Abstract
Background: The present study investigated the effects of Quercus infectoria (QI) gall extract on the proliferation, alkaline phosphatase (ALP), osteocalcin, and the morphology of a human fetal osteoblast cell line (hFOB 1.19).
Methods: The cells were cultured in Dulbecco’s modified eagle medium F12 supplemented with a 10% fetal bovine serum, a 1% penicillin/streptomycin and were treated with QI at various concentrations (0.1 to 99.0 μg/mL) for 72 hours. The levels of ALP and osteocalcin were measured at day 1, 3, 7, 10, and 14 and were compared among the negative control, pamidronate and QI groups.
Results: The median effective concentration (EC50) of hFOB 1.19 treated with QI was 10.30 μg/mL. This concentration was more effective compared to the control drug, pamidronate (EC50 at 16.09 μg/mL). The ALP and osteocalcin levels of hFOB 1.19 treated with QI from day 7 and onwards were significantly increased in a time and concentration-dependent manner. Interestingly, from day 7 until day 14, the ALP and osteocalcin levels were highest in the cells treated with QI compared to the other two groups. The morphology of cells treated with QI was uniformly elongated, higher in number and over-confluent.
Conclusion: After treatment with QI, cell proliferation enhanced and ALP and osteocalcin levels increased.
Keywords: Alkaline phosphatase (ALP), hFOB 1.19 cell line, MTT assay, osteocalcin, Quercus infectoria
Introduction
The human skeleton is an important structural support that has its own homeostatic activity to maintain its structural integrity. This homeostatic activity is carried out by osteoblasts and osteoclasts via a process termed “bone remodeling.” In a healthy adult, under normal circumstances, osteoblast, and osteoclast activity is a balanced process where bone resorption is always followed by an equal degree of bone formation. This process is indicated by the expression of various phenotypic markers such as alkaline phosphatase (ALP) and osteocalcin (1). Osteocalcin is produced by osteoblasts and is a sensitive marker of bone formation (2). ALP has become the most clinically relevant enzyme in the diagnosis of bone disease (3) and is routinely used in in vitro experiments as a relative marker of osteoblastic differentiation (4,5). Other than normal physiological bone growth, bone-specific ALP (BAP) activity also correlates with bone formation rates in metabolic diseases of the bone (6). The association between BAP and bone loss is equivalent between bone mineral density (BMD) and fractures, and suggests the marker can play a valuable role in risk assessment (7). Any imbalance in the activity of osteoblasts and osteoclasts often leads to pathological situations such as osteoporosis or osteopetrosis.
Osteoporosis is a metabolic bone disease characterized by low bone mass and micro-architectural deterioration of bone tissue, and it is a major public health problem because of its association with age-related fractures (8). The occurrence of osteoporosis increases with age, when bone mass starts to decrease at about age 35 and continually decreases thereafter in both men and women. Generally, white or Asian post-menopausal women with thin body frames are considered at risk for developing osteoporosis; additional factors include a lack of estrogen for a significant portion of a woman's lifetime, a sustained lack of calcium in the diet (or a poor absorption of calcium), inadequate exercise, a family history of osteoporosis, excessive alcohol use and smoking (9).
Treatments for osteoporosis are designed to reduce bone loss, increase bone formation or both. The treatment consists of dietary and lifestyle changes along with pharmacologic intervention. Hormone replacement therapy (HRT) has been shown to be effective in preventing osteoporosis. The effective treatment options for women who have been diagnosed with the disease are bisphosphonates, calcitonin, and hormone replacement therapy (HRT) (10). However, the risks of long-term treatment of with these drugs are undesirable in most patients. For example, the use of HRT is associated with increased risks of ovarian cancer (11), endometrial cancer (12), stroke (13), and venous thromboembolism (14).
Musculoskeletal problems can be effectively treated but many cannot be reversed, so early treatment is important to get the best outcome. Presently, many people turn to natural products to treat diseases such as cancer and osteoporosis. Thus, studying these products in a scientific manner to determine their efficacy and safety is important. These natural products can then be developed into clinically acceptable medications for future therapeutic use.
Quercus infectoria (QI) Olivier (Fagaceae) is a small tree widely distributed in Greece, Asia Minor, and Iran. The tree bears galls that emerge on its young branches due to the attack of gall wasps, Cypnis gallae-tincotoriae (15). The galls are mixed with other herbs and ingested by post-partum women as a folk remedy to restore the elasticity of the uterine wall (16). QI galls have been proven to have an astringent effect as well as anti-diabetic, anti-tremorine, local anaesthetic, anti-pyretic, anti-inflammatory, antiviral, antibacterial, and antioxidant properties (17–20). The pharmacological properties of QI gall extract are reportedly due to the presence polyphenols. Additionally, the QI galls also contain calcium, phosphorus, potassium, magnesium, iron, manganese, zinc and nickel (21). These minerals, especially calcium and phosphorus, are essential for bone mineralisation and may be beneficial to prevent bone diseases like osteoporosis.
Furthermore, QI galls also have plenty of phytochemicals that possess an anabolic effect on bone. The main phytochemicals found in QI galls are tannin (50–70%), gallic acid (2–4%), ellagic acid, starch, and sugar (22). Our pevious study by Rina et al. (23), found that water and ethanol extracts of QI contained substantial amounts of phenolic compounds [144.5 to 177.5 mg phosphoenolpyruvate carboxylase (PEPC) /g MKE] but negligible amounts of flavanoids. Recently, Shrestha et a1. (2004), found that preliminary phytochemical screening of leaf galls of QI from water extract showed the presence of phenols, flavanoids, saponins, alkaloids, carbohydrates, and tannins (24). Tannin, which is a phenolic compound, can act directly on the bone (25) by modulating osteoblast proliferation, differentiation, and mineralisation (26).
