Abstract
The considerable advances of genome sequencing over the past decades have had a profound impact on our daily life and opened up new avenues for the public to have access to their genetic information and learn more about their ancestry, genealogy and other traits that make each of us unique individuals. A very large number of individual single nucleotide polymorphisms (SNPs) have been associated to diseases whereas others have no known phenotype. For example, among the SNPs mapped within ccn1(cyr61), ccn2(ctgf), ccn3(nov), ccn4(wisp-1), ccn5(wisp-2) and ccn6 (wisp-3), only mutations within ccn4 were associated to PPD (the autosomal recessive skeletal disorder Progressive Pseudorheumatoid Dysplasia). On the occasion of this JCCS special issue on the roles of hormetic responses in adaptation, and response of living species to the modifications of their environment, it appeared that it was a good time to briefly review a topic that has been the subject of passionate discussions for the past few years, that is Direct to Consumer genetic tests (DTC GT). Based on the use of DNA analysis and identification of polymorphisms, DTC GT have been developed by several companies in the USA and in countries where there was no legal obstacle for customers to have direct access to their genetic information and manage their healthcare. Problems that arose and decisions that have been taken by regulatory agencies are presented and discussed in this editorial. The « freeze » of health-oriented DTC GT in the USA neither implies the end of DNA analysis nor « fun » applications, which are not aimed at providing risks estimates for particular illnesses. As shown in the example which is discussed in this editorial, DTC GT for cosmetic applications might be considered a fun application of great interest for companies such as L’Oréal, who recently developed the Makeup Genius mobile application. Other fun applications of DTC GT are discussed but there is no doubt that nothing will stop progress and it is most probable than within a few years from now all the tensions raised about these procedures will vanish to the profit and benefit of consumers. In any case, this will only be possible through an intensive communication effort, because …communication is the key !
Keywords: DTC GT, SNPs, DNA sequencing, Polymorphisms, Cosmetics, L’Oréal, 23andMe, DeCODE, Knome, Navigenics, Illumina, BGI, Cell communication, Hormesis, FDA, GWAS, Mobile applications, Next generation sequencing, BRCA, Illness risk
Introduction
Autumn has always been a particular time in my life. I cherish the subtile changes that take place in the surrounding Nature, with bird migrations, tree leaves changing colours, and daylight becoming gradually shorter, just as to prepare our mind and our body for the end of another year.
Being in the Northern Hemisphere, November is one of these autumn months when you can enjoy the warmth of family and friends reunions, and discussions in a cosy atmosphere.
It also the time for reflection and writing an editorial that will close a year of hard work from our team. I would like to take this opportunity to thank all authors, members of our editorial board, and managing team who helped us to reach the high scientific standard of the Journal of Cell Communication and Signaling.
With this special issue of JCCS devoted to the role of hormetic responses in fundamental biological processes, guested-edited by Professor Edward Calabrese who was the recipient of the third ICCNS-Springer award,1 we wanted to widen the scope of our journal and address questions about regulatory pathways that are intimately involved in the adaptation and communication of cells, organs and bodies with their environment.
Let me also take this opportunity to thank all the authors who have kindly accepted to contribute to this special issue and Edward Calabrese for his remarkable editing job.
Communication across the whole living world is fascinating
A number of years ago, when I was the Editor in Chief of Cell Communication and Signaling (CCS), an electronic journal that I had created and published with BMC, I wrote an editorial entitled « Communication is the key » (Perbal 2003) 2
At that time, I had stressed that the harmonious development of tissues, organs and bodies, within a plastic environment that is constantly cross talking to cells that it contains, relies on sensing a complex array of biosignals to which cells respond and adapt.
Major steps in the understanding of cell behavioral choices have been made over the past decades and have pointed out the critical importance of microenvironments. This is particularly true for normal development and for appearance and maintenance of pathological situations such as cancer.
The coordination of signaling circuits, involving sophisticated interplay of many regulatory pathways is essential in the ability of unicellular and highly organized living organisms to communicate, be they in their early or late stages of life.
Along this line, evidence has now been obtained in favor of the CCN family of proteins, a new group of cell signaling regulators which was uncovered 20 years ago that play critical roles in the regulation of normal and pathological cell behavior, in various tissues and organs including skin, cartilage and bone, hematopoietic fluids, muscle and connective tissue, reproductive and urinary tracks, nervous sytem and more. In several instances, the dysfunctioning of normal signaling resulted from dysregulation of CCN protein production or function. These aspects have been largely reviewed in various issues of the Journal of Cell Communication and Signaling.
Due to their striking tetramodular structure, the CCN proteins can interact with ligands and regulatory proteins that are involved in major signaling pathways.
As mentioned above, a number polymorphic sites have been detected within the CCN genes. Among them, 13851 SNPs have been detected within the ccn1 gene,3 459 within ccn2,4 393 within ccn3,5 3285 within ccn4,6 1269 within ccn5,7 and 1723 for ccn6.8 As of today, only CCN4 mutations were associated to a human disease that is an autosomal recessive skeletal disorder Progressive Pseudorheumatoid Dysplasia (Hurvitz et al. 1999).
For several years, I have proposed that CCN proteins function as regulatory hubs allowing the coordinated action of independent pathways whose balanced interplay is required for the harmonious development of tissues and organs and sensing of the surrounding microenvironment (Perbal 2001, 2013)
Depending upon the nature and bioavailability of their partners, different combinatorial events would be permitted, hence providing a unique functional flexibility for signaling and coordination of independent pathways.
It is not my intent to review here the progress made in all fields of intra- and intercellular signaling. Many excellent reviews address these topics.
In the present editorial, I would rather like to comment on how DNA sequencing technologies and genome wide association studies (GWAS)9 have had a profound impact on both our scientific vision, our behavior, and our ability to communicate about our own genome. The relatively recent explosion of Direct to Consumer genetics companies providing medically-oriented tests, has raised a considerable amount of passionate reactions.
I will briefly review and discuss a few main aspects of this promising approach.
Direct to consumer genetics (DTC genetics)
The advent of high troughput DNA sequencing technologies has afforded a much better insight into the genome physical constitution of many living species including human. As a consequence, many applications have resulted from the analysis of genomic mutations and their association with several traits of human beings.
It is not the aim of this short article to review the whole field, but rather to give a flavor of what has been or could be done in some areas of wide interest for the public, and briefly discuss some of the problems that these new approaches have already raised at various communication levels between the consumers, the scientific community and the regulatory agencies.
The compilation and comparison of sequence data sets obtained from various independent DNA samples revealed the existence of individual variations at the nucleotide level that were designated Single Nucleotide Plymorphism (SNPs).
These polymorphisms were observed in about 1 % of the population, either within coding or non-coding regions of the human genome. When localized within a coding frame, a SNP may alter the function(s) or the end product. SNPs occuring in the non-coding sequencing may affect the regulation of gene expression and other structural components that are critical for the biology of living organisms.
It was rapidly realized that SNPs occasionally occured at the same positions in the genome of different individuals, therefore suggesting that SNPs might be associated to the expression of certain traits within this group of individuals.
Indeed, the observation that several SNPs were more frequently detected among individuals sharing a common illness, triggered interest in using statistical analysis (Genome Wide Association Studies, GWAS) which aimed at establishing whether a significant difference in SNP representation existed between healthy individuals and patients with disease.
