Abstract
Premature ventricular contractions (PVCs) are early depolarizations of the myocardium originating in the ventricle. PVCs are common with an estimated prevalence of 40% to 75% in the general population on 24- to 48-hour Holter monitoring. Traditionally, they have been thought to be relatively benign in the absence of structural heart disease but they represent increased risk of sudden death in structural heart disease. Especially in ischemic heart disease, the frequency and complexity of PVCs is associated with mortality. Implantable cardioverter defibrillator therapy is indicated in patients with nonsustained ventricular tachycardia (NSVT) due to prior myocardial infarction, left ventricular ejection fraction less than or equal to 40%, and inducible ventricular fibrillation or sustained ventricular tachycardia at electrophysiological study. In congestive heart failure, PVCs did not provide significant incremental prognostic information beyond readily available clinical variables. Consequently, NSVT should not guide therapeutic interventions. Recently, the concept of PVC-induced cardiomyopathy was proposed when pharmacological suppression of PVCs in patients with presumed idiopathic dilated cardiomyopathy subsequently showed improved left ventricular systolic dysfunction. For the treatment PVCs, it is important to consider underlying heart disease, the frequency of the PVCs and the frequency and severity of symptoms.
Keywords: Premature ventricular contractions, Nonsustained ventricular tachycardia, Cardiomyopathy
INTRODUCTION
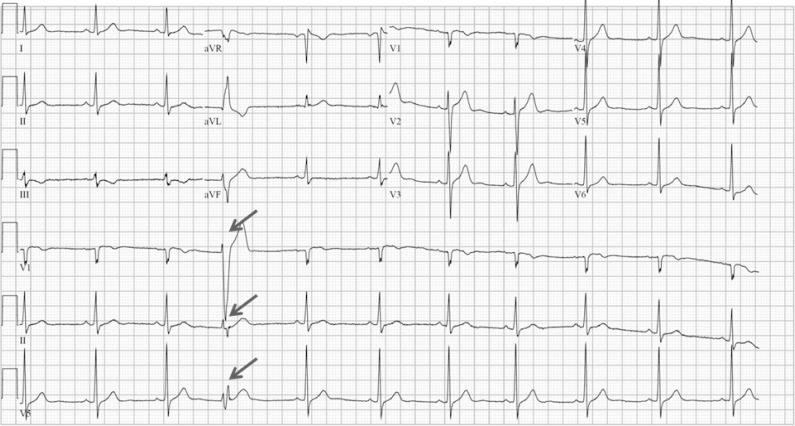
Premature ventricular contractions (PVCs) are early depolarizations of the myocardium originating in the ventricle (Fig. 1). PVCs are common with an estimated prevalence of 1% to 4% in the general population on standard 12-lead electrocardiography and between 40% and 75% of subjects on 24- to 48-hour Holter monitoring [1,2]. Ventricular ectopic activity occurs in a wide variety of clinical settings with a spectrum of clinical implications. They are often seen in association with structural heart disease and represent increased risk of sudden death, yet they are ubiquitous, even in the absence of identifiable heart disease [3,4]. Traditionally, they have been thought to be relatively benign in the absence of structural heart disease [2,5]. Over the last decade, however, PVC-induced cardiomyopathy (CMP) has been a subject of great interest and the evidence for this entity is rapidly emerging. Appropriate clinical evaluation and investigations are important in assessing patients with PVCs so that effective treatment can be targeted when necessary. This article discusses the current knowledge and practice in this commonly encountered clinical cardiological problem.
Fig. 1.
Example of premature ventricular complex.
PROGNOSIS OF PVCs
The incidence, frequency, and complexity of ventricular arrhythmias were greater in the presence of known or suspected heart disease. PVCs and runs of NSVT in subjects with structural heart disease contribute to an increased mortality risk, the magnitude of which varies with the nature and extent of the underlying disease.
