Abstract
Background
Inflammatory bowel disease is characterized by persistent inflammation of the intestinal tissues. Although the usage of biologics has greatly enhanced the management of this disorder, a permanent treatment does not exist. In this study, we investigated whether the cells with anti-inflammatory and healing properties from the omentum could be harnessed to treat colitis in dextran sulfate sodium (DSS)-induced mouse colitis model.
Methods:
C57BL/6 mice were administered 2% DSS for 10 days and then injected in the peritoneum with cells isolated from the murine omentum. Thereafter, body weight change, serum KC levels, and histological analysis of the colon were conducted. We also examined if omentum infused mice were resistant to a lethal challenge of 4% DSS.
Results:
2% DSS-mice injected with omentum cells exhibited a decrease in body weight loss, decreased inflammation in the colon and decreased levels of the inflammatory cytokine KC in the serum compared to mice given 2% DSS alone. In addition, mice administered a lethal dose of 4% DSS exhibited a 50% decrease in mortality when injected with omentum cells.
Conclusion:
Cells from the omentum exert anti-inflammatory and/or healing properties in the acute DSS-induced colitis model.
Keywords: Omentum, Stem cells, Dextran sulfate sodium, Colitis, Inflammatory bowel disease
INTRODUCTION
The omentum is a thin sheath that resides within the peritoneal cavity and has been reported to curtail inflammation and promote tissue healing [1,2]. It was described as the ‘policeman’ of the abdomen over 100 years ago for its ability to seek and attach to sites of injury [3]. Several clinical studies have suggested that omental transposition (i.e., surgical repositioning of the omental pedicle or flap into damaged tissues and organs) could improve healing of injured organs [4–6]. Specifically, omental transposition has been used in brain surgeries and to promote healing of the heart and extremities [7–13]. However, the mechanism by which the omentum exerts its anti-inflammatory and healing properties is unclear.
Several studies in rodents have demonstrated that intraperitoneal introduction of foreign particles can induce a dramatic increase in omentum mass due primarily to expansion of the stromal cell population [14,15]. These stromal cells secrete various growth factors such as fibroblast growth factor and angiogenic factors such as vascular endothelial growth factor. In addition, cells of the omentum express stem cell markers such as Nanog, Oct-4, and SSEA-1 [14,16–18]. The secretion of these cytokines along with the increase in the stem cell population may provide a mechanistic explanation for its regenerative properties.
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and it encompasses both Crohn’s disease and ulcerative colitis. Although the etiology of IBD is unknown it is postulated to result from a dysregulated immune response to the host’s normal intestinal flora in genetically susceptible individuals. Dextran sulfate sodium (DSS) is a chemical administered in water to rodents to induce symptoms and histopathological features similar to ulcerative colitis [19]. In the current study, we examined whether cells from the omentum could be used to treat colonic inflammation in a murine model of DSS-induced colitis.
MATERIALS AND METHODS
1. Mice and DSS-induced colitis
Six-week-old male, C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were maintained under specific pathogen-free conditions and experimental procedures were conducted according to protocols approved by the University of Illinois Chicago Animal Care and Use Committee in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care International. Mice were given either 2% DSS or 4% DSS (30–50 kDa)(MP Biomedicals, Solon, OH, USA) in their drinking water for 10 days and then changed to normal drinking water for the duration of the experiment. Mice were euthanized using carbon dioxide euthanasia.