In a 2005 article published in Science by Klein et al. it was reported that « Among 116,204 single-nucleotide polymorphisms genotyped, an intronic and common variant in the complement factor H gene (CFH) is strongly associated with AMD (nominal P value <10–7) » (Klein et al. 2005). Several thousands of SNPs have now been associated to illness and human traits and are catalogued (Welter et al. 2014).10
Several private companies became interested in the identification of SNPs that could be used for marketing kits to the general public. By providing a saliva sample, any customer would receive in return a set of personal files containing the results of screening their DNA for several hundreds of thousands human SNPs.
The big promise was that screening of DNA samples collected in a very simple way (such as a saliva swab) would permit customers to have a direct handle on their genomic information, DNA sequence and be informed about their risk for predisposition to diseases caused by genetic mutations, and sensitivity to chemicals or drugs that might be prescribed by physicians.
The burgeoning of Direct to Consumer (DTC) DNA testing companies was ramped up between 2006 and 2010 with the promise made to the public that customers’ health would benefit directly from the progress made in the identification of SNPs associated with frequent diseases.
Being part of the first Consumer Genetics Show held in Boston in 2009, I was really impressed by the huge potential offered by these new approaches for medical research and for customers daily life even though the validity of risk prediction based on GWAS has been the matter of intense debate for many years.
In many cases though, despite expectations of consumers, answers could not always be realized, at least at that time from their DNA test.
At the present time, 27 companies are listed as DNA testing companies by the International Society of Genetic Genealogy11 .12 Most of them offer service for deep ancestry, genealogy, and ethnicity. Others, about 10 at this time, specialize in paternity tests, and identity relationships.
The interest of people in their origins and their willingness to share data, is illustrated by the success of « The human Story »,13 a genographic project launched in 2005 by National Geographic14 . According to S. Wells, keynote speaker at the first conference for genetic genalogy this summer,15 two million persons have now had their DNA tested, among whom about 688, 000 for the genographic project.
For several different reasons, the landscape of DTC companies offering health-oriented DNA screening has profoundly changed within the last decade or so.
One of the main reasons resulted from the fast pace of progress made by the scientific community which overran the ability of medical and legal agencies to offer a satisfactory framework that would offer solid guarantees to the customers.
The topic has been discussed in a considerable number of debates and newspapers articles which illustrated profound divergencies expressed by DTC companies and regulatory agencies regarding the usefulness and potential danger of DTC genetic tests whose scientific validity was sometimes questioned.
I will briefly sumarize a few milestones in order to point out the critical impact that communication has had in the evolution of this new field.
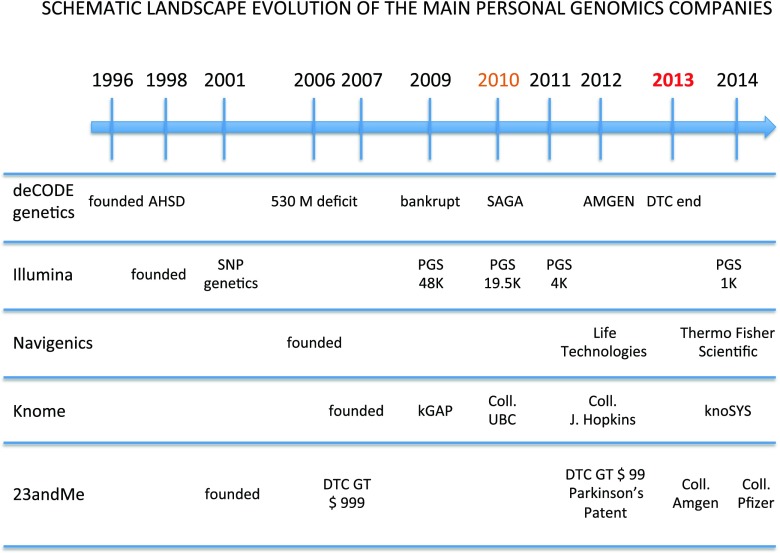
Milestones in the changing landscape of DTC GT (Fig. 1)
Fig. 1.
deCODE Genetics : Founded in 1996, in 1998 developed the Act on Health Sector Database (AHSD), in 2006 DeCODE recognized as non-profit with over 530 million USD deficit, in 2009 went bankrupt, in 2010 acquired by Saga Investments, in 2012 acquired by Amgen, in 2013 website genetic service discontinued. Illumina : Founded in 1998, in 2001 began the SNP genotyping service, in 2009 launched Personal full genome sequencing (PGS), costing 48 K in 2009, 19.5 K in 2010, 4 K in 2011, and 1 K in 2014. Navigenics : Founded in 2006, in 2012 acquired by Life Technologies Inc., in 2014 becomes part of Thermo Fischer Scientific, Inc which acquired Life Technologies Inc. Knome : Founded in 2007, in 2009 launched the kGAP (cloud based engine), in 2010 partnered with University of British Columbia for studies on Parkinson’s Disease, in 2012 partnered with John Hopkins for asthma studies, in 2014 launched a human genome interpretation platform (the knoSYS™ 25). 23andMe : Founded in 2006, in 2007 DTC GTs offered for 999 USD, in 2012 DTC GTs offered for 99 USD, in 2013 DTC offers limited in the US to providing raw genetic data and ancestry-related identification, in 2012 23andMe patent obtained on Parkinson’s Disease, in 2013 partnered with Genentech for studies on metastatic breast cancer, in 2014 partnered with Pfizer Inc. for research on Inflammatory Bowel Disease, in 2014 submitted FDA application for Bloom syndrome. In orange : 2010 date of the warning letter sent to the five companies. In red : 2013 date of FDA ban for medically-oriented DTC tests in the USA
DTC companies offered their customers the possibility to have their DNA assessed for the presence of SNPs associated with selected drug responses or with susceptibility to genetic disorders. The kits became available through the internet, and the samples, collected by the customers in the privacy of their home were sent back to the company performing the tests. Results included a whole set of raw data and comments regarding the known association between SNPs and medical conditions.
Soon, a number of physicians, paramedical practionners, and academic biomedical researchers questioned the societal impact of communicating raw data and their interpretation, to customers who would not be prepared to understand and handle personal information pertaining to their health and other private life traits.
Furthermore, an investigation run by the Government Accountability Office (GAO) in 2010 concluded that these genetic tests sometimes provided conflictual and misleading information to the consumer.16
In June 2010, the Food and Drug Adminstration published online letters to five private companies – deCODE Genetics, Illumina, Knome, Navigenics and 23andMe, which at that time were selling online, a series of DTC genetic testing products that were, based on Section 201(h) of the Federal Food, Drug and Cosmetic Act (FDCA). Section 201(h)(2) of the FDCA, considered as medical devices those marketed without appropriate review and approval of the FDA.
The tests sold by DeCode Genetics, Navigenics and 23andMe were informing customers about their risk to develop diseases such as cancer or diabetes for which SNPs markers had been identified.
Knome’s strategy to evaluate disease was based on complete DNA sequencing and Illumina was also selling DNA chips that some companies used to perform the DNA analysis.
The letter sent to these companies made it clear that the FDA considered that the results sent to the consumer provided clinical interpretation to be used for healthcare decisions.