Recently, we published a review paper (27) which concluded that QI might have a potential anabolic effect on osteoblast function and bone metabolism by upregulating the osteoblast markers [runx2, Osx, IGF-1, bone-specific alkaline phosphatase (BSAP), ALP, osteocalcin, bone mineral content (BMC), and BMD]. Thus, a laboratory study is needed to elucidate the exact mechanism of QI’s action on bone. In this present study, the effects of QI gall extract on the proliferative activity of a human fetal osteoblast cell line (hFOB 1.10 cells) was observed using an enzyme-linked immunosorbent assay (ELISA) and inverted microscopy.
Materials and Methods
Preparation of Quercus infectoria (QI) gall extract
The Quercus infectoria (QI) gall was obtained from the local market. The QI galls were crushed and ground into powder form. The QI gall powder was then diluted in distilled water and extracted by refluxing in a water bath at 50 °C for 24 hours. The extract was filtered by using filter paper and concentrated by using a rotary evaporator (Heidolph Rotavac, Germany). The aqueous QI gall extract was lyophilised in a freeze-drier until it turned into powder; finally, it was stored at -20 °C until use.
Cell culture
Cell Revival and Subculture
Human osteoblast cell lines, hFOB 1.19 (CRL-11372), were purchased from American Type Cell Culture, ATCC (Manassas, USA). The hFOB 1.19 cells were cultured in Dulbecco’s Modified Eagle Medium F-12, DMEM/F12 (Invitrogen GmBH, Germany) which was supplemented with 10% fetal bovine serum (FBS), (Invitrogen GmBH, Germany) and 1% penicillin/streptomycin (Invitrogen GmBH, Germany). The cells were incubated in a 5% CO2 37 °C humidified incubator (Sheldon, United States) and monitored closely for 24 hours. The aseptic work was maintained by using a laminar flow (ESC II Series, Germany) to avoid contamination of the cultured cells. The laminar flow was sterilised by switching on the ultraviolet (UV) (40-watt) germicidal tubes prior to work.
Proliferation assay
The proliferation assay was done to determine the number of hFOB 1.19 cells treated with QI gall extract and pamidronate at days 1, 3, 7, 10, and 14. The cells were plated at 5 × 103 cells/well with a 100 uL culture medium per well in the 96-well microtiter plate and left overnight prior to attachment. QI gall extract and pamidronate were added into each well at different concentrations and incubated at 5% CO2 in a 37 °C humidified incubator. The cells were trypsinised with 100 uL trypsin/EDTA and incubated for 3 minutes for the cells to detach. Then, an inverted microscope was used to view the cells and confirm that a 90% detachment had occurred. In order to stop the trypsinisation process, a 200 uL of culture medium was added and resuspended as well. Automated cell counting was carried out using the Countess Automated Cell Counter (Invitrogen, USA), and the graph of percentage of proliferation versus time (days) was plotted by using GraphPad Prism 5.0 (GraphPad Software Inc., USA).
Cell treatment
QI gall extract and pamidronate treatments were used (Toronto Research Chemicals, Canada). QI gall extract was diluted in dimethyl sulphate (DMSO) (Fisher Scientific, USA). Pamidronate was used as a positive control whereas the cells treated with a complete medium only was used as the negative control. The treated cells were incubated for 72 hours in the 37 °C humidified incubator and supplemented with 5% CO2. The tests were conducted in triplicate and repeated at least three times.
Cell viability and half maximal effective concentration (EC50) determination
In order to determine cell viability, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method was used after the cultured cells were about 80% confluent. In this test, yellow 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide was reduced to a purple formazan. The treated and untreated cells were incubated with a 100 uL culture medium and 15 uL MTT for 4 hours in the incubator (Sheldon, US). After that, 100 uL of stop solution was added into each well and incubated for 1 hour. The optical density was will determined at 570 nm by using a microtiter plate reader (VersaMax, US). The intensity of the wavelength absorbed was proportional to the percentage of viable cells present in the wells. The graph of the percentage of viable cells versus log10 concentration (μg/mL) of QI gall extract was plotted using GraphPad Prism 5.0. From the graph, the half maximal effective concentration (EC50) was determined, and this concentration was used to treat the cells in order to study the cell morphology as well as determining the alkaline phosphatase (ALP) activity and the osteocalcin level.
The percentage of cell viability of the treated cells as compared to the control cells was expressed as the percentage of viable cells. Viable cells will be determined from the formula below:
OD samples = Optical density of treatment samples, and OD value = Optical density of control.
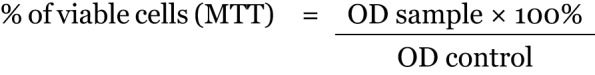
Cell morphology by inverted microscopy
The cell morphology of hFOB 1.19 was observed by using an inverted microscope, Leica DM IL (Wetzlar, Germany). The flask containing the cells were obtained from the incubator and placed on the microscope stage and focused at 4× magnification. For a closer or larger view of the cells, the magnification was changed to 10×, 22×, 40×, and 100×.
Determination of functional activity
Quantification of ALP activity in the medium by ELISA
The ALP activity of hFOB 1.19 treated with QI gall extract and pamidronate was detected through the hydrolysis of p-nitrophenyl phosphate (substrate) into p-nitrophenol at 37 °C with pH 10.3. This was achieved by using ALP ELISA-test kit (Randox, UK). The cells were plated in a 96-well microtiter plate at 5 × 103 cells/well with 115 μL culture medium per well. After 24 hours, the cells were treated with QI gall extract and pamidronate. Then, the treated cells were incubated following the incubation time (days 1, 3, 7, 10, and 14) in the 37 °C humidified incubator supplemented with 5% CO2. The ALP activities were measured using an ELISA plate reader (VersaMax, US) at 405 nm wavelength.
Quantification of osteocalcin level in the medium by ELISA
An osteocalcin ELISA-test kit (BMAssay, US) was used to detect osteocalcin levels. The treated cells were incubated following the incubation time (days 1, 3, 7, 10, and 14) in the 37 °C humidified incubator at 5% CO2. The cells were retrieved by trypsinisation and centrifuged to obtain the cell pellet. The cell pellet was resuspended in 1 mL serum-free medium (DMEM/F12) and stored in -80 °C until used. The serum-free medium was used here instead of a complete culture medium because the presence of serum (FBS) in the culture medium may lead to false positive results. For days 3, 7, 10, and 14, the cell suspensions were stored in –80 °C until used. The osteocalcin levels were measured using an ELISA plate reader (VersaMax, US) at 450 nm.
