Abstract
Background
About 1% of adults suffer from painful osteoarthritis of the ankle. The current literature contains no information on the percentage of such patients who derive long-term relief of symptoms from conservative treatment. Advanced ankle osteoarthritis can be treated with non-joint-preserving measures, such as total ankle replacement and ankle fusion.
Methods
This review is based on selected relevant publications, guidelines from Germany and abroad, and the authors’ personal experience.
Results
Before surgery is considered, conservative measures such as physiotherapy and orthopedic aids should be used to the fullest possible extent. No randomized trials have yet been published comparing total ankle replacement with ankle fusion. Total ankle replacement with newer types of prosthesis yields good to very good intermediate-term and long-term results, with mean success rates of up to 90% at 10 years (range, 68–100%). Independent risk factors for the failure of ankle replacement are age over 70 years (odds ratio [OR] 3.84), primary osteoarthritis (OR 7.19), post-traumatic osteoarthritis (OR 6.2), and type of prosthesis (e.g., single hydroxyapatite coating: OR 15.04). The average range of motion of the replaced ankle joint is 25° to 30°, with values as high as 60°.
Conclusion
Total ankle replacement is a good treatment option for complete, end-stage ankle arthritis. It can restore joint function and make the patient mobile with little or no pain. There are, however, many contraindications to be taken into account. There is a need for further studies of the biomechanics of arthritic and replaced ankle joints and for long-term follow-up studies of total ankle replacement.
Osteoarthritis of the ankle is an increasing issue in the healthcare sector (1, e1, e2). Approximately 1% of the adult population suffers from painful ankle osteoarthritis (2). The psychological and physical limitations associated with advanced ankle osteoarthritis are at least as marked as those of patients with osteoarthritis of the knee or hip (2). Degenerative changes of the ankle, in contrast to osteoarthritis of the knee or hip, are usually posttraumatic (Table 1, eTable 1) (3, e3, e4). Both poorly healed fractures to the lower extremity (4, e5) and repetitive ligament injuries (5) can play a major role. The main causes of secondary osteoarthritis of the ankle include rheumatic diseases, hemophilia, hemochromatosis, gout, avascular necrosis, and postinfectious states (1, e1).
Table 1. Etiology of advanced ankle osteoarthritis, based on a selection of clinical and epidemiological studies*.
| Studya | Study type | Patients (ankles) | Etiology of ankle osteoarthritis, % (absolute values) | ||
|---|---|---|---|---|---|
| Primary | Secondary | Posttraumatic | |||
| (e6) | RS, SC, clinical | 45 (51) | 25.5% (13) | 54.9% (28) | 19.6% (10) |
| (6) | PS, SC, clinical | 684 (722) | 9.5% (69) | 11.4% (82) | 79.1% (571) |
| (e7) | PS, SC, clinical | 47 (50) | 6.0% (3) | 8.0% (4) | 86.0% (43) |
| (e8) | PS, SC, clinical | 49 (50) | 16.0% (8) | 18.0% (9) | 66.0% (33) |
| (e9) | PS, MC register, clinical | 245 (257) | 20.6% (53) | 55.3% (142) | 24.1% (62) |
| (e10) | PS, SC, clinical | 80 (83) | 33.7% (28) | 25.3% (21) | 41.0% (34) |
