Abstract
Innate immunity has evolved as a first line defense against invading pathogens. Cellular and humoral elements of the innate immune system detect infectious parasites, initiate inflammatory resistance reactions and finally contribute to the elimination of the invaders. Repeated attacks by pathogenic agents induce adaptive responses of the innate immune system. Typically, reapplication of pathogens provokes tolerance of the affected organism. However, also stimulatory effects of primary infections on subsequent innate immune responses have been observed. The present overview touches an undervalued aspect in the innate immune response: Its pronounced dependency on pathogen load. In addition to localization and timing of innate immune responses the pathogen dose dependency might be considered as a “fifth dimension of innate immunity”. Experimental results and literature data are presented proposing a hormetic reaction pattern of innate immune cells depending on the dose of pathogens.
Keywords: Innate immunity, Adaptive response, Hormesis
Introduction
Inflammatory responses of the innate immune system include resistance against microorganisms, clearance of damaged cells and regeneration of the affected tissue. These defense reactions are initiated by the interaction of pathogen associated molecular patterns (PAMPs) with pattern recognition receptors (PRR) (Mogensen 2009). Located on the surface and in the cytoplasm of innate immune cells PRR act as sensors for the pathogen attack. Once stimulated they induce resistance processes aimed at eliminating the pathogen. The pleiotropic functions of the innate immune system are driven by the complex interplay of cellular and humoral components.
As a classical model for the investigation of innate immune reactions macrophages and macrophage like cell lines have been established. These cells sense lipopolysaccharides (LPS) as an archetypal PAMP via specific TLR4 receptors. Subsequent signaling reactions induce reprogramming of the expression pattern and inflammatory stimulation of these cells (Mogensen 2009). Many experimental data have revealed different activation states related to diverging functions of the innate immune system. Macrophages are involved in the phagocytosis and elimination of bacteria as well as in the production of pro- and antiinflammatory mediators, the presentation of antigens to the cells of the adaptive immune system and in the control of regeneration processes at the end of the infectious disease (Biswas and Lopez-Collazo 2009).
Immediate reactions of innate immune cells contributing to bacterial resistance have been extensively investigated during the past decades. Less is known about the mechanistic background of slow acting adaptive responses of the innate immune cells. These adaptive immune reactions allow adjustment to the specific pathological challenge and may prevent overreaction to danger signals. Adaptive responses of innate immunity should not be confused with the classical immune reactions of B-cells, T-cells and other elements of the “adaptive immune system”.
Adaptive responses
Repeated or prolonged treatment of any organism with an external stress challenge alternatively provokes either reduced reactivity or sensitization of the affected organs. Both reaction patterns have been observed in the innate immune system and will be addressed in the next paragraphs of this report.
LPS induced tolerance
Reduced reactivity, i.e. tolerance development is of central importance because it keeps the immune reactions in balance and prevents hyperinflammatory responses like septic shock. Experimentally, hyperinflammation can be prevented by “preconditioning” i.e. pretreatment of cells or animals with PAMPs like LPS.
Due to the simple experimental accessibility investigation of LPS induced preconditioning is of special relevance for the mechanistic exploration of tolerance phenomena. As a predominant effect pretreatment of macrophages with LPS provokes suppression of proinflammatory cytokines like TNFα and IL1β and significant stimulation of some anti-inflammatory cytokines including IL10. In addition, an increase in phagocytic activities has been observed (Medzhitov and Horng 2009; West and Heagy 2002).
In a recent study gene chip analysis was used to explore the alterations of macrophage expression pattern after preconditioning with low doses of LPS (Foster et al. 2007). The data reveal that LPS-induced genes fall in two categories on the basis of their pro- and anti-inflammatory functions. Both classes require specific LPS induced chromatin modifications. These chromatin modifications induce on one hand a transient silencing of genes coding proinflammatory mediators whereas on the other hand a second kind of chromatin modifications is associated with the priming of genes, which include antimicrobial effectors. Thus LPS seems to induce a reprogramming of the expression pattern, aimed at reducing the proinflammatory reactions (Medzhitov and Horng 2009). At the same time the expression of some anti-inflammatory mediators but also bactericidal functions are boosted. Together these findings indicate exceptional plasticity of LPS/TLR4 induced signaling pathways in macrophages.