This position triggered a debate between those who considered that the results provided by DTC DNA analysis were indeed a medical test and those who considered that the tests just provided scientific information.17
Companies did not share the same view on DTC genetics.
The rise, success and failure of DTC companies was nicely reviewed a few years ago, with an open discussion on the regulatory evolutions (Borry et al. 2010a, b).
Major changes that occured in the field were the results of DTC companies, leaving the field of medically-oriented DTC to shift towards academic research projects or join bigger biotech companies’projects.
Originally Knome offered DTC whole genome sequencing to its customers. Today, the company defines itself as « the human genome interpretation Company »18 using big data technologies for an accelerated transition of healthcare industry to molecular based medicine.
Navigenics which had decided early to shift away from the DTC area and to move towards partnership with medical institutions and physicians was acquired in 2012 by Life Technologies Corp. who wished to expand its CLIA laboratory services without keeping the DTC activity. In 2014 Thermo Fisher Scientific acquired Life Technologies Corp.19 .
The DeCODE saga
The business path of deCode genetics, a company that had specialized since 1996 in the study of genetics of human diseases was somewhat more chaotic.
DeCODE had originally positioned itself in the study of human genes associated with common diseases through an individual’s genome analysis. The basic profile that was provided to the customers was intended to help them in taking decisions regarding prevention and/or treatment of potential diseases that they were at risk to develop according to their genomic content. Along this line, DeCODE attempted to launch a Health Sector Database for the Icelandic population, by pooling in a wide range study the genealogical, medical and genetic data of all Icelanders. As discussed by D.E. Winickoff, « the HSD Act’s most controversial provision authorized the transfer of all medical record data to the licensee for commercial development without the express consent of individuals, by invoking a rule of “presumed consent.” In addition, information on deceased individuals would be automatically included, despite the potential privacy interests of relatives and individuals »20 . The controversy triggered by this project was intense and resulted in a 2013 judgement of the Supreme Court of Iceland,21,22, who considered that the way the project was run was not in accordance with the right to privacy that people can exercise over the use and disclosure of their own personal information.23
Later on, DeCODE repositioned itself in the DTC business. The company declared bankruptcy in 2009 and most of its assets were purchased by Saga Investments LLC in 2010.24 In 2012, Amgen acquired DeCODE25,26 in order to extend its program for validation of disease targets in humans. Diagnostic tests developed by DeCODE included evluation of risks for prostate cancer, glaucoma, breast cancer and myocardial infarction.
In an effort to communicate and share with the scientific community, deCODE’s production was materialized in several publications among which the recent description of rare mutations and chronic kidney disease (Sveinbjornsson et al. 2014), GWAS on urinary bladder (Rafnar et al. 2014), description of risk factor factors for basal cell carcinoma (Stacey et al. 2014) and type 2 diabetes (Steinthorsdottir et al. 2014) . In the flow of studies aimed at identifying genetic markers for Alzheimer’s Disease (AD) (Kim et al. 2014), DeCODE reported a variant of TREM2 associated with the risk of AD (Jonsson et al. 2013) and a mutation in the Amyloid Precursor Protein (AAP) that protects against age-related cognitive disease and AD (Jonsson et al. 2012).
As expressed in several comments in the press, concerns arose and are still very present regarding the fate of the huge personal data collections that companies have accumulated and are now acquired by investors who do not have the same interests in DTC.27
While major reorganizations took place in the DTC business, 23andMe consolidated its position as a leader in the field by reinforcing a consumer-oriented position and claiming that the strength of the company was to maintain a good communication with the customers28 .
In spite of the warning letter that was sent by the FDA in 2010 to DTC companies, 23andMe was still claiming that « individuals have a right to access their own genetic information and that personal genetics will pave the way for significant advances in healthcare »29 and gave two presentations at the Molecular and Clinical Genetics Panel of the Medical Devices Advisory Committee held in march 2011,30 to explain the 23andMe position and thoughts about the possibility for the regulatory frameworks to move forward.31
Who should decide what you can learn from your DNA ?
As reported on MedPage Today,32 the FDA advisory panel recommended that genetic tests « available for at-home use without a prescription should not be used without the involvement of a physisican or genetic specialist ».
Although the value of the test results indicating genetic predisposition for a disease was questioned, a survey published in the New England Journal of Medicine established that customers using tests were not more anxious when they got their results33 (Bloss et al. 2011; 2013).
An important question that was addressed in these discussions was that of the general public’s ability to appreciate the real meaning of their screening results.
This question has been discussed over and over in the press, media and scientific meetings since the very early days of DTC genetic testing.
On one side, many like the majority of the FDA panelists, who question the ability of the consumer to understand the results without the help of a physician, and on the other side, many others who claim that individuals should have the freedom of choice, and primacy of individual judgment34 without the need of the medical profession.
That tests which are run in different laboratories and using different methods do not always lead to the same type of interpretation is often seen in science. I remember F. Collins himself commenting at a Consumer Genetics meeting on the discrepancies contained in the interpretation of the results that he got from his own DNA analysis. Many others have had the same experience. Therefore it is not surprising that an undercover investigation run by the Government Accountability Office « revealed that home gentic tests often provide incompete or misleading information to consumers ».35
The proponents of DTC genetics based on real and serious scientific data were not ready for an end to DTC genetic testing36 .
Criticisms were directed against a paternalistic position of the panel and the medical professionals 37,38 that has already been observed in the past when a new medical test or device was about to become freely accessible to the general public.39
A major objection to the panelist willing to restrict the interpretation of DTC test to the medical profession was that many physicians themselves admit privately to be unable to do so40 and to have no time for training as a biotech IT.
A key issue in this debate is that people « shouldn’t be misled about the significance of that information and that people should be able to be assured that the claims that are made are accurate and that their privacy will be protected.41 Also, consumers should be « provided with the objective data required to make an informed decision about whether to take a test and how to interpret its results »42
Who said that communication is the key ? ….In a very simple and effective communication exercise, 23andMe clearly states that the company is indeed performing its own research on profiles and « uses the Illumina OmniExpress Plus Genotyping BeadChip . In addition to the variants already included on the chip by Illumina, we’ve included our own, customized set of variants relating to conditions and traits that are interesting. Technical information on the performance of the chip can be found on Illumina’s website. ».
Then what ? 2013 : the big bang ! FDA requests immediate cessation of medically oriented DTC GTs
On Nov 22, 2013, the FDA send a warning letter43 to Anne Wojcicki, CEO of 23andMe, 23andMe requesting the company to « immediatly discontinue marketing PGS (personal genome service) until such time as it receives FDA marketing authorization for the device »
In the passionate context that had developed in the DTC genetics field after the 2011 events quoted above, this letter was received as a real rocket.
Events which took place between 2011 and 2013 might bring explanations to this sharp decision.
After the recommendations expressed by the FDA in 2011, 23andMe pursued its quest to provide customers with medical risk evaluations drawn from DNA analysis based on chip-based assays for known SNPs, rare mutations and variants covered by the plaftform that had been set up by the company (see above).
By offering its customers the feeling that they participated in a profound change in the healthcare system, 23andMe attracted a considerable number of people who wanted to master their personal genetic information the way they want, without needing to rely on a governmental agency.
