Abstract
Insulin-like growth factor binding proteins (IGFBPs) play a key role in insulin and insulin growth factor signaling and are known to be expressed in several tissues. Studies on the transcriptional regulation of IGFBP genes in various cell types have suggested that IGFBPs control both systemic hormones and regulators. Also, the complex regulatory mechanisms involved in both transcription and translation of IGFBPs provide evidence for molecular mechanisms by which growth factor- and hormone-mediated gene expression are regulated. In particular, IGFBP-1 is known to be an important regulator of IGF activity and mainly regulates metabolism in mammals. In this review, we focus on recent progress in elucidating the transcriptional regulation of IGFBP-1 among IGFBP isoforms.
Keywords: IGFBP-1, Metabolic disease, Transcription, IGF
INTRODUCTION
The insulin-like growth factor binding proteins (IGFBPs) are a superfamily comprised of six proteins (IGFBP-1 to 6) that bind to IGFs with high affinity. The existence of IGFBPs was discovered in the 1960s, and most studies were performed after the cloning of six IGFBPs in the early 1990s [1]. IGFBPs are commonly composed of a long amino acid sequence. IGFBPs are not only involved in IGF action, but also appear to regulate IGF-independent functions including inhibition or activation of cell growth and induction of apoptosis [2]. IGFBPs interact with the acid-labile subunits and glycoproteins as well as insulin-like growth factors (IGFs) in serum [3]. Recently, another group of proteins which have a low affinity with IGFs was discovered and named IGFBP- related proteins (IGFBP-rPs) even though their structures are similar to the classical IGFBPs [4].
IGFBPs are regulated by proteases released from several tissues, and their IGF binding affinity is negatively affected by proteolytic cleavage as well as phosphorylation of IGFBPs [4]. The exact physiological importance of these mechanisms, however, is still unclear. The characteristics of the six IGFBPs are summarized in Table 1. When IGFs are secreted and circulate freely, they are unstable and easily degraded. To achieve functional stability, they need to bind IGFBPs for effective circulation in the blood. Lately, IGFBPs have demonstrated multiple functions as potential players in pathophysiological diseases including cancer, metabolic disease and neuronal disease [5].
Table 1.
IGFBP family
| Name | Size (KDa) | Location | Comments |
|---|---|---|---|
| IGFBP-1 | 28 | Chromosome 7 | Synthesized in liver and decidualized endometrium Regulated by insulin |
| IGFBP-2 | 34 | Chromosome 2 | Highly produced in fetal serum |
| IGFBP-3 | 29 | Chromosome 7 | Major form in serum Regulated by growth hormone |
| IGFBP-4 | 26 | Chromosome 17 | Synthesized in bone Low level in serum |
| IGFBP-5 | 29 | Chromosome 2 | Predominant IGFBP in bone |
| IGFBP-6 | 28 | Chromosome 12 | Present in CSF Very low in serum |
In this review, we outline the relationship between IGFBP-1 and metabolic disease. Furthermore, we overview the transcriptional regulation of IGFBP-1 and discuss current evidence that supports the effects of IGFBP-1 gene expression on metabolic signaling. Understanding the transcriptional regulation of IGFBPs will expand our knowledge and research of the pathogenesis of metabolic disease and identify potential therapeutic targets against metabolic diseases.
REGULATORY MECHANISM AND PHYSIOLOGICAL FUNCTION OF IGFBP-1
IGFBP-1, among the IGFBP family, is produced dominantly in the liver and kidney [6]. The N- and C-terminals of IGBFP-1 are highly conserved among the IGFBP isoforms and contribute to binding with IGF [7]. IGFBP-1 has central linker domains affected by post-translational modifications including proteolysis and phosphorylation [8]. IGFBP-1 can affect IGF-1 activity on cellular responses and independently activates IGF-1 action on cell signaling [9,10]. IGFBP-1 not only is an important determinant of IGF activity, but also enhances glucose uptake in peripheral tissues and reduces glucose output in liver. Moreover, it plays a role in lipid metabolism [11].