Tolerance to infections
To survive infections organisms developed two basic strategies: Resistance, which suppresses pathogen spreading and Tolerance, which allows limiting the host expenses for immune reactions. Due to the limited mechanistic understanding, tolerance phenomena recently attracted much interest of investigators in the field. Like LPS-induced tolerance, tolerance of organisms to infections is accompanied by decreased sensitivity of the affected cells to the arriving pathogenic agent. Concomitant suppression of the innate immune cell reactions allows reduction of the impact of the given pathogen (Medzhitov et al. 2012).
As a measure for tolerance to infections the host vitality or fitness in relation to the pathogen burden has been defined (Ayres and Schneider 2012). Excessive decrease of host fitness induced by marginally increased pathogen load signifies low tolerance whereas high vitality in face of high pathogen burden indicates high tolerance to infections.
The current knowledge about tolerance inducing agents is still fragmentary. Recent studies indicate promotion or depression of persistent innate immune responses by toxins or auxiliary infections. Figueiredo et al. recently reported a significant raise of tolerance development in sepsis mouse model induced by cytotoxic anthracyclines (Figueiredo et al. 2013). The tolerance and vitality promoting effect of anthracyclines is strictly dependent on the activation of DNA damage response and autophagy reactions in the lung of the animals. The authors hypothesize that adaptive induction of both repair systems by cytotoxins acts as modulator of immune responses and might be exploited to confer protection to inflammation-driven conditions, including severe sepsis. Suppression of tolerance has been observed after viral-bacterial coinfection of mice. A recent study investigated the effects of Influenza virus on mice infected with Legionella pneumophilia (Jamieson et al. 2013). In a critical time span the virus promotes susceptibility to lethal bacterial coinfection by impairing the ability of the respiratory tissue to tolerate tissue damage.
Trained immunity
In contradiction to tolerance induction primary infections are also able to sensitize the innate immune system. Preexposure of monocytes with bacterial BCG vaccine or fungal cell wall β-glucan induced enhanced production of proinflammatory cytokines after second stimulation (Kleinnijenhuis et al. 2012; Quintin et al. 2012). A prolonged functional reprogramming of the cells was observed after pretreatment of the phagocytes with these pathogenic agents. Strikingly, corresponding reactions could be also observed under in vivo conditions. Pretreatment of immunodeficient mice missing T-cells and B-cells (SCID) with BCG vaccine or β-glucan induced a significantly enhanced and sustained immune response (Ifrim et al. 2014; Quintin et al. 2014). The authors define the adaptive response of innate immune cells as “Trained immunity” and show strict dependence of the reprogramming of innate immune cells on the specific pathogen applied. Alternative preexposure of adherent monocytes with the PAMPs LPS or β-glucan induces opposite effects on the production of inflammatory cytokines. Whereas LPS is able to repress subsequent cytokine production β-glucan induces a strong enhancement.
Together these data indicate differential effects of pathogenic stressors on adaptive reactions of the innate immune system in the affected organism. The first contact with specific pathogens either leads to a repression and tolerance induction or to enhancement of the subsequent immune response. Clearly the innate immune system is able to develop a memory-like plasticity towards specific sequential pathogen attacks. Irrespective of the obvious importance of these immune responses in medicine the mechanistic understanding of these processes is limited. One of the main deficits in the existing data sets seems the limited exploration of dose dependent effects of pathogenic stressors on immune tolerance development.
Dose dependent innate immune responses
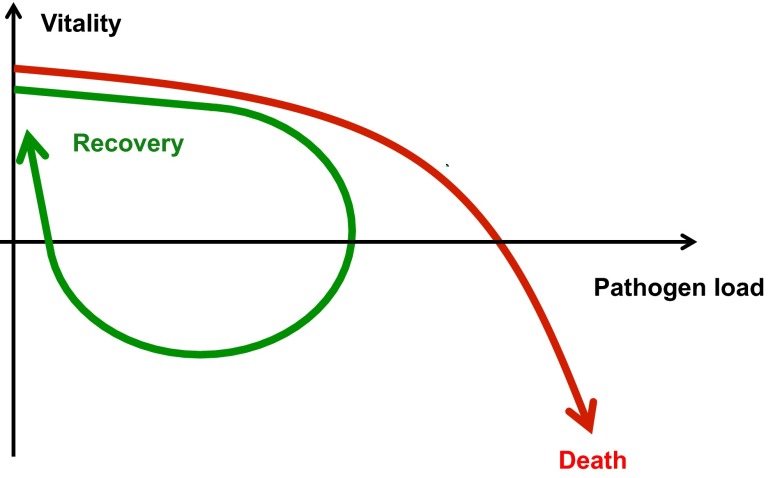
Upon monitoring patients during infections, a strict dependency of the vitality on the pathogen dose becomes apparent. If the resistance and tolerance capacities of the hosts innate and adaptive immune defense systems are exhausted the pathogen load exceeds a critical value and the host dies (Fig. 1) (Schneider 2011). On the other hand reduction of the pathogen by resistance activities of the immune system may allow recovery of the infected organism. This simplified view on the interrelation of host vitality and pathogen dose should be mirrored by the reaction pattern of immune cells under in vitro conditions. One may predict increasing pathogen resistance of these cells at increasing pathogen challenge and at a critical dose saturation and exhaustion of these defense activities.