| (e11) | RS, SC, clinical | 111 (123) | 52.8% (65) | 18.7% (23) | 28.5% (35) |
| (e12) | RS, SC, clinical | 61 (62) | 19.4% (12) | 4.8% (3) | 75.8% (47) |
| (e13) | RS, SC, clinical | 45 (52) | 50.0% (26) | 26.9% (14) | 23.1% (12) |
| (e14) | RS, SC, clinical | 126 (132) | 46.2% (61) | 25.0 (33) | 28.8% (38) |
| (e15) | PS, SC, clinical | 43 (50) | 54.0% (27) | 32.0% (16) | 14.0% (7) |
| (e16) | PS, SC, clinical | 396 (404) | 16.6% (67) | 13.6% (55) | 69.8% (282) |
| (e17) | PS, SC, clinical | 80 (84) | 25.0%(21) | 19.0%(16) | 56.0%(47) |
| (e18) | PS, SC, clinical | 82 (82) | 34.2% (28) | 13.4% (11) | 52.4% (43) |
| (e19) | RS, SC, clinical | 95 (100) | 26.0% (26) | 29.0% (29) | 45.0% (45) |
| (e20) | PS, SC, clinical | 229 (229) | 13.8% (32) | 4.0% (9) | 82.2% (188) |
| (e21) | PS, SC, clinical | 106 (106) | 52.8%(56) | 20.8%(22) | 26.4%(28) |
| (e22) | PS, SC, clinical | 233 (240) | 30.8% (74) | 17.9% (43) | 51.3% (123) |
| (3) | PS, SC, epidemiological | 639 (639) | 7.2% (46) | 23.2% (148) | 69.6% (445) |
| (7) | PS, MC, clinical | 593 (593) | 26.5% (157) | 15.3% (91) | 58.2% (345) |
| (e23) | RS, SC, clinical | 100 (100) | 30.0% (30) | 44.0% (44) | 26.0% (26) |
| (e24) | RS, MC, clinical | 501 (517) | 13.9% (72) | 25.9% (134) | 60.2% (311) |
| (e25) | PS, MC register, clinical | 515 (515) | 19.2% (99) | 59.2% (305) | 21.6% (111) |
| (e26) | RS, SC, clinical | 303 (306) | 25.2% (77) | 10.1% (31) | 64.7% (198) |
| (e27) | RS, SC, clinical | 103 (103) | 71.8% (74) | 18.5% (19) | 9.7% (10) |
| (e28) | PS, SC, clinical | 65 (68) | 13.2% (9) | 16.2% (11) | 70.6% (48) |
| (e3) | RS, SC, epidemiological | 390 (406) | 8.9% (36) | 12.8% (52) | 78.3% (318) |
| (e29) | PS, SC, clinical | 66 (66) | 0.0% (0) | 10.6% (7) | 89.4% (59) |
| (e4) | RS, SC, clinical | 226 (233) | 5.6% (13) | 23.2% (54) | 71.2% (166) |
| (e30) | PS, SC, clinical | 96 (100) | 64.0% (64) | 27.0% (27) | 9.0% (9) |
| (e31) | RS, SC, clinical | 90 (99) | 40.4% (40) | 12.1% (12) | 47.5% (47) |
| Total/mean | 6218 (6389) | 21.5% (1373) | 22.6% (1373) | 56.0% (3575) | |
*Clinical (total ankle replacement) and epidemiological (etiology of ankle osteoarthritis) studies with at least 50 patients were included.
MC, multicenter; PS, prospective; RS, retrospective; SC, single-center
eTable 1. Etiology of advanced ankle osteoarthritis, based on a selection of clinical and epidemiological studies*.
| Study | Study type | Patients (ankles) | Etiology of ankle osteoarthritis, % (absolute values) | ||
|---|---|---|---|---|---|
| Primary | Secondary | Posttraumatic | |||
| (e6) | RS, SC, clinical | 45 (51) | 25.5% (13) | 54.9% (28)
|
19.6% (10) |
| (6) | PS, SC, clinical | 684 (722) | 9.5% (69) | 11.4% (82) | 79.1% (571) |
| (e7) | PS, SC, clinical | 47 (50) | 6.0% (3) | 8.0% (4) |
86.0% (43) |
| (e8) | PS, SC, clinical | 49 (50) | 16.0% (8) | 18.0% (9) |
66.0% (33) |
| (e9) | PS, MC register, clinical | 245 (257) | 20.6% (53) | 55.3% (142) | 24.1% (62)
|
| (e10) | PS, SC, clinical | 80 (83) | 33.7% (28) | 25.3% (21)
|
41.0% (34) |
| (e11) | RS, SC, clinical | 111 (123) | 52.8% (65) | 18.7% (23) | 28.5% (35) |
| (e12) | RS, SC, clinical | 61 (62) | 19.4% (12) | 4.8% (3) |
75.8% (47) |
| (e13) | RS, SC, clinical | 45 (52) | 50.0% (26) | 26.9% (14) | 23.1% (12) |
| (e14) | RS, SC, clinical | 126 (132) | 28.8% (38) | 25.0 (33) |
