess the prognostic significance of high NLR for overall survival (OS) and recurrence-free (RFS)/progress-free survival (PFS) in patients with RCC by pooling outcomes from available data.
Materials and methods
Search strategy
A comprehensive literature search of the PubMed and EMBASE databases (up to March 2014) was conducted to identify relevant studies. The search strategy included terms for: “NLR” (eg, “neutrophil to lymphocyte ratio”, “neutrophil lymphocyte ratio” and “neutrophil-lymphocyte ratio”), “RCC” (eg, “renal cancer”, “renal carcinoma”, “kidney cancer”, clear cell carcinoma”, “non-clear cell carcinoma”, and “renal papillary carcinoma”) and “prognosis” (eg, “recurrence”, “survival” and “outcome”). Abstracts and information from conferences were collected independently. The reference list was also checked for additional articles. Only studies published in English were included.
Study inclusion criteria and definitions
Two independent authors (KH and LL) reviewed the retrieved studies and extracted data from each included study. Discrepancies were resolved by discussion. Studies included in our meta-analysis must meet the following criteria: (1) the diagnosis of RCC was based on the current clinical guidelines; (2) NLR was measured by serum-based methods before formal treatment; (3) studies reported HRs and 95% CIs for pretreatment NLR in OS and (or) RFS/PFS, or allowed for calculation from raw data contained in the article; (4) only primary data or data superseding earlier work were included, and articles were superior to conference abstracts.
NLR was defined as the serum absolute neutrophil count divided by lymphocyte count in peripheral blood.8 OS was defined as the interval between medical treatment and death or last follow-up of patients. RFS (disease-free/metastasis-free survival, DFS/MFS) was measured from the date of curative treatment until the detection of tumour recurrence. PFS was calculated from the date of first treatment to radiologically or histologically confirmed disease progress. If all the patients in the individual study only received curative nephrectomy, the study was classified into the nephrectomy only subgroup, and the studies in which patients were mainly treated by non-surgical intervention were classified into the mixed therapies subgroup.
Data extraction
We extracted data including: (1) study information including name of first author, year of publication, study region, sample size, time of research; (2) patient characters including age, gender, follow-up period and treatment methods; (3) data about RCC including type, size, stage and distal metastasis; (4) NLR data and cut-off value of NLR; (5) survival data including OS and RFS/PFS.
Quality assessment of primary studies
Quality assessment of included studies was evaluated with the Newcastle-Ottawa quality assessment scale (NOS) range from 0 to 8 by two independent investigators (KH and LL). Studies with an NOS score ≥6 were assigned as high-quality studies. Studies from conference abstracts were defined as low-quality studies. Any inconsistencies were resolved by joint discussion.
Statistical analysis
HR greater than one indicated a poorer prognosis in patients with elevated NLR. Multivariate analysis for HR was superior to univariate analysis unless adjustment variables in multivariable analysis significantly interacted with the NLR level. As heterogeneity was detected among primary studies, meta-analysis was pooled using the random effects models with the DerSimonian Laird method.13 Between-study heterogeneity was assessed using the Cochran Q test and I2 statistic. The p value <0.10 was considered statistically significant for the Cochran Q test, I2>50% indicating substantial heterogeneity between studies. Potential sources of heterogeneity were then investigated using subgroup analyses and meta-regression. All statistical tests were two sided and the significance level was set at 0.05. The possibility of publication bias was assessed using the Begg test and visual insection of a funnel plot.14 We also performed the Duval and Tweedie non-parametric ‘trim and fill’ procedure to further assess the possible effect of publication bias in our meta-analysis.15 All statistical manipulations were undertaken using the program STATA V.12.0 (Stata Corporation, College Station, Texas, USA).
Results
Study characteristics
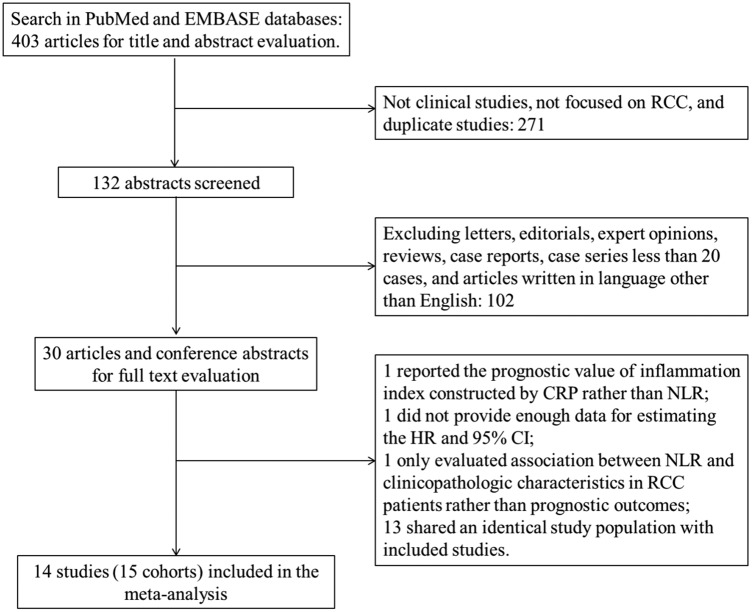
The initial search algorithm retrieved a total of 403 studies. After the title and abstract were reviewed, only 30 records were identified regarding the association of NLR and RCC (figure 1). After a full-text review, a total of 14 retrospective studies12 16–28 (15 cohorts) with 3357 RCCs were included in our meta-analysis. The study by Hatakeyama et al28 reported the HR and 95% CI of two different cohorts separately. If the patients were overlapping or partially overlapping in several studies, only the study with the most complete data was included.