Fig. 1.
Vitality versus pathogen-load plot. (adapted from Schneider (2011) PLOS Biology 9 e1001158)
Typical reactions of innate immune cells like phagocytosis of infectious bacteria or cytokine production are promoted by increasing doses of infectious agents and may be saturated or even decline at a critical level of the PAMPs provided by the infectious agents. Surprisingly, only few recent studies investigated effects of the pathogen dose on innate immune cell responses. Of note, dependence of inflammatory reactions on bacterial tissue load has been recently demonstrated in the intestine of mice (Willer et al. 2012). More recent in vitro studies revealed differential release of pro- and anti-inflammatory mediators by primary macrophages or macrophage cell lines at increasing doses of LPS (Baker et al. 2014; Morris et al. 2014). Low concentrations of LPS preferentially induced production of proinflammatory TNFα or IL-6, whereas high LPS levels increasingly release anti-inflammatory mediators like IL-10 or IL-33. These investigations clearly indicate dose dependency of the release of pro- and anti-inflammatory cytokines by macrophage likes cells.
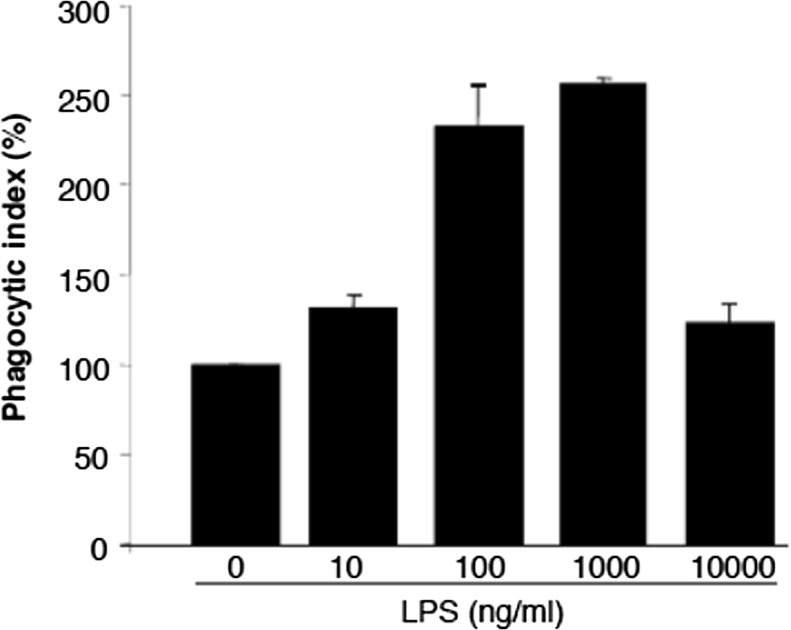
Our group recently investigated the role of PI3Kγ, an intracellular signaling protein in microglia in the control of phagocytosis of bacteria (Schmidt et al. 2013). In connection to this study we were interested in the dose-dependent effects of LPS on the phagocytic activity. As shown in Fig. 2 an optimal LPS dose has been observed, which induces maximal phagocytic response. Interestingly, the data do not show saturation but a pronounced decline of the phagocytic activities at high LPS. The phagocytosis pattern of macrophage-like microglia may reflect the in vivo situation during infections as described above. In fact the dependency of microglia phagocytosis on LPS dose closely mimics a hormetic stress response pattern (Calabrese 2013). Whereas the signaling processes mediating increasing phagocytosis at increasing endotoxin doses are becoming more and more clear (Underhill and Goodridge 2012) the mechanistic background of the drop of phagocytic activity at high LPS is less transparent. Parallel to a saturation of the phagocytic machinery an increasing exhaustion of the energy supply could be predicted. Supplementary investigations of the LPS effects on the expression of the LPS receptor TLR4 revealed saturation pattern including a continuous increased TLR4 expression up to 100 ng/ml LPS followed by nearly constant level until 1,000 ng/ml (data not shown).