46.2% (61) |
| (e15) | PS, SC, clinical | 43 (50) | 54.0% (27) | 32.0% (16) | 14.0% (7) |
| (e16) | PS, SC, clinical | 396 (404) | 16.6% (67) | 13.6% (55) | 69.8% (282) |
| (e17) | PS, SC, clinical | 80 (84) | 25.0% (21) | 19.0% (16) |
56.0% (47) |
| (e18) | PS, SC, clinical | 82 (82) | 34.2% (28) | 13.4% (11) | 52.4% (43) |
| (e19) | RS, SC, clinical | 95 (100) | 26.0% (26) | 29.0% (29) |
45.0% (45) |
| (e20) | PS, SC, clinical | 229 (229) | 13.8% (32) | 4.0% (9) | 82.2% (188) |
| (e21) | PS, SC, clinical | 106 (106) | 52.8% (56) | 20.8% (22) | 26.4% (28) |
| (e22) | PS, SC, clinical | 233 (240) | 30.8% (74) | 17.9% (43) |
51.3% (123) |
| (3) | PS, SC, epidemiological | 639 (639) | 7.2% (46) | 23.2% (148) |
69.6% (445)
|
| (7) | PS, MC, clinical | 593 (593) | 26.5% (157) | 15.3% (91) | 58.2% (345) |
| (e23) | RS, SC, clinical | 100 (100) | 30.0% (30) | 44.0% (44) | 26.0% (26) |
| (e24) | RS, MC, clinical | 478 (489) | 15.3% (75) | 25.8% (126) | 58.9% (228) |
| (e25) | PS, MC register, clinical | 515 (515) | 19.2% (99) | 59.2% (305) | 21.6% (111) |
| (e26) | RS, SC, clinical | 303 (306) | 25.2% (77) | 10.1% (31) | 64.7% (198) |
| (e27) | RS, SC, clinical | 103 (103) | 71.8% (74) | 18.5% (19) | 9.7% (10) |
| (e28) | PS, SC, clinical | 65 (68) | 13.2% (9) | 16.2% (11) |
70.6% (48)
|
| (e3) | RS, SC, epidemiological | 390 (406) | 8.9% (36) | 12.8% (52) |
78.3% (318)
|
| (e29) | PS, SC, clinical | 66 (66) | 0.0% (0) | 10.6% (7) |
89.4% (59)
|
| (e4) | RS, SC, clinical | 226 (233) | 5.6% (13) | 23.2% (54) |
71.2% (166)
|
| (e30) | PS, SC, clinical | 96 (100) | 64.0% (64) | 27.0% (27)
|
9.0% (9)
|
| (e31) | RS, SC, clinical | 90 (99) | 40.4% (40) | 12.1% (12) | 47.5% (47) |
| Total | 6218 (6389) |
21.5% (1373) |
22.6% (1441) |
56.0% (3575) |
|
*Clinical (total ankle replacement) and epidemiological (etiology of ankle osteoarthritis) studies with at least 50 patients were included.MC:
This review article uses the current literature to explain the indications and the absolute and relative contraindications for total ankle replacement. It also presents the results of current clinical studies on postoperative functional outcomes and the probability of success of ankle replacement surgery.
Selective literature search
This review article is based on a selective literature search in established databases. The following medical databases were searched, with no date restriction: Medline, Cochrane, EmbaseTM, Cinahl, Google Scholar, ScienceDirect, and SpringerLink. The search terms used were the following: “total ankle replacement,” “total ankle arthroplasty,” “ankle replacement,” “ankle arthroplasty,” and “ankle prosthesis.” All articles written in languages spoken by the authors (German, English, and French) were included.
The digital indices of the following orthopedic journals were also searched for the above-mentioned search terms: Foot and Ankle International; Journal of Bone and Joint Surgery, American Volume; Bone & Joint Journal (formerly known as the Journal of Bone and Joint Surgery, British Volume); Clinical Orthopaedics and Related Research; Foot and Ankle Clinics of North America; Journal of Foot and Ankle Surgery; and Der Orthopäde. In addition, the bibliographies of the identified original and review articles were searched for further studies.
