Abstract
Obesity is a major health problem throughout the world, and it is increasing both in prevalence and severity. Pharmaceutical approaches developed for the treatment of obesity, despite short-term benefits, often are associated with rebound weight gain after the cessation of drug use and serious side effects deriving from the medication can occur. Resveratrol has been well recognized as an anti-obesity substance for its lipid-lowering function as well as calorie-restriction effect. This polyphenol induces hormetic dose responses in a wide range of biological models, affecting numerous endpoints of biomedical and therapeutic significance. From an hormetic standpoint, we will discuss the potential relevance of resveratrol in the management of obesity and related comorbid conditions, emphasizing its ability to control simultaneously various pathological mechanisms associated with obesity.
Keywords: Obesity, Polyphenols, Resveratrol, Hormesis
Introduction
Obesity is a severe metabolic disorder and it is the major risk factor for the most common human diseases including type 2 diabetes, hypertension, cardiovascular diseases, stroke and certain types of cancer. The number of obese individuals in the world is reaching epidemic proportions. Importantly, obesity is not only an issue of adult life but also children are increasingly affected. This increase in frequency of obese children is mainly attributed to high-energy diets and low activity levels. Obesity is characterized by an excessive amount of adipose tissue and it is not only a health complication on its own, but brings with it significant co-morbidities. These include nonalcoholic fatty liver disease, insulin resistance (Avogaro and de Kreutzenberg 2005), dyslipidaemia, impaired glucose homeostasis, sleep apnea, accelerated skeletal and pubertal development (ageing processes), cancer, orthopedic disorders and psychological consequences (Wellman and Friedberg 2002; Naderali 2009). Although many of these conditions only develop later in life, obese individuals are already setting the scene for their future health concerns when they are young.
Efforts to develop innovative anti-obesity drugs have intensified recently, and a more appropriate, medically based outcome measure for obesity treatment has been advocated (Blackburn et al. 1997). It is well established that weight loss in obese patients will lead to a significant amelioration in obesity-related disorders (Poirier et al. 2006) and the reduction of caloric intake induces weight loss. In higher organisms, calorie restriction (CR) results in a reduction of body fat from white adipose tissue (WAT) and an increase in insulin sensitivity (Picard et al. 2004). The adipose tissue of obese individuals has been found to release pro-inflammatory cytokines and systemic inflammation is a hallmark of obesity and its complications (Fuentes et al. 2013).
To optimize obesity prevention and intervention efforts, many researchers have looked into potential therapeutic effects of naturally occurring phytochemicals (González-Castejón and Rodriguez-Casado 2011; Bracale et al. 2014). The secondary metabolites represent a key component of plant metabolism characterized by dynamic plasticity and adaptive value, because they are involved in the responses to biotic and abiotic stresses. Polyphenols predominate among the secondary plant metabolites and thousands of polyphenolic compounds have been identified in different plant species (Quideau et al. 2011). Polyphenols may represent a novel therapeutic approach to manage or prevent obesity and diet-related disease in view of their ability to exert anti-inflammatory responses and modulate important regulatory factors involved in adipogenesis and lipogenesis. However, even though numerous polyphenols show promising effects in in vitro and animal models, many fail to affect adipogenesis in humans. The lack of results in clinical studies can be explained by pharmacological doses used in vitro, whereas physiological doses used in humans are not carefully designed, considering the hormetic activity of certain polyphenols.
Although polyphenols may interact with molecular and cellular pathways associated with stress resistance and cell survival benefits, it is important to point out that some polyphenols may have carcinogenic or genotoxic effects at high doses or concentrations (Catterall et al. 2000). Further human studies evaluating the potential efficacy of polyphenols for the management of obesity and associated co-morbidities should consider that high-dose of polyphenols can potentially cause adverse effects. Alternatively, a dose that produces a beneficial effect in cell cultures may be poisonous or ineffective when applied in a human setting (Mennen et al. 2005). In this scenario, hormesis represents an essential concept to explain how bioactive secondary metabolites produced by plants may have a biphasic dose–response depending on their concentration and exert anti-adipogenic, anti-lipogenic, anti-inflammatory and/or antioxidant effects when administered at low doses. Moreover, to confirm the anti-obesity benefits of polyphenols the hormetic concept must be taken into account in the design of future human studies since the dosing approach for clinical trials typically involves high-dose treatment, whereas experimental data suggest that intermittent lower doses may be more effective (Lee et al. 2014).