The number of medically-oriented tests proposed to the public significantly increased as the results of research performed « cooperatively with other groups and university scientists obtained by 23andMe » allowed expansion of the panel of SNPs associated with diseases or drug response,44,45,46,47,48,49 .
In 2012, 23andMe filed 510(k)s for some medically-oriented tests50 and launched a series of professional advertizing campaigns to attract more customers.
No doubt that the numerous adds, sometimes qualified as being « business-agressive », might have added to the irritation of the FDA …
In its warning letter of November 2013, the FDA stated « your company has failed to address the issues described during previous interactions with the Agency or provide the additional information » that was requested. « Consequently, the 510(k)s are considered withdrawn ».
One of the main concern expressed by the FDA deserves more attention.
The letter says « Some of the uses for which PGS is intended are particularly concerning, such as assessments for BRCA-related genetic risk and drug responses (e.g., warfarin sensitivity, clopidogrel response, and 5-fluorouracil toxicity) because of the potential health consequences that could result from false positive or false negative assessments for high-risk indications such as these. For instance, if the BRCA-related risk assessment for breast or ovarian cancer reports a false positive, it could lead a patient to undergo prophylactic surgery, chemoprevention, intensive screening, or other morbidity-inducing actions, while a false negative could result in a failure to recognize an actual risk that may exist. »
As a matter of fact an article written by Angelina Jolie in the New York Times 51 has drawn a lot of attention to the DTC tests.
Being diagnosed with a mutated version of the BRCA1 gene, that put her risk to develop breast cancer at 87 %, Angelina Jolie decided to undergo preventive mastectomies.
It was not the first time that such decisions were taken and announced in the media52 but the public audience of the actress, shed light on a decision that was largely commented in the press and among various societal groups53,,54 .55
The delicate management of BRCA mutation carriers has been recently reviewed (Stan et al. 2013).
It is not an easy thing to decide for any kind of invasive treatment based on the sole result of a genetic test and I truly believe that stressful situations and decisions taken in haste could largely be avoided thanks to careful, thorough and objective information provided to the customer.
Again and again, communication is the key !
Although the decision of the FDA appeared excessive to many analysts, one must remember that the FDA enforcement capacity is dependent in the USA, upon Laws passed by Congress.56 To avoid conflictual situations, regulations need to be in place as fast as possible with the scientific progress and with the profound changes that our societies are presently experiencing. 57
What kind of future is there for DTC tests ?
At the present time, it seems that the FDA is willing to enact quite important moves to regulate cancer genetics and other medically-oriented tests58, .59 Along this line, the FDA gave Congress notice that a series of guidelines for the regulation of laboratory developed tests (LDTs) would be submitted shortly.
The anticipated details of the draft guidance for Industry, Food and Drug Administration Staff and clinical laboratories have been recently released.60
Upcoming discussions will need to address the terms of the regulatory regime for LDTs and will set the ground for a reassessement of DTC genetics activities.
In the meantime, DTC tests will still address the fun aspects of our life that a lot of people seem to enjoy such as knowing more about ancestry, eye colour, eyebrow shape (high or low), wet or dry earwax, earlobe shape, sensitivity to motion sickness, filiation, etc.. Readers interested in the activities of DTC companies can easily find information on the internet61 (see also Borry et al. 2010b)
As an Associate at L’Oréal USA, in 1988, I became very much interested in new biological advances that could benefit the consumer. DTC genetics was one.
It then came to my attention that a cosmetic company which would take advantage of these new openings could become a leader for a new type of commercially fruitful interactive communication with their customers.
After discussing the matter with Dr. Andras Pellionisz (Founder and Chief Scientist HolGenTech, Inc.) who was developing new applications for cell phones, I envisioned cosmetics customers using their mobile telephone to check whether the brand they are buying, i) contains any particular component to which they might be sensitive or, ii) is the best suited product for their skin or hair type.
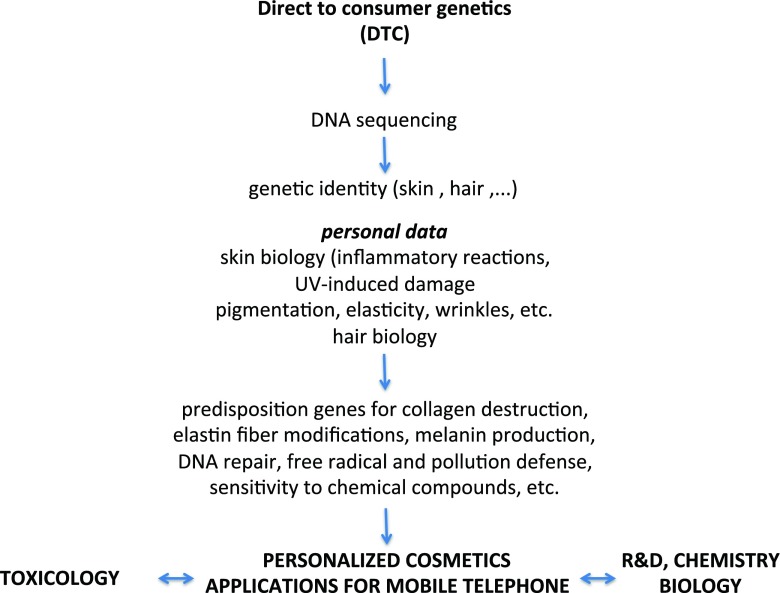
As schematically depicted in Fig. 2, the proposed process was based on the consumer using their previously collected personal genetic information and screening the product composition that would be encrypted in the bar code of each preparation.
Fig. 2.
Is this cosmetic brand good for me
After downloading his/her personal information or accessing it online, the consumer would be able to check whether he/she is carrying specific genetic markers known to be involved in any kind of illness or predisposition for allergic reactions to compounds contained in the preparations.
Along this line, it is worth pointing out that Illumina has now developed an iPad and iPhone application which allows the user to explore a real human genome.62
The increasing scientific knowledge regarding skin and hair biology would be automatically updated and be made accessible to the consumer in order to look for the composition that would contain the right balance of active ingredients for their body.
In a recent article63 where she quoted Datamonitor’s researcher Aleksandrina Yotova, Michelle Yeomans stated that consumers are looking for digital devices that change the way they communicate with the brands, especially when new products use technology progress for a purpose, rather than just offering a complementary advantage.
The development of simple telephone accessible applications such as the one described above would certainly be appreciated by consumers who are interested in profitting from their personal genomic data.
This type of approach might have seemed science fiction a few years ago, but nowadays it is only a few steps away, and one can predict that the consumers will be able to use mobile apps to select cosmetic products as they presently select the food and other items that they use on a daily basis.
Indeed, several studies have identified genetic and environmental factors that are associated with an increased risk of developing Atopic Dermatitis (AD) a frequent chronic inflammatory disease that is characterized by skin itching.
The GWAS studies that have been conducted with different populations have identified several genes associated with AD, among which are those involved in the innate and adaptative immune system responses.
Including these genes and epigenetic factors that are likely linked to the diverse pathophysiology of AD (Tamari and Hirota 2014) the DTC type of approach schematized above would permit customers to avoid using cosmetic preparations that contains compounds favoring inflammatory responses.