1. Ischemic heart disease
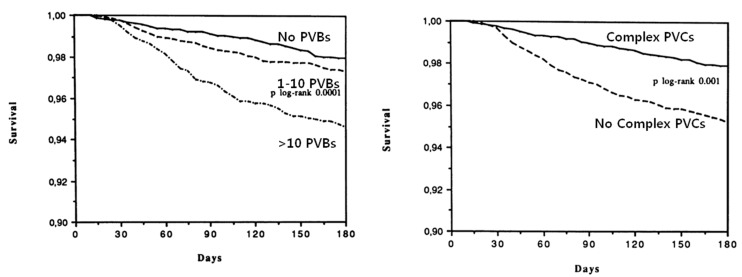
In 1975, Schulze et al. reported results from the first post-infarction studies of left ventricular (LV) dysfunction, ventricular arrhythmias, and death that used radionuclide methods to measure left ventricular ejection fraction (LVEF) and 24 hr electrocardiogram (ECGs) to assess ventricular arrhythmias. All eight deaths in their 81 patients occurred in the group with high-grade ventricular arrhythmias and LVEF below 40% [6,7]. In a multicenter postmyocardial infarction study, 766 patients with acute myocardial infarction had their LVEFs measured by radionuclide methods and a 24 hr ECG analyzed by sensitive and specific methods. Out of these 766 patients, 86 deaths occurred during the 3 year follow-up period. When the variables were analyzed separately, there were strong associations between death and LVEF, frequency of VPCs, or repetitiveness of VPCs [8]. In the GISSI trial, Twenty-four-hour Holter recordings obtained before discharge from the hospital in 8,676 post-myocardial infarction patients were analyzed for the presence of ventricular arrhythmias. The presence of more than 10 PVBs per hour or of complex ventricular arrhythmias was significantly associated with a higher mortality risk regardless of the presence of LV dysfunction (Fig. 2) [9]. In December 1990, investigators initiated prophylactic Multicenter Automatic Defibrillator Implantation Trial (MADIT) in which high-risk patients with coronary heart disease and asymptomatic unsustained ventricular tachycardia (a run of 3 to 30 ventricular ectopic beats at a rate>120 beats per minute) were randomly assigned to receive an implantable cardioverter defibrillator (ICD) or conventional medical therapy. The prophylactic therapy with an implanted defibrillator led to improved survival as compared with conventional medical therapy [10]. The Multicenter Unsustained Tachycardia Trial was initiated in 1989 to test the hypothesis that antiarrhythmic therapy guided by electrophysiologic testing can reduce the risks of sudden death and cardiac arrest among patients with coronary artery disease, LV dysfunction, and spontaneous NSVT. The results of this study established that high risk patients with asymptomatic, NSVT, and inducible sustained ventricular tachyarrhythmia have substantial mortality due to arrhythmia. The rate of death among patients with inducible sustained tachyarrhythmia was reduced by the use of defibrillators [11]. ICD therapy is indicated in patients with NSVT due to prior myocardial infarction, LVEF less than or equal to 40%, and inducible ventricular fibrillation or sustained VT at electrophysiological study [12].
Fig. 2.
6-month survival of patients by premature ventricular contractions (PVCs) per hour. Adapted from Maggioni et al [9].
2. Heart failure
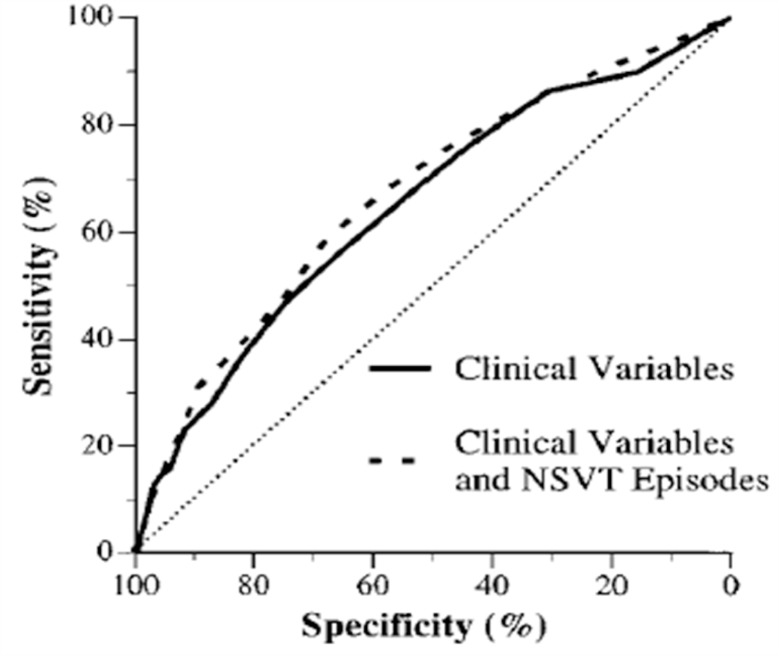
Although we might expect all patients with LV dysfunction to die from progressive heart failure, many die suddenly and unexpectedly without any evidence of recent hemodynamic or functional deterioration. Sudden death is the final event in approximately 35–50% of patients with chronic heart failure [13]. The patient with chronic heart failure is not only at risk of sudden death but is also likely to manifest serious ventricular arrhythmias. In addition to their hemodynamic derangements, patients with chronic heart failure have numerous electrical abnormalities that develop and progress in parallel with the mechanical dysfunction. The prevalence and complexity of ambulatory