Resveratrol, a natural phytoalexin, is a polyphenolic compound mainly found in the skin of grapes and is known to affect a broad range of intracellular pathways (Carrizzo et al. 2013). Resveratrol mimics the metabolic effects of long-term CR and many in vitro studies have demonstrated that resveratrol has an anti-obesity potential by inhibiting preadipocyte differentiation, decreasing adipocyte proliferation, inducing adipocyte apoptosis, decreasing lipogenesis, and promoting lipolysis and fatty acid β-oxidation (Wang et al. 2014). The detailed pharmacokinetics of resveratrol in humans and rodents have been studied (Almeida et al. 2009) and recent studies have shown that resveratrol binds several receptors and signalling molecules, and inhibits the functions of key receptors, kinases, proteinases and other enzymes involved in metabolic pathways (Pervaiz and Holme 2009). It is noteworthy that resveratrol causes a plethora of biological responses in cells and organisms that involve a spectrum of adaptive mechanisms, suggesting that such responses may be mediated by hormetic mechanisms. In this review we will discuss the hormetic mechanisms of action of resveratrol to elucidate its usefulness in the treatment of obesity and related diseases.
Phytochemical hormesis and its relevance in obesity
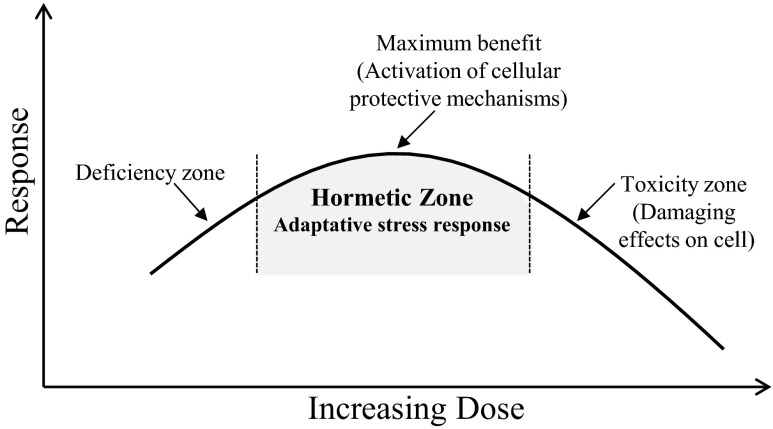
There is considerable potential for the application of hormesis in biomedical sciences and the concept of hormesis may provide important insights to improve the treatment of obese patients. In particular, the emerging field of phytochemical hormesis indicates that the anti-obesity effects of polyphenols may be attributed to the ability of these compounds to interact with adipose tissues (Wang et al. 2014). Phytochemical hormesis is supported by studies showing that polyphenol-rich diets may prevent or mitigate various diseases by activating adaptive stress response signaling pathways (Mattson and Cheng 2006). The concept of hormesis as a significant dose–response model in toxicology and pharmacology has been extensively reviewed elsewhere (Calabrese et al. 2010a). Although many of the most effective drugs and widely used dietary supplements for the management of obesity are naturally occurring phytochemicals, the application of the hormetic dose–response model in obesity research remains elusive. However, a highly conserved feature of the responses of cells to phytochemicals is that they are biphasic (Fig. 1) and nutritional experimental findings provide evidence that the molecular mechanisms of action of phytochemicals involve activation of hormetic pathways (Calabrese et al. 2012).
Fig. 1.
Hormetic response curve Within the hormetic zone, mild or moderate doses of phytochemicals may increase stress resistance and promote beneficial effects by invoking transcription of stress response genes
The pharmacological treatments of obesity have not reduced the disease burden associated with this condition, and this is due to adverse reactions found with many of the anti-obesity compounds used so far. As already mentioned, a correct dose translation from cells, rats or mice to humans is required and the hormetic dose–response model may offer an important approach to identify effective dosages and predict adverse effects of anti-obesity phytochemicals.