The literature search was performed by two of the authors (AB and MDW), independently of each other.
History and implant designs
Most first-generation ankle replacements performed in the 1970s and early 1980s were two-component cemented implants. The rate of aseptic loosening for all first-generation implant types was extremely high, occurring in almost 90% of implants (8).
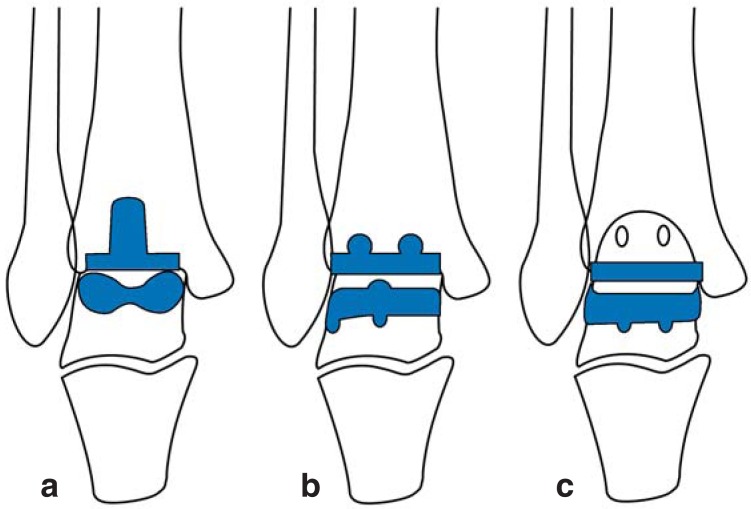
Second-generation ankle implants (from the mid-1980s onwards) show improved implant shapes and better surgical technique: bone-conserving surgical approach and no cementation. Today there are several commercially available ankle implant types (Figure 1). All implant designs can be classified by surgical technique and implant properties (eTable 2) (8).
Figure 1.
Modern ankle implant types
a) Components with tibial stem b) Components with bars c) Flat components
eTable 2. Classification of current ankle implant types.
| Surgical access | Inlay type | Replaced surfaces | Internal implant surfaces | Inlay materials | Sulcus type | Surface morphology | |
|---|---|---|---|---|---|---|---|
| AAA | Anterior | Mobile | Superior | HA | UHMWPE | None | Trapezoidal |
| AES | Anterior | Mobile | Superior | HA | UHMWPE | Deep | Trapezoidal |
| Agility | Anterior | Fixed | Superior/medial/ lateral | Titanium | UHMWPE | None | Trapezoidal |
| BOX | Anterior | Mobile | Superior | HA | UHMWPE | Normal | Ellipsoidal |
| Buechel-Pappas | Anterior | Mobile | Superior | Titanium | UHMWPE | Deep | Ellipsoidal |
| ESKA | Lateral/medial | Fixed | Superior | Titanium | UHMWPE | Normal | Ellipsoidal |
| INBONE | Anterior | Fixed | Superior | Titanium | UHMWPE | Normal | Spheroidal |
| HINTEGRA | Anterior | Mobile | Superior/medial | HA | UHMWPE | None | Conical |
| Mobility | Anterior | Mobile | Superior | Titanium | UHMWPE | Deep | Trapezoidal |
| Ramses | Anterior | Mobile | Superior | HA | UHMWPE | None | Ellipsoidal |
| Salto | Anterior | Mobile | Superior/medial | HA | UHMWPE | Normal | Conical |
| STAR | Anterior | Mobile | Superior | HA | UHMWPE | None | Cylindrical |
| TNK | Anterior | Fixed | Superior | HA | UHMWPE, ceramic | None | Cylindrical |
| TM Total Ankle | Lateral | Fixed | Superior | Porous metal | Highly cross-linked UHMWPE | None | Conical |
AAA: Alpha Ankle Arthroplasty; AES: Ankle Evolutive System; BOX: Bologna–Oxford; HA: hydroxyapatite; STAR: Scandinavian Total Ankle Replacement; TM: Trabecular Metal; UHMWPE: ultra-high-molecular-weight polyethylene
Diagnosis and preoperative planning
A clinical and radiological diagnosis of osteoarthritis of the ankle can be made by the patient’s treating physician on the basis of clinical and radiological examination, as described.