2. Preparation of cells from the omentum
Preparation of the omentum and cell isolation has been described previously [18]. In brief, C57BL/6 mice were given an intraperitoneal injection with 1 ml of Bio-gel P-60 polyacrylamide bead slurry (BioRad Laboratories, Hercules, CA, USA) in PBS (1:1 volume ratio). Seven days later, mice were euthanized and the omentum minced with a scapel, strained through a cell strainer (100 um mesh) and digested with collagenase Type I (1 mg/ml)(Sigma, St. Louis, MO, USA) for 30 minutes at 4°C with gentle agitation. Cells were recovered by several rounds of centrifugation and washing in cold Hank’s balanced salt solution (HBSS). Mononuclear cells were isolated using a Ficoll gradient, washed with HBSS and enumerated using trypan blue dye exclusion assay. Cells were resuspended in PBS and the DSS-treated mice were given a single intraperitoneal injection of cells (5 × 106 cells/1 ml volume) at 7 days post-DSS treatment. Control mice were given a 1 ml injection of PBS alone.
3. Histology and inflammation scoring
Formalin-fixed, paraffin-embedded intestinal tissues were sectioned (5 μm) and stained with hematoxlyin and eosin (H&E). Colonic inflammation was graded as follows: 0, normal; 1, mild increase in inflammatory cells, no mucosal epithelial changes (no proliferation or loss of crypt structure); 2, moderate increase in inflammatory cells, mild scattered mucosal epithelial proliferation ± focal loss of crypt architecture; 3, moderate increase in inflammatory cells, diffuse or nearly diffuse (>2 sites) mucosal epithelial proliferation, edema ± focal loss of crypt architecture; 4, severe increase in inflammatory cells, marked consistent proliferation; extensive loss (>2 sites) of crypt architecture; 5, complete destruction of mucosa. Images were taken using a Olympus camera and rendered using Adobe Photoshop.
4. ELISA
Blood was collected via cardiac puncture and then allowed to clot overnight at 4°C. The serum was harvested and stored at −20°C until analyzed by KC ELISA (R&D Systems, USA).
5. Data analysis
For statistical analysis of inflammation scores, the Mann-Whitney U test was used to compare between group distributions for unpaired data. A p-value of <0.05 was considered statistically significant.
RESULTS
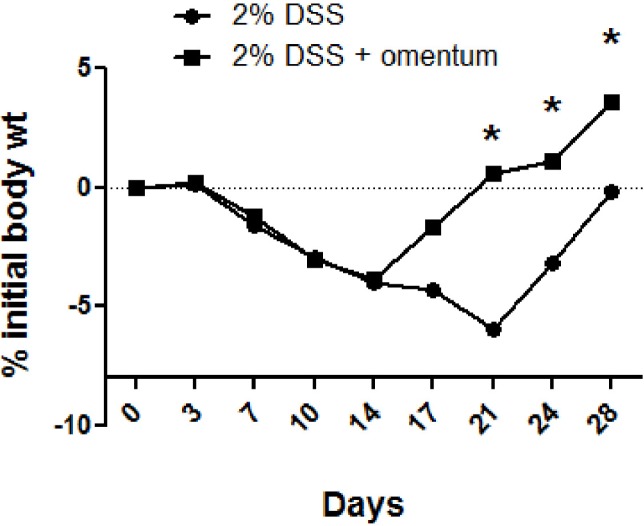
DSS induces diarrhea, gross rectal bleeding, and weight loss in mice [19]. Histologically, DSS induces mucosal ulcerations, dysplasia and formation of crypt abscesses. We tested the effect of omentum cells in colonic tissue repair by using the DSS-induced colitis model. To prepare sufficient numbers of omentum cells, the omentum was expanded in mice by intraperitoneal injection of polyacrylamide beads as reported for rats [14]. Seven days after injection of polyacrylamide beads, omentum mass was grossly increased in the peritoneum (data not shown). To examine if omentum cells could alleviate DSS-induced colitis, mice were given 2% DSS in the drinking water for 10 days and then injected intraperitoneally with mononuclear cells isolated from the omentum (5 × 106 cells/mouse). Control mice (mock group) were given 2% DSS for 10 days and then injected with PBS alone. Both groups of mice exhibited a 3% decrease in initial body weight at day 10 post-DSS treatment and 5% decrease in initial body weight at day 14 (Fig. 1). Thereafter, the body weights of the two groups diverged: the omentum-injected mice had a gradual increase in body weight whereas the mock-injected mice continued to lose weight up to day 21.