Many polyphenols share beneficial effects against a broad range of pathologies, including obesity and diabetes but the concentrations of polyphenols often used in in vitro experiments cannot be achieved in vivo. Therefore, according to the hormesis concept, it seems crucial to reconsider in studies with obese human subjects that polyphenols display hormetic action and the concentrations and the duration of exposure to the polyphenols are essential to have positive physiological outcomes. Since concern has been raised on the safety of the intake of high doses of polyphenols, detailed dose–response analyses are essential for extending polyphenols research to clinical studies related to obesity, overweight, and body composition. It will also be important to investigate whether intermittent dosing with polyphenols may be more effective to improve various metabolic parameters associated with obesity. Although recent evidence supports the capability of polyphenols to modulate pathways related to obesity including neuroregulatory factors that affect food intake (Panickar 2013), clinical data are extremely limited. However, polyphenols are able to promote lipolysis and limit the production of proinflammatory adipokines or influence the activities of hormetic intracellular targets such as adenosine monophosphate activated protein kinase (AMPK), the deacetylating enzyme sirtuin-1 (SIRT-1), and the peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which are altered in metabolic disturbances associated with obesity (Timmers et al. 2011). Thus, the development of hormetic nutritional interventions using polyphenols that activate adaptative signaling pathways is a promising new approach for the preventation and treatment of obesity and related pathologies.
Dose reposonse relationship of resveratrol in obesity
The physiological benefits of resveratrol are currently under intensive investigation and numerous studies report that resveratrol displays hormesis-like biphasic dose responses (Calabrese et al. 2010b). Resveratrol has received widespread attention because it was shown to extend the life span of yeast, flies, and worms (Barger 2013). Findings suggest that resveratrol may prevent the deleterious effects of a high-fat diet on metabolism, lowering inflammation, and enhancing memory in mice fed a high-fat diet (Baur et al. 2006; Jeon et al. 2012). In adipose tissue of genetically obese rats, resveratrol reduced nuclear factor- kβ (NF-kβ) activity and obesity-related inflammation markers (Gómez-Zorita et al. 2013).
To date, the only nonpharmacological intervention known to alleviate age-related chronic diseases such as obesity, diabetes, and cancer is CR. Hormesis has been considered as one of the possible mechanisms of CR and resveratrol modulates gene expression profiles that resemble those induced by CR in multiple tissues of healthy mice (Barger et al. 2008). Although resveratrol mimics the health benefits of CR and can improve adipose tissue function and insulin sensitivity in models of obesity and metabolic disorders, the optimal dose of resveratrol remains an unresolved issue in clinical pratice. Human studies evaluating the efficacy of resveratrol are emerging but different results are reported due to notable differences in the dose of resveratrol administered. In terms of cellular experiments, the most commonly applied resveratrol concentration is 50 μM (≈11,400 ng/mL), and the generally accepted range of in vitro activity is 5–100 μM (Poulsen et al. 2013a). These in vitro concentrations are potentially unachievable in the in vivo setting. Pharmacokinetic studies in healthy volunteers have shown that after repeat dosing of 0.5–5.0 g daily for 28 days, the mean average plasma concentration values of resveratrol and its metabolites across the four dose levels ranged from 0.04 to 0.55 nmol/mL and 0.19 to 4.24 nmol/mL, respectively (Brown et al. 2010).
