Abstract
Aging process is accompanied by hormonal changes characterized by an imbalance between catabolic hormones, such as cortisol and thyroid hormones which remain stable and hormones with anabolic effects (testosterone, insulin like growth factor-1 (IGF-1) and dehydroepiandrosterone sulphate (DHEAS), that decrease with age. Deficiencies in multiple anabolic hormones have been shown to predict health status and longevity in older persons.
Unlike female menopause, which is accompanied by an abrupt and permanent cessation of ovarian function (both folliculogenesis and estradiol production), male aging does not result in either cessation of testosterone production nor infertility. Although the circulating serum testosterone concentration does decline with aging, in most men this decrease is small, resulting in levels that are generally within the normal range. Hormone therapy (HT) trials have caused both apprehension and confusion about the overall risks and benefits associated with HT treatment. Stress-response hormesis from a molecular genetic perspective corresponds to the induction by stressors of an adaptive, defensive response, particularly through alteration of gene expression. Increased longevity can be associated with greater resistance to a range of stressors. During aging, a gradual decline in potency of the heat shock response occur and this may prevent repair of protein damage. Conversely, thermal stress or pharmacological agents capable of inducing stress responses, by promoting increased expression of heat-shock proteins, confer protection against denaturation of proteins and restoration of proteome function. If induction of stress resistance increases life span and hormesis induces stress resistance, hormesis most likely result in increased life span. Hormesis describes an adaptive response to continuous cellular stresses, representing a phenomenon where exposure to a mild stressor confers resistance to subsequent, otherwise harmful, conditions of increased stress. This biphasic dose–response relationship, displaying low-dose stimulation and a high-dose inhibition, as adaptive response to detrimental lifestyle factors determines the extent of protection from progression to metabolic diseases such as diabetes and more in general to hormonal dysregulation and age-related pathologies. Integrated responses exist to detect and control diverse forms of stress. This is accomplished by a complex network of the so-called longevity assurance processes, which are composed of several genes termed vitagenes. Vitagenes encode for heat shock proteins (Hsps), thioredoxin and sirtuin protein systems. Nutritional antioxidants, have recently been demonstrated to be neuroprotective through the activation of hormetic pathways under control of Vitagene protein network. Here we focus on possible signaling mechanisms involved in the activation of vitagenes resulting in enhanced defense against functional defects leading to degeneration and cell death with consequent impact on longevity processes.
Keywords: Hormesis, Hormones, Aging, Heme oxygenase, Vitagenes
Introduction
A critical key to successful medical intervention is getting the dose right, which can be extremely challenging due to human inter-individual variation as affected by age, hormonal balance, as well as nutritional, life style and genetic factors (Calabrese 2013; Hunt et al. 2011). Theophrastus Bombastus von Hohenheim, a Swiss pharmacist born in 1493 also named Paracelsus, developed this revolutionary idea at the Renaissance period: “Alle Ding’ sind Gift, und nichts ohn’ Gift; allein die Dosis macht, daß ein Ding kein Gift ist” freely translated to “the dose makes the poison.” Five centuries later, this notion has been extended to the so-called hormesis. Hormesis is a phenomenon whereby exposure of cells or organs to low levels of a given toxin confers resistance to subsequent contacts to higher concentrations, as such describing an adaptive response to continuous cellular stresses, representing specifically a biphasic dose–response relationship. Hormesis can be viewed as adaptive response to detrimental lifestyle factors and thus determining the extent of protection from progression to metabolic diseases, such as diabetes and more in general to hormonal dysregulation and age-related pathologies (Calabrese et al. 2010; Currò et al. 2014; Davinelli et al. 2014; Cornelius et al. 2014; Mancuso et al. 2013). Hormesis is graphically represented by either an inverted U-shaped dose response or by a J- or U-shaped dose response. The concept of hormesis is central for the biomedical sciences because of its generalizability (Calabrese et al. 2011, 2012, 2013). Hormetic dose responses are independent of biological model, endpoint as well as chemical class and physical agent. The hormetic dose response model, as found in multiple studies with many thousands of dose responses to make far more accurate predicts of low dose responses, describe the fundamental features of several dozen receptor systems, affecting a vast array of biological endpoints (Calabrese et al. 2013). The concepts of pre-conditioning and post-conditioning are also manifestations of hormesis. When these two highly significant and general concepts are analyzed within the dose response framework they conform to the features of the hormetic dose response (Calabrese et al. 2010). Preconditioning and postconditioning are considered specialized forms of hormesis, based on several hundred reports in the biomedical literature in which biphasic dose responses are induced by the range of conditioning doses employed within pre-postconditioning experimental protocols. The quantitative features of the biphasic dose responses for pre- and postconditioning protocols are similar to hormetic dose responses that do not use a pre- and postconditioning experimental framework. That is, the maximum stimulatory response is modest and is usually only about 30–60 % greater than the control group. Additional research on the temporal features and effects indicates that optimized induced effects by the conditioning doses (i.e. prior to the second and more massive exposure) are the same optimized dose for the final preconditioning effect. This demonstrates a temporal linkage between the preconditioning induced response and the subsequent response following the more massive exposure. Furthermore, the blocking of the preconditioning response via an antagonist/pathway inhibitor prior to administering the second and more massive exposure in a preconditioning protocol blocks the preconditioning response, thereby providing a direct mechanistic linkage between the two temporal events. These converging lines of evidence strongly support the conclusion that pre-and postconditioning are manifestations of hormesis. Quantitative features of the hormetic dose response are, importantly, similar across biological models and endpoints. This remarkable general feature of hormetic dose response suggests that the hormetic dose response may represent the first comprehensively based quantitative estimation of biological plasticity. Hence, hormetic dose response provides a quantitative estimate of biological performance, such as the extent to which memory drugs can enhance learning. This concept can be extended to all other areas of biological performance such as bone strengthening, hair growth, decreases in anxiety, plant growth and productivity and others. Of particular relevance to the current paper is the concept that hormesis can be used to understand the biological limits within which pharmaceutical efforts are made to enhance the quality of aging with relevant impact on longevity. For example, the hormesis concept argues that the biological performance can only be modestly improved in all biological systems in most situations. The term modestly is quantitatively constrained to be less than two fold, with most maximal increases being within the 30–60 % range. Such biological constraints are of vital importance to pharmaceutical companies and regulatory agencies as they develop strategies for product development and evaluation. This also suggests that attempts to extend life would also be constrained within the bounds of plasticity, which are quantitatively estimated by the hormetic dose response. Thus, if the normal bounds of human longevity are seen to be approximately as about 100 years, the hormesis concept predicts that it may be possible to extend the human lifespan by 30–60 years at most. The 60 year extension being the upper bound of the hormetic optima, a notion consistent with prediction of evolutionary biology even if theoretical for humans. Hormesis is a highly relevant concept also for hormones since their ability to cause biphasic proliferative responses (Brandes 2005). This is particularly evident for estrogens, but there are also several examples of progestogens hormesis (Inagaki et al. 2010). A better understanding of the hormesis phenomenon may result in an improved future designs of studies involving the use of hormones. Furthermore, hormetic response promotes regulatory changes important in aging processes including increases in stress hormones.
The principle of stress-response hormesis can be seen in action in many contexts. As a classical example, low levels of insecticides can induce chemical resistance by increasing xenobiotic detoxification (Calabrese et al. 2006). Various other phenomena involve stress-response hormesis, though they are not typically described as such. For example, induction of drug metabolizing enzymes by xenobiotic chemicals can provide protection against carcinogenesis, consistent with the notion of chemoprotection and/or chemoprevention (Talalay et al. 2003), and innate and acquired immunity involves pathogen-stimulated resistance. Hormesis is well illustrated by ischemic preconditioning, a situation where short ischemic episodes protect brain and heart from prolonged lack of oxygen and nutrients. Regarding CNS or vulnerable endocrine cells, such as pancreatic or sex hormone cells emerging concepts suggest that efficiency of hormetic responses to detrimental lifestyle factors might set the level of protection, impacting on the progression of age related pathologies. In addition, mitochondrial adaptation and hormesis, or mitohormesis, originally referred to the hypothetical model of cell preservation in response to ROS-induced stresses originating from mitochondria (Calabrese et al. 2010). The concept was substantiated by findings in nematode which revealed how glucose restriction activates mitochondria and ROS formation, thus promoting hormetic extension of life span. In conflict with Harman’s free radical theory of aging, protective effects depend on mitochondrial ROS formation inducing an adaptive response, in turn conferring increased stress-resistance. This might ultimately give rise to long-term cell preservation. In agreement with this model, calorie restriction extend life span in different organisms by increasing mitochondrial ROS production (Calabrese et al. 2010). The ROS-induced endogenous H2O2 generation contributes to prolongation of oxidative attacks days after exposure to exogenous H2O2. This is accompanied by increased expression of genes participating to recovery of mitochondrial function, detoxification, and cell survival stress responsive genes called vitagenes. Vitagenes encode for heat shock proteins (Hsps), thioredoxin and sirtuin protein systems (Cornelius et al. 2013). Nutritional antioxidants, have recently been demonstrated to be neuroprotective through the activation of hormetic pathways under control of Vitagene protein network.