The first step in preoperative diagnosis is to take a clinical history. All available documents should be evaluated: it is important to note which, if any, treatment options have already been administered. Further information such as BMI body mass index), physical activity levels, previous and/or current treatment, severity of pain, limitations in everyday private and/or occupational activities, intake of analgesics, and concomitant diagnoses (diabetes mellitus, osteoporosis, polyneuropathy, etc.) should be recorded.
Clinical examination begins with examination of the foot/hindfoot on standing, sitting, and walking. Hindfoot alignment (valgus, varus, or neutral) is assessed from behind, with the patient standing. Stability is determined with the patient seated, using the talar tilt test (examination of medial and lateral ankle inversion) and the anterior drawer test (which tests for increased anterior translation of the talus) (9). Mobility of the subtalar joint is measured using a goniometer under load (10). Mobility of the ankle is measured manually, with the ankle fixed and free (e32).
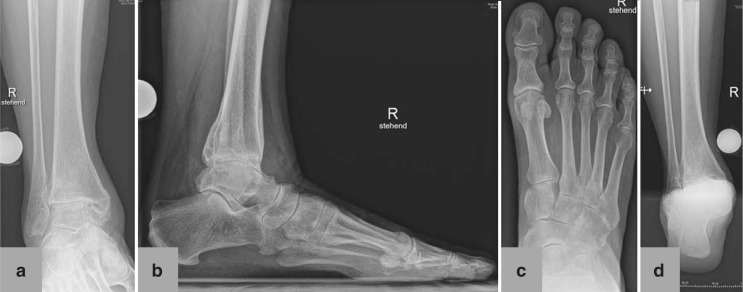
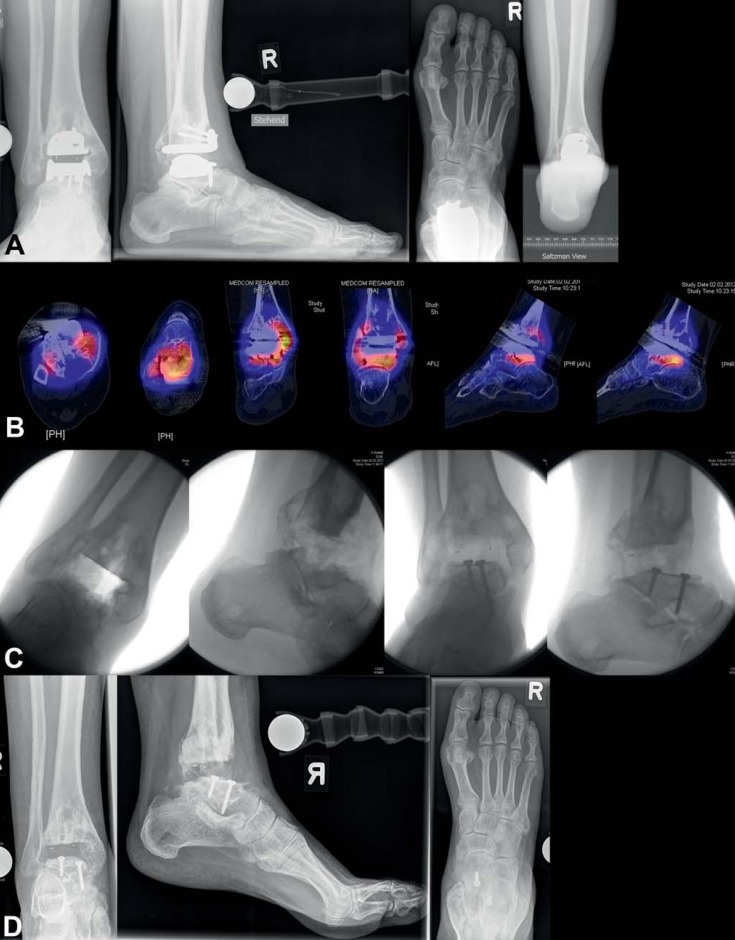
Radiological examination includes conventional weight bearing radiographs: dorsoplantar and lateral views of the foot, anteroposterior (mortise) and lateral views of the ankle, and Saltzman view (hindfoot alignment view to assess inframalleolar alignment [11]) (Figure 2). Supramalleolar alignment is determined using the of medial distal tibial angle (12, e33). In patients with knee deformities, a whole leg radiograph (orthoradiogram) is also taken. Optionally, computed tomography or magnetic resonance imaging may be performed; these can provide important additional information.
