Abstract
It is widely accepted that chronic inflammation contributes to the pathogenesis of obesity. Researchers have recently discovered that increased inflammatory cytokines and the infiltration and activation of macrophage cells in the adipose tissue are related to chronic obesity. This immunologic dysregulation has led to the development of the classical pro-inflammatory paradigm. However, since chronic inflammation associated with obesity is more than just the overproduction of pro-inflammatory cytokines, precise dissection requires beyond the classical pro-inflammatory cytokines. The purpose of this review is to summarize the immunological profiling of obesity for theragnostic convenience, focusing on the cytokine and adipokine network in obesity and the significance of the balance of Th1/Th2 immunity.
Keywords: Adipokine, Cytokine, Immune homeostasis, Immunological profiling, Obesity
INTRODUCTION
Obesity, which involves chronic low-grade inflammation, has become a global epidemic. This poses a large health burden, because excess adiposity can lead to an array of chronic diseases such as type 2 diabetes, stroke, coronary heart disease and high blood pressure [1]. Obesity is associated with an abnormal increase in adipose tissue mass and liver fat (adiposity), as well as dysregulated levels of adipocytokines and imbalance of T helper cell type-1 (Th1) and Th2 cytokines [2]. It is well-established that the inflammatory response associated with obesity is triggered by and predominantly resides in the adipose tissue [3]. Adipose tissue is a biologically-active endocrine tissue that serves as energy storage and secretes various adipocytokines such as adiponectin, leptin, tumor necrosis factor-α (TNF-α), resistin and plasminogen-activator type 1 (PAI-1) [4–8]. Of these adipokines, adiponectin and leptin are novel specific hormones implicated in the pathogenesis of obesity [4]. The immunological profiling of obesity-related biomarkers will allow researchers to better understand the complex interactions between classical immunocytes, adipose tissue macrophages, adipokines and other inflammation-related cytokines implicated in the pathogenesis of chronic low-grade obesity. In summary, this review will highlight the importance of adipokines and the Th1 and Th2 network in obesity.
IMMUNE FEATURES OF OBESITY
There is some controversy surrounding the immune features of obese and non-obese states. The host defense immune response mechanism comprises innate and adaptive immunity. According to Lumeng and Saltiel, “the chronic nature of obesity produces a tonic low-grade activation of the innate system that affects metabolic homeostasis over time” [9]. Obesity triggers an immune response that includes a systemic increase in circulating inflammatory cytokines, the recruitment of immunocytes to inflamed tissues, activation of leukocytes, and the generation of repair tissue responses [10]. Thus, it seems feasible that alterations in immune function induced by obesity would be mirrored through immunological parameters such as leucocyte and lymphocyte subpopulation counts and the secretion of cytokines and adipokines. However, the weaker nature of chronic inflammation such as obesity is often difficult to detect the exact immunologic status, thereby ignoring the serious systemic complications, which would be ascribed to the immunologic dysfunction.
OBESITY AND ADIPOKINES
Adipose tissue and its core cells, adipocytes, were once thought to not have a role in regulating the immune system. However, with an expanded knowledge of innate immunity, this idea is now known to be antiquated and obsolete. According to the principle of innate immunity, adipocytes are innate immunocytes that exert their function via a vast array of chemical messengers such as cytokines and a type of hormokine called “adipokine.” For instance, adipose tissue specifically regulates the expression and secretion of adipokines. Adipokines such as leptin and adiponectin play pivotal roles in the pathogenesis of obesity. The inflammatory state associated with obesity is reflected in increased circulating levels of pro-inflammatory proteins such as leptin, and decreased secretion of anti-inflammatory proteins such as adiponectin [11]. Of these adipokines, researchers have extensively studied the role of the leptin in obesity. Leptin circulates in blood serum and regulates energy intake and expenditure [12]. Starvation conditions trigger a decrease in leptin levels, leading to neural pathways in the hypothalamus that cause increased appetite and decreased energy expenditure, as a means of restoring body fat [13,14]. However, in the obese state, where there is an accumulation of fat, leptin plasma levels increase. Other studies also showed that mice lacking leptin or the leptin receptor were severely obese [15–17]. Leptin can be regarded as a cytokine, since the leptin receptor bears significant homology to type 1 cytokine receptors [18]. Leptin is now considered the master hormone for long-term body weight control [19]. In the obese state, immunocytes are subject to higher circulating leptin concentrations, innate immunity is activated, and there is a predominance of pro-inflammatory Th1 cells and reduced Th2 phenotypes [20]. This pro-inflammatory phenotype switch is beneficial during acute infections. Paradoxically, over the long-term and in chronic conditions, it would be deleterious.