Fig. 1.
Omentum cells alleviate body weight loss. Mice were administerd 2% DSS for 10 days and then either injected intraperitoneally with omentum cells (5 × 106 cells/mouse)(2% DSS + omentum) or with mock injection of PBS alone (2% DSS). The body weight change was monitored for up to 28 days post DSS treatment. The daily body weight of individual mice was normalized to the starting body weight. Shown are pooled data from two independent experiments with a total of 10 mice per group. *p-value <0.05 using the Mann-Whitney U test.
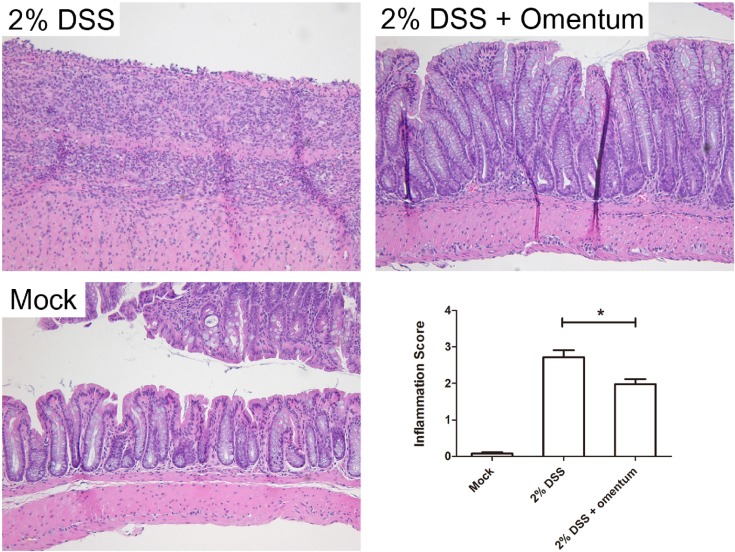
To determine if omentum protects against histological damage induced by DSS treatment, mice were administered 2% DSS for 10 days, injected with omentum cells and then euthanized 14 days later (day 24). Mice given 2% DSS alone for 10 days plus 14 days of water or untreated mice (i.e., no DSS) served as controls. We found that 2% DSS treated mice exhibited colonic inflammation throughout the colon with ulcerations observed in the distal region (Fig. 2). In addition, there was an increased frequency of colonic lymphoid follicles and extensive epithelial hyperplasia. In contrast, 2% DSS + omentum treated mice exhibited minimal ulcerations though they also showed extensive mucosal hyperplasia. Average colonic inflammation scores were significantly lower in the 2% DSS + omentum group compared to the 2% DSS group suggesting that omentum cells accelerated mucosa healings (Fig. 2). In addition, we found that KC levels (functional homolog of interleukin-8) in the serum were also decreased in DSS + omentum mice compared to DSS treated mice (Fig. 3).
Fig. 2.
Omentum cells decreases DSS-induced colitis. Mice were administered 2% DSS for 10 days and then injected intraperitoneally with omentum cells (2% DSS + omentum) or PBS (2% DSS). Representative H&E stained tissue sections are shown compared to mock treated mice (no DSS). The histologic inflammation scores of the colon were examined at day 21. *p-value <0.05 using the Mann-Whitney U test. Data are from 8 mice per group. Bars, 100 μm.
Fig. 3.