Figure 1.
Flow chart of study selection process (CRP, C reactive protein; NLR, neutrophil–lymphocyte ratio; RCC, renal cell carcinoma).
The basic features of the 14 studies were summarised in table 1. The median quality score of the involved studies was 6 (range 4–8). Eight studies were from western countries, including the USA, Italy, Belgium, Austria, Canada and Australia. The rest of the studies were from Turkey and Japan. Seven of these cohorts enrolled more than 200 patients and eight had less than 200 patients. Radical and partial nephrectomy as the only initial treatment for non-metastatic RCC was reported in four studies. Others were treated with mixed therapies, including nephrectomy, immunotherapy, targeted therapy and others. NLR was calculated using the white blood cell differentiated counts in all studies. In the study by Cetin et al,21 some of the adjustment variables used in multivariate analysis was significantly associated with the NLR value, so HR and 95% CI from univariate analysis for PFS and OS were used in our meta-analysis.
Table 1.
Main characteristics of included studies in the meta-analysis
| Study cohort | Year | Study region | Research time | Follow-up (month) | Treatment | M/F (n) | Age (years) | Tumour type | Distal metastasis (n) |
|---|---|---|---|---|---|---|---|---|---|
| De Martino et al16 | 2013 | USA | 1995–2012 | Mean:49; IQR: (15–71) | Radical and partial nephrectomy | 202/79 | Mean: 63; IQR: (54–72) | Non-clear cell RCC | 0 |
| Ohno et al17 | 2012 | Japan | 1990–2008 | Mean±SD: (75±54) | Radical and partial nephrectomy | 186/64 | Mean±SD: (61±12) | Clear cell RCC | 0 |
| Ohno et al12 | 2014 | Japan | 1990–2008 | Mean (range): 20.6 (1–114) | Cytoreductive nephrectomy: Yes 48; No 25 | 61/12 | Cytoreductive nephrectomy (median (range)): yes: 63 (38–79); No: 65 (34–88) | mRCC | 73 |
| Dirican et al18 | 2013 | Turkey | 2006–2011 | Median:13.43; range: (1.97–40.91) | Nephrectomy, INF-α, sunitinib | 17/6 | Median (range): 59 (43–76) | Clear cell RCC:18; non-clear cell RCC: 5 | 23 |
| Keizman et al19 | 2014 | USA, Israel | 2004–2013 | NA | Sunitinib | 186/92 | Median: 63 | mRCC | 278 |
| Santoni et al20 | 2013 | Italy | 2005–2013 | Median:46.9; 95% CI (39.9 to 53.9) | Past nephrectomy: 91; second-line everolimus: 65; third-line everolimus: 32 | 70/27 | Median:64; 95% CI (44 to 82) | mRCC | 97 |
| Cetin et al21 | 2013 | Turkey | 2008–2011 | Median:15; range: (1–53) | First-line therapy with IFN-α; second-line therapy with VEGF targeted TKIs | 76/24 | Median (range): 58 (24–80) | mRCC: clear cell 73; non-clear cell 24; unknown 3 | 100: liver 17; bone 24; lung 65 |
| Forget et al22 | 2013 | Belgium | 1993–2005 | Median:74.5; IQR: (31–112) | Radical nephrectomy | 71/156 | Mean±SD: (63±12) | Clear cell 166; tubulopapillary 29; chromophobe 4; others 28 | 0 |
| Pichler et al23 | 2013 | Austria | 2000–2010 | Mean (range): 44 (0–130) | Curative radical or partial nephrectomy | Total: 678 | Mean±SD: (63.7±11.9) | Clear cell RCC | 0 |
| Kobayashi et al24 | 2013 | Japan | 2008–2012 | Median:12; range: (1.1–48.9) | Radical nephrectomy, cytokine therapy and sorafenib, sunitinib or mTORi | 44/14 | Median (range): 64 (53–81) | mRCC | 26 |
| Templeton et al25 | 2014 | Canada | NA | NA | Targeted therapy | Total: 859 | NA | RCC | NA |
| Fox et al26 | 2013 | Australia | 2002–2005 | NA | As in EGF20001 | 268/94 | Median (range): 62 (19–84) | mRCC | 362 |
| Huang et al27 | 2011 | USA | 2004–2011 | Median: 35 | Sunitinib | Total: 109 | NA | mRCC | 109 |
| Hatakeyama et al28 | 2013 | Japan | 1995–2013 | Surgery: 26; immunotherapy or IFN-α: 5 | Radical nephrectomy with thrombectomy, immunotherapy or IFN-α | 55/30 | Mean±SD: (62±12) | RCC with tumour thrombus | 14 |
