Abstract
Oral supplementation of micronutrients, or functional foods, to prevent aging has gained much attention and popularity as society ages and becomes more affluent, and as science reveals the pathological mechanisms of aging. Aging of the skin combines biologic aging and extrinsic aging caused predominantly by sunlight and other environmental toxins. Anti-aging functional foods exert their influence mostly through their anti-oxidant and anti-inflammatory effects, thereby abrogating collagen degradation and/or increasing procollagen synthesis. Clinical evidence supporting a role in preventing cutaneous aging is available for oral supplements such as carotenoids, polyphenols, chlorophyll, aloe vera, vitamins C and E, red ginseng, squalene, and omega-3 fatty acids. Collagen peptides and proteoglycans are claimed to provide building blocks of the dermal matrix. This review summarizes the current study findings of these functional foods.
Keywords: Functional foods, Photoaging, Anti-oxidant, Collagen
INTRODUCTION
Cutaneous aging is the composite sum of biologic aging and extrinsic aging due to environmental factors including sunlight, smoking, pollution, and inflammation. Regardless of etiology, the aging process essentially involves the generation of reactive oxygen species (ROS) with subsequent signal transduction and activation of transcription factors activator protein 1 (AP-1) and nuclear factor κB (NF-κB). AP-1 increases the secretion of matrix-degrading enzymes called matrix metalloproteinases (MMPs), which results in the degradation of the dermal matrix, including collagen [1]. Functional foods and ‘nutraceuticals’ include all kinds of food with health or medical effects. According to an international survey, about 69% of adults worldwide take vitamins, minerals, or food supplement products daily [2]. There is an ever increasing interest in anti-aging substances derived from food, and since the aging process inevitably involves the generation of reactive oxygen species, oral supplements with antioxidant properties are the most popular. These include botanicals with carotenoids or polyphenols, isoflavones, vitamins, coenzyme Q10, phytoestrogens, probiotics, and omega-3 fatty acids. In addition, collagen peptides and hyaluronic acid, which provide building blocks of the skin, are on the market.
Endogenous antioxidant defenses include non-enzymatic (e.g., uric acid, glutathione, bilirubin, thiols, albumin, and nutritional factors such as vitamins C and E, β-carotene, ubiquinone, and phenols) and enzymatic (e.g., superoxide dismutases, glutathione peroxidases, and catalase) components. Although nutritional antioxidants are all mainly free radical scavengers, they act through different mechanisms and in different compartments: 1) they directly neutralize free radicals, 2) they reduce peroxide concentrations and repair oxidized membranes, 3) they quench iron to decrease ROS production, and 4) they neutralize ROS via lipid metabolism, i.e., short-chain free fatty acids and cholesteryl esters [3].
In contrast to topically applied cosmeceuticals, the effects of dietary bioactive compounds are complicated by the fact that they must go through the gastrointestinal tract, cross the intestinal barrier, reach the blood circulation, and then be distributed to the target tissue, the skin [4].
CAROTENOIDS
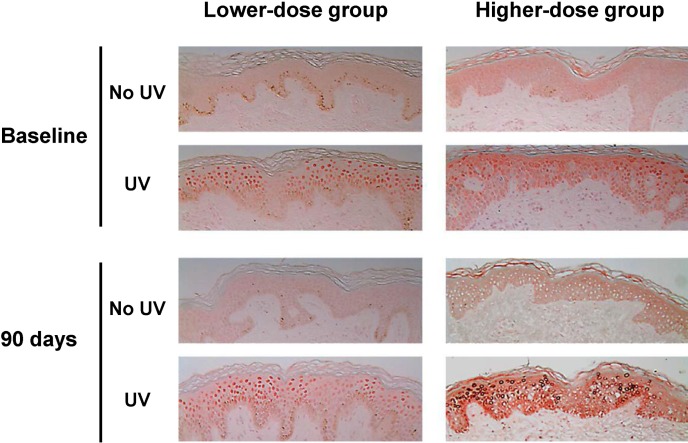
Beta-carotene is a very lipophilic, plant-derived carotenoid that has provitamin A (retinol) ROS-quenching activity [5] and therefore has been used for the treatment of erythropoietic protoporphyria and to increase sunburn threshold. The maximum dosage recommended by the Food and Drug Administration is 300 mg/d [6]. In 30 photoaged female subjects, 90 days of 30 mg/d β-carotene supplementation improved facial wrinkles and elasticity, increased type I procollagen mRNA levels, decreased UV-induced thymine dimer staining, and reduced 8-hydroxy-2’-deoxyguanosine staining, hence demonstrating its anti-photo-aging effects; however, 90 mg/d β-carotene decreased minimal erythema dose (MED) and tended to increase thymine dimer-staining cells after supplementation (Fig. 1) [7]. Since MED is a measure of cutaneous reactivity to UV irradiation, 90 mg/d of β-carotene seems to render the skin more susceptible to UV-induced erythema. In addition, UV-induced direct cutaneous DNA damage, as measured by thymine dimer staining, tended to increase, albeit non-significantly, in the high-dose group. Oxidative DNA damage, as measured by 8-OHdG stain, was not significantly affected by high-dose β-carotene. Taken together, the dosage of β-carotene matters: 30 mg/d has beneficial effects on cutaneous photoaging, but single 90 mg/d supplementation is not recommended.