Statistical analysis
Results were expressed as mean values ± standard deviation (SD). Data were analysed with paired t test for cell viability or EC50 determination by using GraphPad Prism 5.0. In addition, data analysis for the results of proliferation activity, alkaline phosphatase (ALP) and osteocalcin were performed using the Statistical Package of Social Sciences (SPSS) Software, version 20. The Shapiro-Wilk test was used for normality checking. The statistical significance of differences was determined using one-way ANOVA followed by Tukey HSD test as a post-hoc. The result was considered to be statistically significant for a P value of < 0.05.
Results
Half maximal effective concentration (EC50) of Quercus infectoria (QI) gall extract and pamidronate treatments for hFOB 1.19 cells
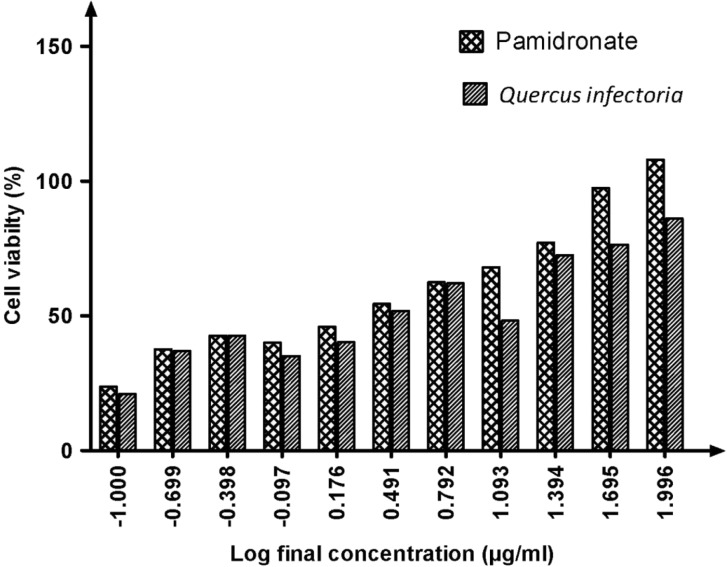
Our results showed that the EC50 for QI gall extract was 10.30 μg/mL, whereas the EC50 for pamidronate was 16.09 μg/mL (Figure 1). The concentration for QI gall extract was more effective compared to pamidronate. This indicated that lower concentrations of QI gall extract are required to enhance the proliferation of osteoblasts (hFOB 1.19 cells) as compared to pamidronate, which is a well-known osteoporotic drug.
Figure 1:
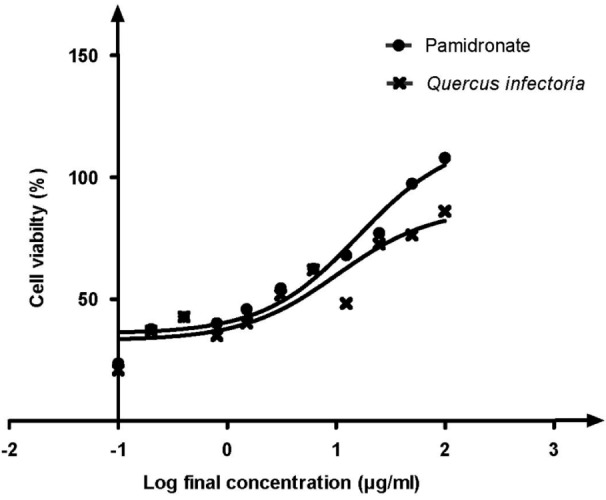
Graph of percentage of cell viability versus log final concentration of pamidronate and Quercus infectoria (QI) gall extract. Paired t test was significant (P < 0.05). EC50 values were calculated by using dose response stimulation curve in GraphPad Prism version 5.00 for Windows. The EC50 for QI gall extract was 10.30 μg/ml, lower than EC50 for pamidronate, 16.09 μg/mL.
The EC50 for both treatments were then used to treat hFOB 1.19 cells to observe the pattern of cell proliferation during 14 days of incubation. The graphs of proliferation versus the time in days were plotted to see the proliferation pattern. The result showed that the number of hFOB 1.19 cells treated with both QI gall extract as well as pamidronate were increased in a dose-dependent manner (Figure 2).
Figure 2:
Bar chart for percentage of cell viability versus log final concentration of pamidronate and QI gall extract. Paired t test was significant (P < 0.05).
Morphology of hFOB 1.19 cells by inverted microscope
The morphology of the hFOB 1.19 cells after seeding appeared to be rounded because they are suspended in the medium and not yet attached to the surface (Fugure 3a). Healthy cultured cells usually take about 24 hours to attach to the surface and live. After incubation for 24 hours, they appeared flat, elongated, and expanded, which indicated that they were already attached to the surface (Figure 3b).
Figure 3:

hFOB 1.19 cells in 96-well microtiter plates viewed at 22× magnification using an inverted microscope. (a) The hFOB 1.19 cells after seeding. (b) Day 1 (after 24 hours incubation) before treatment. (c) Day 3, hFOB 1.19 cells treated with QI gall extract. (d) Day 3, hFOB 1.19 cells treated with pamidronate. (e) Day 3, hFOB 1.19 control cells (without treatment).
On day 3, of the incubation period, the hFOB 1.19 cells that were treated with QI gall extract were observed to have a uniformly elongated shape and overlapped on each other (Figure 3c). This indicated that the cells became confluent on the third day of treatment. Meanwhile, hFOB 1.19 cells treated with pamidronate and control cells (without treatment) on the third day of the incubation period were observed to be elongated, sparsely distributed and less dense (Figure 2d,3e).
In addition, the existence of the spaces in between the hFOB 1.19 cells indicated that on day 7, the cells treated with pamidronate and without treatment were proliferating at a slower rate as compared to the cells treated with QI gall extract (Figure 4a,4b,4c). After 10 days, the hFOB 1.19 cells treated with QI gall extract were over-confluent and overlapped on each other (Figure 4d). Whereas, the condition of hFOB 1.19 cells treated with pamidronate were scattered, rounded and less dense (Figure 4e).