IGFBP-1 has been identified as a phosphoprotein and contains three phosphorylation sites [12]. IGFBP-1 phosphorylation induces the development of retinopathy centers. In addition, activity of IGFBP-1 is regulated by both phosphorylation and dephosphorylation. The phosphorylated form of IGFBP-1 shows an enhanced affinity to IGF-1 and II, thereby reducing the utility of IGFs. Regulation of IGF activity by IGFBPs, however, is very complicated in both stimulatory and inhibitory mechanisms [13]. Because the affinity of IGF for IGF receptor is limited by that of IGFBP-1 to IGFs, IGFBP-1 inhibits free IGF-1 levels and reduces IGF signaling through the IGF receptor. This result indicates that high concentrations of IGFBP-1 limit IGF-1 bioavailability and activity of IGF-1 with regard to metabolism.
IGFBP-1 is also stimulated to very high concentrations during intensive exercise and in catabolic conditions, which is not completely explained by known regulators such as insulin and glucocorticoids [14]. The role of AMP activated protein kinase (AMPK), an important signaling system for lipid and carbohydrate metabolism, in the regulation of IGFBP-1 was studied in H4-II-E rat hepatoma cells. Arsenic (III) oxide and 5-aminoimidazole-4-carboxamide-riboside (AICAR) were used as activators. AMPK was identified as a novel regulatory pathway for IGFBP-1 by stimulating secretion and blocking the inhibitory effect of insulin.
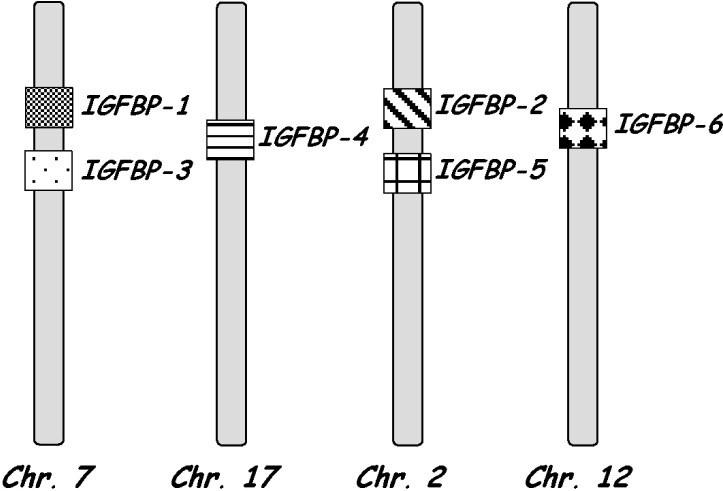
The mammalian IGFBP genes have been duplicated by chromosome copying since 1993 when locations of IGFBP-1 and 2 genes were identified in the same regions as housekeeping gene clusters on human chromosomes 7 and 2 [15]. The location of the IGFBP family is described in Fig. 1. The evolutionary relationship between the different IGFBP isoforms is well explained by genome duplication in vertebrates [16]. Along with the historical aspect for the analysis of IGFBP evolution, there are also functional implications when considering genome duplications. IGFBPs form part of the growth-activating hormonal axis with IGFs, which are released into the blood by the action of growth hormone. IGFBP-1 is located on the short arm of chromosome 7 and encoded by a 5.31 kb gene. IGFBP-1 has a molecular weight of about 25 kDa and circulates in plasma as a heterodimer complex with IGF-1 or II. IGFBP-1 is present in the amniotic fluid at concentrations 100–500 times higher than in plasma [11].
Fig. 1.
Location of IGFBPs.
TRANSCRIPTIONAL REGULATION OF IGFBPs
Although IGFBPs are secreted into the blood and the physiological function of IGFBPs has been studied and is achieved via IGF-1 and II binding, this review focuses on IGFBP gene expression regulated by transcription factors to help achieve metabolic homeostasis. From the point of view of IGFBP gene expression, it is known that various tissues highly induce one or two particular IGFBPs [17]. Regulation of IGFBP gene expression has been shown to be affected by hormones and growth factor-dependent molecular mechanisms, and it has been speculated that the dynamic regulations of IGFBP gene expression may suggest the modulation of IGF actions by systemic local effectors in various tissues [10,18,19]. In this review, we introduce several regulators for IGFBP-1 gene transcription.