Fig. 1.
Thymine dimer immunostaining demonstrating nuclear staining of thymine dimer in UV-irradiated buttock skin 24 hrs after 2 MED of UV irradiation, before and after β-carotene in-take. The figures are representative of data from 6 subjects (original magnification ×400) (from ref. 7).
Astaxanthin is a xanthophyll carotenoid widely distributed in marine organisms and is responsible for the red color of lobsters and shrimp. It has potent anti-oxidant and anti-inflammatory properties, with 10-fold greater anti-oxidant action than that of other carotenoids and 100-fold greater action than that of α-tocopherol [8]. Our group found that dietary astaxanthin (2 mg/d) combined with collagen (3 g/d) improves facial elasticity and skin barrier integrity, upregulates type I procollagen gene expression, and decreases MMP-1 and -12 expression in human subjects compared to placebo (unpublished data).
POLYPHENOLS AND ISOFLAVONES
Natural polyphenols or flavonoids are not only plant pigments but also powerful antioxidants that protect plants from diseases. Flavonoids may be divided into 7 subclasses: flavones (apigenin, luteolin, etc.), flavonols (quercetin, etc.), flavanones (hesperitin, etc.), flavanonols (taxifolin), flavanols (catechin, epigallocatechin gallate, etc.), isoflavones (genistein, daidzein, etc.), anthocyanins, and anthocyanidins.
Resveratrol is a small polyphenol compound found in red grape skin, nuts, fruits, and red wine. Many studies have suggested that this compound has anti-carcinogenic effects that can be attributed to its free radical-scavenging ability [9] and anti-inflammatory effects [10]. It has been shown to protect against depletion of endogenous antioxidant defense enzymes, suppress H2O2 and NO production as well as lipid and protein oxidation, inhibit activation of mitogen-activated protein kinase (MAPK) and NF-κB, and inhibit apoptosis through activation of p53 activity [11]. It has poor bioavailability, and up to 5 g of resveratrol intake has been described as safe [12]; however, in a recent pilot study with 10 healthy volunteers, resveratrol was shown to possess cytokine-potentiating, pro-inflammatory properties with a significant increase in TNF-α and activation of alternative NF-κB signaling, suggesting enhanced immune surveillance as the mechanism behind its anti-carcinogenic effects [13].
Green tea polyphenols have been shown to prevent UVB-induced protein oxidation and MMP expression in mouse skin [14]. Epigallocatechin gallate (EGCG) comprises 59% of total catechins and is responsible for most of the biological activity of tea, and oral EGCG supplementation has been shown to increase MED, skin barrier function, and to reduce UVB-induced skin damage in rats [15]. However, similar studies in human beings have not demonstrated such effects [16,17], presumably because the human dermis forms a stronger barrier to absorption from the vasculature [18], warranting further clinical studies with high methodological quality.
Soybean isoflavone, a well-known anti-aging agent, is also referred to as a phytoestrogen because it has a chemical structure similar to estrogen [19]. In hairless mouse models, dietary soy isoflavones cause less skin wrinkling in UV-irradiated skin than in controls, with a concomitant increase in collagen deposition, which is partly due to the inhibitory effects on UV-induced MMP expression and subsequent collagen degradation [20]. In human subjects, facial fine wrinkles are decreased after 12 weeks of isoflavone aglycone supplementation [21]; however, more robust clinical studies are necessary to substantiate these initial findings.