| Study cohort | NLR value | Cut-off | Elevated NLR (n) | Survival analysis | HR | Adjustment variables | NOS score |
|---|---|---|---|---|---|---|---|
| De Martino et al16 | Median (IQR): 2.6 (1.9–3.6) | 3.6 | NA | RFS (DFS) | R (M) | Age, gender, ECOG performance score, pT stage, TNM group, grade, MVI, subtype, ANC, ALC | 7 |
| Ohno et al17 | Mean±SD: 2.62±1.44 | 2.7 | 84 | RFS | R (M) | Age, presentation, nephrectomy, tumour size, pT, grade, MVI, eastern Cooperative Oncology Group, neutrophil, lymphocytes | 8 |
| Ohno et al12 | Mean±SD: 3.98±2.27 | 4 | NA | OS | R (M) | Age, presentation mode, T stage, ECOG PS, Charlson comorbidity index, haemoglobin, LDH, corrected calcium, CRP, neutrophils, lymphocytes | 5 |
| Dirican et al18 | NA | 3 | NA | OS, PFS | E (U) | / | 4 |
| Keizman et al19 | NA | 3 | NA | OS, PFS | R (M) | Unclear | 5 |
| Santoni et al20 | Median: 2.2 | 3 | 38 | OS, PFS | R (M) | Gender, age, Motzer prognostic group, PFS on first-line therapy, neutrophilia | 6 |
| Cetin et al21 | Median: 3.04 | 3.04 | 50 | OS, PFS | R (U) | Age, tumour history, sex, haemoglobin level, red cell distribution width, albumin level, alkaline phosphatase level, PFS, site and number of metastatic organs, MSKCC score, dose reduction, second-line mTOR inhibitors | 5 |
| Forget et al22 | Median (IQR): 3.01 (1.97–4.49) | 5 | 52 | OS, RFS | R (U) | Age, sex, node status, histological grade, stage | 8 |
| Pichler et al23 | Mean±SD: 3.51±2.49 | 3.3 | 398 | OS,RFS (MFS), CSS | R (M) | Age, gender, T stage, tumour grade, presence of tumour necrosis | 7 |
| Kobayashi et al24 | Mean±SD: sorafenib: 4.25±3.01; sunitinib: 4.50±3.43; mTORi: 4.26±2.87 | 4.41 | Sorafenib: 8; sunitinib: 23; mTORi: 16 | OS, PFS | R (M) in OS, E (U) in PFS | Karnofsky PS, metastasis at presentation, number of metastases, prior nephrectomy, prior cytokine therapy, initial targeted agent, Heng’s risk classification, pretreatment level of haemoglobin, platelet count, albumin, CRP, corrected calcium | 5 |
| Templeton et al25 | Mean: 4.98; Median (95% CI) 3.51 (1.42 to 14.0) |
2.5 | 622 | OS | R (M), E (U) | 6 international metastatic renal cell carcinoma database consortium (IMDC) | / |
| Fox et al26 | NA | 3 | 188 | OS | R (M) | MSKCC and systemic inflammation markers | 7 |
| Huang et al27 | NA | 3 | 57 | OS, PFS | R (U) | / | / |
| Hatakeyama et al28 | Mean±SD: 3.1±1.5 | NA | NA | OS | R (U, M) | Age, ECOG-performance status, gender, thrombus level, distant metastasis, underwent surgery, haemoglobin, serum albumin, eGFR, cholinesterase, serum sodium, correlated calcium, LDH, CRP, Charison comorbidity index, molecular targeted agents | 5 |
HR obtained by reporting in text (R), or estimating (E).
ANC, absolute neutrophil count; ALC, absolute lymphocyte count; CSS; CRP, C reactive protein;
CSS, cancer-specific survival; DFS, disease free survival; ECOG, Eastern Cooperative Oncology Group; PS, ; EGF, epidermal growth factor; eGFR, estimated glomerular filtration rate; IFN-α, interferon α; LDH, lactate dehydrogenase; M/F, male, female; (M), the HR comes from multivariate analysis; MFS, metastasis free survival; mRCC, metastatic renal cell carcinoma; MSKCC, Memorial Sloan Kettering Cancer Center; mTORi, inhibitor of the mammalian target of rapamycin; MVI, microvascular invasion; NA, not available; NLR, neutrophil–lymphocyte ratio; NOS, Newcastle-Ottawa Quality Scale; OS, overall survival; PFS, progress-free survival; PS, performance status; pT, primary tumour; RCC, renal cell carcinoma; RFS, recurrence-free survival; TNM, tumour, node, metastasis; TKIs, tyrosine-kinase inhibitor; (U), the HR comes from univariate analysis; VEGF, vascular endothelial growth factor.