Figure 4:

hFOB 1.19 cell line in 96-well microtiter plates viewed at 22× magnification using an inverted microscope. (a) Day 7, hFOB 1.19 cells treated with pamidronate. (b) Day 7, hFOB 1.19 control cells. (c) Day 7, hFOB 1.19 cells treated QI gall extract. (d) hFOB 1.19 cells treated with QI gall extract on day 10. (e) Day 10, cells treated with pamidronate.
Until day 14, the hFOB 1.19 cells treated with QI gall extract were still alive and actively multiplying (Figure 5a). However, the cells treated with pamidronate and without treatment were decreased in number, and many of them were dead (Figure 5b,5c). Interestingly, the observation of the hFOB 1.19 cell morphology by inverted microscopy revealed that most of the hFOB 1.19 cells treated with pamidronate were dead or had deteriorated at day 10 of the incubation period (Figure 4e).
Figure 5:
hFOB 1.19 cell line in 96-well microtiter plates viewed at 22× magnification using an inverted microscope. (a) Day 14, hFOB 1.19 cells treated with QI gall extract. (b) Day 14, cells treated with pamidronate. (c) Control cells (without treatment) on day 14.
Proliferation Assay of hFOB 1.19 cells
The cell (hFOB 1.19) number was compared among negative control, pamidronate and QI gall extract after 1, 3, 7, 10, and 14 days of incubation. Figure 6 shows that the hFOB 1.19 cells in all the three groups were significantly increased with time (day 3 until day 14). Interestingly, from day 3 onwards, the number of hFOB 1.19 cells were significantly higher in the QI gall extract group as compared to the other two groups. Moreover, from day 3 until day 14, the hFOB 1.19 cells were significantly lower in the pamidronate-treated group as compared to the negative control and QI-treated groups.
Figure 6:
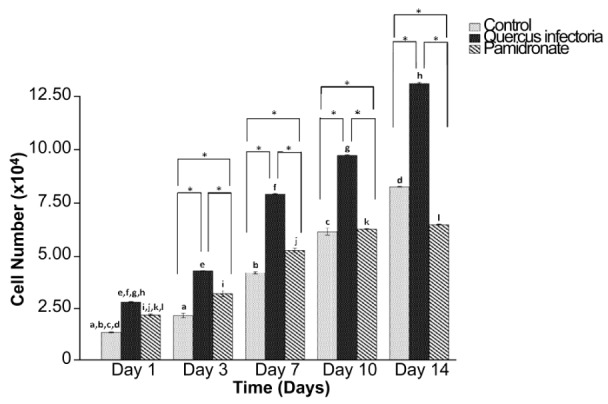
Cell number (×104) after 1, 3, 7, 10, and 14 days of incubation. The values of the bars represented mean ± standard deviation (SD) of the three independent experiments. * Significant difference between the three groups within the same day (P < 0.05) by one-way ANOVA and Tukey’s test. Days that share the same letter showed a significant difference (within the same group) (P < 0.05) by one-way ANOVA and Tukey’s test.
Alkaline Phosphatase (ALP) activity of hFOB 1.19 cells
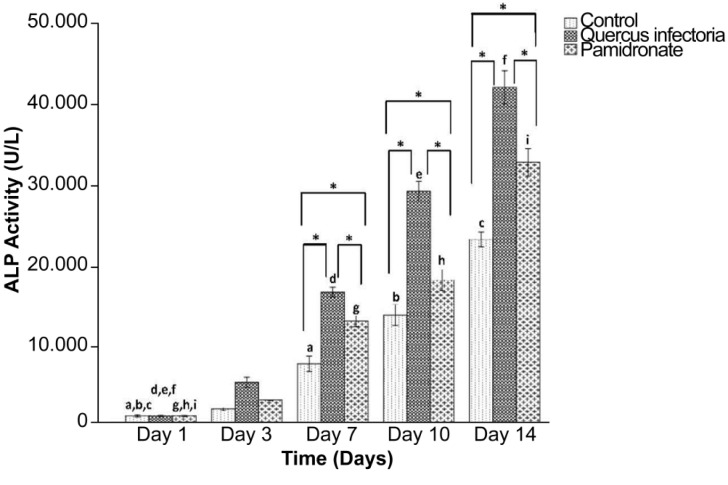
The ALP activities of the treated and untreated hFOB cells increased with time and peaked at day 14. Figure 7 shows that the ALP activities in the hFOB 1.19 cells in both treated and untreated cells were significantly increased with a time course (day 7 until day 14). Subsequently, starting on day 7 and onwards, the ALP activity in the hFOB 1.19 cells treated with QI gall extract was significantly higher compared to the other two groups. On day 14, the highest activity for ALP in the QI gall extract treated cells was recorded as 41.70 U/L.
Figure 7:
Alkaline phosphatase activity by hFOB 1.19 cells; untreated hFOB 1.19 cells (control), hFOB 1.19 cellstreated Quercus infectoria and hFOB 1.19 cells-treated pamidronate after 1, 3, 7, 10, and 14 days of incubation. The values of the bars represented mean (SD) of three independent experiments. * indicates significant difference between the three groups within the same day (P < 0.05) by one-way ANOVA and Tukey’s test. Days that share the same letter show a significant difference (within same group) (P < 0.05) by one-way ANOVA and Tukey’s test.