ventricular arrhythmias increase dramatically as LV function deteriorates [14]. In patients with a LVEF of less than 40%, the prevalence of NSVT rises from 15–20% in patients with class I–II symptoms of heart failure to 40–55% in class II–III patients and 50–70% in class III–IV patients [15]. Numerous studies have shown an independent direct relationship of complex cardiac arrhythmias (repetitive forms) and LV dysfunction with subsequent mortality [16–18]. But in the CHF STAT study, NSVT was frequently seen in patients with heart failure and was associated with worsened survival by univariate analysis. However, after adjusting other variables, especially for EF, NSVT was not an independent predictor of all-cause mortality or sudden death. The suppression of NSVT by amiodarone had no effect on total survival nor on sudden cardiac death [19]. The Prospective Randomized Milrinone Survival Evaluation (PROMISE) study was undertaken to determine whether ventricular arrhythmias were independent and specific predictors of sudden death. In this study, ventricular arrhythmias did not specifically define a group at high risk for sudden death and did not provide significant incremental prognostic information beyond readily available clinical variables (Fig. 3) [20]. The presence of complex ventricular arrhythmias (especially NSVT) on ambulatory monitoring predicts total cardiac mortality but does not identify patients who are destined to die suddenly. This observation suggests that the frequency and complexity of rhythm disturbances in patients with severe heart failure reflect the severity of the underlying disease process rather than a specific arrhythmogenic state. NSVT should not guide therapeutic interventions, such as the institution of antiarrhythmic therapy or implantation of antifibrillatory devices.
Fig. 3.
ROC curves of multivariate logistic regression models. Multivariate model including only clinical variables (age, NYHA class, ejection fraction, systolic blood pressure, cause of heart failure, and treatment group) is denoted by solid line, whereas model including number of episodes of NSVT in addition to clinical variables is denoted by dashed line. Adapted from Teerlink et al [20].
3. Premature ventricular complexes in the absence of heart disease
In the Tecumseh, Michigan, communitywide cardiovascular epidemiology study, PVCs in subjects with structurally normal hearts carried no adverse prognostic significance under the age of 30 years, but in those older than 30 years, PVCs and short runs of NSVT began to influence risk [21]. More recent studies provide conflicting implications regarding risk in asymptomatic subjects. In one study [22], asymptomatic ventricular arrhythmias in the absence of identifiable heart disease predicted a small increase in risk, while another study [23] suggested no increased risk. In 1985, Kennedy et al. published a follow-up study (mean 6.5 years) of 73 apparently healthy subjects with frequent and complex ventricular ectopy. The conclusion was that the long-term prognosis of these patients is similar to that of the healthy population [2]. In another study, patients with a diagnosis of idiopathic right ventricular ectopy were evaluated after a follow-up of at least 12 years to verify the occurrence of sudden death and the possible evolution towards arrhythmogenic right ventricular dysplasia (ARVD). That study reported that no patient developed ARVD, none died suddenly nor had sustained ventricular tachycardia during 12 to 20 years follow up for 61 patients.