OTHER BOTANICALS
We reported that chlorophyll improves facial wrinkles and elasticity in female volunteers over the age of 45 who received two different doses of chlorophyll extract supplement for 90 days. Compared to baseline, type I procollagen synthesis was increased, with a substantial reduction in UV-induced thymine dimer staining and UV-induced apoptosis of keratinocytes in a dose-dependent manner [22]. Considering the pivotal role of ROS in photoaging, chlorophyll’s anti-oxidant properties are speculated to play a role in reducing wrinkles, epidermal DNA damage, and apoptosis.
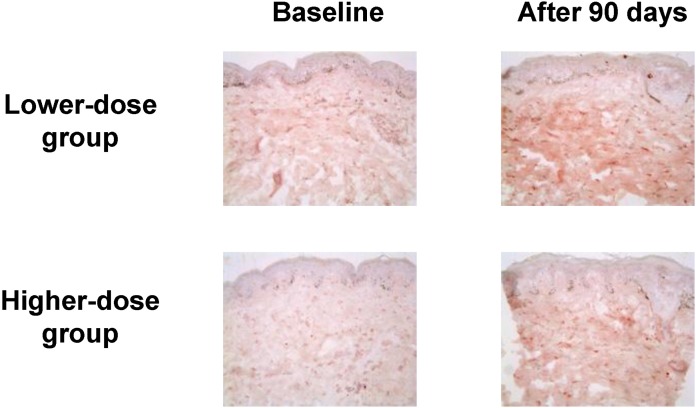
Aloe vera gel is obtained from the pulp of a tropical cactus that belongs to the lily family with purported anti-inflammatory, healing, moisturizing, antibacterial, antifungal, and antiviral properties. Dietary aloe vera gel supplementation (low dose, 1,200 mg/d; high dose, 3,600 mg/d) in 30 photoaged female volunteers for 90 days resulted in improvements in facial wrinkles and elasticity, an increase in type I procollagen mRNA levels, and a reduction in MMP-1 mRNA levels at both doses. Compared to baseline, type I procollagen immunostaining increases throughout the dermis in both groups (Fig. 2) [23]. No dose-response relationship has been found in the tested doses. The known therapeutic effect of aloe vera is due to its immunostimulatory properties attributed to the presence of polysaccharides; the polysaccharides have no significant anti-oxidant activity [24]. An acetylated glucomannan, acemannan, is the biologically active, dominant polysaccharide that has been shown to increase collagen biosynthesis, probably through macrophage immunostimulation [25].
Fig. 2.
Type I procollagen immunostaining in the buttock skin before and after aloe vera intake (original magnification ×200). The results are representative of 6 biopsied subjects in each group (from ref. 23).
VITAMINS C AND E
Vitamin C (ascorbic acid) is the major water-soluble endogenous antioxidant; it is a powerful inhibitor of lipid peroxidation, regenerates vitamin E in lipoproteins and membranes, and is essential for the production of collagen. It has been shown to provide a wide variety of benefits including lowering blood pressure and decreasing infectious episodes in daily doses of 500 mg to 6 g [26]; however, no benefit has been reported regarding skin aging. Vitamin E (α-tocopherol) is a lipid-soluble anti-oxidant vitamin found in cell membranes and circulating lipoproteins. Vitamin E can also enhance immune function, reducing infection rates in elderly subjects [27]. For a systemic photoprotective effect, several hundred mg/d is required, and doses up to 800 mg/d have been taken for years without harm. Vitamin C and vitamin E act synergistically [28]. Simultaneous oral intake of these two vitamins has been shown to reduce UV-induced skin inflammation, in contrast to either vitamin alone, which has shown no protective effects [29]. Several controlled clinical studies have demonstrated that the two vitamins act synergistically to reduce sunburn reaction and increase MED [30]. Unfortunately, oral supplementation of vitamin C and E has proven insufficient in preventing skin aging owing to their poor solubility, inefficient skin permeability, or instability during storage [31]. Chemical modification of the molecules or new delivery systems would make optimized delivery of these molecules to the skin possible in the future. On the other hand, there are some promising reports regarding mixtures of antioxidants: an antioxidant combination containing vitamins C and E, carotenoids, selenium, and proanthocyanidins decrease MMP-1 compared to placebo in humans [32], and a combination of vitamins C and E, pycnogenol, and evening primrose oil also decrease wrinkles and MMP while increasing collagen synthesis in hairless mice [33]. Both studies imply that antioxidants are effective when they work together.
