Abstract
Research over the past few years has highlighted the ability of the unfolded protein response (UPR) to minimize the deleterious effects of accumulated misfolded proteins under both physiological and pathological conditions. The endoplasmic reticulum (ER) adapts to endogenous and exogenous stressors by expanding its protein-folding capacity and by stimulating protective processes such as autophagy and antioxidant responses. Although it is clear that severe ER stress can elicit cell death, several recent studies have shown that low levels of ER stress may actually be beneficial to cells by eliciting an adaptive UPR that ‘preconditions’ the cell to a subsequent lethal insult; this process is called ER hormesis. The findings have important implications for the treatment of a wide variety of diseases associated with defective proteostasis, including neurodegenerative diseases, diabetes, and cancer. Here, we review the physiological and pathological functions of the ER, with a particular focus on the molecular mechanisms that lead to ER hormesis and cellular protection, and discuss the implications for disease treatment.
Keywords: Endoplasmic reticulum, Mitochondria, Unfolded protein response, Neurodegenerative diseases, Cancer, Diabetes
Maintenance of protein homeostasis, or proteostasis, requires an effective means of ensuring that newly synthesized secreted and membrane proteins are correctly folded, assembled, and modified before they exit the endoplasmic reticulum (ER) (Hetz and Mollereau 2014). The unfolded protein response (UPR) senses ER stress caused by the accumulation of unfolded or misfolded proteins, and responds by initiating a transcriptional program dedicated to restoring proteostasis by reducing new protein synthesis, increasing the synthesis of chaperone proteins to assist with protein folding, and degrading aberrantly folded proteins (Walter and Ron 2011; Hetz 2012; Wang and Kaufman 2012). The goal of the UPR is thus to restore optimal ER functioning or, if this is not possible, to initiate programmed cell death as a last resort.
The ER has an extraordinary ability to adapt to increasing physiological demands for protein synthesis by expanding its protein folding ability. For example, pancreatic β-cells, which must secrete insulin promptly in response to physiological and pathological fluctuations in blood glucose concentrations, have a high protein-folding capacity to accommodate the demand for proinsulin synthesis (Biden et al. 2014). Similarly, photoreceptor cells in the retina must synthesize and fold large quantities of opsins, which can reach up to 70 % of the protein content of the cell (Hamm and Bownds 1986). Thus, although ER-associated degradation (ERAD) is sufficient to remove misfolded proteins in most cells under normal physiological conditions, the UPR is constitutively activated in cells with unusually large demands for protein synthesis. In this review, we focus on the mechanisms of ER adaptation in response to stress and how the UPR triggers protective responses that allow the cells to avert pathological conditions.
ER stress and the unfolded protein response
Given the critical role of the ER folding machinery and the UPR in proteostasis, it is not surprising that perturbation of these processes can have pathological consequences (Walter and Ron 2011; Wang and Kaufman 2012). For example, expression of mutant proteins, such as proinsulin C96Y in pancreatic β-cells of the Akita mouse model, induces ER stress and activates the UPR (Zhang et al. 2014). Similarly, the UPR is elicited by mutations in rhodopsins in photoreceptor cells, as observed in Drosophila and mouse models of retinitis pigmentosa (Mendes et al. 2009; Ryoo and Steller 2007; Ryoo et al. 2007 ; Lin et al. 2007). Another example is the accumulation of aggregated mutated proteins, such as β-amyloid, α-synuclein, and superoxide dismutase 1 in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), respectively (Hetz and Mollereau 2014). Cataract formation in the lens can be caused by oxidative stress and the accumulation of misfolded crystallin proteins. Misfolded crystallins promotes ER stress and the UPR, which may contribute to cataract formation (Ikesugi et al. 2006; Elanchezhian et al. 2012). Many chemicals and environmental factors also induce ER stress, and these have proven to be useful tools for research. In cultured cells and animal models, ER stress is commonly induced by treatment with tunicamycin, a glycosylation inhibitor, or with thapsigargin, an inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) that causes depletion of Ca2+ stores in the ER.
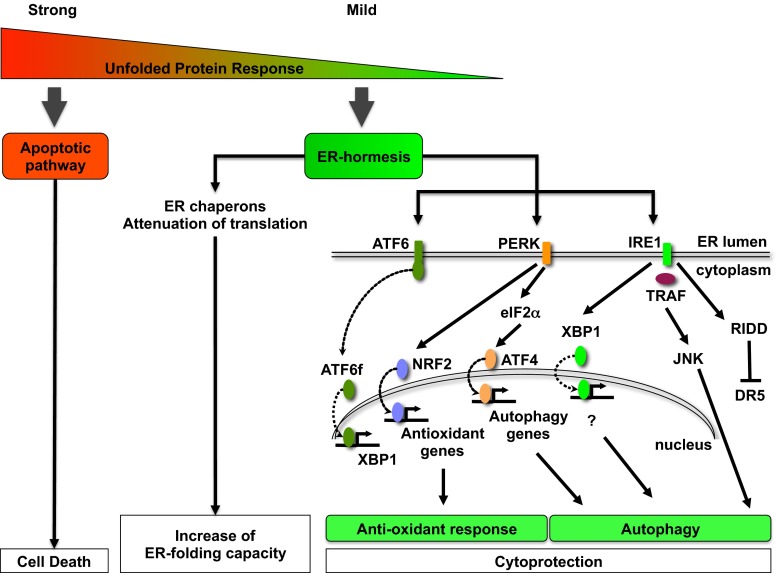
The three membrane-associated ER proteins that act as stress sensors and set in motion the UPR are: protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (Fig. 1). PERK responds to ER stress by phosphorylating and inhibiting the activity of eukaryotic translation initiation factor 2α (eIF2α). This attenuates protein translation and rapidly reduces the influx of nascent proteins into the ER. When homeostasis is restored, eIF2α is dephosphorylated by the UPR-induced phosphatase subunit GADD34, restoring translation (Harding et al. 2009). Although the majority of mRNA translation is halted by eIF2α phosphorylation, translation of selected proteins continues. One example is the transcription factor ATF4, which induces expression of a number of ER-resident chaperones as well as proteins involved in regulating autophagy, the antioxidant response, and amino acid metabolism. ATF4 also induces genes controlling apoptosis, such as C/EBP-homologous protein (CHOP). The second UPR sensor, IRE1 has both endoribonuclease and kinase activities and catalyzes the unconventional splicing of X-box binding protein 1 (XBP1) mRNA, which is then translated into the transcription factor XBP1s. XBP1s activity upregulates the expression of ER chaperones and proteins associated with ERAD and lipogenesis. IRE1 also regulates XBP1-independent pathways; for example, IRE1 phosphorylates and activates c-Jun N-terminal kinase (JNK) via formation of a complex with TRAF2. The endoRNase activity of IRE1 induces a process termed regulated IRE1-dependent decay (RIDD), which induces the rapid decay of mRNAs associated with several processes, including lipogenesis and apoptosis. The final arm of the UPR is ATF6, which translocates to the Golgi and is cleaved by site 1 protease (S1P) and S2P. This releases a cytosolic fragment, ATF6f, which functions as a transcription factor and induces the expression of XBP1 and ERAD-associated proteins.