In contrast to the apparently non-life-threatening implication of PVCs at rest, PVCs elicited during exercise testing, even in apparently normal individuals, appear to imply risk over time. In the study by Jouven et., a total of 6,101 asymptomatic French men free of clinically detectable cardiovascular disease were exercised and persons with frequent VPCs, defined as having >10% of all ventricular depolarisations in any 30s recordings during exercise, were found to have an increase in cardiovascular deaths by a factor of 2.67 after 23 years of follow up [24]. A recently published study in 2885 subjects who are offspring of the original Framingham study participants also presented similar findings [25]. A study by Frolkis et al. focused on the recovery period of the exercise test and showed that frequent PVCs during recovery were associated with an increased risk of death (hazard ratio 2.4) than frequent PVCs during exercise (hazard ratio 1.8) during a mean of 5.3 years of follow-up [26]. A selection bias, based on indications for stress testing, may have influenced these observations. Although additional corroborative data are required from large cohort studies, these results have prompted the suggestion that frequent VPCs associated with exercise testing be considered as a new prognostic criterion in addition to ischemia.
PREMATURE VENTRICULAR CONTRACTION-INDUCED CARDIOMYOPATHY
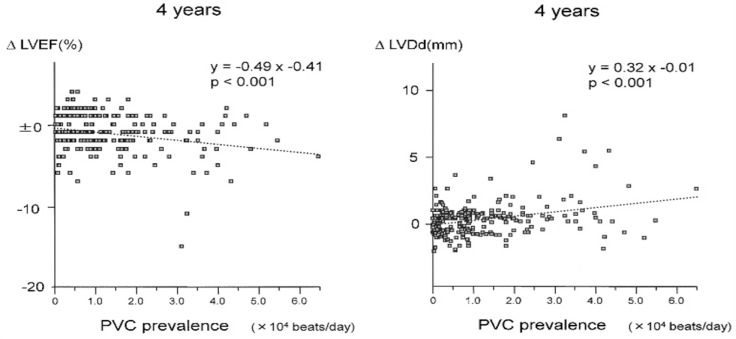
Traditionally, PVCs have been thought to be relatively benign in the absence of structural heart disease [2,5]. Over the last decade, however, PVC-induced CMP has been a subject of great interest and the evidence for this entity is rapidly emerging. The concept of PVC-induced CMP was proposed by Duffee et al. in 1998 when pharmacological suppression of PVCs in patients with presumed idiopathic dilated CMP subsequently improved LV systolic dysfunction [27]. The exact prevalence of PVC-induced CMP is not known; it is an underappreciated cause of LV dysfunction, and it is primarily observed in older patients [28]. Niwano et al. demonstrated progressive worsening of LV function in patients with frequent PVCs (>1,000 beats/day) as measured by the LVEF and LV end-diastolic dimension over a follow-up period of 4 to 8 years (Fig. 4) [29]. Yarlagadda et al. reported the results of repetitive monomorphic ventricular ectopy ablation in 27 patients. In that study, the ventricular function improved in 7 of 8 patients with depressed ventricular function [28].
Fig. 4.
Relationship between the premature ventricular contraction prevalence and change in left ventricular ejection fraction (ΔLVEF) and left ventricular diastolic dimension (ΔLVEDd). Adapted from Niwano et al [29].
1. Risk factors for PVCs induced cardiomyopathy
1). PVC burden:
Several studies have shown that the frequency of PVCs correlates at least modestly with the extent of LV dysfunction and ventricular dilation at the time of initial clinical presentation [29–33]. However, there are no clear-cut points that mark the frequency at which CMP is unavoidable. Baman et al. suggested that a PVC burden of >24% had a sensitivity and specificity of 79% and 78%, respectively, in separating the patient populations with impaired versus preserved LV function [34]. But in another study, the threshold burden of PVCs for reduced LVEF differed between those with a LV and those with a RV site of origin of PVCs. PVCs originating from the RV were associated with a significantly increased prevalence of reduced LVEF at a PVC burden ≥10%, whereas PVCs originating from the LV were associated with reduced LVEF only at a PVC burden ≥20% [35]. In the MOST trial, ventricular pacing in the DDDR mode >40% of the time conferred a 2.6-fold increased risk of heart failure hospitalization compared with less pacing. This provided some evidence that highly paced patients are not only at greater risk for heart failure hospitalization but are also hospitalized for heart failure more often [36]. Even though the burden of PVCs seems to be an important determinant of LV systolic dysfunction, some patients with a high PVC burden do not develop CMP. This suggests that in addition to the PVC burden, other characteristics of PVCs might also be contributory [37].