RED GINSENG
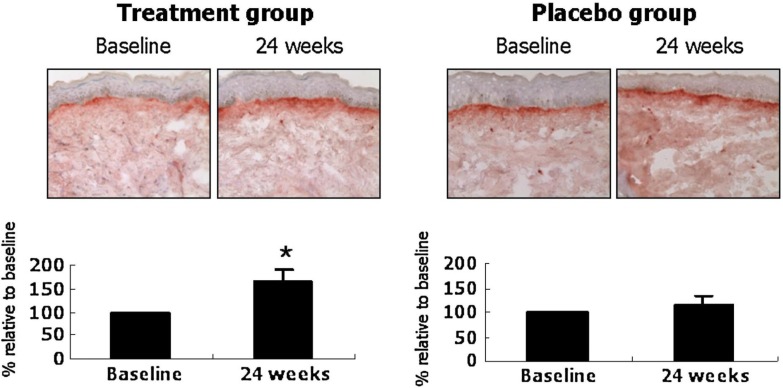
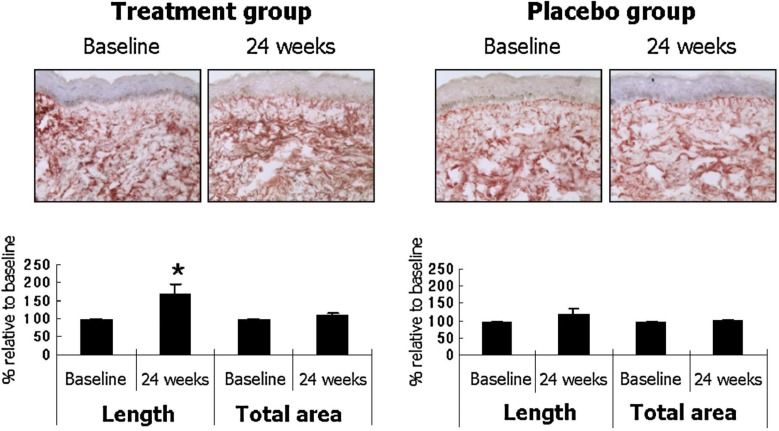
The roots of Panax ginseng have been used as a general tonic in Oriental medicine for several thousand years. Red ginseng is prepared by steaming and air-drying P. ginseng, and reportedly has more bioactivity than white ginseng, which is the peeled and air-dried form [34]. Red ginseng contains various ginsenosides that have antioxidant, immunostimulatory, and anti-aging activity. Our group performed the first controlled human study on 82 female volunteers to assess red ginseng’s effects on photoaged skin. Compared to placebo, the group that took 3 g/d of a red ginseng extract-containing herbal mixture for 24 weeks had decreased facial wrinkles with concomitant increases in type I procollagen synthesis (Fig. 3) and fibrillin-1 fiber length (Fig. 4). Therefore, objective evidence of a reduction in facial wrinkles by long-term ingestion of red ginseng was provided for the first time; the clinical improvement was substantiated by biochemical and histological evidence of increased collagen and elastic fiber synthesis in the dermis [35]. The clinical improvement may be due to the activation of COL1A2 promoter and Smad signaling [36], through ginseng’s estrogen-like activity [37], and/or additionally through increased hyaluronan levels by a metabolite of ginsenosides [38].
FIG. 3.
Ginseng mixture treatment increases type I procollagen immunostaining in human facial skin. Immunohistochemistry for SP1.D8 was performed from punch-biopsied skin samples, and the degree of staining was visually graded by five dermatologists. Data are mean ± SE values (n = 6, treatment group; n = 7, placebo group). *p < 05 by Wilcoxon signed rank test, compared with baseline (from ref. 35).
FIG. 4.
Ginseng mixture treatment increases fibrillin-1 fiber length in the papillary dermis. Total percentage area and fiber length of fibrillin-1 were measured from the dermo-epidermal junction to 15 μm downward. The photographs are representative of six subjects in the ginseng group and seven subjects in the placebo group. Data are mean ± SE values. *p < 05 by Wilcoxon signed rank test, compared with baseline (from ref. 35).