NLR and OS in RCC
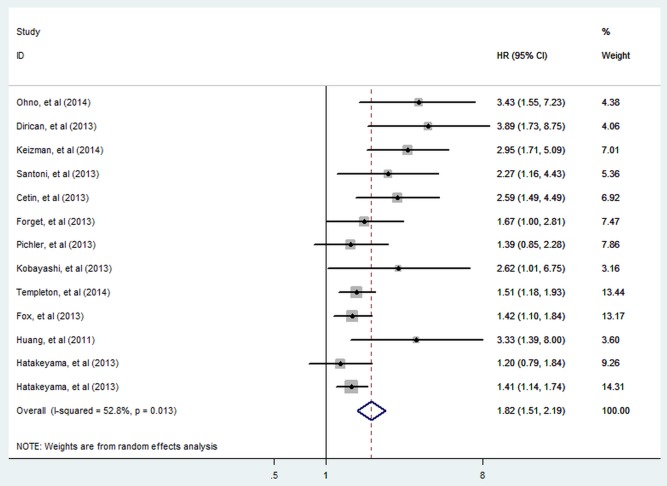
There were 13 cohorts presenting the data of pretreatment NLR and OS in patients with RCC. Elevated NLR was significantly associated with shorter OS (HR=1.82; 95% CI 1.51 to 2.19; p<0.001; figure 2), but there was evidence of moderate heterogeneity between studies (I2=52.8%; p=0.013).
Figure 2.
Meta-analysis of the association between elevated NLR and OS of RCC. Results are presented as individual and pooled HR and 95% CI (NLR, neutrophil-lymphocyte ratio; OS, overall survival; RCC, renal cell carcinoma).
NLR and RFS/PFS in RCC
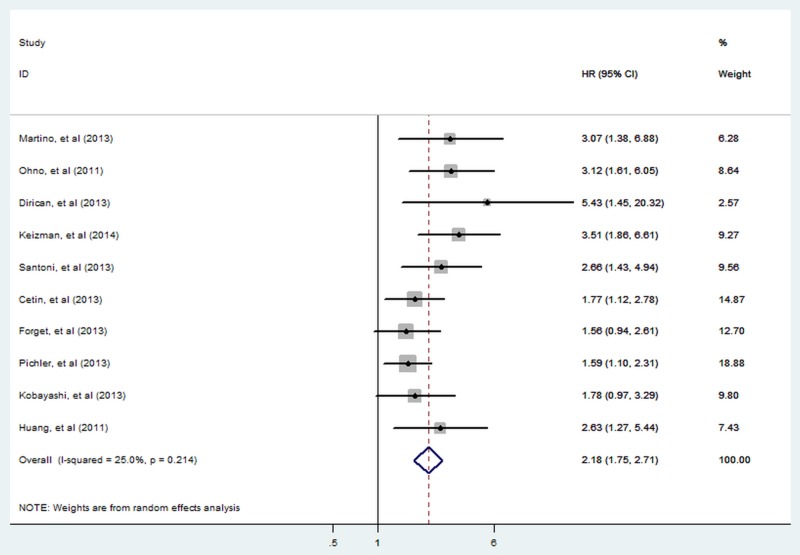
There were 10 cohorts presenting the data of pretreatment NLR and RFS/PFS in patients with RCC. A significant relationship between elevated pretreatment NLR and shorter RFS/PFS (HR=2.18; 95% CI 1.75 to 2.71; p<0.001; figure 3) with non-significant heterogeneity (I2=25.0%; p=0.214) was detected according to our pooled estimates.
Figure 3.
Meta-analysis of the association between elevated NLR and RFS/PFS of RCC. Results are presented as individual and pooled HR and 95% CI (NLR, neutrophil-lymphocyte ratio; RCC, renal cell carcinoma, RFS/PFS, recurrence-free/progress-free survival).
Subgroup analysis and meta-regression
To explore the heterogeneity, subgroup analysis and meta-regression were performed by study region (eastern vs western countries), sample size (≥200 vs <200), cut-off value defining ‘elevated NLR’ (>3 vs ≤3), therapeutic intervention (nephrectomy only vs mixed therapies), type of RCC (clear cell RCC vs non-clear cell RCC/NA; if the majority of patients were those with clear cell RCC in one study, the study was assigned to the clear cell RCC subgroup; NA: not applicable) and NOS score (≥6 vs <6). Subgroup analysis did not alter the prognostic role of NLR in OS or RFS/PFS substantially (table 2), except for stratified analysis29 by cut-off of NLR in PFS/RFS. Meta-regression showed consistent results with subgroup analysis.
Table 2.