Adiponectin is another adipokine that contributes to the chronic low-grade inflammation associated with obesity. Adiponectin, abundantly present in the plasma, is known to modulate metabolic processes and increase insulin sensitivity through regulation of glucose metabolism and induction of fatty acid oxidation [21]. Plasma concentrations of adiponectin decrease with increasing obesity. Convincing evidences showed that overexpression of the adiponectin gene would protect the mice from diabetes and atherosclerosis [22]. Adiponectin is known to enhance IL-10 mRNA expression, thereby augmenting IL-10 release from macrophages [23]. In addition, overexpression of adiponectin leads to differentiation of 3T3-L1 fibroblasts to adipocytes via metabolic effects in an autocrine fashion [24], which also has been proven by another study in vivo [25]. Herein, the important point is that imbalances in the cytokine and adipokine network would affect the inflammatory status of obesity or metabolic syndrome. This is greatly indebted to new concept of immune homeostasis beyond classical immune homeostasis.
IMMUNOLOGIC ACTIVATION BEYOND THE CLASSICAL PROINFLAMMATORY PARADIGM
Chronic inflammation in obesity would more aggravate the inflamed visceral adipose tissue brought via the accumulation of inflammatory immunocytes such as T cells, as well as the activation of macrophages, consequently, leading to the overproduction of pro-inflammatory cytokines [26]. Obesity is associated with moderate but chronic production of these inflammatory factors [27].
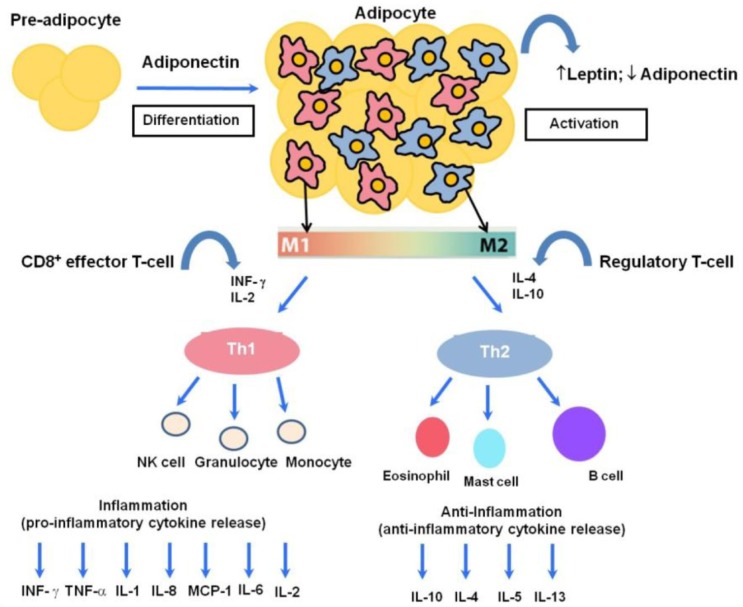
In the point of innate immunology, obesity is widely characterized by macrophage infiltration into the adipose tissue. This tissue-resident macrophage has two different phenotypes: M1 (pro-inflammatory) and M2 (anti-inflammatory) [28–31]. Chronic obesity involves the accumulation of M1-polarized macrophages [29–31] and a switch from the M2 to the M1 phenotype. That is, chronic obesity leads to a more pro-inflammatory profile (Fig. 1) [28]. Thus, the absence of the anti-inflammatory macrophage response is linked to a higher risk of obesity, inflammation and insulin resistance [32]. The M1/M2 polarization paradigm describes the macrophage activation directed against pro-inflammatory M1 driven by the Th-1 type cytokine such as interferon-gamma (IFN-γ) (Fig. 1) [33]. This “phenotype switch” from M2 to M1 made it plausible that T cells and Th1/2 cytokines would act as orchestrators of adipose tissue-resident macrophage responses during the development of obesity-associated adipose tissue inflammation and insulin resistance [28,34,35].