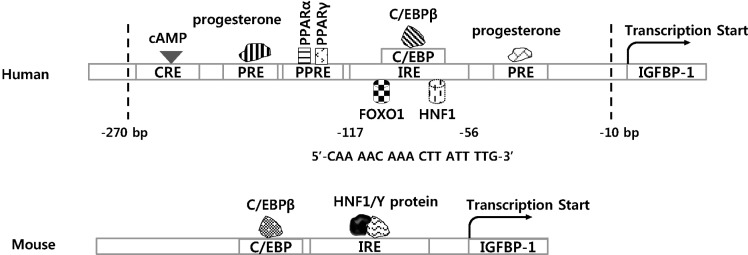
First, Tamura et al. suggested that IGFBP-1 gene expression was regulated by cAMP in human endometrial stromal cells (ESCs) [20]. cAMP is a commonly used decidualization stimulus, which is involved in the process of differentiating fibroblastoid stromal cells of the estrogen-primed endometrium by progesterone [21]. Decidualization of stromal cells is induced by biochemical stimuli in vitro and in vivo cAMP is known to be an activator of IGFBP-1 and increases IGFBP-1 gene expression in ESCs. Two cAMP response elements (CREs) have been found on the IGFBP-1 promoter (Fig. 2). In addition to cAMP, the effect of metformin on decidualization was revealed in ESCs [22]. Metformin has been identified as an anti-diabetic drug. However, Tamura et al. found a new function of metformin during decidualization processing through regulation of IGFBP-1. The Tamura group showed that IGFBP-1 gene expression was reduced by metformin treatment, and IL-8 and IL-1β were stimulated after long-term exposure to metformin. Even though the effect of metformin on IGBFP-1 mRNA expression was revealed, the molecular mechanism was not addressed.
Fig. 2.
Transcription factors on IGFBP-1 promoter.
IGFBP-1 is also induced by progestin and relaxin when human ESCs are decidualized [23]. Two progesterone response elements (PREs) were identified; in a long-term primary culture system, progesterone receptor (PR) directly activates IGFBP-1 gene transcription by binding to PREs (Fig. 2). Mutation of either the 5′ or 3′ half-site of the putative PRE1 site (from −193 to −179 bp) reduced the promoter activity. Endogenous PR alone is insufficient to activate PRE1, indicating that the PRE1 site of the IGFBP-1 promoter mediates direct activation of PR upon transcription, specifically in decidualized stromal cells.
The primary physiological functions of IGFBP-1 appear to be the suppression of IGF action and a role in hypoglycemia. Moreover, IGFBP-1 in liver may act as an IGF-independent survival factor, resulting in pro-apoptotic signal reduction [24]. Recently, Degenhardt et al. found that IGFBP-1 was produced by peroxisome proliferator-activated receptor (PPAR)-γ agonists independently of insulin [25]. The direct activator of gene expression by PPAR ligands requires at least one binding site of PPAR protein as a PPAR response element (PPRE) on the promoter of the PPAR target gene [26].
A previous study has suggested that insulin inhibits hepatic IGFBP-1 gene expression, and that IGFBP-1 levels rapidly declined after feeding [27]. These data indicate that IGFBP-1 level in serum is related to plasma insulin level. Because IGFBP-1 mRNA expression increases during fasting, glucagon seems to stimulate plasma insulin level [28]. These results suggest that IGFBP-1 gene transcription might play a key role in a complicated metabolic regulation. Moreover, IGFBP-1 gene expression is also activated by glucocorticoids as well as hepatocyte nuclear factor (HNF)-1 [29]. Suh and his colleague found a glucocorticoid response element (GRE) located between −91 and −77 from the transcription start site of the rat IGFBP-1 promoter. Also, three accessory regulatory sites for a hepatocyte nuclear factor-1 at −62/−50, an insulin response element at −108/−99, and an upstream site at −252/−236 might be involved in dexamethasone-dependent activation. Specifically, Suh et al. showed that HNF-1α and -1β transcription factors extracted from H4-II-E cells could bind to the palindromic HNF-1 response element. In addition, HNF-1β synergistically stimulates IGFBP-1 gene expression with the glucocorticoid receptor bound to the GRE, suggesting a possible mechanism for glucocorticoid-specific regulation.
In addition to the effect of glucocorticoid as a transcription factor of IGFBP transcription through GRE, IGFBP-1 gene expression is regulated by CAAT/enhancer-binding proteins (C/EBPs) [30] (Fig. 2). In the liver, the effects of insulin for gene expression are mediated through an insulin response element (IRE) on the promoter of the target gene. Ghosh et al. uncovered the regulatory mechanism of C/EBPβ on the IGFBP-1 promoter [30]. C/EBPs are mainly expressed in the liver and adipose tissue where they play key roles in metabolism and cell differentiation [31,32]. They found that C/EBPβ protein forms a complex with other factors and inhibits insulin from binding to IRE without interacting directly with the IRE. Owing to inhibition of IGFBP-1 expression by insulin, as mentioned above, this complex formation correlates with the capacity of insulin to control IGFBP-1 promoter activity.