SQUALENE AND OTHER LIPIDS
Squalene is a polyunsaturated aliphatic hydrocarbon that is abundant in shark liver oil. Our group previously reported that 27 g/day (high dose) oral squalene supplementation for 90 days decreased facial wrinkles, while 13.5 g/day (low dose) increased type I procollagen mRNA levels and MED [39]. Both dosage groups had a significant decrease in facial erythema. Both dosages of squalene protected against UV-induced keratinocytic damage, as shown by reduced thymine dimer-staining cells and apoptotic cells in the skin. The antioxidant action of squalene may be implicated in all these phenomena. However, transient loose stool was experienced by 35% of subjects in the low-dose group and 55% in the high-dose group, making high-dose squalene supplementation unsuitable for the treatment of skin aging.
Lipid compounds from honeybee royal jelly extracts, which are composed of mostly medium-chain aliphatic fatty acids, have been shown to possess in vitro collagen production-promoting effects. 10-hydroxy-2-decenoic acid, a characteristic constituent of lipids in royal jelly, stimulate normal human dermal fibroblast cell lines and produce transforming growth factor(TGF) β1, a cytokine that induces collagen synthesis [40,41]; however, these results are yet to be confirmed in controlled clinical trials.
When consumed in high amounts ranging from 4 to 10 g/d, eicosapentaenoic acid (EPA) and other omega-3 fatty acids reduce levels of pro-inflammatory and immunosuppressive mediators, including PGE2, IL-6, IL-8, and TNF-α, while decreasing UV-induced p53 upregulation in the skin, reducing DNA strand breaks, and increasing sunburn threshold, hence conferring protection against UV-induced cutaneous damage [42,43].
COLLAGEN PEPTIDES
In a study by Proksch et al., oral supplementation of collagen hydrolysate composed of specific collagen peptides (2.5 g/d or 5.0 g/d for 8 weeks) increased skin elasticity in middle-aged women after 4 weeks of supplementation, and a skin moisturizing effect was observed in women over 50 years of age. The supplement had a long-lasting effect, especially in women over 50 years of age [44]. Prior studies have demonstrated that collagen hydrolysate is absorbed in the digestive tract, appears in human blood partly in a small peptide form, and is deposited in the skin for up to 96 hrs [45]. Food-derived collagen peptides in human blood are chemotactic for dermal fibroblasts [46], and they increase the migration and growth of mouse skin fibroblasts [47]. One controlled study found that type I and IV collagen increases while MMP-2 decreases, implying that the effect of collagen is protein-specific [48], and providing evidence for the improved cutaneous elasticity. In animal studies, oral administration of collagen peptide induced an increase in fibroblast density and formation of dermal collagen fibrils in piglet skin [49], and suppressed UVB-induced decreases in skin hydration, epidermal hypertrophy, and soluble type I collagen in mouse skin [50].
PROTEOGLYCANS
Proteoglycans (PG) are a family of complex macromolecules consisting of a core protein with covalently attached glycosaminoglycan chains. In hairless mice, oral administration of high molecular weight PG from salmon nasal cartilage inhibited UVB-induced skin aging, i.e., increased erythema and transepidermal water loss, and decreased hydration, in a molecular weight-dependent manner [51]. From decreased serum and dorsal skin inflammatory cytokine levels, it is speculated that PG acts on gut immunity and improves skin condition by inhibiting surplus inflammatory cytokine production induced by UVB irradiation. Aggrecan, the most abundant PG in cartilage, was shown to bind to hyaluronic acid in vivo [52]; in contrast, chondroitin sulfate, a degradation product of salmon nasal cartilage PG, did not show any beneficial anti-photoaging effects [53]. Therefore, the cutaneous anti-aging effects of PG are molecular weight-dependent [51].