Fig. 1.
Potential for therapeutic modulation of the UPR. Strong ER stress and activation of the UPR triggers apoptosis. In contrast, mild UPR activation induces a beneficial ER hormetic response by reducing the load of misfolded proteins and by activating cellular protective mechanisms such as an antioxidant response and autophagy. The PERK–ATF4 pathway plays a key role in ER adaptation. PERK phosphorylates Nrf2, which promotes transcription of antioxidant genes. PERK mediates phosphorylation of eIF2α and ATF4-dependent transcriptional activation of autophagy genes. ATF6-dependent induction of XBP1 is also important for ER adaptation and is required for cytoprotection via induction of autophagy. Specific degradation of death receptor 5 (DR5) mRNA by RIDD contributes to ER adaptation and cytoprotection
ER hormesis: a cytoprotective response to ER stress
It is becoming increasingly clear that exposure to low levels of ER stress can be beneficial by eliciting hormesis. Hormesis is an adaptive cellular response whereby exposure to low doses of stress activate protective mechanisms that render the cell resistant to a subsequent challenge with higher doses of stress (Mattson 2008). Hormesis is observed clinically when the body is exposed to low levels of stress in the form of ischemic preconditioning or exercise, dietary, and energy restriction (Calabrese et al. 2007; Dirnagl and Meisel 2008). The molecular mechanisms underlying hormesis are still unclear, but have been formerly attributed to changes in mitochondrial function and increased formation of reactive oxygen species (ROS), a process termed mitochondrial hormesis or mitohormesis (Schulz et al. 2007; Calabrese et al. 2010). For example, exposure of Caenorhabditis elegans to the glucose analog 2-deoxy-D-glucose (DOG) inhibits glycolysis but induces a compensatory increase in mitochondrial respiration and ROS production, thus triggering a hormetic response that increases oxidative stress resistance via expression of antioxidant proteins such as catalase (Schulz et al. 2007). Interestingly, the hormetic response and resulting lifespan extension in C. elegans was abolished by the addition of antioxidants, which raises concerns about the potential detrimental effects of antioxidant therapy.
Hormesis can also be induced by ER stress (ER hormesis) (Table 1). ER hormesis was first used to describe cellular protection observed in Drosophila carrying a mutant form of the chaperone protein NinaA in the retina (Mendes et al. 2009). NinaA is an ER-resident chaperone dedicated to the folding of rhodopsin-1 (Rh1) in the photoreceptor cells, but in ninaA mutants, Rh1 proteins are largely misfolded and accumulate in the ER. Here, the loss of a key chaperone protein leads to the accumulation of misfolded proteins and induction of the UPR that is not toxic for the photoreceptors but prompts a cellular protective response that protects cells from subsequent apoptotic insults. A similar ER hormetic response is observed following inhibition of the ERAD effector protein valosin-containing protein (VCP) in Drosophila (Griciuc et al. 2010). In the Rh1P37H/RhP23H fly model of retinitis pigmentosa, loss-of-function mutations in VCP activate the IRE1–XBP1 pathway and efficiently suppress retinal degeneration and blindness resulting from the toxic accumulation of misfolded mutated Rh1 (Griciuc et al. 2010). In the same model, strong suppression of retinal degeneration was also observed following treatment of flies with the VCP/ERAD inhibitor Eeyarestatin I or the proteasome inhibitor MG132. In rodent and human cell lines, ablation of Herp, a component of the ERAD pathway, promoted ER adaptation and elimination of poly-ubiquitin protein aggregates that accumulate in response to glucose to deprivation or proteasome inhibition (Quiroga et al. 2013). In each of these examples, loss of proteins involved in normal ER function prompted a hormetic response leading to resistance to the secondary stressor, such as apoptotic insults or expression of pathological proteins.
Table 1.