Sex hormones and aging
For centuries, the importance of the testes for the maintenance of male phenotype, psychosexual behavior, and physical power has been recognized. Indeed, testicular extracts have been used for the treatment of impotence and, even during the early part of the last century, castration was used as a punishment for sexual offenders. Brown-Sequard, considered by many the Father of Andrology, was so convinced that the age-associated decline in sexual function and frailty was a result of decreased production of “a chemical” from the testes, that in 1885, he self-injected aqueous extracts of guinea pig and dog testes and reported an improvement in his virility and well-being at the Société de Biologie de Paris meeting in 1889 (Brown-Sequard 1889; Basaria 2013). Although the improvements that Brown-Sequard experienced were due to the “placebo effect,” his work ultimately led to the isolation of testosterone, the major testicular androgen, by Butennandt and colleagues almost half a century later (Butenandt et al. 1960). Unlike female menopause, which is accompanied by an abrupt and permanent cessation of ovarian function (both folliculogenesis and estradiol production), male aging does not result in either cessation of testosterone production nor infertility. Although the circulating serum testosterone concentration does decline with aging, in most men this decrease is small, resulting in levels that are generally within the normal range. However, testosterone deficiency does occur in some aging men resulting in symptoms that are reminiscent of “organic hypogonadism,” such as caused by testicular or hypothalamic-pituitary disease. Age-related hypogonadism has been referred to as andropause, viropause, partial androgen deficiency of the aging male, and late-onset hypogonadism (LOH), with LOH considered to be the most suitable term for this condition (Handelsman 2004; Basaria et al. 2001). Although symptoms such as sexual dysfunction, muscle weakness, loss of bone mass, fatigue, cognitive decline, and mood changes are seen in organic hypogonadism, they also occur as part-and-parcel of aging. The similarities between organic hypogonadism and LOH have resulted in a growing interest in testosterone replacement therapy in aging men (Handelsman 2004). This interest is mainly driven by the following 2 factors: (i) lack of understanding among clinicians to differentiate LOH from organic hypogonadism (that may occur at any age), and (ii) the robust marketing campaign of testosterone products by the industry toward physicians and the general public. This campaign has been effective because the marketing volume of testosterone preparations has increased more than 15-fold over the past 2 decades. A large randomized controlled efficacy trial, the T-Trial, is underway and should provide valuable data on the efficacy (and risks) of testosterone replacement in LOH (Handelsman 2004; Basaria et al. 2001).
Androgen physiology in Men
Testosterone is the predominant and biologically most important circulating androgen in men and is secreted almost exclusively by the testes. The other androgens include dihydrotestosterone, androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS). Most dihydrotestosterone (80 %) is derived from 5-a reduction of testosterone in peripheral tissues (by the enzyme 5-a reductase), whereas the remaining 20 % is secreted directly from the testes. Approximately 85 % of serum androstenedione levels are contributed equally by the testes and the adrenal glands, whereas the remainder originates from peripheral conversion of testosterone (via 17b-hydroxysteroid dehydrogenase) and DHEA (via 3b-hydroxysteroid dehydrogenase). Both DHEA and DHEAS are produced almost exclusively from the adrenal glands. Testosterone is largely bound to plasma proteins with approximately 40 % loosely bound to albumin and 58 % tightly bound to the sex hormone binding globulin (SHBG), which leaves only 1 % to 2 % of testosterone unbound, the moiety considered to be “biologically active.” The free testosterone diffuses passively through lipophilic cell membranes into the target cell, where it binds to the androgen receptor and is carried to the nucleus, where it binds to androgen response elements in the DNA to initiate transcription. Due to loose binding, the albumin-bound testosterone dissociates during tissue transit, whereas SHBG-bound testosterone does not. Therefore, the combination of free and albumin-bound testosterone (non-SHBG-bound testosterone) is referred to as the “bioavailable testosterone.” Dihydrotestosterone also exerts its effects via binding to the androgen receptor, whereas androstenedione, DHEA, and DHEAS do not bind substantially to the androgen receptor as they exert their effects via conversion to testosterone (Basaria 2013).
In healthy adult men, rate of testosterone production ranges between 3 and 10 mg/d, which approximately translates into serum levels of 300 to 1,000 ng/dL. Testosterone is secreted in a circadian fashion with the highest levels occurring in the morning and nadir levels seen in the evening. It has been known for more than half a century that testosterone levels decline with age, when reduced testosterone concentrations were found in the spermatic veins of elderly men (compared with young men). Since then, both cross-sectional and longitudinal studies have confirmed an age-related decline in serum testosterone levels.
Although total testosterone levels are lower in older men, there is no clear age-based inflection point at which there is an abrupt cessation of testosterone production. Longitudinal studies have confirmed that testosterone levels peak in the second and the third decade of life and then decline gradually throughout life (at a rate of 1 %–1.5 % per year).
This age-related decline in serum testosterone is due to a decreased rate of production, which is significant considering that the rate of metabolic clearance of testosterone also decreases with aging. At the same time as testosterone levels are declining, there is an age associated increase in serum SHBG levels (1.0 % per year), resulting in an even steeper decline in free and bioavailable fractions (2 %–3 % per year). Indeed, studies show that at age 75 years, mean total serum testosterone level is about two-thirds of the level at age 25, whereas mean serum FT level is only about one-half of that in young men.
In the Baltimore Longitudinal Study of Aging (BLSA), 19, 28, and 49 % of men older than 60, 70, and 80 years of age, respectively, had total testosterone levels below the young reference range (325 ng/dL), whereas 34, 68, and 91 % had subnormal free testosterone index. The trajectory of age-related decline of testosterone is affected by adiposity, comorbid conditions, medications, and some genetic factors.
The European Male Aging Study (EMAS) is the first population study that has proposed a “syndromic” definition of LOH whereby a constellation of symptoms are associated with androgen deficiency. In EMAS, symptoms of poor morning erections, low sexual desire, and erectile dysfunction were associated with total testosterone levels less than 317 ng/dL (Wang et al. 2008).
Contrary to the decline in sex hormones, a man remains fertile throughout his entire post-pubertal life. However, the testicular volume of an older man is lower compared with a young man, reflecting some degree of tubular atrophy. Similarly, there is some decline in the volume of seminal fluid and sperm motility (Basaria 2013; Handelsman 2004).
Hormone-replacement therapy (HRT): advantages and limitations
Hormone-replacement therapy remains the most widely applied anti-aging endocrine therapy in use today. The most commonly used hormones are growth hormone (GH), androgen, estrogen and progesterone. For simplicity and to document potential pharmacological differences, various steroid hormones used for hormone therapy (HT) can be divided into four groups: 1) natural (class A); 2) native to the body and synthesized from natural precursors (class B); 3) native to the body and synthesized from nonsteroidal precursors (class C); and 4) synthetic and not native to the body (class D).
The steroids in class A are found in nature and are formulated into drugs without undergoing any chemical modifications. For example, conjugated equine estrogens are estrogens in the form of sulfate esters, which are simply extracted from pregnant mare’s urine and do not undergo chemical modification. Approximately 50 % of conjugated equine estrogens consist of estrone sulfate, and the remaining approximate 50 % consists of equine estrogens. Equine estrogens are native to the horse but not the human. The class B steroids are semisynthetic. They are steroids that exist in nature and are biosynthesized by the human body, but for these to be formulated as therapeutic agents, they need to be chemically synthesized from a natural starting material, most commonly from a plant source such as the Mexican yam and soybean. These plants contain sterols such as diosgenin and stigmasterol, which are used as precursors for the synthesis of a variety of steroids. Essentially, steroid hormones such as estradiol, estrone, estriol, progesterone, dehydroepiandrosterone, androstenedione, testosterone, cortisol, aldosterone, synthetic conjugated estrogens, etc., have been chemically synthesized from these sources for decades. Contrary to many claims, it is important to note that ingestion of the Mexican yam or soybean does not result in the formation of any of the above steroids because the human body lacks the enzymes needed to convert diosgenin or stigmasterol to those steroids. The chemical synthesis of a steroid hormone such as estradiol from diosgenin requires at least 15 reactions. Another misconception pertains to the occurrence of mammalian steroid hormones in plants. It is only recently that some steroid hormones such as progesterone and androstenedione have been positively identified in plants, using rigorous assay methodology. However, their concentrations are very low. There are no hormonal preparations on the market that are derived by extraction of a steroid hormone from a plant.
It is important to recognize that on a biomolecular level steroids synthesized from plant precursors such as diosgenin by the semisynthetic process are not actually identical to the corresponding endogenous steroids in the human body. There are two naturally occurring isotopes of carbon, 12C and 13C, in the carbons that comprise the chemical structure of steroids in plants and humans. Studies show that semisynthetic steroids consist of a different 13C/12Cratio, when compared with the corresponding human endogenous compounds (Bhavnani and Stanczyk 2012). This observation is based on the fact that endogenous steroid hormones reflect an average of the 12C and 13C isotopes of carbon from vegetal and animal food eaten by humans, whereas plant sterols such as those found in soy exhibit a fixed 13C/12C ratio. However, from a physiological standpoint, there is no evidence showing that there is any difference between a semisynthetic steroid and its corresponding endogenous form in the human. The steroids in the class C group are “synthetic” compounds that exist in nature but are synthesized from simple nonsteroidal starting materials by a process generally called “total synthesis.” This is one of the oldest means by which steroid hormones were first synthesized. Examples include estrone, estradiol, equilenin, progesterone, among others. Without rigorous analytical analysis, it is extremely difficult to differentiate between class B and class C steroids. However, there can be significant differences in both the chemical and biological properties of the steroids made by these two distinct methods. In total synthesis, there is one fundamental requirement and, that is, the steroid synthesized has to have the same precise stereochemistry as that of the naturally occurring hormone. It is only then that the synthesized hormone will have the same biological activity. It is obligatory that the very specific three-dimensional structure be the same for a compound to behave as an agonist because the activity of the compound is expressed by its interaction with its specific endogenous receptor. Unfortunately, total synthesis can give rise to a number of isomers; for example, in the total synthesis of estrone, there are eight possible racemates (16 isomers). Only one of these is the natural hormone; others have different physical properties, and some of them are totally inactive. Because estrogen preparations compounded by pharmacists do not undergo any rigorous testing or approval by regulatory bodies, it is very possible that some mixtures, depending on the route of synthesis, may not be as potent or can be totally inactive therapeutically, as discussed later. In other words, the so-called bioidentical hormones may not be identical to the natural hormones produced in vivo by the human body. The group of class D steroids includes steroids not found in humans, animals, or plants, and includes drugs such as medroxyprogesterone acetate, ethinyl estradiol, norethindrone, norgestrel, among others.