ROLE OF IGFBP-1 IN METABOLIC DISEASES
Among the six IGFBPs, IGFBP-1 represents the most important member with regard to insulin and glucose metabolism. It is mainly produced in the liver and binds insulin-like growth factor-1 and II. Hence, there is considerable evidence that dysregulation of IGFBP-1 may contribute to metabolic disease. In this section, we review the development of metabolic diseases via functional problems of IGBFP-1.
Dysregulation of IGFBP-1 as well as IGFs in serum and cervical secretions induces metabolic disease and cardiovascular disease [33]. Therefore, measurement of IGFBP-1 level in various body fluids might be used in clinical diagnosis for such afflictions as insulin resistance, cardiovascular risk, hypoglycemia, and growth failure including fetal growth, pre-eclampsia, Laron dwarfism, and ruptured fetal membranes [34,35]. IGFBP-1 level is decreased in fasting serum of early NIDDM patients with insulin resistance [36,37]. Overexpression of IGFBP-1 has been shown to improve impaired glucose tolerance. On the other hand, serum IGF-1 is reduced while IGFBP-1 level is increased in type 1 diabetic patients [38].
IGFBP-1 secretion is regulated by a variety of pathological and physiological signals [39]. Serum level of IGFBP-1 is associated with metabolic nutritional factors and increases IGF-1 concentration, resulting in reciprocal regulation of plasma insulin level. Glucagon acts as an activator of serum IGFBP-1 independently of insulin level [28]. This implies a complicated metabolic regulation of IGFBP-1. Various evidences suggest that malfunction of the IGFBP-1 system is involved in the long-term complications of diabetes mellitus [40]. Low levels of IGFBP in serum identify insulin resistance as a marker of insulin sensitivity. Therefore, IGFBP-1 level can correlate well with common indicators of insulin resistance in obese children and adults [41].
Inflammation plays a pivotal role in atherosclerosis development [42]. Recent studies demonstrated that secretion of IGFBP-1 is activated by proinflammatory cytokines like IL-1β and TNFα in HepG2 liver hepatocarcinoma cell lines [43,44]. In addition, IL-6 upregulates mRNA expression and secretion of IGFBP-1 in a dose-dependent manner in hepatocytes which are co-cultured with liver macrophages [45]. Therefore, the concentration of IGFBP-1 regulated by cytokines may be involved in the initiation and progression of cardiovascular disease.
CONCLUSION
This article aimed to discuss the regulation of the IGFBP-1 in metabolic diseases like diabetes and cardiovascular disease. In addition, a complex diagram for cis-elements of transcription factors on the IGFBP-1 promoter have emerged suggesting that the molecular mechanism of their expression is dependent on transcription factors in cells and tissues. Although roles of IGFBP-1 in the pathogenesis and progression of disease are complicated, the literature clearly indicates functional significance of IGFBP-1 gene expression in vivo and in vitro. However, the physiological relevance of IGFBP expression in human pathogenesis requires further study. Therefore, the knowledge of IGFBP-1 system regulation including its gene expression may offer a wide variety of interesting developments that are involved in the future strategy of IGFBP-mediated therapy.
Acknowledgments
We apologize to all contributors in the field of IGFBP-1 whose work due to space limitations could not be cited. This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120864).