in vitro research and preclinical studies have greatly facilitated the characterization of molecular targets activated by resveratrol. Among these, PGC-1α, SIRT1, NF-kβ, AMPK, Forkhead Box O3 (FOXO3), and peroxisome proliferator-activated receptor-γ (PPARγ) appear to be related to obesity pathophysiology and affected by specific hormetic mechanisms (Poulsen et al. 2013b; Barrajón-Catalán et al. 2014). For instance, SIRT1 is the primary molecular target of resveratrol and is an important regulator of many processes influencing obesity, including fatty acid oxidation, gluconeogenesis, adipogenesis, insulin secretion, and glucose and cholesterol homeostasis. SIRT1 and AMPK activation and NF-κβ suppression appear to be the common signatures of the potential beneficial effects of resveratrol in models of obesity (Sun et al. 2014). Adipocyte mitochondrial function is impaired in obesity and SIRT1 plays an essential role in the ability of moderate doses of resveratrol to stimulate AMPK and improve mitochondrial function. Price et al. 2012have shown that resveratrol dosage is a critical factor both in vitro and in vivo and the mechanism by which resveratrol activates AMPK is highly dose dependent. Interestingly, at a low dose, the ability of resveratrol to activate AMPK was blocked by SIRT1 knockout but at a dose that was 10 times higher, activation of AMPK occurred in the SIRT1 knockout mouse (Price et al. 2012). AMPK in the dephosphorylated state is a key signaling compound required for adipogenesis and resveratrol was also found to increase the phosphorylation of AMPK in a dose-dependent fashion. Thus, enhancing the phosphorylation state of AMPK by resveratrol affects the down-regulation of PPARγ expression and the inhibition of the differentiation of pre-adipocytes into mature fat cells (Tang et al. 2014). Furthermore, resveratrol induces apoptosis of adipocytes, decreases their proliferation and causes cell cycle arrest at a concentration range 10 to 100 μM (Kwon et al. 2012). At these concentrations, resveratrol also inhibits preadipocyte differentiation in a dose dependent manner with a concomitant suppression of expression of PPARγ, CCAAT/enhancer binding protein α (C/EBPα), fatty acid binding protein 4 (FABP4) and other biomarker proteins [e.g., AMPK, SIRT1, FOXO1] for adipocytes (Wang et al. 2014). Resveratrol down-regulates lipogenic genes (10–100 μM) including fatty acid synthase (FAS), lipoprotein lipase (LPL), sterol regulatory element binding protein-1c (SREBP-1c) and stearoyl-CoA desaturase-1 (SCD1) (Zhang et al. 2012; Rayalam et al. 2008). Several in vitro and animal studies have examined the anti-inflammatory effects of resveratrol in obesity. At concentrations ranging from 0.1 to 10 μM, resveratrol significantly reduced NF-κβ activation and the expression and release of interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) in 3 T3-L1 adipocytes (Kang et al. 2010). Resveratrol at up to 50 μM also suppressed the expression of matrix metalloproteinase (MMP)-2 and MMP-9 and TNF-α-induced release of IL-6, monocyte chemoattractant protein-1 (MCP-1) and other adipokines in 3 T3-L1 cells (Kang et al. 2012; Wang et al. 2014).
Numerous in vitro studies have shown that resveratrol has anti-obesity properties, however these in vitro results taken together with the findings from obese animal models indicate that most responses triggered by resveratrol may be even more complex than biphasic. Resveratrol has multiple targets (Fig. 2) and their activation or inhibition depends on the doses, models studied, and timing of administration. Morover, these promising in vitro anti-obesity results need to be extensively investigated in human controlled trials. Although several studies examined the efficacy of resveratrol on obesity-related energy expenditure, fat oxidation, plasma lipid profiles, glucose hemostasis and inflammation, sporadic clinical trials have been performed to define the dose–response effects of resveratrol in relation to the activation or inhibition of specific molecular targets involved in obesity. In a randomized double-blind crossover study, Timmers et al. 2011 treated 11 healthy and obese men with placebo and 150 mg/day resveratrol for 30 days. This study revealed that cytokine signaling was significantly decreased after the intervention period and western blotting analysis of skeletal muscle indicated that resveratrol increases AMPK activity and the protein levels of SIRT1 and PGC-1α (Timmers et al. 2011). In contrast to these findings, Poulsen et al. 2013a; b. reported that a high-dose (500 mg for 4 weeks) of resveratrol supplementation in 24 obese men did not affect gene expression for a panel of pivotal metabolic