Models of ER hormesis
| Models | UPR manipulations | Consequences | Refs |
|---|---|---|---|
| Photoreceptor and S2 cell survival: Drosophila | • ninaA mutation • Tunicamycin • XBP1 • BIP |
• Increased antioxidant and autophagic responses • Resistance to apoptosis |
Mendes et al. 2009; Fouillet et al. 2012 |
| Retinitis pigmentosa: Drosophila (Rh1P37H/RhP23H) | • VCP mutation • Eeyarestatin 1 • MG132 |
• Suppression of retinal degeneration and blindness | Griciuc et al. 2010 |
| Parkinson’s disease: 6-OHDA treatment of SH-SY5Y cells and mice Hu-α-synuclein in Drosophila |
• Tunicamycin | • Autophagy induction • DA neuron protection • Locomotor improvement • Locomotor improvement |
Fouillet et al. 2012 |
| Parkinson’s disease: 6-OHDA treatment of SH-SY5Y cells Hu-α-synuclein in C. elegans |
• LRRK2 induction • Bip • Lrk-1 |
• DA neuron protection | Yuan et al. 2011 |
| Parkinson’s disease: 6-OHDA treatment of SH-SY5Y cells |
• Tunicamycin • Thapsigargin • Bip • ATF4 |
• Antioxidant response • Heme oxygenase-1 upregulation |
Hara et al. 2011 |
| Parkinson’s disease: 6-OHDA treatment of mice |
• XBP1 mutation | • Adaptive response • DA neuron protection |
Valdes et al. 2014 |
| Huntington’s disease: Transgenic mice | • XBP1 mutation | • Adaptive response • Autophagy induction • DA neuron protection |
Vidal et al. 2012 |
| ALS: Transgenic mice |
• XBP1 mutation | • Adaptive response • Autophagy induction • Neuron protection |
Hetz et al. 2009 |
| Brain ischemia in rats | • Ischemic preconditioning | • UPR and autophagy induction | Sheng et al. 2012 |
| Myocardial ischemic/reperfusion injury in rats | • Tunicamycin • Thapsigargin |
• UPR and autophagy induction • Reduced cardiomyocyte apoptosis • Reduced infarct size |
Petrovski et al. 2011 |
| Human umbilical vein endothelial cells | • Quercetin | • Protection against tunicamycin toxicity | Suganya et al. 2014 |
| Longevity in C. elegans | • Insulin/IGF-1 pathway mutation | • IRE1 and XBP1 induction • Lifespan extension • Resistance to ER stress |
Henis-Korenblit et al. 2010 |
| Viability in C. elegans | • HPL-2 mutation | • IRE1 and XBP1 induction • Resistance to ER stress |
Kozlowski et al. 2014 |
| Cancer: Constitutive Hedgehog signaling in Drosophila | • Temperature • Thapsigargin |
• Reduction of inappropriate Hedgehog signaling in smo mutants | Marada et al. 2013; Mollereau 2013 |
Although the cytoprotection associated with an ER hormetic response is generally observed in cells submitted to low ER stress, it is also induced in cells exposed to prolonged and lethal ER stress. Indeed, it was proposed that a prolonged ER stress induces a biphasic UPR response that initiates a survival phase, which is followed by the activation of a death response. This is well illustrated in the transgenic rat model of autosomal dominant retinitis pigmentosa that expresses mouse P23H rhodopsin (Lin et al. 2007). In this model, rhodopsin P23H induces the initial combined activation of the three UPR branches IRE1, PERK and ATF6, which results in cytoprotection until post-natal day 12. This early protective phase is then followed by the dampening of IRE1 and the selective activation of the pro-apoptotic factor CHOP and photoreceptor cell death. In this review, we focus on the early and protective phase of the UPR and review the molecular mechanisms that favor cytoprotection while restraining the UPR-induced death response.
Contributions of IRE1–XBP1 to ER hormesis
Recent studies describing the physiological functions of the IRE1–XBP1 arm of the UPR have provided insights into organismal development, stress resistance, and longevity. For example, in cultured murine neurites, XBP1 is induced in response to treatment with brain-derived neurotrophic factor and promotes neurite outgrowth (Hayashi et al. 2007; Hayashi et al. 2008). More recent work in Drosophila showed that RIDD specifically targets mRNAs encoding fatty acid transport protein (Dourlen et al. 2012), an essential process for photoreceptor differentiation (Coelho et al. 2013). In addition, mutations of the C. elegans heterochromatin protein like-2 (HPL-2), the homolog of heterochromatin protein 1, were shown to induce a hormetic response dependent on XBP1. This response was associated with a marked increase in organismal resistance to tunicamycin toxicity, indicating that ER hormesis is under the control of the epigenetic factor HPL-2 (Kozlowski et al. 2014). Interestingly, pretreatment of human endothelial cells with quercetin, a flavonol that binds the kinase–RNAse domain of IRE1 (Wiseman et al. 2010), increased the cellular resistance to subsequent ER stress, suggesting that ER hormesis in these cells may depend on IRE1 activation (Suganya et al. 2014). ER hormesis was also observed in C. elegans insulin/IGF-1 signaling pathway mutants (Henis-Korenblit et al. 2010), which display increased IRE1- and XBP-1–dependent resistance to ER stress and increased longevity. A recent study in C. elegans also showed that expression of XBP-1s in neurons was sufficient to increase longevity and induce UPR signaling in distal, non-neuronal cell types via a cell-nonautonomous mechanism (Taylor and Dillin 2013). This is a particularly exciting finding because it suggests that a cell- and/or tissue-restricted ER hormetic response has remote effects and can be beneficial for the entire organism. Whether such cell-nonautonomous control of the UPR also functions in mammals remains to be determined.
Although the molecular mechanisms of cytoprotection during the ER hormetic response are still being elucidated, it seems clear that the increased folding capacity and attenuated protein translation resulting from UPR activation allow the cell to eliminate potentially pathogenic proteins. In addition, ER hormesis has been shown to activate antioxidant responses and autophagy, both of which are cytoprotective (Higa and Chevet 2012; Hetz and Mollereau 2014) (Fig. 1).