2). PVC origin, morphology and duration:
Theoretically, PVC originating in the right ventricle can cause more severe LV dyssynchrony compared with that originating in left ventricle and PVC duration also affects the LV synchronization. The morphology can, to some extent, determine the site and etiology of the PVCs. Munoz et al. retrospectively studied 70 subjects who underwent PVC ablation. They did not find any association of LVEF with PVC coupling interval, delay in PVC ID, LBBB versus RBBB morphology of the PVC or the site of PVC origin, except for fascicular PVCs. They only reported that PVCs originating in the RV were associated with reduced LVEF at a lower threshold PVC burden than that for LV PVCs as described above [35]. In some recent studies, longer PVC QRS duration was also associated with the presence of CMP. However, in a subgroup of patients with very wide PVCs (mean QRS duration 173 ms), successful suppression of PVCs failed to normalize LV function [38,39].
2. Mechanism of PVC induced cardiomyopathy
The mechanism of PVC-CMP is presumed to be related to PVC-induced LV dyssynchrony. Indeed, every known mechanism of LV dyssynchrony (left bundle branch block, right ventricular pacing, and preexcitation) can produce CMP [40,41]. Although QRS duration is a major determinant of LV dyssynchrony, significant variation in LV activation patterns and degree of dyssynchrony exists between various wide QRS morphologies [42]. To predict the future development of PVC-CMP, methods of direct LV dyssynchrony assessment during PVC might be required. Additional factors such as dyssynchrony-induced papillary muscle dysfunction that can cause mitral regurgitation with additional LV volume overload, and autonomic response modification by variations in ventriculoatrial conduction, can affect CMP development and have not been studied systematically [43,44].
TREATMENT OF PVCs
When considering the need for further intervention and planning treatment for patients with VPCs, it is important to consider: (1) whether there is underlying heart disease; (2) the frequency of the VPCs and if VT has been documented; and (3) the frequency and severity of symptoms.
In the absence of heart disease and if VPCs are infrequent or reduce in frequency on exercise tolerance test, with no documented VT, patients should be reassured and no specific treatment is required-especially if they are relatively asymptomatic. The same patients with significant symptoms should have their blood pressure checked and investigated and treated if high. β-blockers may be used to control symptoms in patients where VPCs arise from multiple sites. It should also be considered in patients with impaired ventricular systolic function and/or heart failure. There is no evidence to support the use of other antiarrhythmic agents simply for the sake of suppressing VPCs, especially considering their proarrhythmic (for example, flecainide) and other side effects (for example, amiodarone) [45]. A therapeutic medical trial or catheter ablation may be considered in patients with LV dysfunction and frequent PVCs (a generally accepted range of >10,000–20,000 or >10% of total heart beats over 24 hours) if the clinical suspicion for PVC-induced CMP is high [29,33–35,46].
CONCLUSION
PVCs are early depolarizations of the myocardium originating in the ventricle. PVCs are frequently observed in the general population. Traditionally, they have been thought to be relatively benign in the absence of structural heart disease but they represent increased risk of sudden death in structural heart disease. Recently the concept of PVC-induced CMP was proposed when pharmacological suppression of PVCs in patients with presumed idiopathic dilated CMP subsequently improved LV systolic dysfunction. The frequency of PVCs correlates at least modestly with the extent of LV dysfunction and ventricular dilation.
For the treatment PVCs, it is important to consider underlying heart disease, the frequency of the PVCs and the frequency and severity of symptoms. Usually asymptomatic PVCs do not need treatment. In case of symptomatic PVCs, β-blockers may be used to control the symptoms. A therapeutic medical trial or catheter ablation may be considered in patients with PVC-induced CMP.