CONCLUSION
The advantage of functional foods is that once they go through systemic circulation in active forms, they can then be distributed to all skin compartments--including the epidermis, dermis, subcutaneous fat, and sebum [4]--of the entire body, which is much more convenient and efficient than applying cosmetics topically. Many manufacturers have already begun to launch strategic combinations of nutraceuticals and cosmeceuticals. Functional foods with some evidence of cutaneous anti-aging properties include carotenoids, polyphenols, other botanicals, vitamins C and E, red ginseng, squalene, omega-3 fatty acids, collagen peptides, and proteoglycans. However, since effective governmental regulation of anti-aging interventions is lacking, consumers may be misled by the manufacturers’ claims. Moreover, long-term supplementation of a single micro-nutrient in higher doses might lead to “flooding” of the organism and be even harmful. In addition, some studies in humans suggest that anti-oxidant supplementation can blunt the beneficial effects of exercise in humans, probably by abrogating beneficial ROS signaling that stimulates mitochondrial biogenesis and expression of oxidant defense biomolecules [54,55]. Manufacturers are therefore presently at work to achieve “nutritargeting”, which means accumulating certain micronutrients selectively in specific target tissues by means of nanocolloids, microemulsions, and other means. The ball is in the court of dermatologists: they should be diligent in testing the scientific veracity of the manufacturers’ claims regarding the health benefits of numerous anti-aging substances, for the good of all consumers and patients. At the same time, physicians should keep educating patients on the importance and superior efficacy of topical sunscreen and retinoid use, compared to functional foods, in preventing cutaneous aging.
REFERENCES
- 1.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–28. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 2.Harris Interactive Inc Anti-aging medicine, vitamins, minerals and food supplements: a public opinion survey conducted for the International Longevity Center. J Anti Aging Med. 2003;6:83–90. doi: 10.1089/109454503769684766. [DOI] [PubMed] [Google Scholar]
- 3.Berger MM. Can oxidative damage be treated nutritionally? Clin Nutr. 2005;24:172–83. doi: 10.1016/j.clnu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Richelle M, Sabatier M, Steiling H, Williamson G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227–38. doi: 10.1079/bjn20061817. [DOI] [PubMed] [Google Scholar]
- 5.Krinsky NI. Carotenoids as chemopreventive agents. Prev Med. 1989;18:592–602. doi: 10.1016/0091-7435(89)90032-7. [DOI] [PubMed] [Google Scholar]
- 6.Mathews-Roth MM. Erythropoietic protoporphyria--diagnosis and treatment. N Engl J Med. 1977;297:98–100. doi: 10.1056/NEJM197707142970207. [DOI] [PubMed] [Google Scholar]
- 7.Cho S, Lee DH, Won CH, Kim SM, Lee S, Lee MJ, Chung JH. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photo-aging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160–71. doi: 10.1159/000305548. [DOI] [PubMed] [Google Scholar]
- 8.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–96. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 9.Frombaum M, Le Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and center dot NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie. 2012;94:269–76. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Imler TJ, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17(+)IL-10(+) T cells, CD4(-) IFN-gamma(+) cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–43. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health - A comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–41. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 12.Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 13.Gualdoni G, Kovarik JJ, Doberer D, Dose F, Steinberger P, Wolzt M, Zlabinger GJ. Differential immunomodulatory effects of resveratrol. Immunology. 2012;137:178–9. [Google Scholar]
- 14.Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol. 2004;122:1480–7. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeon HY, Kim JK, Kim WG, Lee SJ. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and UV-induced skin damage. Skin Pharmacol Physiol. 2009;22:137–41. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 16.Chiu AE, Chan JL, Kern DG, Kohler S, Rehmus WE, Kimball AB. Double-blinded, placebo-controllecl trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol Surg. 2005;31:855–9. doi: 10.1111/j.1524-4725.2005.31731. [DOI] [PubMed] [Google Scholar]