Summary of subgroup analyses results
| Analysis | N | Random effects model |
Heterogeneity |
Interaction revisited |
Meta-regression | ||||
|---|---|---|---|---|---|---|---|---|---|
| References | HR (95% CI) | p Value | I2% | p Value | RHR (95% CI) | p Value | p Value | ||
| OS | 12 (13) | 12 18–28 | 1.82 (1.51 to 2.19) | <0.001 | 52.80 | ||||
| Subgroup 1: study region | |||||||||
| Western countries | 7 | 19 20 22 23 25–27 | 1.73 (1.39 to 2.14) | <0.001 | 39.80 | 0.126 | |||
| Eastern countries | 5 (6) | 12 18 21 24 28 | 2.06 (1.41 to 3.02) | <0.001 | 67.70 | 0.013 | 0.84 (0.54 to 1.30) | 0.434 | 0.680 |
| Subgroup 2: sample size | |||||||||
| ≥200 | 5 | 19 22 23 25 26 | 1.60 (1.30 to 1.96) | <0.001 | 34.60 | 0.190 | |||
| <200 | 7 (8) | 12 18 20 21 24 27 28 | 2.16 (1.55 to 3.01) | <0.001 | 62.80 | 0.013 | 0.74 (0.50 to 1.09) | 0.132 | 0.305 |
| Subgroup 3: cut-off value | |||||||||
| >3 | 5 | 12 21–24 | 2.04 (1.47 to 2.82) | <0.001 | 28.20 | 0.234 | |||
| ≤3 | 6 | 18–20 25–27 | 2.07 (1.51 to 2.83) | <0.001 | 63.60 | 0.017 | 0.99 (0.63 to 1.55) | 0.950 | 0.959 |
| Subgroup 4: therapeutic intervention | |||||||||
| Nephrectomy only | 2 | 22 23 | 1.52 (1.06 to 2.17) | 0.022 | 0 | 0.615 | |||
| Mixed therapies | 10 (11) | 12 18–21 24–28 | 1.92 (1.54 to 2.38) | <0.001 | 60.10 | 0.005 | 0.79 (0.52 to 1.20) | 0.275 | 0.424 |
| Subgroup 5: NOS score | |||||||||
| ≥6 | 4 | 20 22 23 26 | 1.51 (1.24 to 1.84) | <0.001 | 0 | 0.594 | |||
| <6 | 8 (9) | 12 18 19 21 24 27 28 | 2.06 (1.51 to 2.70) | <0.001 | 65.10 | 0.003 | 0.73 (0.51 to 1.04) | 0.083 | 0.313 |
| Subgroup 6: tumour type | |||||||||
| Non-clear cell RCC/NA | 7 (8) | 12 19 20 24 25 27 28 | 1.87 (1.45 to 2.42) | <0.001 | 58.20 | 0.065 | |||
| Clear cell RCC | 5 | 18 21–23 26 | 1.82 (1.32 to 2.50) | <0.001 | 53.70 | 0.067 | 1.03 (0.68 to 1.55) | 0.891 | 0.859 |
| PFS/RFS | 10 | 16 17 18–24 27 | 2.18 (1.75 to 2.71) | <0.001 | 25 | ||||
| Subgroup 1: study region | |||||||||
| Western countries | 6 | 16 19 20 22 23 27 | 2.20 (1.64 to 2.96) | <0.001 | 35.70 | 0.169 | |||
| Eastern countries | 4 | 17 18 21 24 | 2.23 (1.51 to 3.28) | <0.001 | 28.60 | 0.241 | 0.99 (0.61 to1.61) | 0.957 | 0.958 |
| Subgroup 2: sample size | |||||||||
| ≥200 | 5 | 16 17 19 22 23 | 2.25 (1.56 to 3.24) | <0.001 | 51.30 | 0.084 | |||
| <200 | 5 | 18 20 21 24 27 | 2.15 (1.62 to 2.85) | <0.001 | 0 | 0.444 | 1.05 (0.66 to 1.66) | 0.847 | 0.950 |
| Subgroup 3: cut-off value | |||||||||
| >3 | 5 | 16 21–24 | 1.74 (1.39 to 2.17) | <0.001 | 0 | 0.675 | |||
| ≤3 | 5 | 17 18–20 27 | 3.08 (2.24 to 4.24) | <0.001 | 0 | 0.867 | 0.56 (0.38 to 0.83) | 0.004 | 0.020 |
| Subgroup 4: therapeutic intervention | |||||||||
| Nephrectomy only | 4 | 16 17 22 23 | 2.00 (1.40 to 2.85) | <0.001 | 39.90 | 0.172 | |||
| Mixed therapies | 6 | 18–21 24 27 | 2.36 (1.79 to 3.12) | <0.001 | 11.40 | 0.342 | 0.85 (0.54 to 1.33) | 0.472 | 0.404 |
| Subgroup 5: NOS score | |||||||||
| ≥6 | 5 | 16 17 20 22 23 | 2.08 (1.53 to 2.84) | <0.001 | 33.60 | 0.197 | |||
| <6 | 5 | 14 18 19 21 27 | 2.35 (1.67 to 3.32) | <0.001 | 26.50 | 0.245 | 0.89 (0.56 to 1.41) | 0.605 | 0.622 |
| Subgroup 6: tumour type | |||||||||
| Non-clear cell RCC/NA | 5 | 16 19 20 24 27 | 2.62 (1.94 to 3.53) | <0.001 | 0 | 0.644 | |||
| Clear cell RCC | 5 | 17 18 21–23 | 1.92 (1.42 to 2.59) | <0.001 | 34.10 | 0.194 | 1.36 (0.89 to 2.09) | 0.151 | 0.112 |
Subgroup analyses for OS and RFS/PFS were performed by study region (eastern vs western countries), sample size (≥200 vs <200), cut-off value (>3 vs ≤3), therapeutic intervention (nephrectomy only vs mixed therapies), type of RCC (Clear cell RCC vs Non-clear cell RCC/NA) and NOS score (≥6 vs <6). Interactions revisited of estimates between subgroups and meta-regression were also applied to figure out heterogeneity among studies.