Osteocalcin level of hFOB 1.19 cells
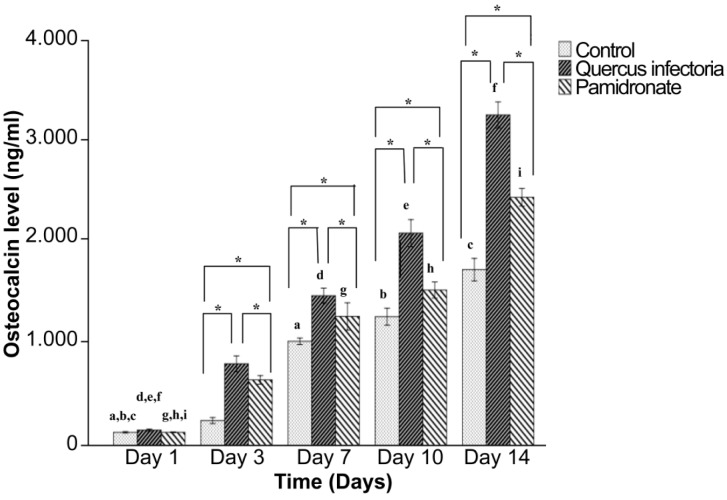
The osteocalcin activities for QI gall extract treated hFOB 1.19 cells and untreated cells were increased in a time-dependent manner and peaked at day 14. Figure 8 shows that the osteocalcin levels in the hFOB 1.19 cells in all three groups increased gradually along the incubation period from day 3 to day 14. In addition, from day 3 until day 14, the osteocalcin levels in the hFOB 1.19 cells treated with QI gall extract were significantly higher than in the other two groups. On day 14, the highest osteocalcin level in the QI gall treated cells was 3.43 ng/mL.
Figure 8:
Osteocalcin level of hFOB 1.19 cells; untreated hFOB 1.19 cells (control), hFOB 1.19 cells- treated Quercus infectoria and hFOB 1.19 cellstreated pamidronate after 1, 3, 7, 10, and 14 days of incubation. The values of the bars represented mean (SD) of the three independent experiments. * Indicates significant difference compared to day one within the same group by one-way ANOVA and Tukey’s test. Days that share the same letter show a significant difference (within similar group) (P < 0.05) by one-way ANOVA and Tukey’s test.
Discussion
This study evaluated the effects of Quercus infectoria (QI) gall extract treatment on the biochemical analysis of bone formation markers (ALP and osteocalcin) and light microscopy on bone-forming cells by using a human osteoblast cell line (hFOB 1.19) model. Prior to observing the proliferative effects of the QI gall extract, we have to determine the half maximal effective concentration (EC50) of QI gall extract and pamidronate which was used as the positive control for this study. The percentage of hFOB 1.19 cell viability increased in a concentration and time-dependent manner after treatment with QI gall extract and the anti-osteoporotic drug, pamidronate.
Interestingly, we have found that the EC50 of QI gall extract (10.30 μg/mL) needed to induce cell proliferation is lower than pamidronate (EC50 = 16.09 μg/mL). This finding showed that the proliferative activity of hFOB 1.19 cells were much greater when treated with QI gall extract, whereas the proliferative activity of hFOB 1.19 cells treated with pamidronate were not as effective as the QI gall extract. This may be due to the presence of polyphenols, the main phytochemical contained in the QI gall extract (28). Furthermore a previous study had shown that a polyphenolic phytoestrogen enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells (29).
To support biochemical evidence, the histological study was done using an inverted microscope to evaluate the morphological changes of the hFOB 1.19 cells before and after treatment with the QI gall extract or pamidronate. Our biochemical data seemed to be congruent with the morphological study. Various types of cells have different morphological characteristics. The most important morphological characteristic of osteoblasts is that they are spindle-shaped and elongated with only slight areas of spreading at the end of long lamellipodia (30). However, the morphology of hFOB 1.19 cells was altered following treatment with QI gall extract and pamidronate.
The hFOB 1.19 cells that were treated with QI gall extract were observed to have a uniformly elongated shape, parallel orientation (31), and an overlap on each other due to rapid cell multiplication. On the other hand, the hFOB 1.19 cells treated with pamidronate were observed to also have elongated shape but were sparsely distributed and less dense. The evaluation of cellular morphology by inverted microscopy revealed that the hFOB 1.19 cells treated with pamidronate were dead or deteriorated. This may be because long term use of pamidronate may reduce bone growth (32). Therefore, the histological study of the cells had proven the ability of QI gall extract to stimulate the hFOB 1.19 cells’ multiplication and the morphological appearance was better than pamidronate.
As for further evidence of the proliferative effect of QI gall extract on hFOB 1.19 cells, the alkaline phosphatase (ALP) activity was measured by using ELISA method. The results showed an increase in ALP activity during the 14 days for both treatments. The hFOB 1.19 cells treated with QI gall extract showed higher activity of ALP as compared to the cells treated with pamidronate as well as untreated cells. This result was consistent with a study where ALP activity in hFOB/pvc cells was found to be significantly increased as a function of time in cultured cells (and peaked on day 12) (33). In addition, the ALP was upregulated and increased in the presence of QI gall extract just after 24 hours, and it continued to have an almost sixfold increase on day 3 of incubation (0.85 U/L on day 1 and 5.05 U/L on day 3). From day 7 until day 14, the ALP activity in the hFOB 1.19 cells was significantly higher in the QI gall-treated group when compared with control groups. This condition was in line with a study done by Wanachewin et al. (34), where they found that the ALP activity of hFOB 1.19 cells significantly increased after 3, 7, and 14 days of sesamin treatment, while the ALP activity reached its highest levels on day 7 of the culture. Sesamin is a class of phytoestrogens isolated from the oil of sesame seeds (Sesamum indicum) and has been shown to exhibit a variety of properties (35). This result agreed with previous findings that ALP is most highly expressed during the onset of the mineralisation process which lasts for 7 days, and hFOB cell mineralisation begins to decrease by day 14 of the culture (36).
The osteocalcin level was measured by using the ELISA method on days 1, 3, 7, 10, and 14. After treatment with the QI gall extract, the osteocalcin levels gradually and persistently increased until day 14 of the incubation period. These results showed that the osteocalcin secretion increased fivefold on day 3 (0.16 ng/mL on day 1 and 0.85 ng/mL on day 3). This data was similar to Hsu et al. (37), who reported that myricetin (a flavonoid compound in vegetables and fruits) caused a significant increase in osteocalcin and the ALP activity of both hFOB human osteoblasts and MG-63 (human osteosarcoma cell line). The expression or upregulation of osteocalcin by osteoblasts suggested that the osteoblastic cells had undergone maturation (38). Our results showed that osteocalcin secretion was high in the presence of QI gall extract, and that implied that the extract promoted the differentiation and maturation of hFOB 1.19 cells.