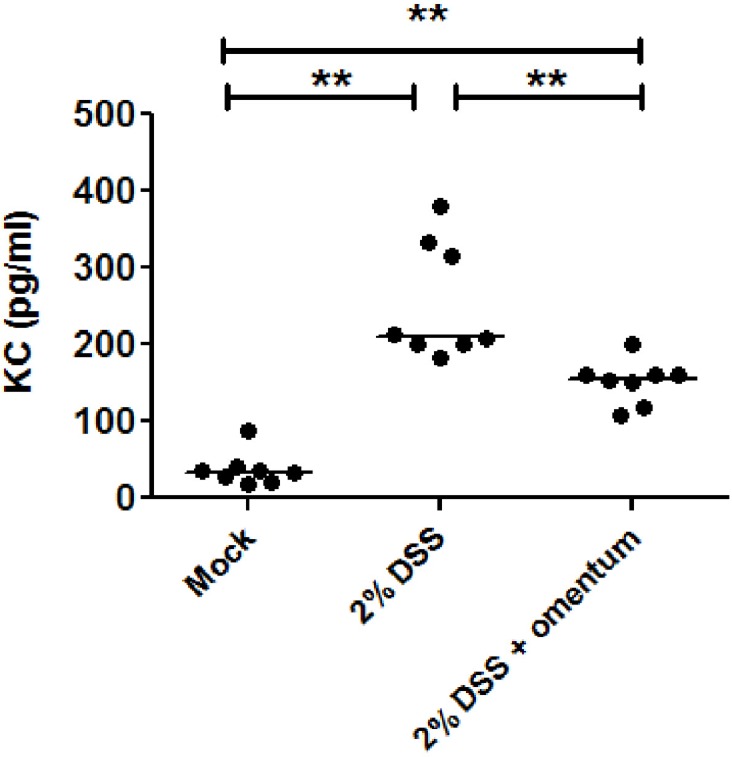
Omentum cells decrease serum KC levels. Mice were administered 2% DSS for 10 day and then either injected intraperitoneally with omentum cells (5 × 106 cells/mouse)(2% DSS + omentum) or with mock injection of PBS alone (2% DSS). Serum was collected at day 21 and analyzed by KC ELISA. **p-value <0.01 using the Mann-Whitney U test. Data are from 8 mice per group.
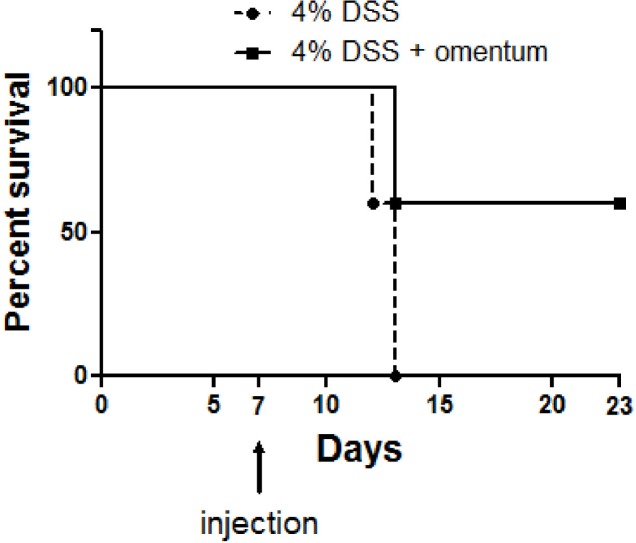
The results thus far suggest that injection of omentum cells accelerated colonic healing in the DSS-colitis model. We next determined if omentum cells could decrease DSS-induced death. Mice were given 4% DSS for 7 days and then given normal water for the duration of the experiment. One group of mice was injected with omentum cells (5 × 106 cells/mouse) while another group was given a mock injection into the peritoneum and survival monitored for up to 23 days. We found that 4% DSS was 100% lethal in mock injected mice at day 13 post DSS treatment whereas the 4% DSS + omentum treated group exhibited 60% survival (Fig. 4). This result suggests that the omentum cells provided protection against a lethal dose of DSS.
Fig. 4.