N, number of studies (cohorts); NOS, Newcastle-Ottawa Quality Scale; OS, Overall survival; PFS/RFS, progress-free/recurrence-free survival; RCC/NA, renal cell carcinoma not applicable; RHR, Ratio of HR.
Sensitivity analyses
Each single cohort included in our meta-analysis was deleted every time to investigate the influence of individual data sets on the pooled HR. Results of sensitivity analyses indicated the robustness of our findings (data not shown).
Publication bias
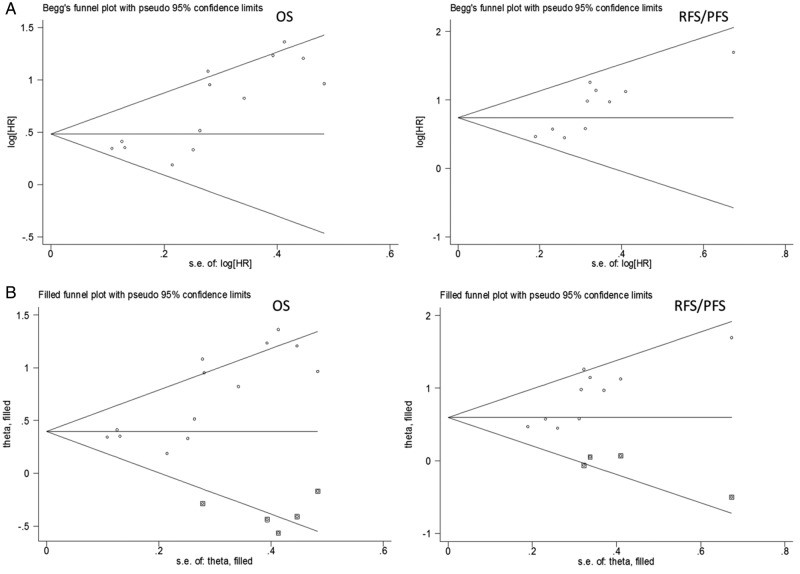
Visual inspection of the Begg funnel plot revealed an asymmetry (p=0.001 in OS and p=0.003 in RFS/PFS; figure 4A), which raised the possibility of publication bias. As a result, we undertook sensitivity analysis using the trim and fill method, which conservatively imputes hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry. The imputed studies produced a symmetrical funnel plot (figure 4B). The pooled analysis incorporating the hypothetical studies continued to show a statistically significant association between elevated NLR and prognosis of patients with RCC (HR=1.54, 95% CI 1.25 to 1.88; p<0.001 in OS and HR=1.85, 95% CI 1.45 to 2.36; p<0.001 in RFS/PFS).
Figure 4.
Funnel plots without and with trim and fill. The pseudo 95% CI is computed as part of the analysis that produces the funnel plot, and corresponds to the expected 95% CI for a given SE (OS, overall survival; RFS/PFS, recurrence-free/progress-free survival).
Discussion
Since the TNM staging and Robson's staging system cannot estimate the outcomes of patients with RCC precisely or guide clinical practice appropriately, lots of patients in the same stage turned out to be quite different in prognosis. Therefore, the introduction of a new laboratory index as a supplementary item to the current RCC risk stratification system, which mainly focuses on the biological characteristics of the tumour itself, is really urgent for personalising the optimal treatment strategy.
As haematological tests are routinely conducted in patients with RCC before medical intervention, NLR acts as a simple, robust and convenient parameter of the inflammatory response. To the best of our knowledge, the present study is the first meta-analysis to systemically and comprehensively determine the exact relationship between elevated NLR and clinical outcomes of patients with RCC. We found that increased NLR has an unfavourable effect on OS and RFS/PFS in patients with RCC. As there was heterogeneity existing among included studies, we also conducted subgroup analyses based on study region, sample size, cut-off value of NLR, therapeutic intervention, type of RCC and NOS score. No significant change was found according to subgroups. According to the results above, NLR is a promising prognostic biomarker to help physicians make appropriate treatment decisions and estimate clinical outcomes of patients with RCC.
We tried to figure out the source of heterogeneity observed among included studies by meta-regression and interaction revisited between subgroup estimates analyses. Although meta-regression did not find any possible reasons for heterogeneity in our meta-analysis for OS, sample size (p=0.132) and NOS score (p=0.083) according to results of interaction revisited between subgroup estimates may partially explain the interstudy heterogeneity. In the same way, we found that the NLR cut-off value (p=0.004) and tumour type (p=0.151) were responsible for the mild heterogeneity in RFS/PFS. It is inevitable that studies with a smaller sample size or lower NOS score are more likely to gain statistic heterogeneity. Authors of included studies defined the cut-off value of NLR, which best discriminated between good and poor survival, on the basis of different methods. A pooled analysis of studies with a cut-off value no more than 3 played a far more superior prognostic role in patients with RCC than studies with a cut-off value higher than 3. We suppose that some patients with poor outcomes were wrongly classified into the low-risk group if the cut-off was too large, which leads to an underestimate of the role of NLR in outcomes of patients with RCC. Although NLR is a sensitive prognostic indicator in retrospective researches, prospective clinical trials are still warranted to evaluate the exact value of NLR in predicting the prognosis of patients with RCC.