- 17.Janjua R, Munoz C, Gorell E, Rehmus W, Egbert B, Kern D, Chang ALS. A two-year, double-blind, randomized placebo-controlled trial of oral green tea polyphenols on the long-term clinical and histologic appearance of photoaging skin. Dermatol Surg. 2009;35:1057–65. doi: 10.1111/j.1524-4725.2009.01183.x. [DOI] [PubMed] [Google Scholar]
- 18.Dvorakova K, Dorr RT, Valcic S, Timmermann B, Alberts DS. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother Pharmacol. 1999;43:331–5. doi: 10.1007/s002800050903. [DOI] [PubMed] [Google Scholar]
- 19.Basaria S, Wisniewski A, Dupree K, Bruno T, Song MY, Yao F, Ojumu A, John M, Dobs AS. Effect of high-dose isoflavones on cognition, quality of life, androgens, and lipoprotein in post-menopausal women. J Endocrinol Invest. 2009;32:150–5. doi: 10.1007/BF03345705. [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Kim SJ, Lee JY, Kim WG, Park WS, Sim YC, Lee SJ. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J Am Coll Nutr. 2004;23:157–62. doi: 10.1080/07315724.2004.10719356. [DOI] [PubMed] [Google Scholar]
- 21.Izumi T, Saito M, Obata A, Arii M, Yamaguchi H, Matsuyama A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J Nutr Sci Vitaminol (Tokyo) 2007;53:57–62. doi: 10.3177/jnsv.53.57. [DOI] [PubMed] [Google Scholar]
- 22.Cho S, Lee DH, Won CH, Kim SM, Lee S, Lee MJ, Chung JH. Drink containing chlorophyll extracts improves signs of photoaging and increases type I procollagen in human skin in vivo. Korean J Invest Dermatol. 2006;13:111–9. [Google Scholar]
- 23.Cho S, Lee S, Lee MJ, Lee DH, Won CH, Kim SM, Chung JH. Dietary Aloe Vera Supplementation Improves Facial Wrinkles and Elasticity and It Increases the Type I Procollagen Gene Expression in Human Skin in vivo. Ann Dermatol. 2009;21:6–11. doi: 10.5021/ad.2009.21.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strickland FA. Immune regulation by polysaccharides: implications for skin cancer. Journal of Photochemistry and Photobiology B-Biology. 2001;63:132–40. doi: 10.1016/s1011-1344(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 25.Lindblad WJ, Thul J. Sustained increase in collagen biosynthesis in acemannan impregnated PVA implants in the rat [abstract] Wound Repair Regen. 1994;2:84. [Google Scholar]
- 26.Janson M. Orthomolecular medicine: the therapeutic use of dietary supplements for anti-aging. Clin Interv Aging. 2006;1:261–5. doi: 10.2147/ciia.2006.1.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meydani SN, Han SN, Hamer DH. Vitamin E and respiratory infection in the elderly. Vitamin E and Health. 2004;1031:214–22. doi: 10.1196/annals.1331.021. [DOI] [PubMed] [Google Scholar]
- 28.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among Vitamin-C, Vitamin-E, and Beta-Carotene. Am J Clin Nutr. 1995;62:S1322–S6. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006–12. doi: 10.1016/s0891-5849(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 30.Bialy TL, Rothe MJ, Grant-Kels JM. Dietary factors in the prevention and treatment of nonmelanoma skin cancer and melanoma. Dermatol Surg. 2002;28:1143–52. doi: 10.1046/j.1524-4725.2002.02114.x. [DOI] [PubMed] [Google Scholar]
- 31.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Greul AK, Grundmann JU, Heinrich F, Pfitzner I, Bernhardt J, Ambach A, Biesalski HK, Gollnick H. Photoprotection of UV-irradiated human skin: an anti-oxidative combination of vitamins E and C, carotenoids, selenium and proanthocyanidins. Skin Pharmacol Appl Skin Physiol. 2002;15:307–15. doi: 10.1159/000064534. [DOI] [PubMed] [Google Scholar]
- 33.Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, Kang S, Cho WG, Park HJ, Oh KW, Hong JT. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 2007;23:155–62. doi: 10.1111/j.1600-0781.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee FC. Facts about Ginseng : the elixir of life. Hollym International Corp.; 1992. Elizabeth. [Google Scholar]