Fig. 2.
LPS-dependent phagocytosis of wild type microglia. Concentration dependent effects of LPS on phagocytotic activity of primary microglia. Primary microglia cells were seeded into 6-well plates and incubated at 37 °C (5 % CO2) for 24 h. After attachment cells were incubated for 24 h in DMEM without FCS in the presence of the indicated LPS concentrations. Phagocytosis assay was performed using GFP producing Escherichia coli. Forty microliters of the suspended bacteria was added to the microglial cells and incubated 1 h at 37 °C (5 % CO2). After incubation the cells were harvested, washed and re-suspended in phosphate buffer solution. The phagocytic activity of the cells was measured by flow cytometry using FACS Canto (BD, Heidelberg, Germany)
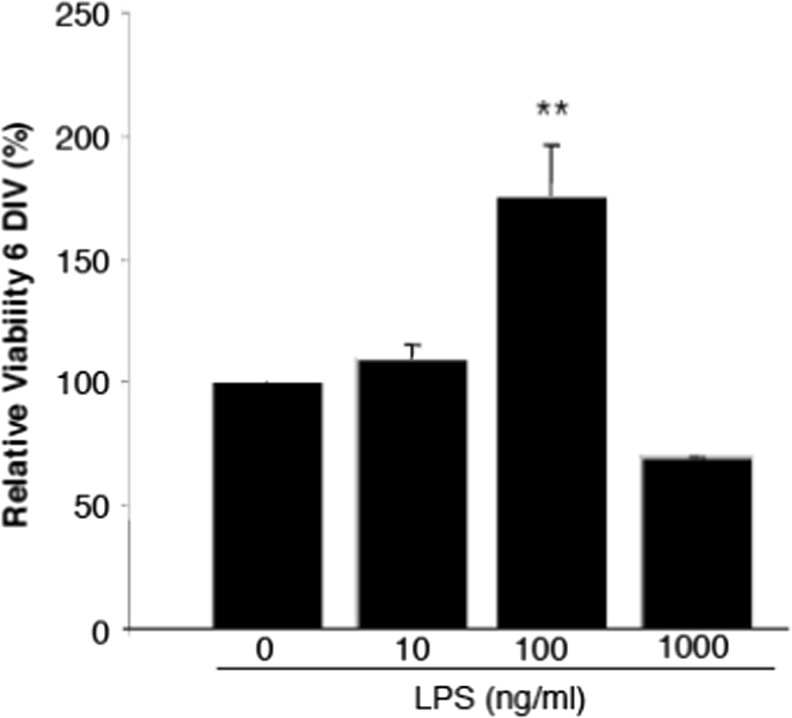
Looking at the LPS-dependence of the microglia vitality in the same concentration range there is an even more pronounced decline at high LPS concentrations (Fig. 3). Ongoing experiments of our group are directed at the identification of signaling proteins controlling the stimulatory and inhibitory branch of this hormetic pattern. Preliminary data clearly reveal PI3Kγ in addition to reactive oxygen species (ROS) as essential mediators of the hormetic response (Schmidt C. et al. unpublished data). Our study suggests LPS-induced increase of PI3Kγ expression and the subsequent stimulation of NADPH oxidases (Lehmann et al. 2009) as a cause of increased ROS. Stimulatory effects of ROS on cell proliferation and vitality have been described in many biological systems (Ostman et al. 2011). The decline of microglia ROS production at high LPS doses still awaits explanation. Together these data hint to a limited capacity of macrophage-like cells to perform efficient immune reactions at high bacterial load and/or endotoxin dose.
Fig. 3.