Our findings indicated that QI gall extract produced better effects on osteoblast growth compared to the well established osteoporotic drug, pamidronate. Whereby, on day 7 until day 14, the ALP and osteocalcin levels were significantly higher in the QI treated group as compared to the other two control groups, including the pamidronate-treated group. Pamidronate belongs to a group of drugs called the bisphosphonates. The bisphosphonates are the most widely used drugs for the treatment of established primary and secondary osteoporosis (39). Although bisphosphonates are commonly used clinically to treat bone diseases, the mechanism of action of these compounds on bone is not completely understood. The effects of pamidronate on osteoblasts varies among studies. Ponader et al. (40), have found that pamidronate enhanced osteoblastic (hFOB) proliferation and differentiation, while Reinholz et al. (36), stated that the direct treatment of hFOB cells with pamidronate and zoledronate was found to decrease their cellolar cellular proliferation but enhanced cellular differentiation.
In addition, Marolt et al. (41), observed that pamidronate treatment over extended periods could negatively affect bone balance by reducing ALP activity, cell proliferation and the viability of alveolar osteoblasts. In this current study, cell proliferation was increased in a time-dependent manner during pamidronate treatment, however, the hFOB cell numbers are lower compared to other groups (Figure 6). It can be assumed that hFOB cells proliferate better without the administration of pamidronate, and the possible reason may be due to the absence of pamidronate’s anabolic effects of on hFOB cells.
In a recent report, Lee and colleagues (2) found that ALP levels were significantly decreased after pamidronate treatments in children with low BMD during and after chemotherapy; however, no changes were observed in the levels of calcium, phosphate, magnesium, type I collagen c-terminal telopeptide (ICTP), and osteocalcin. Bauman et a1. (42), also demonstrated that osteocalcin levels displayed non-significant changes after adminstering pamidronate for more than 24 months in patients with acute spinal cord injuries. On the other hand, previous observations suggested that pamidronate induced a significant increase in bone formation markers, bone alkaline phosphatase (BAP) and osteocalcin in patients with multiple myeloma (43). Moreover, Cauze et al. (44) reported that the efficacy of pamidronate treatment for postmenopausal osteoporosis was reflected in a decrease in circulating biochemical markers including ALP and osteocalcin. The results of these human studies suggest that the effects of pamidronate on bone formation markers vary according to the types of of disease, drug dosage, gender, and age.
To the best of our knowledge, the studies done regarding the effects of QI gall extract on bone metabolism are scarce. Hence, this research contributed more data on the positive role of QI gall extract on hFOB 1.19 cells. As mentioned in the literature review, QI gall extract contains many phytochemicals and minerals that are required in bone metabolism by enhancing the osteoblastic activity. Over a time course, these phytochemicals are believed to enhance the ALP activity, osteocalcin secretion, and calcium deposition (45). The ALP and osteocalcin are bone formation markers for early-stage differentiated osteoblasts and terminally differentiated osteoblasts, respectively. As proven by the results of this study, QI gall extract stimulates the differentiation of hFOB 1.19 cells at various stages, starting from maturation to terminally differentiated osteoblasts.
Pertaining to the effect of QI gall extract on the morphology of hFOB 1.19, this study demonstrated that the extract promoted changes in osteoblast morphology by the increase of cell volume as well as by the maintenance of its flat and elongated shape. This result was consistent with another study using the alcoholic extract of Tinospora cordifolia (TC) where the cell morphology clearly showed an increase in cell numbers and the absence of adverse changes in cell morphology (46). However, these findings are still inadequate, and further studies are needed to provide more evidence on QI gall extract’s effects on the proliferation of hFOB 1.19 cells.
Conclusion
In summary, the proliferative effects of QI gall extract on hFOB 1.19 cells were better than pamidronate. Small concentrations of QI gall extract were able to enhance bone formation better compared to pamidronate. In addition, QI gall extract increased cell proliferation by increasing the level of bone formation markers (ALP and osteocalcin) secreted by the osteoblast cells. Thus, this study suggested that QI gall extract might be a potent anabolic agent that may significantly stimulate osteoblastic activity. Further studies are needed to confirm the efficacy of QI gall extract on hFOB 1.19 cells. In the future, we hope that QI gall extract will be developed as a safe and effective drug for the treatment and prevention of osteoporosis.
Acknowledgments
The authors were grateful to the Universiti Sains Malaysia (USM) for funding this work under USM short term grant number 304/PPSK/61312042.
Footnotes
Conflict of interest
None.
Funds
USM short term grant number 304/PPSK/61312042.