and inflammatory biomarkers. More importantly, resveratrol failed to activate AMPK and no significant effect was recorded on blood pressure, resting energy expenditure, and oxidation rates of lipid, ectopic, and visceral fat content (Poulsen et al. 2013a). Consistent with these results Yoshino et al. 2013., conducted a randomized, double-blind, placebo-controlled trial to evaluate the metabolic effects of 12 weeks of resveratrol supplementation (75 mg/day) in non-obese, postmenopausal women with normal glucose tolerance. This sudy showed that resveratrol did not affect its putative molecular targets, including AMPK, SIRT1, and PGC-1α, in either skeletal muscle or adipose tissue (Yoshino et al. 2013). Regarding cardiovascular effects and anti-inflammatory potential of resveratrol, Tomé-Carneiro et al. 2013 demonstrated that chronic daily consumption of resveratrol may exert cardiovascular benefits in stable coronary artery disease patients. Interestingly, 75 participants were assigned to placebo, low-, or high dose (8 mg daily) resveratrol groups and resveratrol supplementation in the high-dose group significantly decreased adiponectin, TNF-α, and IL-6/IL-10 ratio (Tomé-Carneiro et al. 2013). Human safety studies indicate that high doses of resveratrol as well as chronic exposure to lower doses have not induced adverse effects, but few clinical trials have been undertaken to date and detailed dose–response analyses in clinical settings are essential to consider resveratrol supplementation as an effective nutritional intervention in metabolically abnormal individuals.
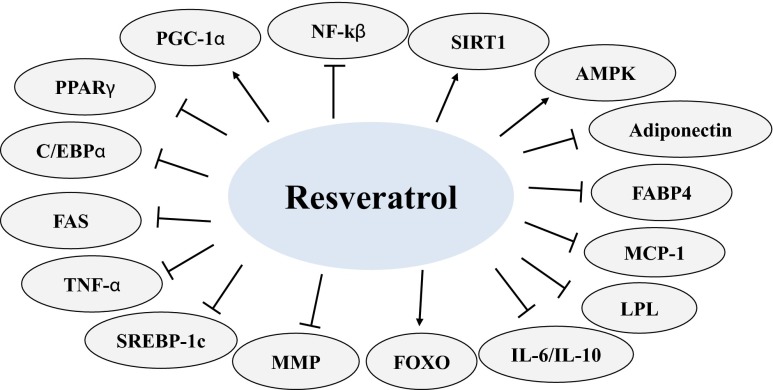
Fig. 2.
Some of the main molecular targets modulated by resveratrol in obesity models. For purposes of simplicity, this figure do not differentiate results based on the dose administered, and highlight only those studies where resveratrol was tested as an anti-obesity agent. Abbreviations: Nuclear factor- kβ (NF-kβ); sirtuin-1 (SIRT-1); adenosine monophosphate activated protein kinase (AMPK); fatty acid binding protein 4 (FABP4); monocyte chemoattractant protein-1 (MCP-1); lipoprotein lipase (LPL); interleukin (IL)-; forkhead Box O (FOXO); metalloproteinase (MMP); sterol regulatory element binding protein-1c (SREBP-1c); tumor necrosis factor-alpha (TNF-α); fatty acid synthase (FAS); CCAAT/enhancer binding protein α (C/EBPα); peroxisome proliferator-activated receptor-γ (PPARγ); peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)
Conclusions
Obesity is associated with various diseases such as diabetes, hypercholesterolemia, hyperlipidemia or metabolic syndrome. Currently, obesity and its related diseases have become a major health problem throughout the world and is increasing in both prevalence and severity. In spite of serious efforts to treat and prevent obesity and its related diseases, an ideal solution for obesity has not been developed. Considering efficacy and side effects, current pharmaceutical drugs do not provide a solution for obesity and its related diseases so that only 6 % of obese patients are treated pharmacologically. Obesity is a strong risk factor for type 2 diabetes and appears generally in subjects developing β cell dysfunction and death in response to metabolic and inflammatory stresses. However, about half of obese individuals do not develop diabetes, due to efficient long-term adaptation to insulin resistance by increasing β cell mass and insulin secretion. In these resistant individuals, β cells may develop adaptive stress responses to prevent their loss, at least transiently. Converging evidence suggests that stresses can induce specific responses rendering β cells more resistant to the stress-molecule, or even to other toxins. Thus, β cells possess hormetic mechanisms in response to inflammatory and metabolic stresses. Stressors are not merely toxic; they can also prime the stressed cell to future pathogenic challenges by rendering them more resistant. Mitochondrial adaptation or mitohormesis, originally described the hypothetical model of cell preservation in response to ROS-induced stresses originating from mitochondria. In agreement with this model, calorie restriction extends life span in different organisms by increasing mitochondrial ROS production. In pancreatic β cells, the mitohormetic response is suggested by adaptation to dietary fat-induced insulin resistance attributed to increased mitochondrial function, an effect correlated with elevated ROS levels secondary to fatty acid treatment of insulin-secreting cells. The ROS-induced endogenous H2O2 generation is accompanied by increased expression of genes participating to recovery of mitochondrial function, detoxification, and cell survival.