LPS-dependent proliferation of microglial cells. Effects of increasing concentrations of LPS on proliferation of primary microglia. Cells were seeded into 96-well plates and incubated at 37 °C (5 % CO2) for 24 h. After attachment cells were treated with different concentrations of LPS for 6 days under in vitro (DIV) conditions. MTT-solution (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid) was added to each well and incubated 4 h at 37 °C (5 % CO2). Afterwards the supernatant was aspirated and ethanol (100 % v/v) was added for 30 min. Proliferation was measured by the intensity of blue colored cells at 570 nm in a plate reader. **P < 0.01, compared to unstimulated cells; n = 6
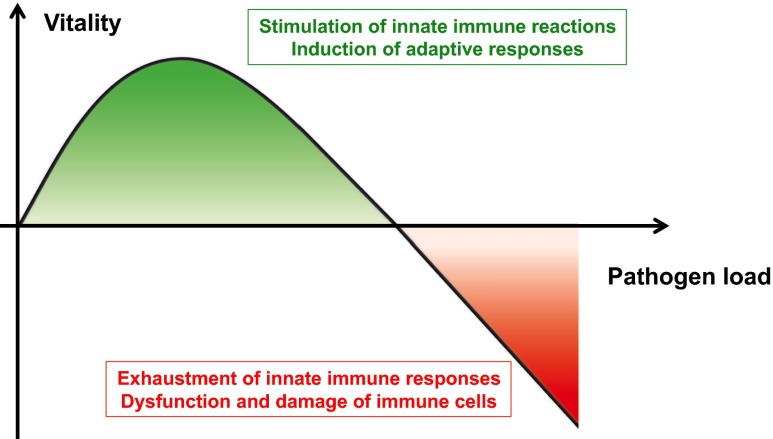
The schema in Fig. 4 is aimed at correlating the individual segments of the hormesis plot (Calabrese 2013) to defined responses of innate immune cells induced by pathogens. The illustration underlines the importance of investigations directed to dose-dependent effects of PAMPs like LPS on the immunogenic functions of the innate immune cell.
Fig. 4.
Hormesis of innate immune-cell responses
Conclusion
In sum the presented data and considerations strongly suggest that strengthening the experimental investigations on innate immune reactions in dependency on the load of the invading pathogen or on the dose of PAMPs is highly warranted. In addition to location and time relations of innate immune responses the dependency on pathogen doses appears as a crucial fifth dimension of innate immunity.
References
- Ayres, J. S., & Schneider, D. S. (2012). Tolerance of infections. [Research Support, N.I.H., Extramural Review]. Annu Rev Immunol, 30, 271-294. doi: 10.1146/annurev-immunol-020711-075030 [DOI] [PubMed]
- Baker B, Maitra U, Geng S, Li L. Molecular and cellular mechanisms responsible for cellular stress and low-grade inflammation induced by a super-low dose of endotoxin. J Biol Chem. 2014;289(23):16262–16269. doi: 10.1074/jbc.M114.569210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- Figueiredo N, Chora A, Raquel H, Pejanovic N, Pereira P, Hartleben B, Moita LF. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity. 2013;39(5):874–884. doi: 10.1016/j.immuni.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Ifrim DC, Quintin J, Joosten LA, Jacobs C, Jansen T, Jacobs L, Netea MG. rained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol. 2014;21(4):534–545. doi: 10.1128/CVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340(6137):1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Muller JP, Schlott B, Skroblin P, Barz D, Norgauer J, Wetzker R. PI3Kgamma controls oxidative bursts in neutrophils via interactions with PKCalpha and p47phox. [Research Support, Non-U.S. Gov’t] Biochem J. 2009;419(3):603–610. doi: 10.1042/BJ20081268. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9(10):692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Medzhitov, R., Schneider, D. S., & Soares, M. P. (2012). Disease tolerance as a defense strategy. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Science, 335(6071), 936-941. doi: 10.1126/science.1214935 [DOI] [PMC free article] [PubMed]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Gilliam EA, Button J, Li L. Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J Biol Chem. 2014;289(31):21584–21590. doi: 10.1074/jbc.M114.583518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman A, Frijhoff J, Sandin A, Bohmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150(4):345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Netea MG. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12(2):223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29C:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schneble N, Muller JP, Bauer R, Perino A, Marone R, Wetzker R. Phosphoinositide 3-kinase gamma mediates microglial phagocytosis via lipid kinase-independent control of cAMP. Neuroscience. 2013;233:44–53. doi: 10.1016/j.neuroscience.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Schneider DS. Tracing personalized health curves during infections. PLoS Biol. 2011;9(9):e1001158. doi: 10.1371/journal.pbio.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Goodridge H. Information processing during phagocytosis. [Review] Nat Rev Immunol. 2012;12(7):492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(1 Supp):S64–S73. doi: 10.1097/00003246-200201001-00009. [DOI] [PubMed] [Google Scholar]
- Willer Y, Müller B, Bumann D. Intestinal inflammation responds to microbial tissue load independent of pathogen/non-pathogen discrimination. PLoS ONE. 2012;7(5):e35992. doi: 10.1371/journal.pone.0035992. [DOI] [PMC free article] [PubMed] [Google Scholar]