Figure 2.
Preoperative conventional X-ray in standing position of 67-year-old female patient with posttraumatic ankle osteoarthritis following open reduction and internation fixation for trimalleolar luxation fracture 4 years earlier: a) mortise view of ankle; b) lateral view of foot and ankle; c) dorsoplantar view of foot; d) Saltzman view of hindfoot
Indication for surgery
Conservative therapy should be administered before surgery is indicated. This includes intensive physiotherapy (local antiphlogistic therapy, muscle and movement exercises to prevent stiffness of the joint, muscle strength development, gait training) and possibly intra-articular hyaluronic acid viscosupplementation and orthopedic adaptation of footwear (13, 14).
The ideal indication for total ankle replacement is advanced, complete osteoarthritis of the ankle (primary, secondary, or posttraumatic) with good bone quality, neutral alignment, good stability, and preserved mobility of the ankle. Further special indications include patients with bilateral osteoarthritis of the ankle (15, e34).
Total joint replacement can also be performed as revision arthroplasty in patients with failed ankle prosthesis (16, 17, e35). However, revision ankle arthroplasty, like revision joint replacement in general, is a technically demanding surgical procedure. Patients with painful non-union or malunion of previous ankle arthrodesis are another specific indication for total ankle replacement (18, e36, e37).
Absolute contraindications include acute or chronic infections, with or without osteomyelitis or osteitis; severe osteonecrosis of the talus (more than one third of the talus); neuromuscular diseases; neuroarthropathies (e.g. patients with Charcot foot); and patients with severe circulatory disorders (19). In patients with concomitant significant ligament instabilities and/or deformities that cannot be corrected intraoperatively, arthrodesis of the ankle should be performed instead of joint replacement. Metal allergies are also a contraindication (20, 21).
Relative contraindications include severe osteoporosis, poor bone quality (e.g. due to steroid treatment), diabetes mellitus, smoking, and excess weight, although the literature shows that good outcomes can be achieved in some of these cases [22]). There may be an increased rate of aseptic loosening of implant components in patients who engage in high levels of sporting activity (23, 24). Low-impact exercise (walking, swimming, cycling, golf), however, is recommended postoperatively (19, 24).
Surgical technique
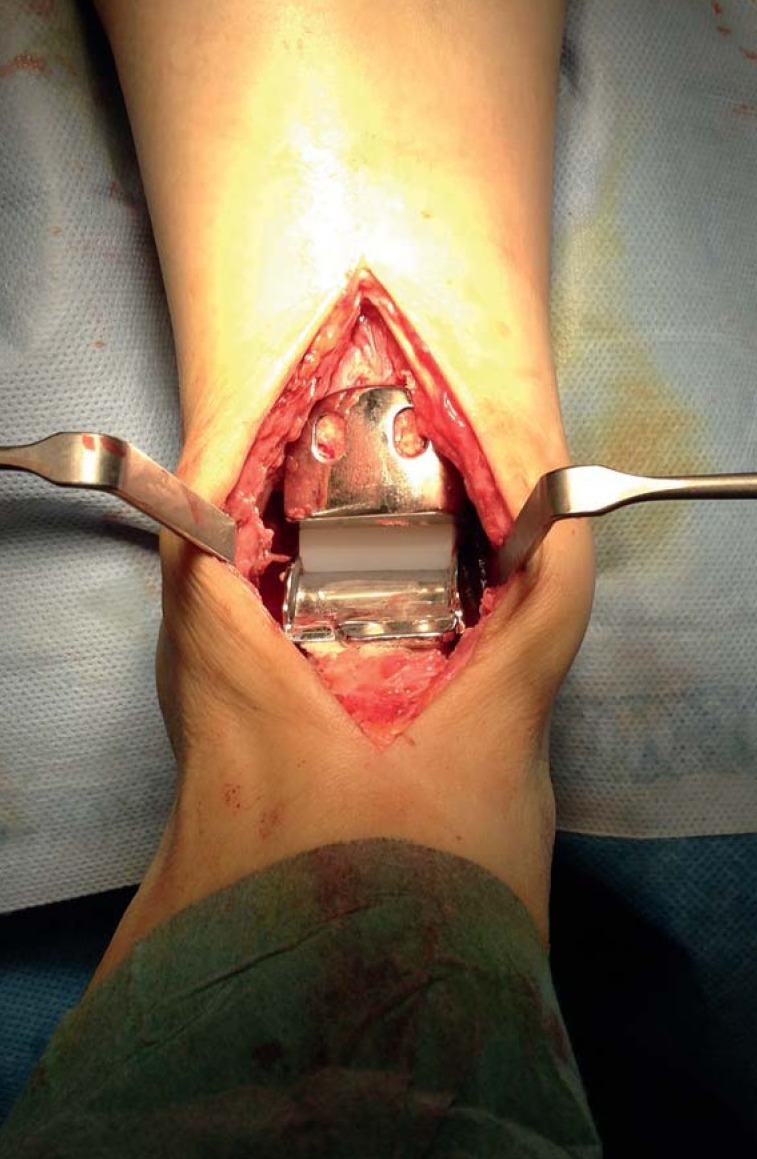