Numerous studies have reported a remarkable ability of resveratrol to inhibit weight gain, improve endothelial function, and reduce inflammatory responses, representing a promising candidate to manage obesity and obesity-induced secondary sequels (Wong et al. 2011). Hormesis can account for numerous types of adaptive responses such as pre-conditioning and post-conditioning in a broad spectrum of biomedical models, as well as for adaptive responses to radiation exposure. Resveratrol causes a plethora of biological responses in cells and organisms that involve a spectrum of adaptive mechanisms, suggesting that such responses may be mediated by hormetic mechanisms. Resveratrol induces hormetic dose responses in a wide range of biological models, affecting numerous endpoints of biomedical and therapeutic significance.
Society is still generally guided by the assumption that the nature of the dose response follows a threshold model with respect to non-cancerous outcomes or a linear model for carcinogens. In these instances, the guidance that can be offered is rather straight forward. In the case of threshold-acting agents, the goal has been to keep the exposure below the toxic threshold with an adequate margin of safety. In the case of carcinogens, in which risk has been assumed to be proportionate to dose, the lower the exposure the better. However, voluminous findings over the past decade have revealed that these standard dose–response models fail to make accurate predictions in the low-dose zone, the zone where people tend to live. However, the hormetic dose response has been shown to commonly occur and to make far more accurate predictions of biological responses in the low zone than either the threshold or linear models. The key challenge for the biomedical and regulatory communities is to take advantage of these developments in the area of dose–response biology in terms of how to improve study designs so as not to miss important low-dose effects of a beneficial or undesirable nature, and how to use the information provided by the hormetic framework to improve the preclinical and clinical testing of chemotherapeutic and chemopreventive agents and to enhance the likelihood of ‘getting the dose right.’
Abbreviations
- (CR)
Calorie restriction
- (WAT)
White adipose tissue
- (NF-kβ)
Nuclear factor- kβ
- (SIRT-1)
Sirtuin-1
- (AMPK)
Adenosine monophosphate activated protein kinase
- (FABP4)
Fatty acid binding protein 4
- (MCP-1)
Monocyte chemoattractant protein-1
- (LPL)
Lipoprotein lipase
- (IL)-
Interleukin
- (FOXO)
Forkhead Box O
- (MMP)
Metalloproteinase
- (SREBP-1c)
Sterol regulatory element binding protein-1c
- (TNF-α)
Tumor necrosis factor-alpha
- (FAS)
Fatty acid synthase
- (C/EBPα)
CCAAT/enhancer binding protein α
- (PPARγ)
Peroxisome proliferator-activated receptor-γ
- (PGC-1α)
Peroxisome proliferator-activated receptor γ coactivator-1α.