Progress made in the understand of this illness points to an increasing number of cytokines which control cell signaling and communication, suggesting that each patient shows a particular pattern of response and therefore stresses the need for a personalized approach at a medical level but also opens the road for personalized choice offered to customers who wish to use cosmetics at no risk.
Similar types of information have been obtained regarding psoriasis susceptibility genes, antioxidant activity and lipid peroxidation-related skin diseases, skin pigmentation, senecence and aging, photodamaging and repair genes in skin quality and turn over.
The extraordinary growth of sequencing technologies has already led to the replacement of previous protocols with Next Generation Sequencing that considerably reduces both time and cost of the process (Lai-Cheong and McGrath 2011). Considering the very strong pressure that will be brought to bear by personalized medicine based on genome sequencing, one can expect that affording personal genome sequencing will not be restricted to wealthy people in the very near future.
Furthermore, it is expected that application of these new technologies will increase the size and number of datasets, that will become available to the scientific community and eventually to the customers. The compilation of various independent data libraries should ease the identification of DNA rearrangements, and other mutations that are of interest in the order to assess the potential predictive value of polymorphisms for the benefit of customers.
Discussion
In updates published by 23andMe,64,65 after the suspension decision of the FDA, one could read that « i) customers who received health-related results prior to November 22, 2013 will continue to have access to that information. ii) Customers who purchased kits before November 22, 2013 will still receive health-related results. iii) Customers who purchase or have purchased 23andMe’s Personal Genome Service (PGS) on or after November 22, 2013, the date of the Warning Letter from the FDA, will receive ancestry information, as well as their raw genetic data without interpretation. These new customers may receive additional health-related results in the future, dependent upon FDA marketing authorization. » Instructions for possible refund were provided. »
Later on, 23andMe published a press release66 in which the company diclosed an agreement signed with Pfizer Inc. to collaborate on Research Genetics of ulcerative colitis and Crohn’s disease, two manifestations of Inflammatory Bowel Disease (IBD). Crohn’s can affect any part of the digestive system, whereas ulcerative colitis affects the rectum and colon.
In this study, 23andMe aims at recruitment of 10, 000 people with IBD. Customers who will send their saliva samples for the test, will receive information regarding IBD studies but will not receive any risk assessments in addition to their raw data and ancestry information,67 in accordance with the FDA restrictions discussed above.
In August 2014, 23andMe submitted for approval by the FDA, a health report regarding assessement of customers for Bloom syndrome.68 Other DTC companies, such as Natera, and Counsyl and Pathway Genomics, offer genetic tests which do not need FDA approval because they are provided through medical doctors. Therefore, the 23andMe application might be a stone in the garden of its competitors, and the first step towards getting back to better relationships with the agency and opening the way for approval of other DTC tests in the future.
Concerns regarding the mode of collection, storage and use of personal data have been expressed very early in the media and scientific meetings. It is still a matter of intense debate, on both economic and legal standpoints69 .
The success of 23 and Me in identifying polymorphisms associated with Parkinson’s disease had confirmed that the great number of sequences analysed in a DTC context could lead to significant scientific achievements.
Even though personal data collected by 23andMe had been used with the consent of the participants, the economic impact of this information had not been fully measured by consumers who voluntarily accepted to participate in the advancement of scientific and medical knowledge.
The social dimension of this problem was highlighted recently when the company 23andMe announced70 on May 28, 2012 that it would get the next day its first patent 71 on “ polymorphisms associated with Parkinson’s Disease . ”
This announcement triggered an awareness of consumers, many of whom reacted very strongly to the idea of having been deceived by the company 23andMe when there were told that providing the authorization to use their personal data would give them the opportunity to contribute to fundamental discoveries for the benefit of the whole society.
With the consent mentioned above, 23andMe showed a clear willingness to protect the use of results whose diffusion would have potentially opened new paths for competitors.
What seems to have offended a large number of participants in this study is the fact that they were not told about the ultimate commercial purpose of the data acquisition. Furthermore, the data were published within a timeframe compatible with the filing of the patent. Some participants even said that if they had been aware of the goals, they would not have participated and not suggested family members to follow suit.
The relationships established by consumers and 23andMe when signing the contract and the breach of trust issues raised by this patent were discussed elsewhere (Sterckx et al. 2013).
As recently suggested 72 the real value of 23andMe might be in the collection of data that has been accumulated over the years.
Mishap or not, this example was worth considering, because it highlighted the type of problems that may underly collection and usage of personal data for DTC, as was expected from previous examples in rapidly developing scientific fields, when legal issues are rapidly raised as the result of the fast pace of technical progress that overwhelms regulatory committees.
As of today, it appears that 23andMe is the sole DTC company still fighting for the freedom of consumers to know about their genome, and to manage as they wish the information that they can get about their genes. As the leader, and survivor in the field of DTC in the USA, 23andMe faced agressive critiscisms regarding the 99USD price of its DNA-analysis kit that was considered as unfair for the competition in consumer genetics (see Dorfman 2013 and response by Wojcicki 2013).
In a very lively talk that she gave at Austin,73 in which she commented on « ups and downs » that pioneers always face in their way to progress, Anne Wojcicki presented her views regarding « The Future of Genetics in People’s Lives » and the impact that the sequencing technologies that we can expect in a not so far off future.
I strongly recommend our readers to watch the video of this inspiring and extremely informative presentation.
23andMe in a worldwide competition
A key message that I gathered is that the 23andMe business model is based on respectful consideration of customers, protection of their personal data and reciprocal trust. As clearly stated by Anne Wojcicki, the main goal of the company is to provide all customers with a better healthcare system in which they will be real actors.
Although Anne Wojcicki said that she is quite happy that 23andMe presently has the largest database of genotyped human DNA74, she pointed out that while FDA’s decision puts a hold on DTC genetics in the USA, a few foreign countries including China,75 Saudi Arabia, UK and Scotland, have put a lot of effort in getting human DNA databases. The situation in the European community has been reviewed recently (Borry et al. 2012). In France, Germany, Switzerand and Portugal, genetic tests can be performed only by a medical doctor, whereas Belgium and the United Kingdom allow DTC genetic tests. Netherlands’ legislation allows a governmental ban on tests whose benefits would not be in balance with potential health risks.
An instructive example is provided by the China’s Beijing Genomics Institute (BGI-Shenzhen), a leading international organization for research in genetics that has acquired the US Complete Genomics company in 2013, and has already analysed the genome of nearly 60, 000 people76 with the aim of achieving « the million human project ».
For information, « the 1000 genomes project consortium aims to provide a deep catalog of human genome sequence variation as a foundation for investigating the relationship between genotype and phenotype ».77
The BGI company claims that the data, that will be obtained with DNA genetics, will cover many unforseen applications such as improvement of average lifespan, decoding of human genetic diseases, understanding the influence of genetics on intelligence, evolution, and the effects of epigenetics in genes expression.
Whether the 1 million genomes database will be accessible to researchers around the world or remain under the sole control of BGI is a critical issue.
The generation and accumulation of biomedical genetic data by private companies has often been presented as a source of concern. Indeed, it is important to have a clear idea of what will be the fate of all the private information that is collected and stored by a private company when it is acquired by another group, as discussed previously in this text.