REFERENCES
- 1.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–87. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 2.Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170:63–70. doi: 10.1677/joe.0.1700063. [DOI] [PubMed] [Google Scholar]
- 4.Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 5.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–33. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 6.Powell DR, Lee PD, Suwanichkul A. Multihormonal regulation of IGFBP-1 promoter activity. Adv Exp Med Biol. 1993;343:205–14. doi: 10.1007/978-1-4615-2988-0_20. [DOI] [PubMed] [Google Scholar]
- 7.Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA. 2006;103:13028–33. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sala A, Capaldi S, Campagnoli M, Faggion B, Labo S, Perduca M, Romano A, Carrizo ME, Valli M, Visai L, Minchiotti L, Galliano M, Monaco HL. Structure and properties of the C-terminal domain of insulin-like growth factor-binding protein-1 isolated from human amniotic fluid. J Biol Chem. 2005;280:29812–9. doi: 10.1074/jbc.M504304200. [DOI] [PubMed] [Google Scholar]
- 9.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–76. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 10.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 11.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–31. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 12.Jones JI, Busby WH, Jr, Wright G, Smith CE, Kimack NM, Clemmons DR. Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J Biol Chem. 1993;268:1125–31. [PubMed] [Google Scholar]
- 13.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DG, Marino EM, Thule PM, Seneviratne CK, Murphy LJ. Insulin and glucocorticoids regulate IGFBP-1 expression via a common promoter region. Biochem Biophys Res Commun. 1994;200:226–32. doi: 10.1006/bbrc.1994.1438. [DOI] [PubMed] [Google Scholar]
- 15.Lundin LG. Evolution of the vertebrate genome as reflected in paralogous chromosomal regions in man and the house mouse. Genomics. 1993;16:1–19. doi: 10.1006/geno.1993.1133. [DOI] [PubMed] [Google Scholar]
- 16.Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–89. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- 17.Rechler MM. Insulin-like growth factor binding proteins. Vitam Horm. 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- 18.Collett-Solberg PF, Cohen P. Genetics, chemistry, and function of the IGF/IGFBP system. Endocrine. 2000;12:121–36. doi: 10.1385/ENDO:12:2:121. [DOI] [PubMed] [Google Scholar]
- 19.Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol. 1996;28:619–37. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 20.Tamura I, Asada H, Maekawa R, Tanabe M, Lee L, Taketani T, Yamagata Y, Tamura H, Sugino N. Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology. 2012;153:5612–21. doi: 10.1210/en.2012-1420. [DOI] [PubMed] [Google Scholar]
- 21.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–72. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 22.Germeyer A, Jauckus J, Zorn M, Toth B, Capp E, Strowitzki T. Metformin modulates IL-8, IL-1beta, ICAM and IGFBP-1 expression in human endometrial stromal cells. Reprod Biomed Online. 2011;22:327–34. doi: 10.1016/j.rbmo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Mazella J, Suwanichkul A, Powell DR, Tseng L. Activation of the insulin-like growth factor binding protein-1 promoter by progesterone receptor in decidualized human endometrial stromal cells. Mol Cell Endocrinol. 1999;153:11–7. doi: 10.1016/s0303-7207(99)00096-9. [DOI] [PubMed] [Google Scholar]
- 24.Leu JI, Crissey MA, Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J Clin Invest. 2003;111:129–39. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degenhardt T, Matilainen M, Herzig KH, Dunlop TW, Carlberg C. The insulin-like growth factor-binding protein 1 gene is a primary target of peroxisome proliferator-activated receptors. J Biol Chem. 2006;281:39607–19. doi: 10.1074/jbc.M605623200. [DOI] [PubMed] [Google Scholar]
- 26.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–4. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 27.Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266:18868–76. [PubMed] [Google Scholar]
- 28.Hilding A, Brismar K, Thoren M, Hall K. Glucagon stimulates insulin-like growth factor binding protein-1 secretion in healthy subjects, patients with pituitary insufficiency, and patients with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1142–7. doi: 10.1210/jcem.77.5.7521339. [DOI] [PubMed] [Google Scholar]