REFERENCES
- 1.Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707–12. doi: 10.1136/hrt.2005.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–7. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 3.Messineo FC. Ventricular ectopic activity: prevalence and risk. Am J Cardiol. 1989;64:53J–6J. doi: 10.1016/0002-9149(89)91200-9. [DOI] [PubMed] [Google Scholar]
- 4.Kostis JB, McCrone K, Moreyra AE, Gotzoyannis S, Aglitz NM, Natarajan N, Kuo PT. Premature ventricular complexes in the absence of identifiable heart disease. Circulation. 1981;63:1351–6. doi: 10.1161/01.cir.63.6.1351. [DOI] [PubMed] [Google Scholar]
- 5.Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, Brusin MC, Molinari G, Trevi G. Long-term follow-up of right ventricular monomorphic extrasystoles. J Am Coll Cardiol. 2001;38:364–70. doi: 10.1016/s0735-1097(01)01403-6. [DOI] [PubMed] [Google Scholar]
- 6.Schulze RA, Jr, Strauss HW, Pitt B. Sudden death in the year following myocardial infarction. Relation to ventricular premature contractions in the late hospitals phase and left ventricular ejection fraction. Am J Med. 1977;62:192–9. doi: 10.1016/0002-9343(77)90314-x. [DOI] [PubMed] [Google Scholar]
- 7.Schulze RA, Jr, Rouleau J, Rigo P, Bowers S, Strauss HW, Pitt B. Ventricular arrhythmias in the late hospital phase of acute myocardial infarction. Relation to left ventricular function detected by gated cardiac blood pool scanning. Circulation. 1975;52:1006–11. doi: 10.1161/01.cir.52.6.1006. [DOI] [PubMed] [Google Scholar]
- 8.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–8. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 9.Maggioni AP, Zuanetti G, Franzosi MG, Rovelli F, Santoro E, Staszewsky L, Tavazzi L, Tognoni G. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI-2 results. Circulation. 1993;87:312–22. doi: 10.1161/01.cir.87.2.312. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 11.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 12.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm. 2008;5:934–55. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681–5. doi: 10.1161/01.cir.72.4.681. [DOI] [PubMed] [Google Scholar]
- 14.Califf RM, McKinnis RA, Burks J, Lee KL, Harrell FE, Jr, Behar VS, Pryor DB, Wagner GS, Rosati RA. Prognostic implications of ventricular arrhythmias during 24 hour ambulatory monitoring in patients undergoing cardiac catheterization for coronary artery disease. Am J Cardiol. 1982;50:23–31. doi: 10.1016/0002-9149(82)90004-2. [DOI] [PubMed] [Google Scholar]
- 15.Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am J Cardiol. 1990;65:42I–8I. doi: 10.1016/0002-9149(90)90125-k. [DOI] [PubMed] [Google Scholar]
- 16.Meinertz T, Hofmann T, Kasper W, Treese N, Bechtold H, Stienen U, Pop T, Leitner ER, Andresen D, Meyer J. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1984;53:902–7. doi: 10.1016/0002-9149(84)90522-8. [DOI] [PubMed] [Google Scholar]
- 17.Holmes J, Kubo SH, Cody RJ, Kligfield P. Arrhythmias in ischemic and nonischemic dilated cardiomyopathy: prediction of mortality by ambulatory electrocardiography. Am J Cardiol. 1985;55:146–51. doi: 10.1016/0002-9149(85)90317-0. [DOI] [PubMed] [Google Scholar]
- 18.Unverferth DV, Magorien RD, Moeschberger ML, Baker PB, Fetters JK, Leier CV. Factors influencing the one-year mortality of dilated cardiomyopathy. Am J Cardiol. 1984;54:147–52. doi: 10.1016/0002-9149(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 19.Singh SN, Fisher SG, Carson PE, Fletcher RD. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol. 1998;32:942–7. doi: 10.1016/s0735-1097(98)00338-6. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink JR, Jalaluddin M, Anderson S, Kukin ML, Eichhorn EJ, Francis G, Packer M, Massie BM. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation. 2000;101:40–6. doi: 10.1161/01.cir.101.1.40. [DOI] [PubMed] [Google Scholar]
- 21.Chiang BN, Perlman LV, Ostrander LD, Jr, Epstein FH. Relationship of premature systoles to coronary heart disease and sudden death in the Tecumseh epidemiologic study. Ann Intern Med. 1969;70:1159–66. doi: 10.7326/0003-4819-70-6-1159. [DOI] [PubMed] [Google Scholar]
- 22.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framing-ham Heart Study. Ann Intern Med. 1992;117:990–6. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 23.Engstrom G, Hedblad B, Janzon L, Juul-Moller S. Ventricular arrhythmias during 24-h ambulatory ECG recording: incidence, risk factors and prognosis in men with and without a history of cardiovascular disease. J Intern Med. 1999;246:363–72. doi: 10.1046/j.1365-2796.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 24.Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med. 2000;343:826–33. doi: 10.1056/NEJM200009213431201. [DOI] [PubMed] [Google Scholar]