An anterior approach is usually used for ankle replacement surgery (eFigure 1). In patients with a history of previous ankle surgery, the surgical approach can be modified in order not to compromise postoperative wound healing (e38, e39). Depth preparation is performed beneath the tendon of the tibialis anterior muscle in order to preserve the anterior neurovascular bundle, which in most cases lies behind the tendon of the extensor hallucis longus muscle or between the tendons of the extensor hallucis longus and extensor digitorum longus muscles (e40). Bone resection is performed using an oscillating saw. Additional procedures for patients with concomitant deformities and/or instabilities should be performed after insertion of the implant components (eTable 3) (25, 26, e41, e42).
eFigure 1.
Total ankle replacement (HINTEGRA implant) in situ, implanted via anterior approach to ankle. Talar metal components (bottom), polyethylene inlay (white, center), and tibial metal components (top) are visible
eTable 3. Additional procedures in patients with concomitant valgus or varus hindfoot deformity.
| Patients with valgus hindfoot deformity | |
| Supramalleolar valgus deformity | Supramalleolar tibial osteotomy: |
| Isolated valgus defective heel position | Corrective calcaneal osteotomy:
|
| Flexible pes planovalgus et abductus deformity | Corrective calcaneal osteotomy:
Tendon transfer:
|
| Rigid pes planovalgus et abductus deformity | Corrective arthrodesis of the hindfoot:* |
| Medial instability | Medial ligament stabilization: |
| Patients with varus hindfoot deformity | |
| Supramalleolar varus deformity | Supramalleolar tibial osteotomy: |
| Flexible varus abnormal heel position | Corrective calcaneal osteotomy:M |
| Rigid inframalleolar varus alignment | Corrective arthrodesis of the hindfoot:
|
| Lateral instability | Lateral ligament stabilization: |
*Depends on extent of deformity and degenerative changes
Aftercare
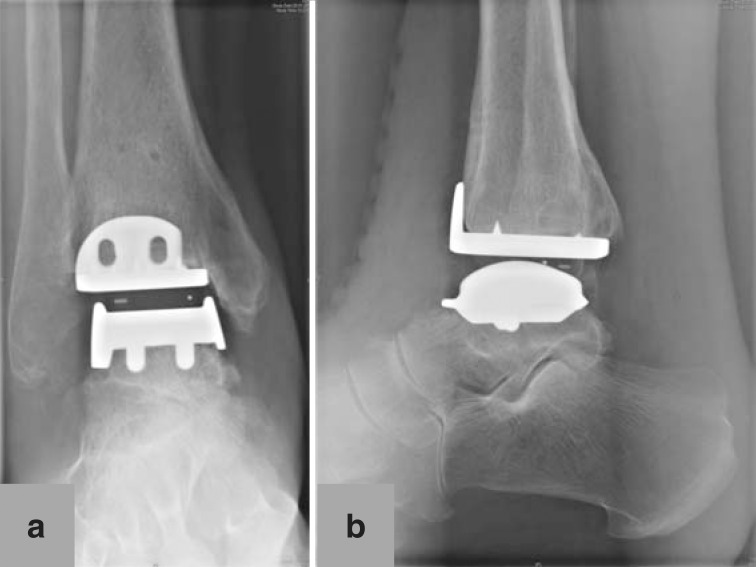
We recommend immobilization using plaster cast of the lower leg or a stabilizing boot for six weeks after surgery. During this period full weight may be borne with the aid of two elbow crutches, depending on the severity of the patient’s complaints. In patients with reduced bone quality and/or who have undergone additional procedures such as corrective osteotomy, we recommend 15 kg partial weightbearing for six weeks after surgery. Thromboprophylaxis is administered during immobilization (27). Clinical and radiological follow-up examination is performed after six weeks (Figure 3). After this, intensive outpatient physiotherapy begins: gait training, proprioception exercises, gradual increase to full weightbearing, local antiphlogistic therapy including lymph drainage, active and passive ankle mobility therapy, extension exercises, and therapy to strengthen the triceps surae muscle.
Figure 3.
Postoperative X-ray of 67-year-old female patient 6 weeks after total ankle replacement: a) mortise view of ankle; b) lateral view of ankle