Authors’ Contributions
Conception and design, obtaining of funding, critical revision of the article for the important intellectual content: HH, HA, INS
Analysis and interpretation of the data, provision of study materials or patient, collection and assembly of data: HH, DR, WNWH
Drafting of the article: HH, DR
Final approval of the article: HH, DR, HA, WNWH, INS
Statistical expertise, administrative, technical or logistic support: HH, WNWH
References
- 1.Aubin JE, Liu F. The osteoblast lineage. Principles of Bone Biology. In: Bilezikian JP, Raisz LG, Rodan GA, editors. San Diego (SD): Academic Press; 1996. pp. 51–67. [Google Scholar]
- 2.Lee JM, Kim JE, Bae SH, Hah JO. Efficacy of pamidronate in children with low bone mineral density during and after chemotherapy for acute lymphoblastic leukemia and non-Hofgkin lymphoma. Blood Res. 2013;48(2):99–106. doi: 10.5045/br.2013.48.2.99. doi: 10.5045/br.2013.48.2.99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Hoof VO, De Broe ME. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci. 1994;31:197–293. doi: 10.3109/10408369409084677. doi: 10.3109/10408369409084677 . [DOI] [PubMed] [Google Scholar]
- 4.Martin TJ, Findlay DM, Heath JK, Ng KW. Osteoblasts: diferentiation and function. Handbook of experimental pharmacology. In: Mundy JR, Martin TJ, editors. Berlin (BER): Springer; 1993. pp. 149–183. [Google Scholar]
- 5.Aubin JE, Turksen K, Heersch JNM. Osteoblastic cell lineage. Cellular and molecular biology of bone. In: Noda M, editor. Tokyo (JA): Academic Press Inc; 1993. pp. 1–45. [Google Scholar]
- 6.Gundberg CM. 2nd ed. New York (NY): American Society of Bone and Mineral Research; 1993. Alkaline phosphatase and osteocalcin. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism; pp. 74–76. [Google Scholar]
- 7.Ross PD, Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res. 1998;13:297–302. doi: 10.1359/jbmr.1998.13.2.297. doi: 10.1359/jbmr.1998.13.2.297 . [DOI] [PubMed] [Google Scholar]
- 8.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. doi: 10.1001/jama.296.24.2947 . [DOI] [PubMed] [Google Scholar]
- 9.Scheiber LB II, Torregrosa L. Evaluation and treatment of postmenopausal osteoporosis. Semin Arthritis Rheum. 1998;27(4):245–261. doi: 10.1016/s0049-0172(98)80004-8. doi: 10.1016/S0049-0172(98)80004-8 . [DOI] [PubMed] [Google Scholar]
- 10.Davidson MR. Pharmacotherapeutics for osteoporosis prevention and treatment. J Midwifery Womens Health. 2003;48(1):39–52. doi: 10.1016/s1526-9523(02)00359-8. doi: 10.1016/S1526-9523(02)00359-8 . [DOI] [PubMed] [Google Scholar]
- 11.Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369(9574):1703–1710. doi: 10.1016/S0140-6736(07)60534-0. doi: 10.1016/S0140-6736(07)60534-0 . [DOI] [PubMed] [Google Scholar]
- 12.Emons G, Huschmand-Nia A, Krauss T, Hinney B. Hormone replacement therapy and endometrial cancer. Onkologie. 2004;27(2):207–210. doi: 10.1159/000076914. doi: 0.1159/000076914 . [DOI] [PubMed] [Google Scholar]
- 13.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113(20):2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. doi: 10.1161/CIRCULATIONAHA.105.594077 . [DOI] [PubMed] [Google Scholar]
- 14.Curb JD, Prentice RL, Bray PF, Langer RD, Van Horn L, Barnabei VM, et al. Venous thrombosis and conjugated equine estrogen in women without uterus. Arch Intern Med. 2006;166(7):772–780. doi: 10.1001/archinte.166.7.772. doi: 10.1001/archinte.166.7.772 . [DOI] [PubMed] [Google Scholar]
- 15.Samuelson G. Stockholm (SWE): Swedish Pharmaceutical Press; 1992. Drugs of Natural Origin; p. 86. [Google Scholar]
- 16.Muhammad Z, Mustafa AM. Chapter 6. Kuala Lumpur (MY): Penerbit Fajar Bakti Sdn. Bhd; 2010. Traditional Malay Medicine Plants; pp. 29–32. [Google Scholar]
- 17.Dar MS, Ikram M. Studies on Quercus infectoria; isolation of syringic acid and determination of its central depressive activity. Planta Med. 1979;35(2):156–161. doi: 10.1055/s-0028-1097197. doi: 10.1055/s-0028-1097197 . [DOI] [PubMed] [Google Scholar]
- 18.Hwang JK, Kong TW, Baek NI, Pyun YR. α-Glycosidase Inhibitory Activity of hexagalloylglucose from the gall of Quercus Infectoria. Planta Med. 2000;66(3):273–274. doi: 10.1055/s-2000-8569. doi: 10.1055/s-2000-8569 . [DOI] [PubMed] [Google Scholar]
- 19.Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus protease. Phytother Res. 2000;14(7):510–516. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. doi: 10.1002/1099-1573(200011)14:7<510::AID-PTR646>3.0.CO;2-B . [DOI] [PubMed] [Google Scholar]
- 20.Kaur G, Athar M, Alam MS. Quercus infectoria gall posses antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages. Chem Biol Interact. 2008;171(3):272–282. doi: 10.1016/j.cbi.2007.10.002. doi: 10.1016/j.cbi.2007.10.002 . [DOI] [PubMed] [Google Scholar]
- 21.Vermani A, Navneet Prabhat, Chauhan A. Physicochemical analysis of ash of some medicinal plants growing in Uttarakhand, India. Nature and Sci. 2010;8(6):88–91. [Google Scholar]
- 22.Bruneton J. 2nd ed. Hampshire (UK): Intercept; 1999. Pharmacognosy, Phytochemistry, Medicinal Plants. [Google Scholar]
- 23.Shrestha S, Kaushik VS, Eshwarappa RS, Subaramaihha SR, Ramanna LM, Lakkappa DB. Pharmacognostic studies of insect gall of Quercus infectoria Olivier (Fagaceae) Asian Pac J Trop Biomed. 2014;4(1):35–39. doi: 10.1016/S2221-1691(14)60205-7. doi: 10.1016/S2221-1691(14)60205-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rina R, Rafiquzzaman M, Hasmah A. Spectrophotometer determination of total phenol and flavanoid content in manjakani (Quercus infectoria) extracts. Health Environ J. 2011;2(1):9–13. [Google Scholar]