- 25.Morshedi-Meibodi A, Evans JC, Levy D, Larson MG, Vasan RS. Clinical correlates and prognostic significance of exercise-induced ventricular premature beats in the community: the Framingham Heart Study. Circulation. 2004;109:2417–22. doi: 10.1161/01.CIR.0000129762.41889.41. [DOI] [PubMed] [Google Scholar]
- 26.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–90. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 27.Duffee DF, Shen WK, Smith HC. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin Proc. 1998;73:430–3. doi: 10.1016/S0025-6196(11)63724-5. [DOI] [PubMed] [Google Scholar]
- 28.Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–7. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 29.Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, Hatakeyama Y, Izumi T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230–7. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- 30.Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–65. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 31.Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, Jongnarangsin K, Marine JE, Chugh A, Pelosi F, Oral H, Morady F. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–7. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Taieb JM, Maury P, Shah D, Duparc A, Galinier M, Delay M, Morice R, Alfares A, Barnay C. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J Interv Card Electrophysiol. 2007;20:9–13. doi: 10.1007/s10840-007-9157-2. [DOI] [PubMed] [Google Scholar]
- 33.Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2008;13:81–5. doi: 10.1111/j.1542-474X.2007.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F, Jr, Crawford T, Ebinger M, Oral H, Morady F, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–9. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Del Carpio Munoz F, Syed FF, Noheria A, Cha YM, Friedman PA, Hammill SC, Munger TM, Venkatachalam KL, Shen WK, Packer DL, Asirvatham SJ. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–8. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 37.Rhee KH, Jung JY, Rhee KS, Kim HS, Chae JK, Kim WH, Ko JK. Tachycardiomyopathy induced by ventricular premature complexes: complete recovery after radiofrequency catheter ablation. Korean J Intern Med. 2006;21:213–7. doi: 10.3904/kjim.2006.21.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokokawa M, Kim HM, Good E, Crawford T, Chugh A, Pelosi F, Jr, Jongnarangsin K, Latchamsetty R, Armstrong W, Alguire C, Oral H, Morady F, Bogun F. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–4. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Deyell MW, Park KM, Han Y, Frankel DS, Dixit S, Cooper JM, Hutchinson MD, Lin D, Garcia F, Bala R, Riley MP, Gerstenfeld E, Callans DJ, Marchlinski FE. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm. 2012;9:1465–72. doi: 10.1016/j.hrthm.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 40.van Geldorp IE, Delhaas T, Gebauer RA, Frias P, Tomaske M, Friedberg MK, Tisma-Dupanovic S, Elders J, Fruh A, Gabbarini F, Kubus P, Illikova V, Tsao S, Blank AC, Hiippala A, et al. Impact of the permanent ventricular pacing site on left ventricular function in children: a retrospective multicentre survey. Heart. 2011;97:2051–5. doi: 10.1136/heartjnl-2011-300197. [DOI] [PubMed] [Google Scholar]
- 41.Udink ten Cate FE, Kruessell MA, Wagner K, Trieschmann U, Emmel M, Brockmeier K, Sreeram N. Dilated cardiomyopathy in children with ventricular pre-excitation: the location of the accessory pathway is predictive of this association. J Electrocardiol. 2010;43:146–54. doi: 10.1016/j.jelectrocard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Bleeker GB, Schalij MJ, Molhoek SG, Verwey HF, Holman ER, Boersma E, Steendijk P, Van Der Wall EE, Bax JJ. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–9. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 43.Ypenburg C, Lancellotti P, Tops LF, Boersma E, Bleeker GB, Holman ER, Thomas JD, Schalij MJ, Pierard LA, Bax JJ. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008;29:757–65. doi: 10.1093/eurheartj/ehn063. [DOI] [PubMed] [Google Scholar]
- 44.Havranek S, Stovicek P, Psenicka M, Wichterle D, Linhart A. Heart rate turbulence after ventricular pacing trains during programmed ventricular stimulation. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S170–3. doi: 10.1111/j.1540-8159.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- 45.Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321:406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 46.Hasdemir C, Ulucan C, Yavuzgil O, Yuksel A, Kartal Y, Simsek E, Musayev O, Kayikcioglu M, Payzin S, Kultursay H, Aydin M, Can LH. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663–8. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]