The use of estrogen and progesterone in HRT is both popular and controversial. The Women’s Health Initiative (WHI) results suggested that a regiment of estrogen with progesterone increased the risk factor for coronary heart disease (CHD), breast cancer, stroke and pulmonary embolism, but showed significant benefit for fractures and colon cancer (Bao et al. 2014). Five years later, the WHI published a follow up study that showed that women who initiated hormone therapy closer to menopause (<10 years) tended to have reduced CHD risks as compared to those who received the therapy more distant from menopause, but the risk of stroke was elevated regardless of years since menopause. A RCT trial conducted in Denmark with 1,006 healthy women aged from 45 to 58 (mean age 50 y) confirmed the protective effect of estrogen and progesterone on CHD. The result was consistent with WHI trial within the same age range, which indicated that women receiving hormone replacement therapy early after menopause had a significantly reduced risk of mortality, heart failure, or myocardial infarction. Nonetheless, the results from the Million Women Study, Collaborative Group on Hormonal Factors in Breast Cancer Study and Finland Case Control Study suggested an increased risk of breast cancer and endometrial cancer in women using HRT (Bao et al. 2014). HRT can ameliorate age-related diseases such as osteoporosis, decrease fracture and colon cancer. However, it also increases the risk of breast cancer, endometrial cancer, stroke and venous thrombo-embolism, and shows time-dependent safety-risk effect of coronary heart disease. It is suggested that for the application of estrogen and progesterone treatment in HRT, it is important to use them at the lowest possible dose that alleviates symptoms, for the shortest time period needed, and only by prescription. Although patients have described “feeling good” after HRT, and most of the long-term controlled randomized studies and case control studies are convincing, the objective data suggested only limited benefits when balanced with the many risks identified. This general strategy is not advised in anti-aging therapy.
Misconception and concerns about bioidentical hormones used for custom-compounded hormone therapy
Hormone therapy (HT) trials have caused both apprehension and confusion about the overall risks and benefits associated with HT treatment (Clark 2006). In this recent period patient and clinician interest in potential alternatives to conventional HT has grown immensely and continues to grow (Bhavnani and Stanczyk 2012). This appears to be particularly true for products or regimens that claim to have fewer risks and side effects than commercially available HT preparations. One commonly used alternative HT involves customcompounded hormone preparations. The hormones in these preparations include estrogen [17β-estradiol (estradiol), estrone, and/or estriol], progesterone, testosterone, androstenedione, and dehydroepiandrosterone. The products can be prepared in individualized dosages and forms such as creams, gels, lotions, sublingual tablets, troches (lozenges) for buccal administration, and suppositories by compounding pharmacies from a clinician’s prescription. They are often touted by advocates as safer than commercially prepared HT products. Proponents also claim that custom-compounded HT is associated with fewer side effects and may provide better symptom relief than conventional HT because the hormones used in the preparations are “bioidentical,” i.e. identical to those made in the body. The majority of these claims are unsubstantiated, and no rigorous randomized control trials have been carried out to support any of the claims (Bhavnani and Stanczyk 2012).
Because the custom-compounding of HT products is not regulated, a particular concern is that some patients may be overdosed, or treated with ineffective products, or subject to unidentified risk. Here we present 2 major concerns about bioidentical hormones: 1) the so-called “bioidentical” hormones in custom-compounded HT preparations may not be identical to those made in the body; and 2) the lack of regulation of these hormone products can cause adverse effects in postmenopausal women using them. The U.S. Food and Drug Administration (FDA) regards the use of the terminology “bioidentical hormone” as a marketing ploy, implying a benefit for a drug for which there is no medical or scientific basis (FDA Consumer Health Information). Although drug compounding is generally subject to FDA oversight, the agency relies on states to regulate the practice as part of their overall regulation of the practice of pharmacy. However, the ability of states to oversee the quality and safety of compounded drugs is influenced and limited by the availability of resources for standard inspections and enforcement. A major concern about the custom-compounded products is that the practice of drug compounding is not subject to the same degree of regulations and oversight by the FDA and by regulatory authorities in other countries, which is required for the commercial production and marketing of prescription medicines. Unlike commercially available drugs, compounded medicines are not tested routinely by any regulatory agency for quality, purity and potency. In addition, there are no product labeling requirements for the custom formulations. This differs from commercially available drugs, which are required to be sold with a package insert that details the product’s indications for use, contraindications, pharmacokinetics, and adverse events, using language approved by regulatory authorities. Health care providers need to diligently consider the scientific evidence to determine the safety and efficacy of all hormonal preparations used for HT. Use of the misleading term “bioidentical hormone” is inappropriate, and its use should be discouraged (Bhavnani and Stanczyk 2012). Aging represents an important health issue not only for the individual, but also for society in general. Burdens associated with aging are expanding as longevity increases. This has led to an enhanced focus on issues related to aging and age-related diseases. Until recently, anti-aging endocrine-therapy has been largely limited to hormone-replacement therapy (HRT) that is associated with multiple side effects, including an increased risk of cancer. This has greatly limited the application of HRT in anti-aging therapy. Recently, the focus of anti-aging research has expanded from endocrine signaling pathways to effects on regulatory gene networks. However, newly identified genes, regulatory pathways and networks that are closely associated with endocrine biology may offer new hope for expanded anti-ageing therapy.
Xenoestrogens and phytoestrogens
Xenoestrogens (XEs) are chemicals derived from a variety of natural and anthropogenic sources that can interfere with endogenous estrogens by either mimicking or blocking their responses via non-genomic and/or genomic signaling mechanisms. Disruption of estrogens’ actions through the less-studied non-genomic pathway can alter such functional end points as cell proliferation, peptide hormone release, catecholamine transport, and apoptosis, among others. Studies of potentially adverse effects due to mixtures and to low doses of endocrine-disrupting chemicals have recently become more feasible, though few so far have included actions via the non-genomic pathway. Physiologic estrogens and XEs evoke non-monotonic, hormetic dose responses, with different compounds having different patterns of actions dependent on concentration and time, making mixture assessments all the more challenging.
Trace levels of industrial and naturally occurring chemicals have been shown to perturb endocrine systems. These endocrine disrupting chemicals (EDCs) are currently the subject of intense research and regulatory action. A large number of these EDCs act via the estrogen receptor (ER), imperfectly mimicking and interfering with the physiologic actions of endogenous estrogens (Viñas et al. 2012). Xenoestrogens (XEs) can bind to ERs in the cell nucleus, where the complex recognizes DNA response elements and alters gene expression; in the non-genomic pathway XEs can bind to membrane-bound ERs and rapidly initiate signaling cascades that culminate in kinase and phosphatase activations, ultimately influencing cellular function by post-translational modifications of a variety of proteins. Functional consequences observed at the organismal level include decreased fecundity in aquatic organisms, altered sexual behavior and memory in rats, and malformations and decreased mobility of human sperm (Viñas et al. 2012). XEs have also been implicated in the development of such chronic diseases as obesity, diabetes mellitus, asthma, and cancer.
Some plant-derived components of the diet can act as either estrogenic agonists or antagonists of mERs, depending on concentration and tissue specificity. Common sources of phytoestrogens include soy-based products such as tofu (isoflavones and their metabolites); sprouts, red clover, coumestans and flaxseed, sesame seed, or nut products such as lignans. In Asian cultures traditional culinary dishes are rich in phytoestrogens that are a major component of dietary intake. The intake of soy can be as high as 50 g a day, with measured genistein plasma concentrations from 0.1 to 10 μM. In contrast, Western diets typically have ten-fold lower concentrations. Another phytoestrogen, the stilbenoid resveratrol, present in red wine and other grape products, has emerged for its potential anti-diabetic, anti-cardiovascular disease, and cancer prevention effects, especially in cultures with a rich wine heritage. Phytoestrogens may prevent the increased risk of cancer that can occur from taking other estrogenic hormone supplements. Though high concentrations of phytoestrogens may not cause adverse effects in an adult individual, for an infant, the effects could lead to adverse developmental repercussions. Serum levels of genistein have been detected in a range of 1–10 μM in infants exclusively fed soy-based formulas. Phytoestrogens are capable of inducing MAPK signaling via the membrane ER (mERα) at doses far below or equivalent to the reported plasma concentrations achieved with even Western diets. Unless taken individually as dietary supplements, phytoestrogens are typically found in the diet as chemical mixtures. Therefore the health benefits of resveratrol, for example, could be the result of an additive effect with one of the other hundreds of “minor phenols” found in red wine, and also the grape species type that affects the wine’s chemical composition. Mixtures of phytoestrogens and their combinations with endogenous estrogens need to be examined more carefully for their beneficial (and deleterious) effects, as well as the cross-talk between genomic and non-genomic pathways. Xenoestrogens and phytoestrogens have been found to be “weak” inducers of estrogenic activity via the genomic pathway in comparison to estradiol which shows a 1,000-fold more potent activity, however, they exhibit an equipotent biological potential, in their ability to initiate rapid non-genomic responses from membrane receptors (Viñas et al. 2012). Non-genomic signaling can occur within seconds-minutes of the initial steroid-receptor contact, yet sustained activation of cell signaling can influence more permanent changes such as cell proliferation, differentiation, movement, or apoptosis. Membrane steroid receptor-mediated signals include the activation of kinases that regulate the phosphorylated states of important functional proteins, each linked to different pathways of actions.