Although the funnel plot analysis showed some asymmetry in our meta-analysis suggesting the possibility of publication bias, the trim and fill sensitivity analysis did not change the general result, suggesting that the association of higher NLR value with a poorer prognosis of patients with RCC is not an artefact of unpublished negative studies.
In our analysis, subgroup defined as nephrectomy only also represented the patients’ group with a clinically localised disease, while patients with metastatic disease were stratified to the mixed therapies subgroup. According to our results, elevated NLR was associated with both increased risk of future recurrence in localised disease and accelerated disease progression as well as shortened OS in advanced disease. Therefore, we should take a more active attitude in treatment of patients with RCC, for example, consolidation and maintenance therapy, cytoreductive nephrectomy, especially in patients with elevated NLR before treatment.
Owing to limited data from available studies, we did not conduct pooled analysis on the correlation between elevated NLR and the clinicopathological parameters of RCC. As reported in several studies,21 23 26 high NLR was closely correlated with more malignant tumour characteristics, as well as changed blood and biological indexes. Taking all these into consideration, there may be a significant association between NLR and pathological features and other known risk factors of RCC, but more clinical studies focusing on these relationships are still needed to help us better understand how NLR influences prognosis of patients with RCC.
There are other laboratory markers of systemic inflammation reaction besides NLR, such as CRP30 and modified Glasgow prognostic score,31 32 playing a prognostic role in patients with RCC. What is more, gene polymorphisms33 and biological markers34 35 are also suggested to be predictors of prognosis in patients with RCC. However, factoring in cost-effective analysis and accessibility, NLR stands out for its low-economic costs and wide availability even in primary hospitals. The results of our meta-analysis encourage the routine monitoring of NLR to predict recurrence, progress and survival outcomes in patients with RCC, irrespective of the detailed therapeutic intervention, stage and type of tumour and geographic region.
NLR is an inflammation marker. High NLR represents systemic and local inflammatory response to tumour, which provides a favourable microenvironment for tumour invasion and metastasis.5 As traditional chemotherapy and immunotherapy are with limited benefit in metastatic RCC, treatment remains quite a challenge for clinicians. Now targeted therapy on vascular endothelial growth factor (VEGF) is generally recognised as the first choice for metastatic patients.36 A major difficulty in developing anti-VEGF therapies is tumour intrinsic refractoriness and the emergence of treatment-induced resistance. Tumour-associated macrophages (TAMs) are identified to mediate refractoriness to anti-VEGF treatment recently.37 TAMs promote systemic neutrophilia via secreting cytokines such as interleukin 6,38 so high NLR is associated with high infiltration of TAMs.39 However, tumours can produce immunosuppressive cytokines and reduce cytotoxic T-lymphocyte infiltration.40 Thus, NLR not only reflects system immune status but also a tumour microenvironment which favours tumour invasion and suppresses the host immune surveillance. Hence, NLR acts as an effective prognostic predictor for VEGF-targeted therapy in metastatic patients.
In conclusion, the present meta-analysis demonstrates that elevated NLR is closely associated with poorer prognostic outcome of patients with RCC in different stages. NLR is a widely available, robust and convenient predictor. It helps to figure out patients with high risk and not sensitive to targeted therapy for whom clinicians are urged to adjust the management accordingly. Further research on the best therapeutic schedule fitted with patients of high NLR is needed in the near future.
Footnotes
Contributors: All authors were involved in study concept and design and critical revision of the manuscript for important intellectual content. KH and LL for acquisition, analysis or interpretation of data. KH for drafting of the manuscript. KH and LL for statistical analysis. SZ for obtaining funding and administrative, technical or material support. SZ and JY for study supervision.