- 35.Cho S, Won CH, Lee DH, Lee MJ, Lee S, So SH, Lee SK, Koo BS, Kim NM, Chung JH. Red ginseng root extract mixed with Torilus fructus and Corni fructus improves facial wrinkles and increases type I procollagen synthesis in human skin: a randomized, double-blind, placebo-controlled study. J Med Food. 2009;12:1252–9. doi: 10.1089/jmf.2008.1390. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Jung E, Lee J, Huh S, Kim J, Park M, So J, Ham Y, Jung K, Hyun CG, Kim YS, Park D. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. 2007;109:29–34. doi: 10.1016/j.jep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Palmer BV, Montgomery AC, Monteiro JC. Gin Seng and mastalgia. Br Med J. 1978;1:1284. doi: 10.1136/bmj.1.6122.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Kang BY, Cho SY, Sung DS, Chang HK, Yeom MH, Kim DH, Sim YC, Lee YS. Compound K induces expression of hyaluronan synthase 2 gene in transformed human keratinocytes and increases hyaluronan in hairless mouse skin. Biochem Biophys Res Commun. 2004;316:348–55. doi: 10.1016/j.bbrc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 39.Cho S, Choi CW, Lee DH, Won CH, Kim SM, Lee S, Lee MJ, Chung JH. High-dose squalene ingestion increases type I procollagen and decreases ultra-violet-induced DNA damage in human skin in vivo but is associated with transient adverse effects. Clin Exp Dermatol. 2009;34:500–8. doi: 10.1111/j.1365-2230.2008.03133.x. [DOI] [PubMed] [Google Scholar]
- 40.Koya-Miyata S, Okamoto I, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Bioscience Biotechnology and Biochemistry. 2004;68:767–73. doi: 10.1271/bbb.68.767. [DOI] [PubMed] [Google Scholar]
- 41.Park HM, Hwang E, Lee KG, Han SM, Cho Y, Kim SY. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J Med Food. 2011;14:899–906. doi: 10.1089/jmf.2010.1363. [DOI] [PubMed] [Google Scholar]
- 42.Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-alpha-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol. 2005;124:248–55. doi: 10.1111/j.0022-202X.2004.23543.x. [DOI] [PubMed] [Google Scholar]
- 43.Shahbakhti H, Watson REB, Azurdia RM, Ferreira CZ, Garmyn M, Rhodes LE. Influence of eicosapentaenoic acid, an omega-3 fatty acid, on ultraviolet-B generation of prostaglandin-E-2 and proinflammatory cytokines interleukin-1 beta, tumor necrosis factor-alpha, inter-leukin-6 and interleukin-8 in human skin in vivo. Photochem Photobiol. 2004;80:231–5. doi: 10.1562/2004-01-27-RA-066. [DOI] [PubMed] [Google Scholar]
- 44.Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47–55. doi: 10.1159/000351376. [DOI] [PubMed] [Google Scholar]
- 45.Oesser S, Adam M, Babel W, Seifert J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL) J Nutr. 1999;129:1891–5. doi: 10.1093/jn/129.10.1891. [DOI] [PubMed] [Google Scholar]
- 46.Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci USA. 1978;75:871–5. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C, Taira T, Park EY, Nakamura Y, Sato K. Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. 2009;57:444–9. doi: 10.1021/jf802785h. [DOI] [PubMed] [Google Scholar]
- 48.Zague V, de Freitas V, da Costa Rosa M, de Castro GA, Jaeger RG, Machado-Santelli GM. Collagen hydro-lysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J Med Food. 2011;14:618–24. doi: 10.1089/jmf.2010.0085. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda N, Koyama YI, Hosaka Y, Ueda H, Watanabe T, Araya T, Irie S, Takehana K. Effects of ingestion of collagen peptide on collagen fibrils and Glycosaminoglycans in the dermis. J Nutr Sci Vitaminol (Tokyo) 2006;52:211–5. doi: 10.3177/jnsv.52.211. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci Biotechnol Biochem. 2009;73:930–2. doi: 10.1271/bbb.80649. [DOI] [PubMed] [Google Scholar]
- 51.Goto M, Yamazaki S, Kato Y, Yamamoto K, Katagata Y. Anti-aging effects of high molecular weight proteoglycan from salmon nasal cartilage in hairless mice. Int J Mol Med. 2012;29:761–8. doi: 10.3892/ijmm.2012.918. [DOI] [PubMed] [Google Scholar]
- 52.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 53.Goto M, Ito S, Kato Y, Yamazaki S, Yamamoto K, Katagata Y. Anti-aging effects of extracts prepared from salmon nasal cartilage in hairless mice. Mol Med Report. 2011;4:779–84. doi: 10.3892/mmr.2011.498. [DOI] [PubMed] [Google Scholar]
- 54.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–59. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendelsohn AR, Larrick JW. Trade-offs between anti-aging dietary supplementation and exercise. Rejuvenation Res. 2013;16:419–26. doi: 10.1089/rej.2013.1484. [DOI] [PubMed] [Google Scholar]