- 29.Suh DS, Rechler MM. Hepatocyte nuclear factor 1 and the glucocorticoid receptor synergistically activate transcription of the rat insulin-like growth factor binding protein-1 gene. Mol Endocrinol. 1997;11:1822–31. doi: 10.1210/mend.11.12.0021. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh AK, Lacson R, Liu P, Cichy SB, Danilkovich A, Guo S, Unterman TG. A nucleoprotein complex containing CCAAT/enhancer-binding protein beta interacts with an insulin response sequence in the insulin-like growth factor-binding protein-1 gene and contributes to insulin-regulated gene expression. J Biol Chem. 2001;276:8507–15. doi: 10.1074/jbc.M008541200. [DOI] [PubMed] [Google Scholar]
- 31.Shi XM, Blair HC, Yang X, McDonald JM, Cao X. Tandem repeat of C/EBP binding sites mediates PPARgamma2 gene transcription in glucocorticoid-induced adipocyte differentiation. J Cell Biochem. 2000;76:518–27. doi: 10.1002/(sici)1097-4644(20000301)76:3<518::aid-jcb18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–12. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 33.Ezzat VA DE, Wheatcroft SB, Kearney MT. The role of IGF-1 and its binding proteins in the development of type 2 diabetes and cardiovascular disease. Diabetes Obes Metabol. 2008;10:198–211. doi: 10.1111/j.1463-1326.2007.00709.x. [DOI] [PubMed] [Google Scholar]
- 34.Borai A, Livingstone C, Ghayour-Mobarhan M, Abuosa A, Shafi S, Mehta S, Heidari A, Emadzadeh A, Wark G, Ferns G. Serum insulin-like growth factor binding protein-1 (IGFBP-1) phosphorylation status in subjects with and without ischaemic heart disease. Atherosclerosis. 2010;208:593–8. doi: 10.1016/j.atherosclerosis.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Borai A, Livingstone C, Mehta S, Zarif H, Abdelaal F, Ferns G. Biological variation in fasting serum insulin-like growth factor binding protein-1 (IGFBP-1) among individuals with a varying glucose tolerance. Clin Biochem. 2009;42:1270–4. doi: 10.1016/j.clinbiochem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Leinonen ES, Salonen JT, Salonen RM, Koistinen RA, Leinonen PJ, Sarna SS, Taskinen MR. Reduced IGFBP-1 is associated with thickening of the carotid wall in type 2 diabetes. Diabetes Care. 2002;25:1807–12. doi: 10.2337/diacare.25.10.1807. [DOI] [PubMed] [Google Scholar]
- 37.Gibson JM, Westwood M, Young RJ, White A. Reduced insulin-like growth factor binding protein-1 (IGFBP-1) levels correlate with increased cardiovascular risk in non-insulin dependent diabetes mellitus (NIDDM) J Clin Endocrinol Metab. 1996;81:860–3. doi: 10.1210/jcem.81.2.8636318. [DOI] [PubMed] [Google Scholar]
- 38.Salgado LR, Semer M, Nery M, Knoepfelmacher M, Lerario AC, Povoa G, Jana S, Villares SM, Wajchenberg BL, Liberman B, Nicolau W. Effect of glycemic control on growth hormone and IGFBP-1 secretion in patients with type I diabetes mellitus. J Endocrinol Invest. 1996;19:433–40. doi: 10.1007/BF03349888. [DOI] [PubMed] [Google Scholar]
- 39.Wolk K, Larsson SC, Vessby B, Wolk A, Brismar K. Metabolic, anthropometric, and nutritional factors as predictors of circulating insulin-like growth factor binding protein-1 levels in middle-aged and elderly men. J Clin Endocrinol Metab. 2004;89:1879–84. doi: 10.1210/jc.2003-031349. [DOI] [PubMed] [Google Scholar]
- 40.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–69. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 41.Motaghedi R, Gujral S, Sinha S, Sison C, Ten S, Maclaren NK. Insulin-like growth factor binding protein-1 to screen for insulin resistance in children. Diabetes Technol Ther. 2007;9:43–51. doi: 10.1089/dia.2006.0056. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoudi M, Curzen N, Gallagher PJ. Atherogenesis: the role of inflammation and infection. Histopathology. 2007;50:535–46. doi: 10.1111/j.1365-2559.2006.02503.x. [DOI] [PubMed] [Google Scholar]
- 43.Lang CH, Nystrom GJ, Frost RA. Regulation of IGF binding protein-1 in hep G2 cells by cytokines and reactive oxygen species. Am J Physiol. 1999;276:G719–27. doi: 10.1152/ajpgi.1999.276.3.G719. [DOI] [PubMed] [Google Scholar]
- 44.Frost RA, Nystrom GJ, Lang CH. Stimulation of insulin-like growth factor binding protein-1 synthesis by interleukin-1beta: requirement of the mitogen-activated protein kinase pathway. Endocrinology. 2000;141:3156–64. doi: 10.1210/endo.141.9.7641. [DOI] [PubMed] [Google Scholar]
- 45.Lelbach A, Scharf JG, Ramadori G. Regulation of insulin-like growth factor-I and of insulin-like growth factor binding protein-1, -3 and -4 in cocultures of rat hepatocytes and Kupffer cells by interleukin-6. J Hepatol. 2001;35:558–67. doi: 10.1016/s0168-8278(01)00170-2. [DOI] [PubMed] [Google Scholar]