PERK-induced antioxidant responses contribute to ER hormesis
Signaling through the PERK–ATF4 pathway can both promote and reduce cell survival. On the one hand, prolonged ER stress leads to PERK-dependent induction of ATF4 and activation of the pro-apoptotic factor CHOP (Harding et al. 2000a). On the other hand, PERK also promotes cell survival by increasing the antioxidant capacity (Maas and Diehl 2014). The transcription factor NF-E2-Related Factor 2 (Nrf2) induces an antioxidant response (Cullinan et al. 2003). Nrf2 is normally retained within the cytoplasm by association with Keap1 (Kelch-like ECH-associated protein 1); however, PERK phosphorylation of Nrf2 induces its release from Keap1 and subsequent translocation to the nucleus (Itoh et al. 2004), where it binds to antioxidant response elements in the promoters and induces transcription of numerous antioxidant genes, including thioredoxins, glutathione synthetase, glutathione S-transferase and ferritin (Motohashi and Yamamoto 2004). Hence, deletion of PERK or Nrf2 has deleterious consequences by abolishing the antioxidant response of cells subjected to chronic ER stress (Cullinan and Diehl 2006; Harding et al. 2000b). In addition, activation of the PERK arm of the UPR by tunicamycin suppresses TNFα-induced ROS accumulation and decreases cell death by preventing reduction in cellular glutathione levels (Xue et al. 2005).
Maintaining a redox balance is essential for protein folding within the ER and is achieved via the ER oxidoreductin enzymes Ero1α and Ero1β, protein disulfide isomerases, and the GSH/GSSG redox couple. Perturbation of optimal redox conditions by deregulation of these components can trigger ER stress and compromise survival (Higa and Chevet 2012). Thus, the strong interplay between the UPR and the antioxidant response is essential for proper ER function and the adaptive response to ER stress.
ER stress-induced autophagy plays a cytoprotective role in ER hormesis
Protein quality control mechanisms recognize misfolded proteins and mediate their degradation via proteasome and macroautophagy pathways. Macroautophagy (hereafter autophagy), a conserved catabolic pathway through which the cell degrades and recycles cytoplasmic components, functions as a survival pathway under stress conditions (Klionsky et al. 2012). Crosstalk between the UPR and autophagy is an important component of the cellular response to stress (Vidal and Hetz 2012; Deegan et al. 2013). UPR signaling stimulates autophagy, which is cytoprotective in several ER hormetic paradigms (Matus et al. 2012 ; Hetz and Mollereau 2014). For example, inhibition of autophagy reduces neuroprotection mediated by the ER hormetic response in photoreceptors of Drosophila ninaA mutants (Fouillet et al. 2012). Similarly, inactivation of autophagy in human neuroblastoma SH-SY5Y cells by LC3-targeted siRNA or treatment with the (PI3K) inhibitor 3-methyl adenine abolishes ER-mediated protection induced by tunicamycin against 6-hydroxydopamine (6-OHDA) (Fouillet et al. 2012). Autophagy is also involved in ER hormesis in a mouse model of Parkinson’s disease. Thus, in mice pretreated with tunicamycin, subsequent treatment with 6-OHDA elicits an autophagic response in dopaminergic neurons, suggesting that stress-induced autophagy is cytoprotective (Fouillet et al. 2012). Autophagy and cytoprotection were also observed during ischemic preconditioning of primary cultured murine cortical neurons by oxygen/glucose deprivation (Sheng et al. 2012). Inhibition of the ER stress response by the selective eIF-2α inhibitor salubrinal suppressed autophagy and diminished neuroprotection induced by brain ischemic preconditioning in rat (Gao et al. 2013). Similarly, prior treatment with tunicamycin or thapsigargin induced autophagy and protected against subsequent myocardial ischemic/reperfusion injury in rats (Petrovski et al. 2011). Collectively, these findings demonstrate that autophagy plays a critical role in the ER hormetic response.
Several UPR genes and downstream targets have been shown to regulate autophagy. Interestingly, whereas IRE1 and JNK are required for autophagy regulation, ATF6 appears to be dispensable (Ogata et al. 2006). During ER stress, the IRE1 kinase domain recruits the adaptor molecule TRAF2, and the IRE1–TRAF2 complex activates the apoptosis signal-regulating kinase (ASK1). In turn, ASK1 activates the MAP kinases JNK and p38, and JNK phosphorylates the anti-apoptotic and anti-autophagic proteins Bcl2 and Bcl-XL. These proteins normally bind and sequester the key autophagy regulator Beclin; thus, their phosphorylation by JNK liberates Beclin, allowing the associated class III PI3K to stimulate autophagy (Wei et al. 2008). The PERK–ATF4 pathway also plays an essential role in autophagy activation by promoting the transcription of the conserved autophagy genes ATG5, ATG12, and ATG8/LC3 (Rouschop et al. 2010; Kouroku et al. 2007). Finally, PERK phosphorylates and promotes the activity of the transcription factor FOXO-1, which is a potent activator of autophagy (Xu et al. 2011; Zhao et al. 2010; Vidal and Hetz 2012). Whereas nuclear FOXO-1 induces the transcriptional activation of ATG5, ATG8/LC3, and Beclin, ER stress induces cytosolic FOXO1 to bind to ATG7 and increase autophagy independently of its transcriptional activity (Zhao et al. 2010). Interestingly, XBP1 deficiency induces neuroprotection in a mouse model of Huntington’s disease via upregulation of FOXO-1 and autophagy (Vidal et al. 2012).