References
- Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53:7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clin Chim Acta. 2005;360:9–26. doi: 10.1016/j.cccn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Barger JL. An adipocentric perspective of resveratrol as a calorie restriction mimetic. Ann N Y Acad Sci. 2013;1290:122–129. doi: 10.1111/nyas.12212. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrajón-Catalán E, Herranz-López M, Joven J, Segura-Carretero A, Alonso-Villaverde C, Menéndez JA, Micol V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: far beyond their antioxidant properties. Adv Exp Med Biol. 2014;824:141–159. doi: 10.1007/978-3-319-07320-0_11. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn GL, Miller D, Chan S. Pharmaceutical treatment of obesity. Nurs Clin North Am. 1997;32:831–838. [PubMed] [Google Scholar]
- Bracale R, Petroni ML, Davinelli S, Bracale U, Scapagnini G, Carruba MO, Nisoli E. Muscle uncoupling protein 3 expression is unchanged by chronic ephedrine/caffeine treatment: results of a double blind, randomised clinical trial in morbidly obese females. PLoS ONE. 2014;9:e98244. doi: 10.1371/journal.pone.0098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP, Calabrese V. Dose response biology: the case of resveratrol. Hum Exp Toxicol. 2010;29:1034–1037. doi: 10.1177/0960327110383641. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta. 2012;1822:753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, Maciag A, Puca AA, Vecchione C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Catterall F, Souquet JM, Cheynier V, de Pascual-Teresa S, Santos-Buelga C, Clifford MN, Ioannides C. Differential modulation of the genotoxicity of food carcinogens by naturally occurring monomeric and dimeric polyphenolics. Environ Mol Mutagen. 2000;35:86–98. doi: 10.1002/(SICI)1098-2280(2000)35:2<86::AID-EM3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediat Inflamm. 2013;2013:136584. doi: 10.1155/2013/136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Zorita S, Fernández-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013;29:1374–1380. doi: 10.1016/j.nut.2013.04.014. [DOI] [PubMed] [Google Scholar]
- González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacol Res. 2011;64:438–455. doi: 10.1016/j.phrs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Heng W, Yuan A, Baolin L, Fang H. Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: Relative to inhibition of inflammatory responses. Biochimie. 2010;92:789–796. doi: 10.1016/j.biochi.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3 T3-L1 preadipocytes. Nutr Res Pract. 2012;6:499–504. doi: 10.4162/nrp.2012.6.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Seo SG, Yue S, Cheng JX, Lee KW, Kim KH. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutr Res. 2012;32:607–616. doi: 10.1016/j.nutres.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Lee J, Jo DG, Park D, Chung HY, Mattson MP. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev. 2014;66:815–868. doi: 10.1124/pr.113.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mennen LI, Walker R, Bennetau-Pelissero C, Scalbert A. Risks and safety of polyphenol consumption. Am J Clin Nutr. 2005;81:326S–329S. doi: 10.1093/ajcn/81.1.326S. [DOI] [PubMed] [Google Scholar]
- Naderali EK. Obesity and cardiovascular dysfunction: a role for resveratrol? Obes Res Clin Pract. 2009;3:45–52. doi: 10.1016/j.orcp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Panickar KS. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol Nutr Food Res. 2013;57:34–47. doi: 10.1002/mnfr.201200431. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Jørgensen JO, Jessen N, Richelsen B, Pedersen SB. Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann N Y Acad Sci. 2013;1290:74–82. doi: 10.1111/nyas.12141. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3 T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li J, Xiao N, Wang M, Kou J, Qi L, Huang F, Liu B, Liu K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol Res. 2014;89:19–28. doi: 10.1016/j.phrs.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Tang PC, Ng YF, Ho S, Gyda M, Chan SW (2014) Resveratrol and cardiovascular health - Promising therapeutic or hopeless illusion? Pharmacol Res pii:S1043-6618(14)00138-8 [DOI] [PubMed]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA, García-Conesa MT, Espín JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman NS, Friedberg B. Causes and consequences of adult obesity: health, social and economic impacts in the United States. Asia Pac J Clin Nutr Suppl. 2002;8:705–709. doi: 10.1046/j.1440-6047.11.s8.6.x. [DOI] [Google Scholar]
- Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2013;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3 T3-L1 adipocytes differentiation. Nutr Res Pract. 2012;6:286–293. doi: 10.4162/nrp.2012.6.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]