Omentum cells decrease DSS-induced death. Mice were administered 4% DSS for 7 days and then either injected intraperitoneally with omentum cells (5 × 106 cells/mouse)(4% DSS + omentum) or with mock injection with PBS alone (4% DSS). Survival was assessed daily. Mice which lost >20% of initial body weight were euthanized. *p-value <0.05 using the Mann-Whitney U test. Data are from 9–10 mice were group.
DISCUSSION
A remarkable feature of omentum is that the total mass of the omentum enlarges in response to foreign particles and also shows expansion of immunomodulatory cells as well as cells with stem cell potential. The data reported here suggest that injection of omental cells into the peritoneum of colitis-induced mice promote healing of the damaged colon as well as reduced colitis-induced mortality. The decrease in inflammation was observed at the histological level in the colon, which was further confirmed by a decrease in the IL-8 homologue, KC, in the serum. Although this current study demonstrates that omentum cells can promote healing, it is unclear as to the mechanisms and the cell types involved.
Omentum expansion was performed by activation of the omentum via intraperitoneal injection of biologically inert polyacrylamide beads. It has been reported that polyacrylamide beads elicit a mild inflammatory response as observed by the increase in omentum mass and formation of activated cells [20]. The initiation of the immune response is thought to be due to recruitment of macrophages but the mechanism by which the omentum recognizes polyacrylamide beads is unclear. Regardless, in clinical observations, it has been reported that the omentum responds to foreign objects such as catheter tubing during peritoneal dialysis [21].
The resting omentum consists of connective and adipose tissues. Within the omentum, there are scattered ‘milky spots’ predominantly consisting of macrophages [22]. After exposure to foreign particles, the non-adipose tissues increase in size and a dramatic increase in CD45+ cells is observed. A recent report by Shah et al. [18] demonstrated, using an intratracheal bleomycin lung injury model system in mice, that at least three phenotypically distinguishable cells from the omentum could promote healing in damaged tissues: CD45+Gr1+ myeloid-derived suppressor cells (MDSCs), TH17 suppressive CD45− cells and CD45−CD34+ cells exhibiting mesenchymal stem cell (MSC)-type potential. MSCs were originally isolated from the bone marrow and similar cells can also be found in adipose tissue [23]. MSCs secrete numerous factors exerting both regenerative properties and immunosuppressive functions [24,25]. MSCs cultured in vitro exhibit a fibroblast-like morphology but how these cells migrate or expand in vivo is not completely understood [26]. MDSCs are other cell types that are increased in the omentum and also in sites of inflammation [18,27]. Whether these cells are involved in the healing of the DSS-colitis model is presently unknown.
The omentum has the ability to promote tissue regeneration and may be beneficial for treatment of various diseases. Our data suggests that omentum cells can also be used to treat colitis. In our model, it is currently unclear which cell type(s) is involved in colonic healing. Further experiments utilizing injection of individual cell subsets in the DSS-colitis model would be necessary to elucidate the mechanism of colonic healing by omentum cells.