According to the International Dose–response Society the occurrence of non-linear responses to endocrine disrupting chemicals at low concentrations (below the so-called toxic threshold) has in recent years gained increasing awareness by the scientific and regulatory community. Typical dose–response studies in regulatory testing involve in-vivo or in-vitro models exposed to high concentrations of chemicals uncommonly found in human populations or the environments to which they are exposed. Past evaluations assumed that all chemical responses follow a linear monotonic path that eventually reaches an asymptote; safe doses for humans or wildlife were then determined to be just below the lowest measurable response-causing concentrations or the no-observed-effect-level (NOEL). However, most EDCs exposures occur at low doses and exhibit non-monotonic responses that make it difficult to predict low-dose effects from high-dose effects. Furthermore, because EDCs are rarely present at concentrations that produce immediate death or illness, traditional toxicology testing is irrelevant, and in any case insufficient for understanding EDCs mechanisms. Therefore, more recent EDCs studies have begun to investigate low dose exposures focusing on very sensitive endpoints such as cell signaling or gene expression that could have dire repercussions on tissue and whole-animal functioning and health over time. EDCs are capable of initiating multiple receptor-proximal signaling cascades, responding with different rates and dose dependencies; these eventually contribute to composite response patterns of downstream phospho-activated MAPKs (i.e., pERK, pJNK, p38). Other plausible explanations for non-linear dose-responses include receptor down-regulation or desensitization, changes in receptor selectivity when going from low (selective ER binding) concentrations to high (non-selective) concentrations, the presence of co-factors or co-regulators that influence hormone-receptor binding at certain selective concentrations, and the presence of multiple receptor subtypes that bind to the same EDCs, but each with a different (stimulatory or inhibitory) response pattern. In addition, evidence exists for EDCs inducing biological effects even at very low analytically undetectable concentrations. In this case “No-threshold” responses can be related to the presence of endogenous or exogenous mimetic hormones already present in the biological milieu.
Cellular stress response, HSF biology and the vitagene network
During aging, a gradual decline in potency of the heat shock response occur and this may prevent repair of protein damage. Conversely, thermal stress or pharmacological agents capable of inducing stress responses, by promoting increased expression of heat-shock proteins, confer protection against denaturation of proteins and restoration of proteome function. If induction of stress resistance increases life span and hormesis induces stress resistance, hormesis most likely result in increased life span. Cellular stress response is the ability of a cell to counteract stressful conditions. This phenomenon, which includes heat shock response (HSR), represents an ancient and highly conserved cytoprotective mechanism (Calabrese et al. 2010). Production of heat shock proteins, including protein chaperones, is essential for the folding and repair of damaged proteins, serving thus to promote cell survival conditions that would otherwise result in apoptosis (Perluigi et al. 2010; Di Domenico et al. 2010). Besides their role during stress, chaperones have multiple roles under normal conditions, as such they promote the transport of macromolecules (e.g. proteins or RNA) and participate in remodelling events involving larger protein complexes, including signaling, transcription, cell division, migration and differentia. It is generally recognized that biological systems possess integrated responses to detect and control the various forms of stress. This is accomplished by a complex network of the so-called longevity assurance processes, which are composed of several genes termed vitagenes. Vitagenes encode for heat shock proteins (Hsps) such as Hsp70 or enzymatic protein such as gamma-glutamyl cystein liase and heme oxygenase or Hsp32, as well as thioredoxin/thioredoxin reductase and sirtuin protein systems (Calabrese et al. 2010). Vitagenes produce molecules (heat shock proteins, glutathione, bilirubin) endowed with anti-oxidant and anti-apoptotic activities (Di Paola et al. 2011; Pennisi et al. 2011; Scapagnini et al. 2011). Recent studies have shown that the heat shock response contributes to establishing a cytoprotective state in a wide variety of human diseases, including inflammation, cancer, aging and neurodegenerative disorders (Bellia et al. 2011; Siciliano et al. 2011). Given the broad cytoprotective properties of the heat shock response there is now strong interest in discovering and developing pharmacological agents capable of inducing the heat shock response (Zhang et al. 2011). Molecular chaperones are known to disrupt aggregates but also to promote active aggregation when the concentration of the aggregating protein is high. Consistent with this notion, although protein aggregation is hazardous under certain circumstances, the creation of apparently less-toxic large aggregates is protective. This hypothesis is the basis of the therapeutic potential of Hsps, which prevent protein misfolding and aggregation (Westerheide et al. 2012).
Cellular stress response is regulated at the transcriptional, translational and post-translational levels by a family of heat shock transcription factors (HSFs) that are expressed and maintained in an inactive state under non-stress conditions (Calabrese et al. 2011). HSFs, essential for all organisms to survive to acute or chronic stress, are also important for normal development and lifespan-enhancing pathways, and the repertoire of HSF targets has thus expanded well beyond the heat shock genes. Post-translational regulation of HSFs is emerging to integrate the metabolic state of the cell with stress biology, whereby controlling fundamental aspects of the health of the proteome and ageing. At the transcriptional level the heat shock response is mediated by cis-acting sequences called heat shock elements (HSEs) present in multiple copies upstream of the HSP genes (Calabrese et al. 2011). Hence, pharmacologic modulation of HSF-mediated gene regulation is an emerging area of research which is increasing its potential based on the current knowledge of small-molecule activators and inhibitors of HSFs, so that the impact of HSFs is further extending beyond the HSR, which possesses the potential for attracting growing interest (Akerfelt et al. 2012; Fujimoto et al. 2010). HSFs, like other transcription factors, are composed of functional domains which have been well characterized for HSF1. The DNA-binding domain (DBD) belongs to the family of winged helix-turn-helix DBDs. The DBD, the signature domain of HSFs for recognition of target-genes, forms a compact globular structure, except for a flexible wing or loop, located between β-strands 3 and 4, which is responsible for protein-protein interactions between adjacent subunits of the trimerized HSF, conferring high-affinity binding to DNA and mediating interactions with other factors to modulate the transactivating capacity of HSFs. The trimerization of HSFs is mediated by arrays of hydrophobic heptad repeats (HR-A and HR-B) forming coiled coil, characteristic for many Leu zippers (Fujimoto et al. 2010). Although the regulatory domain of HSF has an intrinsic competence to sense heat stress, its inducibility is modulated through posttranslational modifications (Alam and Cook 2007; Calabrese et al. 2011; Fujimoto et al. 2010). Consistent with the proposed model, HSR-1 undergoes a conformational change in response to heat stress facilitating, together with eEF1A, HSF1 trimerization. In this activation cycle, HSF1 undergoes extensive post translational modifications, such as phosphorylation, sumoylation and acetylation. While phosphorylation and sumoylation of HSF1 occur rapidly on heat shock, the kinetics of acetylation are delayed and coincide with the attenuation phase of the HSF1 activation cycle. Consistent with this notion, acetylation of HSF1 is regulated by the balance of acetylation by p300–CBP (CREB-binding protein) and deacetylation by the NAD + −dependent sirtuin, SIRT1. Increased expression and activity of SIRT1 enhances and prolongs the DNA-binding activity of HSF1 at the human Hsp70.1 promoter, whereas downregulation of SIRT1 enhances the acetylation of HSF1 and the attenuation of DNA-binding without affecting the formation of HSF1 trimers (Calabrese et al. 2011). Thus SIRT1 maintains HSF1 in a state that is competent for DNA binding by counteracting acetylation . In the light of current knowledge, the attenuation phase of the HSF1 cycle is regulated by a dual mechanism: a dependency on the levels of Hsps that feedback directly by weak interactions with HSF1, and a parallel step that involves the SIRT1-dependent control of the DNA-binding activity of HSF1. Because SIRT1 has been implicated in caloric restriction and ageing, the age-dependent loss of SIRT1 and impaired HSF1 activity correlate with an impairment of the HSR and proteostasis in senescent cells, connecting the heat shock response to nutrition and aging (Calabrese et al. 2011).