- 25.Habauzit V, Horcajada MN. Phenolic phytochemicals and bone. Phytochem Rev. 2008;7(2):313–344. doi: 10.1007/s11101-007-9078-9 . [Google Scholar]
- 26.Trzeciakiewicz A, Habauzit V, Hocajada MN. When nutrition interacts with osteoblast function: molecular mechanisms of polyphenols. Nutrition Res Rev. 2009;22(1):68–81. doi: 10.1017/S095442240926402X. doi: 10.1017/S095442240926402X . [DOI] [PubMed] [Google Scholar]
- 27.Hapidin H, Abdullah H, Soelaiman IN. The potential role of Quercus infectoria gall extract on osteoblast function and bone metabolism. Open J Endocrine and Metabolic Diseases. 2012;2(4):81–88. doi: 10.4236/ojemd.2012.24013 . [Google Scholar]
- 28.Haghi G, Safaei A. Identification and determination of polyphenols and tannin in the gall and in the extract of Quercus infectoria. Iran J Pharm Res. 2004;3:85–86. [Google Scholar]
- 29.Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806–814. doi: 10.1016/j.phymed.2007.04.003. doi: 10.1016/j.phymed.2007.04.003 . [DOI] [PubMed] [Google Scholar]
- 30.Baxter LC, Frauchiger V, Textor M, ap Gwynn I, Richards RG. Fibroblast and osteoblast adhesion and morphology on calcium phosphate surfaces. Eur Cell Mater. 2002;4:1–17. doi: 10.22203/ecm.v004a01. [DOI] [PubMed] [Google Scholar]
- 31.Passeri G, Cacchioli A, Ravanetti F, Galli C, Elezi E, Macaluso GM. Adhesion pattern and growth of primary human osteoblastic cells on five commercially available titanium surfaces. Clin Oral Implants Res. 2010;21(7):756–65. doi: 10.1111/j.1600-0501.2009.01906.x. doi: 10.1111/j.1600-0501.2009.01906.x . [DOI] [PubMed] [Google Scholar]
- 32.Evans KD, Sheppard LE, Grossman DI, Rao SH, Martin RB, Oberbauer AM. Long Term Cyclic Pamidronate Reduces Bone Growth by Inhibiting Osteoclast Mediated Cartilage-to-Bone Turnover in the Mouse. Open Orthop J. 2008;2:121–125. doi: 10.2174/1874325000802010121. doi: 10.2174/1874325000802010121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Zhou Z, Saunders MM, Donahue HJ. Modulation of connexin43 alters expression of osteoblastic differentiation markers. Am J Physiol Cell Physiol. 2006;290(4):C1248–C1255. doi: 10.1152/ajpcell.00428.2005. doi: 10.1152/ajpcell.00428.2005 . [DOI] [PubMed] [Google Scholar]
- 34.Wanachewin O, Boonmaleerat K, Pothacharoen P, Reutrakul V, Kongtawelert P. Sesamin stimulates osteoclast differentiation through p38 and ERK1/2 MAPK signaling pathway. BMC Complement Altern Med. 2012;12:17. doi: 10.1186/1472-6882-12-71. doi: 10.1186/1472-6882-12-71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampe JW, Atkinson C, Hullar MA. Assessing exposure to lignans and their metabolites in humans. J AOAC Int. 2006;89(4):1174–1181. [PubMed] [Google Scholar]
- 36.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60(21):6001–6007. [PubMed] [Google Scholar]
- 37.Hsu YL, Chang JK, Tsai CH, Chien TT, Kuo PL. Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem Pharmacol. 2007;73(4):504–514. doi: 10.1016/j.bcp.2006.10.020. doi: 10.1016/j.bcp.2006.10.020 . [DOI] [PubMed] [Google Scholar]
- 38.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151(4):931–944. doi: 10.1083/jcb.151.4.931. doi: 10.1083/jcb.151.4.931 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putnam SE, Scutt AM, Bicknell K, Priestley CM, Williamson EM. Review article: Natural products as an alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother Res. 2007;21:99–112. doi: 10.1002/ptr.2030. doi: 10.1002/ptr.2030 . [DOI] [PubMed] [Google Scholar]
- 40.Ponader S, Brandt H, Vairaktaris E, von Wilmowsky C, Nkenke E, Schlegel KA, et al. In vitro response of hFOB cells to pamidronate modified sodium silicate coated cellulose scaffolds. Colloids Surf B Biointerfaces. 2008;64(2):275–283. doi: 10.1016/j.colsurfb.2008.02.002. doi: 10.1016/j.colsurfb.2008.02.002 . [DOI] [PubMed] [Google Scholar]
- 41.Marolt D, Cozin M, Vunjak-Novakovic G, Cremers S, Landesberg R. Effects of pamidronate on human alveolar osteoblasts in vitro. J Oral Maxillofac Surg. 2012;70(5):1081–1092. doi: 10.1016/j.joms.2011.05.002. doi: 10.1016/j.joms.2011.05.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N, Cirnigliaro C, et al. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305–313. doi: 10.1682/jrrd.2004.05.0062. doi: 10.1682/JRRD.2004.05.006 . [DOI] [PubMed] [Google Scholar]
- 43.Terpos E, Palermos J, Viniou N, Vaiopoulos G, Meletis J, Yataganas X. Pamidronate increases markers of bone formation in patients with multiple myeloma in plateau phase under interferon-alpha treatment. Calcif Tissue Int. 2001;68(5):285–290. doi: 10.1007/BF02390835. doi: 10,1007/s00223-001-0001-x . [DOI] [PubMed] [Google Scholar]
- 44.Cauza E, Etemad M, Winkler F, Hanusch-Enserer U, Partsch G, Noske H, et al. Pamidronate increases bone mineral density in women with postmenopausal or steroid-induced osteoporosis. J Clin Pharm Ther. 2004;29(5):431–436. doi: 10.1111/j.1365-2710.2004.00584.x. doi: 10.1111/j.1365-2710.2004.00584.x . [DOI] [PubMed] [Google Scholar]
- 45.Ming LG, Ge BF, Wang MG, Chen KM. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells' osteogenic differentiation in vitro. Cell Prolif. 2012;45(6):508–515. doi: 10.1111/j.1365-2184.2012.00844.x. doi: 10.1111/j.1365-2184.2012.00844.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abiramasundari G, Sumalatha KR, Sreepriya M. Effects of Tinospora cordifolia (Menispermaceae) on the proliferation, osteogenic differentiation and mineralization of osteoblast model systems in vitro. J Ethnopharmacol. 2012;141(1):474–480. doi: 10.1016/j.jep.2012.03.015. doi: 10.1016/j.jep.2012.03.015 . [DOI] [PubMed] [Google Scholar]