These data illustrate the critical role played by autophagy in ensuring survival during ER stress, allowing the cell to escape the toxic consequences of an accumulation of pathogenic proteins. A recent study showed that opposing UPR signals converge on death receptor 5 (DR5) to dictate cell survival or death in response to ER stress (Lu et al. 2014). In this scenario, IRE1–ATF4-dependent activation of the transcription factor CHOP induces expression of DR5 and subsequent caspase 8-dependent apoptosis. However, DR5 levels are kept in check by RIDD-dependent mRNA degradation, allowing time for cellular adaptation. Thus, the UPR can decide the cell fate, depending on the duration and intensity of stress.
Mitochondrial function in ER hormesis
The ER is structurally and functionally connected to the mitochondria and influences mitochondrial functions via modulation of Ca2+ metabolism and stress signaling (Walter and Hajnoczky 2005). Although sustained ER stress can induce mitochondria-dependent apoptosis, adaptation to moderate stress involves modulation of key mitochondrial functions, including Ca2+ metabolism, ATP synthesis, mitochondrial dynamics, and quality control. Early in the ER stress response to tunicamycin and thapsigargin, a Ca2+-dependent increase in mitochondrial metabolism is observed that involves induction of CHOP expression and phosphorylation of eIF2α and JNK (Bravo et al. 2011). In addition, microtubule-dependent perinuclear re-localization of reticular and mitochondrial networks and enhancement of ER–mitochondria coupling take place, accompanied by a transient stimulation of ATP synthesis that fuels the UPR. Whether in the early phases of ER stress the Ca2+ leak from the ER functions as the signal triggering the relocalization of mitochondrial networks remains to be established. As mitochondrial perinuclear clustering and ER-juxtaposition, and increased Ca2+ transfer, can be observed also under ER-stress induced cell death, the mechanistic aspects of how these processes result in different outputs will need further investigation. A PERK-dependent increase in mitochondrial biogenesis has also been reported to be a component of the ER stress response (Zheng et al. 2012). In this study, ER stress-induced Nrf2-mediated transcription of heme oxygenase-1, increased the transcription of complexes of the electron transport chain, and upregulated mitochondrial DNA biogenesis. Further support for direct crosstalk between UPR signaling and mitochondrial function comes from a recent study demonstrating that the mitochondrial fusion protein mitofusin 2 (Mfn2), a regulator of ER stress, is required for PERK-dependent activation of apoptosis and autophagy during the ER stress response (Munoz et al. 2013). Ablation of Mfn2 induced massive ER expansion and excessive activation of all three UPR branches, but reduced activation of apoptosis and autophagy during ER stress. Mfn2 physically interacts with PERK, and Mfn2-ablated cells showed sustained activation of PERK under basal conditions, leading to mitochondrial swelling, enhanced ROS production, Ca2+ overload, and reduced respiration. These defects were suppressed by PERK silencing, suggesting that Mfn2 acts as a direct PERK repressor under basal conditions (Munoz et al. 2013).
PERK has also been proposed to play a structural role in the regulation of mitochondrial-dependent apoptosis induced by ER stress–ROS signaling (Verfaillie et al. 2012). In this study, PERK was shown to be enriched at the mitochondrial-associated ER membranes (MAMs; the sites of ER–mitochondria coupling) and to be required for apoptosis after ROS-induced ER stress. PERK−/− cells displayed altered ER morphology and Ca2+ signaling, as well as weaker ER–mitochondrial contacts (Verfaillie et al. 2012). PERK re-expression overcame these defects and sensitized PERK−/− cells to ROS-mediated stress. Interestingly, these effects required the cytoplasmic domain of PERK, but not its kinase activity, suggesting a structural and functional role for MAM-associated PERK in the propagation of ROS signals to neighboring mitochondria and in the initiation of apoptosis in response to ROS-induced ER stress. Together, these studies demonstrate the existence of functional ER–mitochondrial interactions during the UPR and raise the intriguing but as-yet-untested possibility that ER hormesis and mitohormesis may be linked.
ER hormesis and disease
The many examples of ER hormesis described in cell culture and animal models raises questions about its relevance to human diseases and whether the process could be exploited for the development of therapeutics.
ER hormesis in diabetes
Chronic hyperlipidemia and hyperglycemia play a causal role in the development of type 2 diabetes, a disease characterized by peripheral insulin resistance and/or insufficient insulin secretion by pancreatic β-cells. Type 2 diabetes has been linked to ER stress (Ozcan et al. 2004; Back and Kaufman 2012) and oxidative stress-associated mitochondrial dysfunction (Lowell and Shulman 2005). During ER stress imposed by high production of insulin, PERK induces an adaptive response that protects β-cells from ROS accumulation (Back et al. 2009; Scheuner et al. 2001). Mutations in PERK are linked to the human Wolcott–Rallison diabetic syndrome (Senee et al. 2004), and PERK is also a regulator of WSF1, an ER membrane protein that contributes to the UPR and maintenance of ER homeostasis (Fonseca et al. 2005). Inactivating mutations in the WFS1 gene cause Wolfram syndrome, which is associated with type 1 diabetes, optic atrophy, and deafness (Fonseca et al. 2009). β-cells carrying mutated WFS1 show increased levels of ER stress, reduced insulin secretion, and a failure to respond to alterations in glucose concentrations (Shang et al. 2014; Lemaire and Schuit 2012). Interestingly, it was observed in rodent and human cell lines that wild type WFS1 negatively regulates ATF6 via the proteasome and reduced the UPR. WFS1 stabilized the ubiquitin ligase HRD1, which induced ATF6 degradation via the proteasome (Fonseca et al. 2010). Thus, PERK-dependent regulation of WFS1 is important for the maintenance of ER homeostasis in pancreatic β-cells.