In this economical and sociological context it may seem rather surprising that the FDA still allows the American customers to receive their raw data from 23andMe, when it is very easy nowadays to send it for interpretation to another company anywhere else in the world, without any guaranty for accuracy, quality and privacy.78
The creation of an open web resource which allows DTC customers to share at no cost both their genetic data and their phenotypic information, and to receive information related to their genotypes (Greshake et al. 2014) might represent an interesting alternative to these issues.
Fantasy and facts
The rapid development of high throughput DNA analysis techniques and the huge correlative decrease in sequencing cost has revolutionized the fields of DNA genomics and human biology.
Since the very first days of the human genome project, people have fantasized about the power that could be drawn from knowing the whole sequence of a human being, including transfer of human genetic information in « humanized robots »79 .
Claims that the whole genome sequencing coupled to the Big Data technologies will open the way to human eternal life, ignore how epigenetic factors, independent of DNA structure, are involved in adaptation, learning and creating the new communication networks that underly species evolution.
The idea spread by a few people in the business of DNA sequencing that the sole manipulation of the genetic information contained in the human DNA will permit people to avoid aging sounds like a nice science fiction and philosophical topic, but does not take into account the basics of cell biology.
In this context I personally like the provocative idea that « the immortal phenotype exists by default and that cell mortality is an evolutionary phenomenon acquired by eukaryotes (Takagi 1999). Mortality would have been created during evolution through sex with outcrossing and recombination » (Macieira-Coelho 2003)
On a more realistic ground, recent exome sequencing results of 90, 000 human genomes allowed the identification of about 150, 000 genes that were naturally knocked-out in the cohort that was studied, therefore opening the way to the identification of genomic variations that are associated to worse or better conditions in the whole population.80
The reduction in the whole genome sequencing cost to 1000 USD, that was announced by Illumina, is expected to open the way to more extensive genome sequencing programs and pave the road to « breakthroughs in personalized medicine ».81 Even more promising is the prospect of a 30 USD genome sequencing cost based on GnuBio 82sequencing technology developed by by David Weitz.83
Even though the use of SNPs-based genome analysis for predictive tests can be very accurate and informative, there is no doubt that within a few years, DTC companies will migrate to more comprehensive sequencing of exomes and whole genomes.
Switching from SNP analysis of genetic inheritable disorders to next-generation sequencing (NGS) is expected to reduce considerably the cost of DNA analysis and at the same time deliver a significant increase in the information that can be made available to the customers and patients.
Interpretation of a such large mass of data accurately places it within the context of the increasing number of other sequenced genomes, communicating large amounts of complex information to the customer or patient.
Securely storing the data to allow its useful accessibility will be among the big challenges to face.
Now that we are in the era of « Big Data » treatment, the fear of re-identification of anonymized personal data from internet resources that are publicly accessible becomes a real concern (Erlich et al. 2014) and needs to be addressed, both on scientific, medical and legal grounds.84
Perspectives
Whether communicating personal health information to a patient is a good thing or not has been the subject of debate for decades. The extraordinary impact of the internet on our inter-cultural communication cannot be ignored. People want to communicate about themselves, about their lives, about their aspirations, about their problems and much more.
The societal impact of DNA genetics is considerable and must trigger thoughtful decisions based on discussions involving all parties.
I do not think that it would be reasonable to avoid having people knowing about their genes. We should let them decide what they want to do with this private information that they own. This is an ongoing societal modification.
I believe that human beings are able to cope with the information regarding their healthcare, whether or not it is provided by a physician, as long as the information that is provided is accurate, well explained, and presented in a realistic and practical way.
Is it or is it not going to change our life to know if we are the carrier of a genetic heritable defect that might increase our chances to develop an illness and affect our offspring ?
The answer will mainly depend upon the means that are available and provided at that time to evaluate the risk and to possibly interfere with the illness.
Understanding the notion of risk is a critical point.
As a reminder, the BRCA mutations that are associated with increased risk of developing breast and uteral cancers are not uniformly detected in patients with cancers.
In conclusion, I am confident that webs of communications involving consumers, patients, physicians, and DTC companies, will bring keys to establish new ways of tackling and actively managing our own healthcare in the near future.
Acknowledgments
I am deeply indebted to my friend and colleague Dr. H. Yeger for his critical review of the manuscript and to Annick for her daily support.
Footnotes
At the time the present manuscript is written, the 2003 editorial has been downloaded 17,000 times, a sign that the topic is of great interest to our scientifc community and redership.
According to the NIH (http://gds.nih.gov/pdf/PTC_for_IRBs_and_Institutions_revised5-31-11.pdf) A genome-wide association study (GWAS) is a study of genetic variation across the entire human genome that is designed to associate genetic variations (SNPs) with traits (such as blood pressure or weight) or with the presence or absence of a disease or condition. This type of study is a comprehensive measurement of all or nearly all variation in all human chromosomes, and sometimes mitochondrial DNA as well. GWAS typically involve hundreds of markers, rather than, for example, studies of candidate genes or targeted chromosomal regions. To meet the definition of a GWAS, the density of genetic markers and the extent of linkage disequilibrium should be sufficient to capture (by the r2 parameter) a large proportion of the common variation in the genome of the population under study, and the number of samples (in a case-control or trio design) should provide sufficient power to detect variants of modest effect.
Hindorff LA, MacArthur J (European Bioinformatics Institute), Morales J (European Bioinformatics Institute), Junkins HA, Hall PN, Klemm AK, and Manolio TA. A Catalog of Published Genome-Wide Association Studies. Available at: www.genome.gov/gwastudies. Accessed november 3rd, 2014.
International Society of Genetic Genealogy. http://www.isogg.org/
National Geographic Society. www.nationalgeographic.com/about/
http://www.thegeneticgenealogist.com/2014/03/05/the-2014-international-genetic-genealogy-conference/
Direct-to-Consumer Genetic Tests. Misleading Test Results Are Further Complicated by Deceptive Marketing and Other Questionable Practices
Is a DNA Scan a Medical Test or Just Informational? Views Differ http://www.nytimes.com/2010/03/20/business/20consumergenebar.html?_r=0
Thermo Fisher Scientific Completes Acquisition of Life Technologies Corporation http://www.marketwatch.com/story/thermo-fisher-scientific-completes-acquisition-of-life-technologies-corporation-2014-02-03
Genome and Nation, Iceland's Health Sector Database and its Legacy
http://ourenvironment.berkeley.edu/wp-content/uploads/2011/09 GENOME_NATION.pdf
Meyer, Michelle N., Comparative Law - Genetic Privacy - Icelandic Supreme Court Holds that Inclusion of an Individual’s Genetic Information in a National Database Infringes on the Privacy Interests of His Child (December 1, 2004). Harvard Law Review, Vol. 118, p. 810, 2004; Harvard Public Law Working Paper. Available at SSRN: http://ssrn.com/abstract=2106154
Icelandic Supreme Court No. 151/2003.
The topic of right to privacy regarding personal data will be the subject of a forthcoming editorial.
Big biotech buys iconic genetics firm
http://www.nature.com/news/big-biotech-buys-iconic-genetics-firm-1.12068
Amgen to Acquire deCODE Genetics, a Global Leader in Human Genetics
This topic will be adressed and discussed in a forthcoming editorial.