Fig. 1.
The human host immune response to a variety of inflammatory states involves a balance between pro-inflammatory and anti-inflammatory cytokines. Adiponectin can act in an autocrine fashion and leads to proliferation, differentiation and activation from preadipocytes to adipocytes. One hallmark of obesity is inflamed adipose tissue leading to macrophage recruitment. Activated adipose tissue is responsible for the secretion of leptin and adiponectin, while increased numbers of macrophages cause phenotypic and morphological changes. These changes include the polarization of adipose tissue macrophages toward a classical M1 or alternatively-activated M2 phenotype, which are associated with potent pro-inflammatory and anti-inflammatory activities, respectively. M1 arises in response to IFN-γ and IL-2, while M2 macrophages arise in response to various immunomodulatory factors such as IL-4, IL-10 and IL-13. In low-grade chronic inflammation associated with obesity, there is a phenotypic shift from M2 to M1, with a prominent M1 macrophage infiltration, consequently augmenting the release of T helper type-1 cytokines such as IL-1, IL-2, IL-6, TNF, monocyte chemo-attractant protein (MCP)-1 and interferon gamma (IFN-γ). Adipocytes can directly and indirectly interact with a wide variety of circulating immunocytes such as granulocytes, NK cells, and monocytes as part of the pro-inflammatory response; and eosinophils, mast cells and B cells as part of the anti-inflammatory response, eventually leading to aberrant immune homeostasis.
Inflammatory cytokines can be functionally divided into two groups, namely Th1 and Th2. Th1-type cytokines are hormonal messengers that produce pro-inflammatory responses implicated in killing intracellular pathogens. Th1-1 type cytokines include IFN-γ, interleukin 2 (IL-2), and TNF-α. Th2-type cytokines promote anti-inflammation, and include IL-4, IL-5, IL-9, IL-10 and IL-13. The balance between the immunological net effects of Th1 and Th2 cytokines would predict the outcome of an inflammatory disease. In that context, a Th1/Th2 paradigm may provide the basis for the development of new therapeutic strategies against inflammation-related diseases. The adipose tissue’s production of TNF-α, a potent pro-inflammatory cytokine exhibits a strong link between pro-inflammatory cytokine and the development of insulin resistance implicated in obesity [27]. Consistent study demonstrated that neutralization of TNF-α with a TNF-α soluble antibody resulted in improved insulin sensitivity in an obese mouse model [36]. TNF-α was also found to augment the release of pro-inflammatory cytokines such as IL-6 and IL-1β in a paracrine fashion [37]. Higher circulating levels of TNF-α and IL-6 are found in overweight pediatric populations [29–31, 38–40]. The pleiotropic IL-6 cytokine is associated with regulation of weight gain, which is supported by the finding that deletion of the IL-6 gene in mice led to mature-onset obesity [41]. Since obesity is associated with increased production of TNF-α and IL-6, inhibiting the expression and release of these cytokines might help treat cytokine imbalances found in obesity. Another important pro-inflammatory cytokine with increased expression in obesity is monocyte chemo-attractant protein (MCP)-1, which contributes to insulin resistance and macrophage infiltration [42]. IL-4, a Th2-type cytokine, has marked inhibitory effects on the expression and release of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-8 [43]. Another potent anti-inflammatory Th-2 type cytokine is IL-10, which is primarily synthesized by CD4+ Th2 cells, monocytes and B cells [44, 45], and has a strong inhibitory effect against Th1 cytokines. Several lines of evidence have verified the beneficial effect of the gain and loss of IL-10 function on infectious and inflammatory-related diseases [46–51]. IL-13, which is secreted by activated T lymphocytes [52], is an additional anti-inflammatory cytokine that has been mapped in proximity to the IL-4 gene and down-regulates TNF-α, IL-1 and IL-8 [53,54]. Given these, IL-4, IL-10, and IL-13 might be a potential anti-inflammatory therapies against chronic low-grade obesity. The veiled roles of these anti-inflammatory cytokines in clinical medicine remain to be elucidated.