ER hormesis in diseases of the CNS
The role of the ER adaptation in CNS diseases was described in three elegant studies showing that ER hormesis induced by XBP1 inactivation was neuroprotective (Hetz et al. 2009; Vidal et al. 2012; Valdes et al. 2014). These authors showed that conditional ablation of XBP1 in models of ALS, Parkinson’s disease, and Huntington’s diseases induced an adaptive ER stress response that protected against subsequent disease-inducing insults. In the ALS mouse model, XBP1 deficiency protected from death induced by mutated SOD1 expression in motoneurons (Hetz et al. 2009). In the mtHttQ128 transgenic mouse model of Huntington’s disease, ablation of XBP1 improved neuronal survival in the striatum (Vidal et al. 2012). Similarly, ablation of XBP1 protected dopaminergic neurons against 6-OHDA-induced cell death in the mouse model of Parkinson’s disease (Valdes et al. 2014). However, XBP1 inhibition is not beneficial in all models of neurodegeneration; XBP1 deficiency enhances the severity of spinal cord injury in mice (Valenzuela et al. 2012) and does not affect prion pathogenesis (Hetz et al. 2008).
The neuroprotective function of ER hormesis in Parkinson’s disease has been studied in detail in cell culture and animal models treated with the dopamine analog 6-OHDA, which induces loss of dopaminergic neurons via oxidative stress and apoptosis (Przedborski et al. 1991; Tanaka et al. 2006). In these models, ER hormesis was induced by pretreatment with low doses of ER stressors such as tunicamycin or thapsigargin. For example, Drosophila S2 cells and human SH-SY5Y neuroblastoma cells are protected against 6-OHDA toxicity by pretreatment with tunicamycin (S2 and SH-SY5Y) and thapsigargin (SH-SY5Y) (Mendes et al. 2009; Fouillet et al. 2012 ; Hara et al. 2011). Importantly, this effect is also observed in vivo, as shown by the neuroprotective effect of tunicamycin treatment in the 6-OHDA mouse model of Parkinson’s disease (Fouillet et al. 2012; Matus et al. 2012). Collectively, these studies have established the cytoprotective property of hormesis induced by low levels of ER stress, suggesting that this process could be exploited for the development of much needed therapies for neurodegenerative diseases.
ER hormesis in cancer
Cancer cells, particularly solid cancers, are often exposed to unfavorable growth conditions such as nutrient and oxygen deprivation, and it is becoming increasingly clear that cancer cells take advantage of the ER stress response and UPR signaling to ensure their survival under these conditions. Given that prolonged ER stress generally leads to cell death, a survival advantage would be conferred on cells that show enhanced pro-survival UPR signaling and reduced pro-apoptotic signaling. In support of this, tumor cell survival is increased when expression of the PERK-induced pro-apoptotic transcription factor CHOP is reduced by either microRNA mir211 (Chitnis et al. 2012) or the co-chaperone p58IPK (Huber et al. 2013). Similarly, deletion of CHOP in mouse models of lung cancer (Huber et al. 2013) and hepatocellular carcinoma (Nakagawa et al. 2014) is associated with increased tumor incidence. On the other hand, cancer cells appear to retain PERK-mediated ER hormetic responses. These include Nrf2-dependent antioxidant responses (Cullinan and Diehl 2004) and ATF4-dependent activation of cytoprotective autophagy (Harding et al. 2003; Hart et al. 2012). Interestingly, recent studies have identified mutations in IRE1α that impair endoribonuclease function but still allow XBP1 mRNA splicing; cancer cells expressing the mutated forms of IRE1α cannot induce RIDD and therefore exhibit enhanced survival (Ghosh et al. 2014). Another well-described mechanism of cancer cell survival is via ATF6-induced expression of the chaperone BiP. Increased BiP expression alleviates ER stress and is positively correlated with malignant cell proliferation and tumor histologic grade (Lee 2007).
Importantly, a recent study has provided evidence that ER hormesis could be exploited for the treatment of some medulloblastomas and basal cell carcinomas induced by mutation of Smoothened (Smo), a G protein-coupled receptor in the Hedgehog (Hh) signaling pathway (Marada et al. 2013; Mollereau 2013). Mutations in a conserved extracellular loop of Drosophila Smo induces its retention in the ER and increases Smo-dependent signaling downstream of Hh (Carroll et al. 2012). An ER adaptive response induced by thapsigargin or heat treatment in Drosophila wing and cancer cell lines destabilized the Smo mutant and reduced its signaling activities (Marada et al. 2013). Moreover, destabilization was dependent on the ERAD-specific ubiquitin ligase Hrd1. Thus, activation of the UPR elicits a complex signaling network that can either support or repress tumorigenesis, with the final outcome likely depending on the chronicity of ER stress and the cellular context.
Conclusions
In this review, we have discussed some of the mechanisms by which ER hormesis could be beneficial for the treatment of a variety of diseases. These include a wide range of protein misfolding disorders such as Parkinson’s and Huntington’s diseases, retinitis pigmentosa, ALS, diabetes and cancers. We have also highlighted the pleiotropic nature of the ER adaptive response. First, the response increases the ER folding capacity to efficiently remove mutated proteins that are retained in the ER, as in the case of mutated forms of Smo (cancer), opsin (retinitis pigmentosa), and insulin (diabetes). Second, the ER adaptive response triggers additional protective pathways such as the antioxidant response and autophagy. Collectively, these responses act as barriers to the deleterious effects of exogenous stimuli, such as cell death insults, or to the expression of endogenous pathogenic proteins. Importantly, these protective responses will also take place in cells submitted to prolonged or acute ER stress but in which the pro-death phase of the UPR will eventually lead to cell demise. The goal of future research will be to further characterize the molecular switches that dictate whether the cell should live or die in response to ER stress to identify therapeutic drug targets and drugs for stimulating an ER adaptive response while minimizing adverse side effects.