Importance of HO-1 in stress tolerance and aging
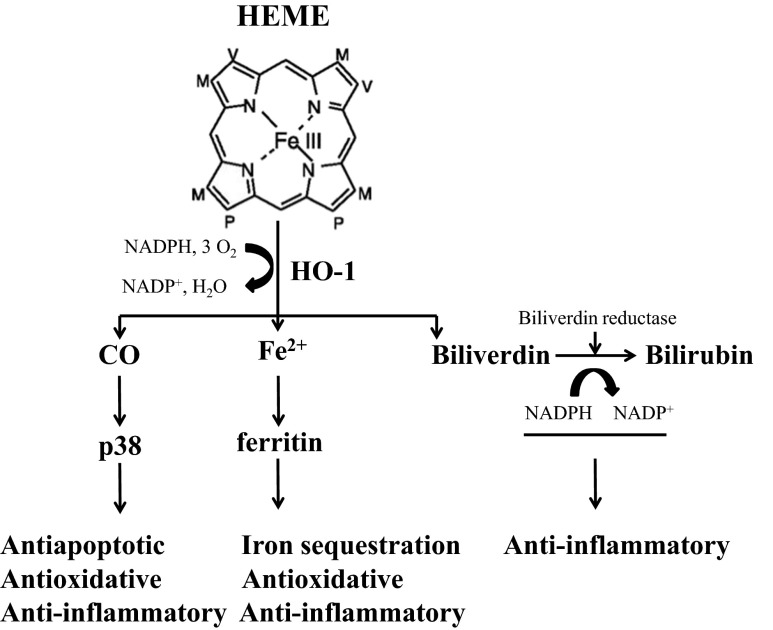
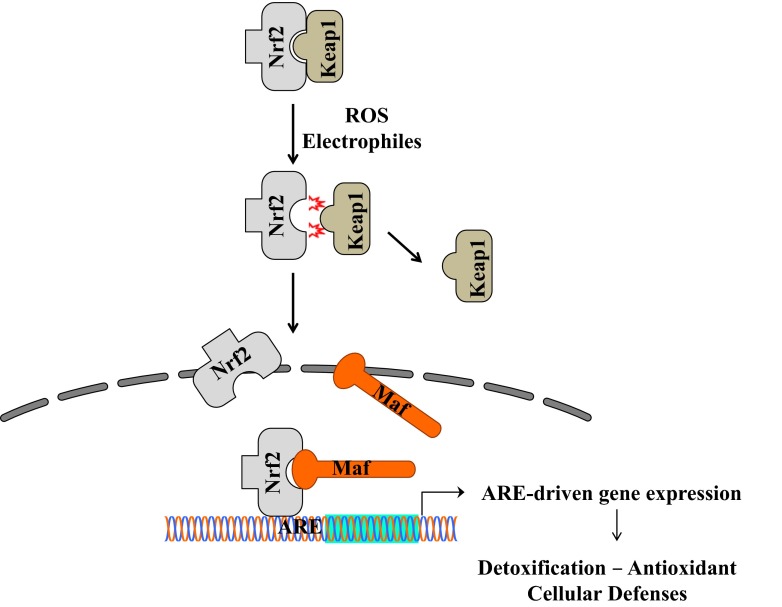
HO-1 is a 32-kDa protein considered an early stress-response agent and is involved in the degradation of heme to generate carbon monoxide (CO), biliverdin and free iron. HO-1 expression is induced in all tissues in response to multiple insults such as heme, ultraviolet light, heavy metals, cytokines, hydrogen peroxide, nitric oxide (NO), and glutathione depletion (Ryter et al. 2006). Induction of HO-1 occurs as an adaptive and beneficial response and its activity plays an important role in the pathophysiology of several disease states. In particular, the cytoprotective properties of HO-1 have taken relevance in recent years since it has been shown that HO-1 has anti-inflammatory, anti-apoptotic and anti-proliferative effects and the products of heme metabolism, bilirubin, ferritin and CO also functions as antioxidants (Morse and Choi 2002). Generally, HO-1 expression is very low in normal tissues, except for liver and spleen where it is involved in detoxification processes and in the protection against oxidative damage (Tenhunen et al. 1968). Moreover, even though in the brain there is a low basal expression of HO-1, its cerebroprotective activity can be highly up-regulated by a variety of environmental stimuli and the expression is more induced in astrocytes and microglia than in neurons (Dwyer et al. 1995; Matsuoka et al. 1999). The enzymatic products of HO-1 enzyme activity (Fig. 1) have crucial roles in the control of brain redox homeostasis and neuroinflammation and provide protection against various neurotoxic insults. However, upregulation of the HO-1 system may not be always beneficial because heme depletion, accumulation of CO and bilirubin may cause toxic effects. HO-1 is one of the main antioxidant response element (ARE)-regulated phase II detoxifying enzymes and it is regulated by the redox-sensitive transcription factor nuclear factor erythroid 2-related factor (Nrf2) (Itoh et al. 1996; Lee et al. 2005; Motohashi and Yamamoto 2004) (Fig. 2). A potential link between physiological aging and HO-1 expression has been reported in brain and liver, even though these results were contradictory depending on differences in the species used and tissues investigated (Schipper 2000; Lavrovsky et al. 2000; Ewing and Maines 2006; Patriarca et al. 2007). Recently, particular attention is given to the emerging approach for slowing down ageing by stimulating the maintenance and repair systems through hormesis. Hormetic findings suggest that mechanism of dose response may be a manifestation of the plasticity of biological systems and hormetic phenomena are mediated by endogenous cellular defense pathways, including Nrf2/HO-1 system, that integrate adaptive stress responses in the prevention of age-related diseases (Calabrese et al. 2010). The involvement of HO-1 system in anti-degenerative mechanisms operating during aging has received considerable attention. The cytoprotective mechanisms induced by HO-1 and its intermediates allow eukaryotic organisms to counteract the damaging effects of oxidants, electrophiles, and inflammation, major agents involved in the pathogenesis of cancer, diabetes, atherosclerosis, neurodegeneration, and aging (Chapple et al. 2012; Calabrese et al. 2006). Most studies addressing the molecular mechanisms responsible for the beneficial effects of HO-1 have been done in the context of aging and even though HO-1 induction has a predominantly cytoprotective outcome, it is essential to point out that protracted or repeated up-regulation of HO-1 may contribute to cellular dysfunction in many chronic degenerative conditions (Chou et al. 2005; Kitamuro et al. 2003). Although previous studies have reported an increase in HO-1 expression with aging (Abraham et al. 1985; Kang et al. 2005), it is now becoming evident that aging alters stress-induced expression of HO-1 in a cell-specific manner, which may contribute to the diminished stress tolerance observed in older organisms (Bloomer et al. 2009). Consistent with a hormesis mechanism, over activation of HO-1 may lead to exacerbation of a pathological state. Indeed, significant increases in HO-1 levels have been reported in Alzheimer’s disease (AD) brains associated with neurofibrillary tangles. AD is a neurodegenerative disorder involving a chronic inflammatory response associated with oxidative brain injury and an increase in HO-1 was also found in AD neocortex and cerebral vessels (Schipper 2000). The rising level of HO-1 in AD may reflect a direct response to an increase in free heme concentrations, associated with neurodegeneration, during which brain cells try to convert the highly toxic heme into the antioxidants CO and bilirubin (Calabrese et al. 2008). For this reason, it is crucial to investigate the hormetic role of HO-1 in terms of activation, biological function, and regulation. The diverse roles of HO-1 in senescence and aging-related disorders have progressed during the last decade but the Janus-faced behaviour of HO-1 imposes to apply the plasticity of the hormesis concept to HO-1 activity in order to provide a common mechanistic denominator for the physiological effects of HO-1.
Fig. 1.
Heme metabolism and HO-1 enzyme activity. HO-1 catalyze the rate-limiting step in heme metabolism. Heme is cleaved by HO-1 to yield equimolar quantities of iron, CO, and biliverdin. The antiinflammatory actions of CO can be in large measure mediated through p38 mitogen-activated protein kinase (MAPK) pathway. Biliverdin is converted to bilirubin by biliverdin reductase. At low physiological concentrations, these compounds trigger anti-oxidant, antiapoptotic and anti-inflammatory activities
Fig. 2.