IMMUNOASSAY OF OBESITY BIOMARKERS
It has been well-documented that important adipokine bio-markers of obesity are adiponectin, leptin, C-peptide, and resistin as well as valid cytokine biomarkers such as TNF-α and IL-6. Additional cytokine biomarkers that may contribute to the pathogenesis of obesity are IFN-γ, IL-1β, IL-4, IL-5 and IL-10. Considering there is insufficient knowledge about biomarkers related to obesity, a more sensitive and accurate immunological profiling technique should be employed to better gauge the dynamic response of cytokines. The multiplex bead suspension array system is a sensitive assay that was recently developed with a limit of detection of less than 1 pg/mL for all cytokines. In addition, in less than 5 hrs this immunoassay is able to simultaneously detect multiple biomarkers in a single sample with as little as 12.5 μL of serum, plasma, cell or tissue lysates. Furthermore, the multiplex suspension array system is known for its high sensitivity, precision, accuracy and linearity of dilutions in sample medium matrices. These multiplex immunoassays are ideal tools for obesity screening in clinical research, and as an in vivo system that produces sensitive, precise and accurate results. The basic principle of the multiplex suspension array technique is based on the sandwich immunoassay technique, employing 100 distinct polystyrene beads filled with different ratios of two fluorescent dyes. Briefly, each set of beads can be conjugated with a different capture molecule (including enzyme substrate, DNA, receptors, antigens and antibodies), which is prepared and incubated with the sample in a microplate to react with specific analytes. Then, a fluorescently-labeled reporter molecule that specifically binds the analyte is added to detect and quantitate each captured analyte. Finally, the contents of each microplate well are drawn into the array reader, and precision fluidics align the beads in single file and pass them through the lasers one at a time.
CONCLUSION
This review emphasizes that the net effects from the interaction of a complex network of pro-inflammatory and anti-inflammatory cytokines determines the nature of an immune response in the host. The alterations between this cytokine and adipokine network can lead to inflammation in a vulnerable host. Immunological profiling of chronic low-grade inflammation associated with obesity reveals the importance of imbalance in cytokine and adipokine network. Further, this review might broaden our knowledge on the significance of the Th1/Th2 network and the importance of cytokine profiling in the treatment of inflammatory diseases. Furthermore, this paper highlights additional obesity biomarkers that help predict the degree of adiposity and possible therapeutic targets for restoring cytokine imbalance in obesity. Therapeutic intervention with specific anti-inflammatory/Th-2 type cytokines might hold promise as a treatment against the excess pro-inflammatory response in obesity. Last, for theragnostic convenience, we introduce our highly sensitive and precise immunoassay as a means of detecting adipokines and cytokine concentrations in preclinical and clinical settings.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea, funded by the Korean Government (NRF-2012K1A2B1A03000595).