Is a DNA Scan a Medical Test or Just Informational? Views Differ http://www.nytimes.com/2010/03/20/business/20consumergenebar.html?_r=0
Molecular and Clinical Genetics Panel of the Medical Devices Advisory Committee. http://www.regulations.gov/#!documentDetail;D=FDA-2011-N-0066-0001; March 8-9, 2011: Molecular and Clinical Genetics Meeting Announcement http://www.fda.gov/AdvisoryCommittees/Calendar/ucm242537.htm
the presentation given by Ashley Gould, General Counsel at 23andMe is available on line at http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-adcom/documents/document/ucm248565.pdf
FDA Panel Says Home Gene Tests Need MD Input http://www.medpagetoday.com/Genetics/GeneticTesting/25280
MIT technology review 12 JAN 2011 Consumer Genetic Tests Have Little Impact http://www.technologyreview.com/news/422373/consumer-genetic-tests-have-little-impact/
see libertarianism as an example of philosophical movement for maximal freedom and minimal governemental interference in people life style choices
FDA Panel Says Home Gene Tests Need MD Input http://www.medpagetoday.com/Genetics/GeneticTesting/25280
The End for Direct-to-Consumer Genetic Testing? http://www.technologyreview.com/view/423270/the-end-for-direct-to-consumer-genetic-testing/
Mary Pendergast’s testimony during the public hearing cited FDA « Panel Says Home Gene Tests Need MD Input http://www.medpagetoday.com/Genetics/GeneticTesting/25280 »
MacArthur wrote in his blog (Snippets From Day One Of The FDA Meeting On Consumer Genetics
http://www.wired.com/2011/03/snippets-from-day-one-of-the-fda-meeting-on-consumer-genetics/ . American Medical Association: You Can’t Look At Your Genome Without Our Supervision
http://www.wired.com/2011/02/american-medical-association-you-cant-look-at-your-genome-without-our-supervision/) that the recommendation of the panel was « an obsolete, paternalistic vision for genomic medicine that flies in the face of primary physicians’ self-admitted ignorance of genetics »
many observers and analysts pointed out the difficulties that general public faced when claiming the free choice to buy pregnancy tests for use at home or the freedom to now about their blood tests results.
American Medical Association: You Can’t Look At Your Genome Without Our Supervision. http://www.wired.com/2011/02/american-medical-association-you-cant-look-at-your-genome-without-our-supervision/
Jeremy Gruber in Topic Update: Direct-To-Consumer Genetic Testing Criticized at FDA Hearing. http://www.councilforresponsiblegenetics.org/genewatch/GeneWatchPage.aspx?pageId=327
American Medical Association: You Can’t Look At Your Genome Without Our Supervision
Inspections, Compliance, Enforcement, and Criminal Investigations WARNING LETTER 2013 23ANDME http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm376296.htm
Direct-to-consumer genomics reinvents itself Malorye Allison Nature Biotechnology30,1027–1029 (2012)
http://www.nature.com/nbt/journal/v30/n11/full/nbt.2409.html
Just the facts, please http://www.nature.com/nbt/journal/v31/n12/full/nbt.2771.html
23andMe Myeloprofiferative Neoplasm (MPN) Research Initiative
23andMe Research Findings https://www.23andme.com/about/factoid/factoid_mpn_jak2/
23andMe and Genentech Expand Cancer Study http://blog.23andme.com/23andme-research/23andme-and-genentech-expand-cancer-study/
Incorporating Technology and DNA Testing into Clinical Trials
Google-Backed 23andMe Asks FDA to Clear DNA Evaluation Service
My Medical Choice By Angelina Jolie Published: May 14, 2013
http://www.nytimes.com/2013/05/14/opinion/my-medical-choice.html?_r=0
“I remember the appointment so vividly” – carrying the BRCA1 gene
The FDA Just Ruined Your Plans To Buy 23andMe’s DNA Test As A Christmas Present
http://www.forbes.com/sites/kashmirhill/2013/11/25/fda-23andme/
Celebrity cancer stories: help or hindrance?
http://scienceblog.cancerresearchuk.org/2013/07/05/celebrity-cancer-stories-help-or-hindrance/
Scientists inch closer to personal risk prediction – for some
Why The FDA Can’t Be Flexible With 23andMe, By Law
http://www.forbes.com/sites/davidkroll/2013/11/28/why-the-fda-cant-be-flexible-with-23andme-by-law/
these issues will be addressed and commented in a forthcoming editorial.
FDA To Regulate Thousands Of Cancer, Genetic, And Other Diagnostics
Long-Awaited Announcement from the FDA on LDTs
FDA notification to the congress
List of DNA testing companies last modified on 26 October 2014 http://www.isogg.org/wiki/List_of_DNA_testing_companies,
MyGenome By Illumina, Inc.
https://itunes.apple.com/us/app/mygenome/id516405838?mt=8
Illumina Launches MyGenome(R) App for iPad(R)First Tool of Its Kind for Visualizing the Human Genomehttp://investor.illumina.com/phoenix.zhtml?c=121127&p=irol-newsArticle&ID=1686310
What’s the next step in ‘smart & connected’ product development? By Michelle Yeomans, 23-Oct-2014
Contemporary consumers expect technology to make their lives easier and more comfortable and the cosmetics sector is taking inspiration from the internet and cashless vending machines to drive more sophisticated and advanced innovations.
23andMe Stops Offering Genetic Tests Related To Health
23andMe, INC. Provides Update on FDA Regulatory Review
http://mediacenter.23andme.com/blog/2013/12/05/23andme-inc-provides-update-on-fda-regulatory-review/
23andMe Announces Agreement with Pfizer Inc. to Research Genetics of Ulcerative Colitis and Crohn’s Disease
Pfizer continues to explore virtual trials with 23andMe pact http://www.fiercebiotechit.com/story/pfizer-continues-explore-virtual-trials-23andme-pact/2014-08-18
Seven Months After FDA Slapdown, 23andMe Returns With New Health Report Submission
these aspects will be discussed in another Editorial shortly .
(U.S. 8,187,811 B2)
For 23andMe, The Real Value Could Be In Its Data
The Future of Genetics in People’s Lives http://blog.23andme.com/news/the-future-of-genetics-in-peoples-lives/
650 000 genotyped customers at the time of the conference
the gene factory in the new yorker jan 6, 2014
http://www.newyorker.com/magazine/2014/01/06/the-gene-factory
the gene factory in the new yorker jan 6, 2014
http://www.newyorker.com/magazine/2014/01/06/the-gene-factory
see Anne WOjcicki comment on this point at : 23andMe Tries to Woo the FDA. MIT Technology Review. This topic will also be discussed in a forthcoming editorial
see the 2012 excellent swedish TV series « Real Humans » based on a story written Lars Lundström and directed by Harald Hamrell and Levan Akin
geneticists tap human knockouts
http://www.nature.com/news/geneticists-tap-human-knockouts-1.16239
Is the $1000 genome for real?