Funding: This work was supported by the Natural Science Foundation of Zhejiang Province (Y13H140006) and the National Natural Science Foundation of China (Grant Number: 30471987).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119–32. 10.1016/S0140-6736(09)60229-4 [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. 10.1038/nrurol.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 4.MacDonald N. Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol 2007;5:157–62; discussion 164–156, 183. [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutterer GC, Stoeckigt C, Stojakovic T et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 2014;32:1041–8. 10.1016/j.urolonc.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. 10.2217/fon.09.136 [DOI] [PubMed] [Google Scholar]
- 8.Proctor MJ, Morrison DS, Talwar D et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633–41. 10.1016/j.ejca.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 9.Pichler M, Hutterer GC, Stojakovic T et al. High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br J Cancer 2013;109:1123–9. 10.1038/bjc.2013.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MX, Liu XM, Zhang XF et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014;134:2403–13. 10.1002/ijc.28536 [DOI] [PubMed] [Google Scholar]
- 11.Xiao WK, Chen D, Li SQ et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:117–26. 10.1186/1471-2407-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno Y, Nakashima J, Ohori M et al. Clinical variables for predicting metastatic renal cell carcinoma patients who might not benefit from cytoreductive nephrectomy: neutrophil-to-lymphocyte ratio and performance status. Int J Clin Oncol 2014;19:139–45. 10.1007/s10147-012-0514-5 [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 16.De Martino M, Pantuck AJ, Hofbauer S et al. Prognostic impact of preoperative neutrophil-to-lymphocyte ratio in localized nonclear cell renal cell carcinoma. J Urol 2013;190:1999–2004. 10.1016/j.juro.2013.06.082 [DOI] [PubMed] [Google Scholar]
- 17.Ohno Y, Nakashima J, Ohori M et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 2012;187:411–17. 10.1016/j.juro.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Dirican A, Kucukzeybek Y, Erten C et al. Prognostic and predictive value of hematologic parameters in patients with metastatic renal cell carcinoma: second line sunitinib treatment following IFN-alpha. Asian Pac J Cancer Prev 2013;14:2101–5. 10.7314/APJCP.2013.14.3.2101 [DOI] [PubMed] [Google Scholar]
- 19.Keizman D, Gottfried M, Ish-Shalom M et al. Active smoking may negatively affect response rate, progression-free survival, and overall survival of patients with metastatic renal cell carcinoma treated with sunitinib. Oncologist 2014;19:51–60. 10.1634/theoncologist.2012-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoni M, De Giorgi U, Iacovelli R et al. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer 2013;109:1755–9. 10.1038/bjc.2013.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetin B, Berk V, Kaplan MA et al. Is the pretreatment neutrophil to lymphocyte ratio an important prognostic parameter in patients with metastatic renal cell carcinoma? Clin Genitourin Cancer 2013;11:141–8. 10.1016/j.clgc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Forget P, Machiels JP, Coulie PG et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20(Suppl 3):S650–60. 10.1245/s10434-013-3136-x [DOI] [PubMed] [Google Scholar]
- 23.Pichler M, Hutterer GC, Stoeckigt C et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 2013;108:901–7. 10.1038/bjc.2013.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Kubo T, Komatsu K et al. Changes in peripheral blood immune cells: their prognostic significance in metastatic renal cell carcinoma patients treated with molecular targeted therapy. Med Oncol 2013;30:556 10.1007/s12032-013-0556-1 [DOI] [PubMed] [Google Scholar]
- 25.Templeton AJ, Heng DYC, Choueiri TK et al. Neutrophil to lymphocyte ratio (NLR) and its effect on the prognostic value of the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model for patients treated with targeted therapy (TT). J Clin Oncol 2014;32. [Google Scholar]
- 26.Fox P, Hudson M, Brown C et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 2013;109:147–53. 10.1038/bjc.2013.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P, Carducci MA, Eisenberger MA et al. The association of pretreatment (pre-tx) neutrophil to lymphocyte ratio (NLR) with outcome of sunitinib tx in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2011;29(15 Suppl):4621. [Google Scholar]
- 28.Hatakeyama S, Yoneyama T, Hamano I et al. Prognostic benefit of surgical management in renal cell carcinoma patients with thrombus extending to the renal vein and inferior vena cava: 17-year experience at a single center. BMC Urol 2013;13:47 10.1186/1471-2490-13-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagdev SP, Gregory W, Vasudev NS et al. Improving the accuracy of pre-operative survival prediction in renal cell carcinoma with C-reactive protein. Br J Cancer 2010;103:1649–56. 10.1038/sj.bjc.6605973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb GW, Aitchison M, Ramsey S et al. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer 2012;106:279–83. 10.1038/bjc.2011.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai CG, Johnson TV, Abbasi A et al. External validation of the modified Glasgow prognostic score for renal cancer. Indian J Urol 2014;30:33–7. 10.4103/0970-1591.124203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Martino M, Haitel A, Schatzl G et al. The protease activated receptor 1 gene variation IVSn -14 A>T is associated with distant metastasis and cancer specific survival in renal cell carcinoma. J Urol 2013;190:1392–7. 10.1016/j.juro.2013.03.041 [DOI] [PubMed] [Google Scholar]
- 34.Vilella-Arias SA, Rocha RM, da Costa WH et al. Loss of caspase 7 expression is associated with poor prognosis in renal cell carcinoma clear cell subtype. Urology 2013;82:974.e1–7. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Jiang J, Ye X et al. Prognostic prediction and diagnostic role of intercellular adhesion molecule-1 (ICAM1) expression in clear cell renal cell carcinoma. J Mol Histol 2014;45:427–34. 10.1007/s10735-014-9568-1 [DOI] [PubMed] [Google Scholar]
- 36.Diamond E, Riches J, Faltas B et al. Immunologics and chemotherapeutics for renal cell carcinoma. Semin Intervent Radiol 2014;31:91–7. 10.1055/s-0033-1363848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber HP, Olazoglu E, Grewal IS. Targeting inflammatory cells to improve anti-VEGF therapies in oncology. Recent Results Cancer Res 2010;180:185–200. 10.1007/978-3-540-78281-0_11 [DOI] [PubMed] [Google Scholar]
- 38.Santoni M, Massari F, Amantini C et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2013;62:1757–68. 10.1007/s00262-013-1487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mano Y, Shirabe K, Yamashita Y et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 2013;258:301–5. 10.1097/SLA.0b013e318297ad6b [DOI] [PubMed] [Google Scholar]
- 40.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]