REFERENCES
- 1.Collins D, Hogan AM, O’Shea D, Winter DC. The omentum: anatomical, metabolic, and surgical aspects. J Gastrointest Surg. 2009;13:1138–46. doi: 10.1007/s11605-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 2.Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am. 2000;80:275–93. xii. doi: 10.1016/s0039-6109(05)70406-0. [DOI] [PubMed] [Google Scholar]
- 3.Morison R. Remarks on some functions of the omentum. Br Med J. 1906;1:76–8. doi: 10.1136/bmj.1.2350.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannaday JE. Some uses of undetached omentum in surgery. Am J Surg. 1948;76:502–5. doi: 10.1016/s0002-9610(48)90236-0. [DOI] [PubMed] [Google Scholar]
- 5.Vineberg AM, Kato Y, Pirozynski WJ. Experimental revascularization of the entire heart. Evaluation of epicardiectomy, omental graft, and/or implantation of the internal mammary artery in preventing myocardial necrosis and death of the animal. Am Heart J. 1966;72:79–93. doi: 10.1016/0002-8703(66)90630-2. [DOI] [PubMed] [Google Scholar]
- 6.Vernik J, Singh AK. Omentum: power to heal and regenerate. Int J Artif Organs. 2007;30:95–9. doi: 10.1177/039139880703000203. [DOI] [PubMed] [Google Scholar]
- 7.Athanassiadi K, Theakos N, Benakis G, Kakaris S, Skottis I. Omental transposition: the final solution for major sternal wound infection. Asian Cardiovasc Thorac Ann. 2007;15:200–3. doi: 10.1177/021849230701500305. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Rosmarakis ES. Recurrent post-sternotomy mediastinitis. J Infect. 2006;52:e151–4. doi: 10.1016/j.jinf.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Moreschi AH, Macedo Neto AV, Barbosa GV, Saueressig MG. Aggressive treatment using muscle flaps or omentopexy in infections of the sternum and anterior mediastinum following sternotomy. J Bras Pneumol. 2008;34:654–60. doi: 10.1590/s1806-37132008000900004. [DOI] [PubMed] [Google Scholar]
- 10.Asai S, Kamei Y, Torii S. One-stage reconstruction of infected cranial defects using a titanium mesh plate enclosed in an omental flap. Ann Plast Surg. 2004;52:144–7. doi: 10.1097/01.sap.0000100898.68014.6f. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith HS, Chen WF, Duckett SW. Brain vascularization by intact omentum. Arch Surg. 1973;106:695–8. doi: 10.1001/archsurg.1973.01350170061015. [DOI] [PubMed] [Google Scholar]
- 12.Vatansev C, Ustun ME, Ogun CO, Tastekin G, Karabacakoglu A, Yilmaz H. Omental transposition decreases ischemic brain damage examined in a new ischemia model. Eur Surg Res. 2003;35:388–94. doi: 10.1159/000070612. [DOI] [PubMed] [Google Scholar]
- 13.Maloney CT, Jr, Wages D, Upton J, Lee WP. Free omental tissue transfer for extremity coverage and revascularization. Plast Reconstr Surg. 2003;111:1899–904. doi: 10.1097/01.PRS.0000056874.31920.7D. [DOI] [PubMed] [Google Scholar]
- 14.Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487–97. doi: 10.1007/s00441-006-0356-4. [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G. Stromal cells cultured from omentum express pluripotent markers, produce high amounts of VEGF, and engraft to injured sites. Cell Tissue Res. 2008;332:81–8. doi: 10.1007/s00441-007-0560-x. [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Pancholi N, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Kraus M, Dunea G, Arruda JA. Omentum facilitates liver regeneration. World J Gastroenterol. 2009;15:1057–64. doi: 10.3748/wjg.15.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Gomez I, Goldsmith HS, Angulo J, Prados A, Lopez-Hervas P, Cuevas B, Dujovny M, Cuevas P. Angiogenic capacity of human omental stem cells. Neurol Res. 2005;27:807–11. doi: 10.1179/016164105X63674. [DOI] [PubMed] [Google Scholar]
- 18.Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, Sethupathi P, Seki Y, Takami M, Byrne K, Wigfield C, Love RB, Iwashima M. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7:e38368. doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Gin H, Dupuy B, Bonnemaison-Bourignon D, Bordenave L, Bareille R, Latapie MJ, Baquey C, Bezian JH, Ducassou D. Biocompatibility of polyacrylamide microcapsules implanted in peritoneal cavity or spleen of the rat. Effect on various inflammatory reactions in vitro. Biomater Artif Cells Artif Organs. 1990;18:25–42. doi: 10.3109/10731199009117287. [DOI] [PubMed] [Google Scholar]
- 21.Crabtree JH, Fishman A. Laparoscopic omentectomy for peritoneal dialysis catheter flow obstruction: a case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 1999;9:228–33. [PubMed] [Google Scholar]
- 22.Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–74. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 23.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 24.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–9. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–33. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]