Schematic representation of cellular protection mechanisms conferred by Nrf2-ARE pathway
Pharmacological activators of the Keap1/Nrf2/ARE pathway
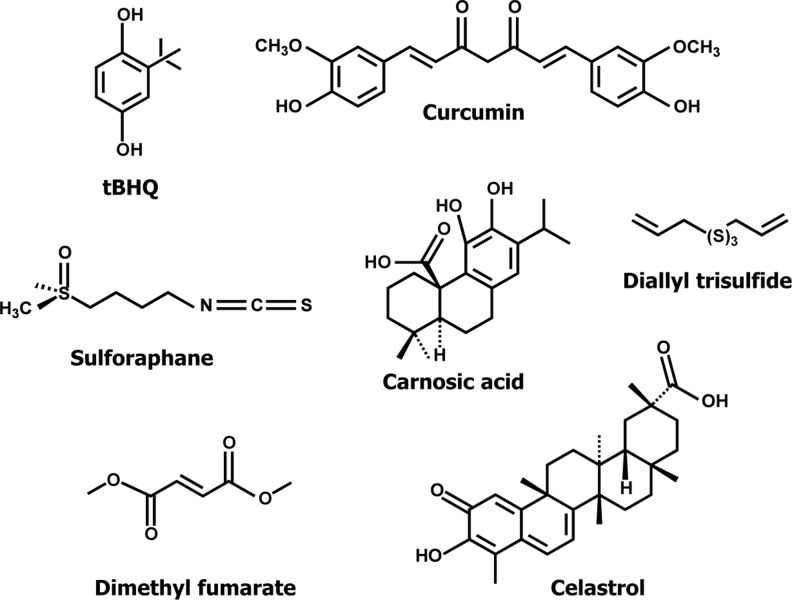
tert-butylhydroquinone (tBHQ)
Numerous inducers of the Keap1/Nrf2/ARE pathway (Fig. 3) have been shown to be cytoprotective. Most of them are either bioactive products present in the human diet or synthetic compounds. In particular, tert-butylhydroquinone (tBHQ) is one of the widely used activators of the Keap1/Nrf2/ARE and this compound is also one of the early inducers able to elevate cytoprotective enzymes in several tissues (De Long et al. 1985; Prochaska et al. 1985; Prochaska and Santamaria 1988). tBHQ is a more potent inducer compared to other phenolic compounds in which the phenolic groups are alkylated. Furthermore, it was recently showed that compounds with hydroquinone and catechol moieties may be novel cytoprotective agents based on their ability to activate the Keap1/Nrf2/ARE pathway (Satoh et al. 2009). Noteworthy, these molecules have the advantage that their action is sustained and amplified by transcription-mediated signalling pathways (Satoh and Lipton 2007). Since hydroquinones and catechols are not electrophilic may be viewed as pro-drugs (Bensasson et al. 2008). Redox imbalance is a crucial component of different neurodegenerative states and a novel strategy against these conditions has been proposed using these pro-drugs (Lipton 2007). For instance, catechol-containing carnosic acid induces Nrf2-dependent genes by binding to specific cysteine residues of Keap1 and it was also reported that carnosic acid crosses the blood–brain barrier, increases the level of reduced glutathione in the brain, and protects brain against ischemia/reperfusion (Satoh et al. 2008).
Fig. 3.
Pharmacological activators of Nrf-2 pathway
Sulforaphane
One of the most potent naturally occurring inducers of the Keap1/Nrf2/ARE pathway is the isothiocyanate sulforaphane. The cytoprotective properties of sulforaphane in various animal models have been recently reviewed (Dinkova-Kostova 2008). Interestingly, in brain of male Long–Evans rats, the levels of HO-1 mRNA were elevated following intraperitoneal administration of sulforaphane (5 mg/kg) (Zhao et al. 2006). The protein expression of HO-1 was increased by ~1.5-fold in both neurons and astrocytes. In a model of brain ischemia/reperfusion, administration of sulforaphane 15 min after the onset of ischemia reduced the infarct volume (which was evaluated 3 days later) by ~50 %. The same dose of sulforaphane administered to Sprague–Dawley rats 6 h after traumatic brain injury attenuated the loss of the water channel aquaporin-4 (AQP4) in the injury core and additionally, it also increased the levels of AQP4 in the penumbra region (Zhao et al. 2005). The protective effects were long-lasting and even 3 days post-injury there was a significant, although modest, reduction in brain edema. The mRNA levels of several cytoprotective genes in the parietal cortex and brain microvessels were increased 24 h after dosing (Zhao et al. 2007a). Postinjury (6 h later) administration of sulforaphane reduced the loss of endothelial cell markers and tight junction proteins and preserved the blood brain barrier function. The protective effects were evident only in wild-type, but not in nrf2-knockout mice. Moreover, compared to their wild-type counterparts, nrf2-knockout mice were hypersensitive to injury-induced blood brain barrier permeability.
In a model of intracerebral hemorrhage in Sprague–Dawley rats, sulforaphane administration caused induction of several Nrf2-dependent genes in brain 3 h later, concomitantly with reduction in markers of oxidative damage (3′-nitrotyrosine and 4-hydroxynonenal) in the perihematoma area (Zhao et al. 2007b). Three days following intracerebral hemorrhage, the perihematoma neutrophil count was reduced by ~60 %, and the total intracerebral hemorrhage-affected striatum neutrophil count was reduced by 33 %. The protective effects were long-lasting; 10 days after intracerebral hemorrhage, the neurologic deficits (a composite score from several behavioral tests, including foot fault, forelimb placing, postural reflex, cylinder, and circling) were substantially reduced. Conversely, Nrf2 deficiency resulted in exacerbated neurologic deficits and loss of the protection. In addition, treatment of organotypic nigrostriatal co-cultures with tBHQ or sulforaphane protected dopaminergic neurons against 6-hydroxydopamine (6-OHDA)-induced toxicity (Siebert et al. 2009).
Inducers of the Keap1/Nrf2/ARE pathway also have anti-inflammatory properties. The protective effects of sulforaphane against brain inflammation were recently reported in C57BL/6 mice that received endotoxin injection (Innamorato et al. 2008). Sulforaphane treatment decreased microglial activation and the upregulation of inflammatory markers in response to lipopolysaccharide (LPS). The same study also showed that compared to wild-type, nrf2-knockout mice were hypersensitive to LPS-induced neuroinflammation.
Dimethyl fumarate
Fumaric acid is the active principal of shepherd’s purse (Capsella bursa-pastoris), a cruciferous weed that has been used as a traditional herbal medicine. Fumaric acid is protective against tumorigenic effects (Kuroda and Takagi 1968; Kuroda and Akao 1980) and it was shown that addition of dimethyl fumarate to the diet of CD-1 mice and Sprague–Dawley rats elevated cytosolic glutathione transferases (GST) and quinone reductase (NQO1) (Spencer et al. 1990). In a model of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice, treatment with dimethyl fumarate reduced inflammation (Schilling et al. 2006). In human keratinocytes and peripheral blood mononuclear cells stimulated with LPS, dimethyl fumarate inhibits the production of chemokines that may be involved in the development of psoriatic lesions (Stoof et al. 2001). In patients with relapsing-remitting multiple sclerosis, dimethyl fumarate reduced the formation of new inflammatory lesions in both a pilot study (Schimrigk et al. 2006) and a multicentre, randomised, double-blind, placebo-controlled, dose-escalation, Phase IIb study (Kappos et al. 2008).
Diallyl trisulfide
The volatile organosulfur compounds from Allium (garlic) plants are also inducers of the Keap1/Nrf2/ARE pathway (Dinkova-Kostova et al. 2004). In particular, the unsaturated allyl or propenyl functionalities in their structures are critical determinants of inducer potency (Talalay 2000). In the 1980s such compounds were found to elevate GST activity and provide protection against carcinogenic effects (Sparnins et al. 1988). Recently using rat spinal cord explants, it was demonstrated that pre-treatment for 48 h with diallyl trisulfide elevates NQO1 and protects motor neurons against glutamate-induced excitotoxicity (Sun et al. 2009).
Celastrol
The quinone methide triterpene celastrol is derived from the traditional Chinese medicinal plant Celastrol is a potent inhibitor of nuclear factor kB (NF-kB) (Corson and Crews 2007; Sethi et al. 2007) and activates heat shock gene transcription synergistically with other stresses providing cytoprotection (Der Sarkissian et al. 2014). In mice, celastrol administration significantly attenuated the loss of dopaminergic neurons and the depletion in dopamine concentration following MPTP treatment (Cleren et al. 2005). In rats, celastrol decreased the striatal lesion volume induced by 3-nitropropionic acid, a neurotoxin used to model Huntington’s disease (Cleren et al. 2005). Hsp70 within dopaminergic neurons was induced by celastrol, whereas NF-kB and astrogliosis were suppressed. Global gene expression profiling in yeast cells revealed that celastrol treatment induced genes that fall in two major categories; (i) xenobiotic metabolism/clearance, and (ii) protein folding, processing, and turnover (Trott et al. 2008). Many of these genes are targets for both HSF1 and transcription factor Yap1, the functional yeast homologue of mammalian Nrf2 strongly suggesting that both activation of Hsps and the Keap1/Nrf2/ARE pathway by celastrol play roles in its protective mechanism(s) (Trott et al. 2008).
Curcumin
The cytoprotective role of curcumin, another naturally-occurring activator of the Keap1/Nrf2/ARE pathway (Dinkova-Kostova and Talalay 2000; Dinkova-Kostova et al. 2001), has been a subject of extensive investigation. Curcumin (diferuloylmethane) is a yellow spice extracted from the rhizome of the East Indian herb Curcuma longa L. Treatment of astrocytes with curcumin induced the cytoprotective proteins HO-1, NQO1 and GST and provided protection against the damaging effects of glucose oxidase-mediated toxicity (Scapagnini et al. 2006). Interestingly, curcumin was reported to bind to amyloid plaques, and to reduce amyloid levels and plaque burden in aged Tg2576 mice (a model of familial Alzheimer’s disease) with advanced amyloid accumulation (Yang et al. 2005). Moreover, curcumin mediates anti-inflammatory responses through the downregulation of inflammatory transcription factors (such as NF-kB), enzymes (such as cyclooxygenase 2 and 5 lipoxygenase) and cytokines (Anand et al. 2010), as well as upregulation of cyprotective vitagenes. Curcumin has chemopreventive properties, which are mainly due to its ability to arrest cell cycle and to induce apoptosis. The anti-carcinogenic properties of curcumin in animals have been demonstrated by its inhibition of tumor initiation and tumor promotion (Anand et al. 2010). The number of signaling pathways and molecular targets involved is growing and consequently the picture is becoming more and more complex, also because the results often appear to be cell-type specific and dose-dependent (Anand et al. 2010). Preclinical studies have shown that administration of 1 g/kg of curcumin to the rat allows the polyphenol to reach plasma concentrations around 1.3 μM. Several studies have shown that curcumin has strong antioxidant activity and its metabolites interact with several intracellular systems and members of the vitagene family. Notably, curcumin increased the expression of HO-1 in human cardiac myoblasts, hepatocytes, monocytes and endothelial cells (Motterlini et al. 2000), as well as rat neurons and astrocytes (Scapagnini et al. 2006). In several rodents and human cells, the curcumin-induced HO-1 overexpression was correlated with production of mitochondrial reactive oxygen species, activation of transcription factors Nrf2 and NF-kB, induction of MAPK p38 and inhibition of phosphatase activity (McMahon et al. 2006). Moreover, curcumin up-regulated Hsp70 in human colorectal carcinoma cells (Chen et al. 1996).