Acknowledgments
This work was supported by grants from the Fondation ARC pour la Recherche sur le Cancer (SFI20121205951) and the Centre national de la recherche scientifique to BM, and from the Ligue Nationale Contre le Cancer (Comité du rhône) and Fondation ARC pour la Recherche sur le Cancer (PJA20131200334) to SM.
Abbreviations
- 6-OHDA
6-Hydroxydopamine
- ALS
Amyotrophic lateral sclerosis
- ASK1
Apoptosis signal-regulating kinase
- ATF
Activating transcription factor
- CHOP
C/EBP-homologous protein
- CNS
Central nervous system
- DOG
2-deoxy-D-glucose
- DR5
Death receptor 5
- eIF2α
Eukaryotic translation initiation factor 2α
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- HPL-2
Heterochromatin protein like-2
- IRE1
Inositol-requiring enzyme 1
- JNK
Jun N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- MAM
Mitochondrial-associated ER membranes
- PERK
Protein kinase RNA-like ER kinase
- PI3K
Phosphoinositide 3-kinase
- Rh1
Rhodopsin-1
- RIDD
Regulated IRE1-dependent decay
- ROS
Reactive oxygen species
- S1P
Site 1 protease
- UPR
Unfolded protein response
- VCP
Valosin-containing protein
- XBP1
X-box binding protein 1
Contributor Information
Bertrand Mollereau, Phone: 33 4 72 72 81 63, Email: bertrand.mollereau@ens-lyon.fr.
Francesco Napoletano, Phone: 33 4 72 72 81 63, Email: francesco.napoletano@ens-lyon.fr.
References
- Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab. 2014 doi: 10.1016/j.tem.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, Lavandero S. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124(Pt 13):2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CE, Marada S, Stewart DP, Ouyang JX, Ogden SK. The extracellular loops of Smoothened play a regulatory role in control of Hedgehog pathway activation. Development. 2012;139(3):612–621. doi: 10.1242/dev.075614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis NS, Pytel D, Bobrovnikova-Marjon E, Pant D, Zheng H, Maas NL, Frederick B, Kushner JA, Chodosh LA, Koumenis C, Fuchs SY, Diehl JA. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol Cell. 2012;48(3):353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho DS, Cairrao F, Zeng X, Pires E, Coelho AV, Ron D, Ryoo HD, Domingos PM. Xbp1-independent ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in Drosophila. Cell Rep. 2013;5(3):791–801. doi: 10.1016/j.celrep.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279(19):20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38(3):317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan S, Saveljeva S, Gorman AM, Samali A. Stress-induced self-cannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell Mol Life Sci. 2013;70(14):2425–2441. doi: 10.1007/s00018-012-1173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55(3):334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Dourlen P, Bertin B, Chatelain G, Robin M, Napoletano F, Roux MJ, Mollereau B. Drosophila fatty acid transport protein regulates rhodopsin-1 metabolism and is required for photoreceptor neuron survival. PLoS Genet. 2012;8(7):e1002833. doi: 10.1371/journal.pgen.1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T. Age-related cataracts: homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012;200(1):1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280(47):39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009;9(6):763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA, Urano F. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest. 2010;120(3):744–755. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B. ER stress inhibits neuronal death by promoting autophagy. Autophagy. 2012;8(6):915–926. doi: 10.4161/auto.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Zhang XY, Han R, Zhang TT, Chen C, Qin ZH, Sheng R. The endoplasmic reticulum stress inhibitor salubrinal inhibits the activation of autophagy and neuroprotection induced by brain ischemic preconditioning. Acta Pharmacol Sin. 2013;34(5):657–666. doi: 10.1038/aps.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, Weiberth KF, Gliedt MJ, Alavi MV, Hari SB, Mitra AK, Bhhatarai B, Schurer SC, Snapp EL, Gould DB, German MS, Backes BJ, Maly DJ, Oakes SA, Papa FR. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158(3):534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Aron L, Roux MJ, Klein R, Giangrande A, Ueffing M. Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm HE, Bownds MD. Protein complement of rod outer segments of frog retina. Biochemistry. 1986;25(16):4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- Hara H, Kamiya T, Adachi T. Endoplasmic reticulum stress inducers provide protection against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int. 2011;58(1):35–43. doi: 10.1016/j.neuint.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106(6):1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122(12):4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Kasahara T, Iwamoto K, Ishiwata M, Kametani M, Kakiuchi C, Furuichi T, Kato T. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J Biol Chem. 2007;282(47):34525–34534. doi: 10.1074/jbc.M704300200. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Kasahara T, Kametani M, Kato T. Attenuated BDNF-induced upregulation of GABAergic markers in neurons lacking Xbp1. Biochem Biophys Res Commun. 2008;376(4):758–763. doi: 10.1016/j.bbrc.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107(21):9730–9735. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15(4):233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci U S A. 2008;105(2):757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23(19):2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012;24(8):1548–1555. doi: 10.1016/j.cellsig.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Huber AL, Lebeau J, Guillaumot P, Petrilli V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, Chevet E, Manie SN. p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant progression upon low glucose. Mol Cell. 2013;49(6):1049–1059. doi: 10.1016/j.molcel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83(3):508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36(10):1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14(2):230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Kozlowski L, Garvis S, Bedet C, Palladino F. The Caenorhabditis elegans HP1 family protein HPL-2 maintains ER homeostasis through the UPR and hormesis. Proc Natl Acad Sci U S A. 2014;111(16):5956–5961. doi: 10.1073/pnas.1321698111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67(8):3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Lemaire K, Schuit F. Integrating insulin secretion and ER stress in pancreatic beta-cells. Nat Cell Biol. 2012;14(10):979–981. doi: 10.1038/ncb2594. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas NL, Diehl JA. Molecular Pathways: the PERKs and pitfalls of targeting the unfolded protein response in cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marada S, Stewart DP, Bodeen WJ, Han YG, Ogden SK. The unfolded protein response selectively targets active smoothened mutants. Mol Cell Biol. 2013;33(12):2375–2387. doi: 10.1128/MCB.01445-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus S, Castillo K, Hetz C. Hormesis: protecting neurons against cellular stress in Parkinson disease. Autophagy. 2012;8(6):997–1001. doi: 10.4161/auto.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy ML, Gambis A, Ryoo HD, Steller H, Mollereau B. ER stress protects from retinal degeneration. Embo J. 2009;28(9):1296–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B. Establishing links between ER-hormesis and cancer. Mol Cell Biol. 2013 doi: 10.1128/MCB.00315-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, Diaz-Ramos A, Hernandez-Alvarez MI, Sebastian D, Mauvezin C, Palacin M, Zorzano A. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. Embo J. 2013;32(17):2348–2361. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, Koike K, Kaufman RJ, Karin M. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26(3):331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14(11):2191–2200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Kostic V, Jackson-Lewis V, Dollison A, Gash DM, Fahn S, Cadet JL. Sham transplantation protects against 6-hydroxydopamine-induced dopaminergic toxicity in rats: behavioral and morphological evidence. Brain Res. 1991;550(2):231–238. doi: 10.1016/0006-8993(91)91323-S. [DOI] [PubMed] [Google Scholar]
- Quiroga C, Gatica D, Paredes F, Bravo R, Troncoso R, Pedrozo Z, Rodriguez AE, Toro B, Chiong M, Vicencio JM, Hetz C, Lavandero S. Herp depletion protects from protein aggregation by up-regulating autophagy. Biochim Biophys Acta. 2013;1833(12):3295–3305. doi: 10.1016/j.bbamcr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Steller H. Unfolded protein response in Drosophila: why another model can make it fly. Cell Cycle. 2007;6(7):830–835. doi: 10.4161/cc.6.7.4064. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Domingos PM, Kang MJ, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. Embo J. 2007;26(1):242–252. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/S1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Senee V, Vattem KM, Delepine M, Rainbow LA, Haton C, Lecoq A, Shaw NJ, Robert JJ, Rooman R, Diatloff-Zito C, Michaud JL, Bin-Abbas B, Taha D, Zabel B, Franceschini P, Topaloglu AK, Lathrop GM, Barrett TG, Nicolino M, Wek RC, Julier C. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes. 2004;53(7):1876–1883. doi: 10.2337/diabetes.53.7.1876. [DOI] [PubMed] [Google Scholar]
- Shang L, Hua H, Foo K, Martinez H, Watanabe K, Zimmer M, Kahler DJ, Freeby M, Chung W, LeDuc C, Goland R, Leibel RL, Egli D. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63(3):923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, Zhang XY, Qin ZH. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8(3):310–325. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, Paulmurugan R, Ramkumar KM. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014;47(3):231–240. doi: 10.1111/cpr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Ogawa N, Asanuma M. Molecular basis of 6-hydroxydopamine-induced caspase activations due to increases in oxidative stress in the mouse striatum. Neurosci Lett. 2006;410(2):85–89. doi: 10.1016/j.neulet.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153(7):1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci U S A. 2014;111(18):6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19(11):1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal RL, Hetz C. Crosstalk between the UPR and autophagy pathway contributes to handling cellular stress in neurodegenerative disease. Autophagy. 2012;8(6):970–972. doi: 10.4161/auto.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, Caballero B, Kiffin R, Segura-Aguilar J, Cuervo AM, Glimcher LH, Hetz C. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr. 2005;37(3):191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30(6):678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RL, Zhang Y, Lee KP, Harding HP, Haynes CM, Price J, Sicheri F, Ron D. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell. 2010;38(2):291–304. doi: 10.1016/j.molcel.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Das M, Reilly J, Davis RJ. JNK regulates FoxO-dependent autophagy in neurons. Genes Dev. 2011;25(4):310–322. doi: 10.1101/gad.1984311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280(40):33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Cao P, Smith MA, Kramp K, Huang Y, Hisamoto N, Matsumoto K, Hatzoglou M, Jin H, Feng Z. (2011) Dysregulated LRRK2 signaling in response to endoplasmic reticulum stress leads to dopaminergic neuron degeneration in C. elegans. PLoS One. 6(8):e22354. [DOI] [PMC free article] [PubMed]
- Zhang L, Nosak C, Sollazzo P, Odisho T, Volchuk A. IRE1 inhibition perturbs the unfolded protein response in a pancreatic beta-cell line expressing mutant proinsulin, but does not sensitize the cells to apoptosis. BMC Cell Biol. 2014;15(1):29. doi: 10.1186/1471-2121-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12(7):665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- Zheng M, Kim SK, Joe Y, Back SH, Cho HR, Kim HP, Ignarro LJ, Chung HT. Sensing endoplasmic reticulum stress by protein kinase RNA-like endoplasmic reticulum kinase promotes adaptive mitochondrial DNA biogenesis and cell survival via heme oxygenase-1/carbon monoxide activity. Faseb J. 2012;26(6):2558–2568. doi: 10.1096/fj.11-199604. [DOI] [PubMed] [Google Scholar]