REFERENCES
- 1.Anderson F, Game BA, Atchley D, Xu M, Lopes-Virella MF, Huang Y. IFN-gamma pretreatment augments immune complex-induced matrix metalloproteinase-1 expression in U937 histiocytes. Clin Immunol. 2002;102:200–7. doi: 10.1006/clim.2001.5161. [DOI] [PubMed] [Google Scholar]
- 2.Lee IS, Shin G, Choue R. Shifts in diet from high fat to high carbohydrate improved levels of adipokines and pro-inflammatory cytokines in mice fed a high-fat diet. Endocr J. 2010;57:39–50. doi: 10.1507/endocrj.k09e-046. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135–42. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 1999;892:146–54. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 6.Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, Arita Y, Kihara S, Matsuzawa Y. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38:202–6. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- 7.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 8.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–3. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 9.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–27. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 12.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. doi: 10.1093/ajcn/68.4.794. [DOI] [PubMed] [Google Scholar]
- 13.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 16.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 17.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 18.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 19.Lu JY, Huang KC, Chang LC, Huang YS, Chi YC, Su TC, Chen CL, Yang WS. Adiponectin: a biomarker of obesity-induced insulin resistance in adipose tissue and beyond. J Biomed Sci. 2008;15:565–76. doi: 10.1007/s11373-008-9261-z. [DOI] [PubMed] [Google Scholar]
- 20.Lord G. Role of leptin in immunology. Nutr Rev. 2002;60(10 Pt 2):S35–8. doi: 10.1301/002966402320634913. discussion S68–84, 5–7. [DOI] [PubMed] [Google Scholar]
- 21.Trayhurn P. Adipose tissue in obesity--an inflammatory issue. Endocrinology. 2005;146:1003–5. doi: 10.1210/en.2004-1597. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–79. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–83. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 25.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 26.Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin Inhibits IL-10-Mediated Regulatory T Cell Function: Implications for Obesity. J Immunol. 2014;192:623–9. doi: 10.4049/jimmunol.1302181. [DOI] [PubMed] [Google Scholar]
- 27.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. Bjog. 2006;113:1141–7. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 28.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halle M, Korsten-Reck U, Wolfarth B, Berg A. Low-grade systemic inflammation in overweight children: impact of physical fitness. Exerc Immunol Rev. 2004;10:66–74. [PubMed] [Google Scholar]
- 30.Reinehr T, Stoffel-Wagner B, Roth CL, Andler W. High-sensitive C-reactive protein, tumor necrosis factor alpha, and cardiovascular risk factors before and after weight loss in obese children. Metabolism. 2005;54:1155–61. doi: 10.1016/j.metabol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Ten S, Anhalt H. Serum levels of soluble tumor necrosis factor-alpha receptor 2 are linked to insulin resistance and glucose intolerance in children. J Pediatric Endocrinol Metab. 2005;18:75–82. doi: 10.1515/jpem.2005.18.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 34.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–25. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 37.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–13. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 38.Aeberli I, Molinari L, Spinas G, Lehmann R, l’Allemand D, Zimmermann MB. Dietary intakes of fat and anti-oxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006;84:748–55. doi: 10.1093/ajcn/84.4.748. [DOI] [PubMed] [Google Scholar]
- 39.Giordano P, Del Vecchio GC, Cecinati V, Delvecchio M, Altomare M, De Palma F, De Mattia D, Cavallo L, Faienza MF. Metabolic, inflammatory, endothelial and haemostatic markers in a group of Italian obese children and adolescents. Eur J Pediatr. 2011;170:845–50. doi: 10.1007/s00431-010-1356-7. [DOI] [PubMed] [Google Scholar]
- 40.Gobel RJ, Jensen SM, Frokiaer H, Molgaard C, Michaelsen KF. Obesity, inflammation and metabolic syndrome in Danish adolescents. Acta Paediatr. 2012;101:192–200. doi: 10.1111/j.1651-2227.2011.02493.x. [DOI] [PubMed] [Google Scholar]
- 41.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–9. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 42.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 44.Howard M, O’Garra A. Biological properties of inter-leukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 45.Opal SM, Wherry JC, Grint P. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis. 1998;27:1497–507. doi: 10.1086/515032. [DOI] [PubMed] [Google Scholar]
- 46.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A, Haslett C. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125:191–6. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 47.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 48.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–67. [PubMed] [Google Scholar]
- 49.van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 50.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–9. [PubMed] [Google Scholar]
- 51.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 52.McKenzie AN, Li X, Largaespada DA, Sato A, Kaneda A, Zurawski SM, Doyle EL, Milatovich A, Francke U, Copeland NG. Structural comparison and chromosomal localization of the human and mouse IL-13 genes. J Immunol. 1993;150:5436–44. [PubMed] [Google Scholar]
- 53.de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, de Vries JE. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 54.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]