http://www.nature.com/news/is-the-1-000-genome-for-real-1.14530
Bio-Rad Acquires GnuBIO, Developer of Droplet-Based DNA Sequencing Technology
Consumer Genetics Puts on a Show
http://www.bio-itworld.com/2010/issues/jul-aug/consumer-genetics.html
this issue will be discussed in a forthcoming editorial
References
- Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS, Wineinger NE, Darst BF, Schork NJ, Topol EJ. Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet. 2013;50(6):393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- Borry P, Cornel MC, Howard HC. Where are you going, where have you been: a recent history of the direct-to-consumer genetic testing market. J Community Genet. 2010;1(3):101–106. doi: 10.1007/s12687-010-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borry P, Howard HC, Sénécal K, Avard D. Health-related direct-to-consumer genetic testing: a review of companies’ policies with regard to genetic testing in minors. Fam Cancer. 2010;9(1):51–59. doi: 10.1007/s10689-009-9253-9. [DOI] [PubMed] [Google Scholar]
- Borry P, van Hellemondt RE, Sprumont D, Jales CF, Rial-Sebbag E, Spranger TM, Curren L, Kaye J, Nys H, Howard H. Legislation on direct-to-consumer genetic testing in seven European countries. Eur J Hum Genet. 2012;20(7):715–721. doi: 10.1038/ejhg.2011.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman R. Falling prices and unfair competition in consumer genomics. Nat Biotechnol. 2013;31:785–786. doi: 10.1038/nbt.2693. [DOI] [PubMed] [Google Scholar]
- Erlich Y, Williams JB, Glazer D, Yocum K, Farahany N, Olson M, Narayanan A, Stein LD, Witkowski JA, Kain RC. Redefining genomic privacy: trust and empowerment. PLoS Biol. 2014;12(11):e1001983. doi: 10.1371/journal.pbio.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greshake B, Bayer PE, Rausch H, Reda J. OpenSNP--a crowdsourced web resource for personal genomics. PLoS One. 2014;9(3):e89204. doi: 10.1371/journal.pone.0089204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV, Rezai-Delui H, Legius E, Le Merrer M, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23(1):94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson KN. Variant of TREM2 associated with the risk of Alzheimer’s disease. Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, Ock MS, Eo J, Kim HS, Cha HJ. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014;545(2):185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor h polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, McGrath JA. Next-generation diagnostics for inherited skin disorders. J Investig Dermatol. 2011;131(10):1971–1973. doi: 10.1038/jid.2011.253. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A. Historical and current concepts of the mechanisms of aging. Berlin: Springer; 2003. [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. Communication is the key. Cell Commun Signal. 2003;1(1):3. doi: 10.1186/1478-811X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: a centralized communication network. J Cell Commun Signal. 2013;7(3):169–177. doi: 10.1007/s12079-013-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnar T, Sulem P, Thorleifsson G, Vermeulen SH, Helgason H, Saemundsdottir J, Gudjonsson SA, Sigurdsson A, Stacey SN, Gudmundsson J, Johannsdottir H, Alexiusdottir K, Petursdottir V, Nikulasson S, Geirsson G, Jonsson T, Aben KK, Grotenhuis AJ, Verhaegh GW, Dudek AM, Witjes JA, van der Heijden AG, Vrieling A, Galesloot TE, De Juan A, Panadero A, Rivera F, Hurst C, Bishop DT, Sak SC, Choudhury A, Teo MT, Arici C, Carta A, Toninelli E, de Verdier P, Rudnai P, Gurzau E, Koppova K, van der Keur KA, Lurkin I, Goossens M, Kellen E, Guarrera S, Russo A, Critelli R, Sacerdote C, Vineis P, Krucker C, Zeegers MP, Gerullis H, Ovsiannikov D, Volkert F, Hengstler JG, Selinski S, Magnusson OT, Masson G, Kong A, Gudbjartsson D, Lindblom A, Zwarthoff E, Porru S, Golka K, Buntinx F, Matullo G, Kumar R, Mayordomo JI, Steineck DG, Kiltie AE, Jonsson E, Radvanyi F, Knowles MA, Thorsteinsdottir U, Kiemeney LA, Stefansson K. Genome-wide association study yields variants at 20p12.2 that associate with urinary bladder cancer. Hum Mol Genet. 2014;23(20):5545–5557. doi: 10.1093/hmg/ddu264. [DOI] [PubMed] [Google Scholar]
- Stacey SN, Sulem P, Gudbjartsson DF, Jonasdottir A, Thorleifsson G, Gudjonsson SA, Masson G, Gudmundsson J, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Fuentelsaz V, Corredera C, Grasa M, Planelles D, Sanmartin O, Rudnai P, Gurzau E, Koppova K, Hemminki K, Nexø BA, Tjønneland A, Overvad K, Johannsdottir H, Helgadottir HT, Thorsteinsdottir U, Kong A, Vogel U, Kumar R, Nagore E, Mayordomo JI, Rafnar T, Olafsson JH, Stefansson K. Germline sequence variants in TGM3 and RGS22 confer risk of basal cell carcinoma. Hum Mol Genet. 2014;23(11):3045–3053. doi: 10.1093/hmg/ddt671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan DL, Shuster LT, Wick MJ, Swanson CL, Pruthi S, Bakkum-Gamez JN. Challenging and complex decisions in the management of the BRCA mutation carrier. J Women’s Health (Larchmt) 2013;22(10):825–834. doi: 10.1089/jwh.2013.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Sulem P, Helgason H, Grarup N, Sigurdsson A, Helgadottir HT, Johannsdottir H, Magnusson OT, Gudjonsson SA, Justesen JM, Harder MN, Jørgensen ME, Christensen C, Brandslund I, Sandbæk A, Lauritzen T, Vestergaard H, Linneberg A, Jørgensen T, Hansen T, Daneshpour MS, Fallah MS, Hreidarsson AB, Sigurdsson G, Azizi F, Benediktsson R, Masson G, Helgason A, Kong A, Gudbjartsson DF, Pedersen O, Thorsteinsdottir U, Stefansson K. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46(3):294–298. doi: 10.1038/ng.2882. [DOI] [PubMed] [Google Scholar]
- Sterckx S, Cockbain J, Howard H, Huys I, Borry P. “Trust is not something you can reclaim easily”: patenting in the field of direct-to-consumer genetic testing. Genet Med. 2013;15(5):382–387. doi: 10.1038/gim.2012.143. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsson G, Mikaelsdottir E, Palsson R, Indridason OS, Holm H, Jonasdottir A, Helgason A, Sigurdsson S, Jonasdottir A, Sigurdsson A, Eyjolfsson GI, Sigurdardottir O, Magnusson OT, Kong A, Masson G, Sulem P, Olafsson I, Thorsteinsdottir U, Gudbjartsson DF, Stefansson K (2014) Rare mutations associating with serum creatinine and chronic kidney disease. Hum Mol Genet. [Epub ahead of print] [DOI] [PubMed]
- Takagi Y. Clonal life cycle of paramecium in the context of evolutionary acquired mortality. In: Macieira-Coelho A, editor. Progress in molecular and subcellular biology. Berlin Heidelberg New York: Springer; 1999. pp. 81–101. [DOI] [PubMed] [Google Scholar]
- Tamari M, Hirota T. Genome-wide association studies of atopic dermatitis. J Dermatol. 2014;41:213–220. doi: 10.1111/1346-8138.12321. [DOI] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicki A. Just the facts, please. Nat Biotechnol. 2013;31:1075–1076. doi: 10.1038/nbt.2771. [DOI] [PubMed] [Google Scholar]