Ferulic acid
Ferulic acid is a phenolic compound and one of the major component of fruit and vegetables with strong antioxidant and anti-infammatory properties. Recently, it has been demonstrated that ferulic acid ethyl ester, a more hydrophobic form of ferulic acid, protects synaptosomal membrane system and neuronal cell culture systems against hydroxyl and peroxyl radical oxidation, as well as mice against Aβ-induced microglial activation (Perluigi et al. 2006; Calabrese et al. 2007). In addition to this direct antioxidant property, ferulic acid ethyl ester has been shown to increase HO-1 activity in rat astrocytes and neurons (Scapagnini et al. 2004), thus corroborating the hypothesis that HO-1 activation is a common pathway through which phenolic compounds can exert neuroprotective effects.
Conclusion
Average lifespan has increased over the last centuries, as a consequence of medical and environmental factors, but maximal life span remains unchanged (Cornelius et al. 2013). Extension of maximal life span is currently possible in animal models with measures such as genetic manipulations and caloric restriction (CR). CR appears to prolong life by reducing reactive oxygen species (ROS)-mediated oxidative damage. But ROS formation, which is positively implicated in cellular stress response mechanisms, is a highly regulated process controlled by a complex network of intracellular signaling pathways. By sensing the intracellular nutrient and energy status, the functional state of mitochondria, and the concentration of ROS produced in mitochondria, the longevity network regulates life span across species by coordinating information flow along its convergent, divergent and multiply branched signaling pathways, including vitagenes which are genes involved in preserving cellular homeostasis during stressful conditions. Vitagenes encode for heat shock proteins (Hsp) Hsp32, Hsp70, the thioredoxin and the sirtuin protein systems (Cornelius et al. 2014; Trovato Salinaro et al. 2014). Dietary antioxidants, have recently been demonstrated to be neuroprotective through the activation of hormetic pathways, including vitagenes. Aging represents an important health issue not only for the individual, but also for society in general. Burdens associated with aging are expanding as longevity increases. This has led to an enhanced focus on issues related to aging and age-related diseases. Consistent to this notion, given the broad cytoprotective properties of the heat shock response there is now strong interest in discovering and developing pharmacological agents capable of inducing stress responses in conditions where this protective response is blunted, such as during agingand age-related pathologies. Until recently, anti-aging endocrine-therapy has been largely limited to hormone-replacement therapy (HRT) that is associated with multiple side effects, including an increased risk of cancer. This has greatly limited the application of HRT in anti-aging therapy. Recently, the focus of anti-aging research has expanded from endocrine signaling pathways to effects on regulatory gene networks. However, newly identified genes, regulatory pathways and networks that are closely associated with endocrine biology may offer new hope for expanded anti-ageing therapy (Cornelius et al. 2013).
The hormetic dose–response, challenges long-standing beliefs about the nature of the dose–response in a lowdose zone, having the potential to affect significantly the design of pre-clinical studies and clinical trials as well as strategies for optimal patient dosing in the treatment of numerous diseases (Mancuso et al. 2013; Currò et al. 2014). Reports exist of enhanced longevity via treatment with a large number of agents in a wide range of animal models displaying hormetic dose responses (Calabrese et al. 2012). The generality of the hormetic dose response, being independent of biological model, endpoint, inducing agent and mechanism and with its quantitative features being a measure of plasticity constrained biological performance, strongly suggests that attempts to extend normal lifespan will be likewise limited to the 30–60 % as has been typically reported. Thus, hormesis has a fundamental role in aging research, affecting both the quality and the length of life as well as affecting the research methods (e.g. study design, statistical power, etc.) by which such biological concepts are studied.
Abbreviations
- LOH
Late-onset hypogonadism
- CBP
CREB-binding protein
- DHEA
Dehydroepiandrosterone
- SIRT
Sirtuin
- DHEAS
Dehydroepiandrosterone sulfate
- CO
Carbon monoxide
- SHBG
Sex hormone binding globulin
- NO
Nitric oxide
- HT
Hormone therapy
- ARE
Antioxidant response element
- HSR
Heat shock response
- Nrf2
Nuclear factor erythroid 2-related factor
- Hsp
Heat shock protein
- Keap1
Kelch ECH Associating Protein 1
- HO-1
Heme oxygenase-1
- Maf
Musculoaponeurotic fibrosarcoma
- HSF
Heat shock transcription factor
- AD
Alzheimer’s disease
- HSE
Heat shock elements
- DBD
DNA-binding domain
- HR
Hydrophobic heptad repeats
- TAD
Trans-activation domain
- eEF1A
Eukaryotic elongation factor 1A
- HSR-1
Heat shock RNA-1
- MAP kinases
MAPK
References
- Abraham NG, Levere RD, Freedman ML. Effect of age on rat liver heme and drug metabolism. Exp Gerontol. 1985;20:277–284. doi: 10.1016/0531-5565(85)90053-1. [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Vihervaara A, Laiho A, Conter A, Christians ES, Sistonen L, Henriksson E. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J Biol Chem. 2012;285:34469–34476. doi: 10.1074/jbc.M110.157552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bao Q, Pan J, Qi H, Wang L, Qian H, Jiang F, Shao Z, Xu F, Tao Z, Ma Q, Nelson P, Hu X. Aging and age-related diseases - from endocrine therapy to target therapy. Mol Cell Endocrinol. 2014;394:115–118. doi: 10.1016/j.mce.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Basaria S. Reproductive aging in Men. Endocrinol Metab Clin N Am. 2013;42:255–270. doi: 10.1016/j.ecl.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Basaria S, Wahlstrom JT, Dobs AS. Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- Bellia F, Vecchio G, Cuzzocrea S, Calabrese V, Rizzarelli E. Neuroprotection in oxidative driven diseases by carnosine. Mol Asp Med. 2011;32:258–266. doi: 10.1016/j.mam.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Bensasson RV, Zoete V, Dinkova-Kostova AT, Talalay P. Two-step mechanism of induction of the gene expression of a prototypic cancer-protective enzyme by diphenols. Chem Res Toxicol. 2008;21:805–812. doi: 10.1021/tx7002883. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR, Stanczyk FZ. Misconception and concerns about bioidentical hormones used for custom-compounded hormone therapy. J Clin Endocrinol Metab. 2012;97:756–759. doi: 10.1210/jc.2011-2492. [DOI] [PubMed] [Google Scholar]
- Bloomer SA, Zhang HJ, Brown KE, Kregel KC (2009) Differential regulation of hepatic heme oxygenase-1 protein with aging and heat stress. J Gerontol A Biol Sci Med Sci 64419–25 [DOI] [PMC free article] [PubMed]
- Brandes LJ. Hormetic effects of hormones, antihormones, and antidepressants on cancer cell growth in culture: in vivo correlates. Crit Rev Toxicol. 2005;35:587–592. doi: 10.1080/10408440500246801. [DOI] [PubMed] [Google Scholar]
- Brown-Sequard CE. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;2:105–107. doi: 10.1016/S0140-6736(00)64118-1. [DOI] [Google Scholar]
- Butenandt A, Guenther H, Turba F. On the primary metabolic action of testosterone. Hoppe Seylers Z Physiol Chem. 1960;322:28–37. doi: 10.1515/bchm2.1960.322.1.28. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–460. doi: 10.1016/j.envpol.2013.07.046. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006;8:444–477. doi: 10.1089/ars.2006.8.444. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield AD, Giuffrida Stella AM. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Asp Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13:215–235. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Iavicoli I, Calabrese V. Hormesis: its impact on medicine and health. Hum Exp Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Chen YC, Kuo TC, Lin-Shiau SY, Lin JK. Induction of HSP70 gene expression by modulation of Ca(+2) ion and cellular p53 protein by curcumin in colorectal carcinoma cells. Mol Carcinog. 1996;17:224–234. doi: 10.1002/(SICI)1098-2744(199612)17:4<224::AID-MC6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Chou YH, Ho FM, Liu DZ, Lin SY, Tsai LH, Chen CH, Ho YS, Hung LF, Liang YC. The possible role of heat shock factor-1 in the negative regulation of heme oxygenase-1. Int J Biochem Cell Biol. 2005;37:604–615. doi: 10.1016/j.biocel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Clark JH. A critique of Women’s health initiative studies. Nucl Recept Signal. 2006;4:1–10. doi: 10.1621/nrs.04023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a “chi”. Immun Ageing. 2013;10:15. doi: 10.1186/1742-4933-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C, Koverech G, Crupi R, Di Paola R, Koverech A, Lodato F, Scuto M, Salinaro AT, Cuzzocrea S, Calabrese EJ, Calabrese V. Osteoporosis and Alzheimer pathology: role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front Pharmacol. 2014;5:120. doi: 10.3389/fphar.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currò M, Trovato-Salinaro A, Gugliandolo A, Koverech G, Lodato F, Caccamo D, Calabrese V, Ientile R. Resveratrol protects against homocysteine-induced cell damage via cell stress response in neuroblastoma cells. J Neurosci Res. 2014 doi: 10.1002/jnr.23453. [DOI] [PubMed] [Google Scholar]
- Davinelli S, Scapagnini G, Denaro F, Calabrese V, Benedetti F, Krishnan S, Curreli S, Bryant J, Zella D. Altered expression pattern of Nrf2/HO-1 axis during accelerated-senescence in HIV-1 transgenic rat. Biogerontology. 2014 doi: 10.1007/s10522-014-9511-6. [DOI] [PubMed] [Google Scholar]
- De Long MJ, Prochaska HJ, Talalay P. Tissue-specific induction patterns of cancer-protective enzymes in mice by tert-butyl-4-hydroxyanisole and related substituted phenols. Cancer Res. 1985;45:546–551. [PubMed] [Google Scholar]
- Der Sarkissian S, Cailhier JF, Borie M, Stevens LM, Gaboury L, Mansour S, Hamet P, Noiseux N (2014) Celastrol protects ischemic myocardium through heat shock response with upregulation of heme oxygenase-1. Br J Pharmacol. Epub ahead of print [DOI] [PMC free article] [PubMed]
- Di Domenico F, Perluigi M, Butterfield DA, Cornelius C, Calabrese V. Oxidative damage in Rat brain during aging: interplay between energy and metabolic Key target proteins. Neurochem Res. 2010;35:2184–2192. doi: 10.1007/s11064-010-0295-z. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Impellizzeri D, Trovato Salinaro A, Mazzon E, Bellia F, Cavallaro M, Cornelius C, Vecchio G, Calabrese V, Rizzarelli E, Cuzzocrea S. Administration of carnosine in the treatment of acute spinal cord injury. Biochem Pharmacol. 2011;82:1478–1489. doi: 10.1016/j.bcp.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT (2008) The isothiocyanate sulforaphane induces the phase 2 response by signaling of the Keap1-Nrf2-ARE pathway: Implications for dietary protection against cancer. In: Dietary Modulation of Cell Signaling Pathways, edited by YJ Surh, Z. Dong, E. Cadenas and L. Packer, pp. 205–229
- Dinkova-Kostova AT, Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–240. doi: 10.1016/S0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res Mol Brain Res. 1995;30:37–47. doi: 10.1016/0169-328X(94)00273-H. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Maines MD. Regulation and expression of heme oxygenase enzymes in aged-rat brain: age related depression in HO-1 and HO-2 expression and altered stressresponse. J Neural Transm. 2006;113:439–454. doi: 10.1007/s00702-005-0408-z. [DOI] [PubMed] [Google Scholar]
- FDA Consumer Health Information. Bio-identicals: sorting myths from facts. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm049311.htm
- Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Tan K, Inouye S, Takii R, Nakai A. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol Biol Cell. 2010;21:106–116. doi: 10.1091/mbc.E09-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman DJ. Trends and regional differences in testosterone prescribing in Australia, 1991–2001. Med J Aust. 2004;181:419–422. doi: 10.5694/j.1326-5377.2004.tb06364.x. [DOI] [PubMed] [Google Scholar]
- Hunt PR, Son TG, Wilson MA, Yu QS, Wood WH, Zhang Y, Becker KG, Greig NH, Mattson MP, Camandola S, Wolkow CA (2011) Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One e21922. doi: 10.1371/journal.pone.0021922 [DOI] [PMC free article] [PubMed]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato NG, Rojo AI, García-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1996;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, Kim KW, Baik HS, Yoo MA, Yu BP, Chung HY. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology. 2005;6:27–37. doi: 10.1007/s10522-004-7381-z. [DOI] [PubMed] [Google Scholar]
- Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, O’Neill GN. BG-12 Phase IIb Study Investigators. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, Hattori T, Shirato K, Igarashi K, Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Akao M. Reduction by fumaric acid of side effects of mitomycin C. Biochem Pharmacol. 1980;29:2839–2844. doi: 10.1016/0006-2952(80)90020-9. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Takagi K. Physiologically active substance in Capsella bursa-pastoris. Nature. 1968;220:707–708. doi: 10.1038/220707a0. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Song CS, Chatterjee B, Roy AK. Agedependent increase of heme oxygenase-1 gene expression in the liver mediated by NFkappaB. Mech Ageing Dev. 2000;114:49–60. doi: 10.1016/S0047-6374(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Santangelo R, Calabrese V. The heme oxygenase/biliverdin reductase system: a potential drug target in Alzheimer’s disease. J Biol Regul Homeost Agents. 2013;27:75–87. [PubMed] [Google Scholar]
- Matsuoka Y, Okazaki M, Kitamura Y. Induction of inducible heme oxygenase (HO-1) in the central nervous system: is HO-1 helpful or harmful? Neurotox Res. 1999;1:113–117. doi: 10.1007/BF03033275. [DOI] [PubMed] [Google Scholar]
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate Cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- Morse D, Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- Patriarca S, Furfaro AL, Cosso L, Pesce Maineri E, Balbis E, Domenicotti C, Nitti M, Cottalasso D, Marinari UM, Pronzato MA, Traverso N. Heme oxygenase 1 expression in rat liver during ageing and ethanol intoxication. Biogerontology. 2007;8:365–372. doi: 10.1007/s10522-006-9079-x. [DOI] [PubMed] [Google Scholar]
- Pennisi G, Cornelius C, Cavallaro MM, Trovato Salinaro A, Cambria MT, Pennisi M, Bella R, Milone P, Ventimiglia B, Migliore MR, Di Renzo L, De Lorenzo A, Calabrese V. Redox regulation of cellular stress response in multiple sclerosis. Biochem Pharmacol. 2011;82:1490–1499. doi: 10.1016/j.bcp.2011.07.092. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Cini C, Butterfield DA. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. J Neurosci Res. 2006;84:418–426. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Giorgi A, Schininà ME, Coccia R, Cini C, Bellia F, Cambria MT, Cornelius C, Butterfield DA, Calabrese V. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res. 2010;88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Prochaska HJ, De Long MJ, Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985;82:8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Saitoh S, Hosaka M, Kosaka K. Simple ortho- and para-hydroquinones as compounds neuroprotective against oxidative stress in a manner associated with specific transcriptional activation. Biochem Biophys Res Commun. 2009;379:537–541. doi: 10.1016/j.bbrc.2008.12.106. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Butterfield DA, Colombrita C, Sultana R, Pascale A, Calabrese V. Ethyl Ferulate, a Lipophilic Polyphenol, Induces HO-1 and Protects Rat Neurons Against Oxidative Stress. Antioxid Redox Signal. 2004;6:811–818. doi: 10.1089/ars.2004.6.811. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Caruso C, Calabrese V. Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders. Adv Exp Med Biol. 2011;698:27–35. doi: 10.1007/978-1-4419-7347-4_3. [DOI] [PubMed] [Google Scholar]
- Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, Hoffmann V, Pöhlau D, Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. 2006;13:604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–830. doi: 10.1016/S0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- Siciliano R, Barone E, Calabrese V, Rispoli V, Butterfield DA, Mancuso C. Experimental research on nitric oxide and the therapy of Alzheimer disease: a challenging bridge. CNS Neurol Disord Drug Targets. 2011;10:766–776. doi: 10.2174/187152711798072356. [DOI] [PubMed] [Google Scholar]
- Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131–134. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- Spencer SR, Wilczak CA, Talalay P. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990;50:7871–7875. [PubMed] [Google Scholar]
- Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001;144:1114–11120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- Sun MM, Bu H, Li B, Yu JX, Guo YS, Li CY. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol Res. 2009;31:23–27. doi: 10.1179/174313208X332959. [DOI] [PubMed] [Google Scholar]
- Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzym Regul. 2003;43:121–134. doi: 10.1016/S0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott A, West JD, Klaić L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA (2008) Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell19:1104–1112 [DOI] [PMC free article] [PubMed]
- Trovato Salinaro A, Cornelius C, Koverech G, Koverech A, Scuto M, Lodato F, Fronte V, Muccilli V, Reibaldi M, Longo A, Uva MG, Calabrese V. Cellular stress response, redox status, and vitagenes in glaucoma: a systemic oxidant disorder linked to Alzheimer’s disease. Front Pharmacol. 2014;5:129. doi: 10.3389/fphar.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Jeng YJ, Watson CS. Non-genomic effects of xenoestrogen mixtures. Int J Environ Res Public Health. 2012;9:2694–2714. doi: 10.3390/ijerph9082694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Raynes R, Powell C, Xue B, Uversky VN. HSF transcription factor family, heat shock response, and protein intrinsic disorder. Curr Protein Pept Sci. 2012;13:86–103. doi: 10.2174/138920312799277956. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ahn YH, Benjamin IJ, Honda T, Hicks RJ, Calabrese V, Cole PA, Dinkova-Kostova AT. HSF1-dependent upregulation of Hsp70 by sulfhydryl-reactive inducers of the KEAP1/NRF2/ARE pathway. Chem Biol. 2011;18:1355–1361. doi: 10.1016/j.chembiol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]