Compression stockings are used for patients with persistent edema or soft-tissue swelling. The following sports can be recommended after full mobilization and full weightbearing ability have been attained: low-impact (e.g. walking, swimming, cycling, golf) or medium-impact (e.g. jogging, tennis, skiing) (24). Contact sports and sports that involve jumping should be avoided (24).
Clinical and radiological follow-up examinations are performed six weeks, three months, six months, and one year after surgery and then annually. The most important tools/questionnaires (28) that can be used to record functional postoperative outcomes following total ankle replacement are the American Orthopaedic Foot and Ankle Society (AOFAS) Ankle–Hindfoot Score (29) (score composed of pain, function, and alignment; minimum score, 0 points; maximum score, 100 points); and the Kofoed Ankle Score (e58) (score composed of pain, function, and range of motion; minimum score, 0 points; maximum score, 100 points). Pain level is determined using the visual analogue scale (VAS) from 0 (no pain) to 10 (worst possible pain) (e59). Quality of life can be analyzed using the SF-36 questionnaire (36-Item Short Form Health Survey) (e60).
Results/literature review
For a long time arthrodesis of the ankle was the first-line treatment for patients with advanced osteoarthritis of the ankle, which is not surprising given how uniformly disappointing the results of first-generation total ankle replacement were. Precise analysis of failures led to the development of new implant designs, acceptance of which is steadily increasing among orthopedic surgeons.
However, it is difficult to find well conducted, controlled, prospective studies in the literature, and in particular there are no comparisons of two-component and three-component implant types (30). Saltzman et al. (7) published the first results of a prospective study comparing ankle arthrodesis (66 cases) and total ankle replacement (593 patients) and demonstrated that patients with total ankle replacement had less pain and better functional outcomes postoperatively, with comparable postoperative complication rates. Although postoperative complications (poor wound healing, infections) were observed more frequently in patients undergoing ankle replacement than in those undergoing arthrodesis of the ankle—6.2% versus 1.5%— the difference was not statistically significant (p = 0.087). The Buechel–Pappas score (score composed of pain, function, deformity, and mobility; minimum score, 0 points; maximum score, 100 points) (e61) was used to assess functional outcome. Patients with total ankle replacement had significantly better functional outcomes: Buechel–Pappas score 46.7 ± 13.0 versus 26.3 ± 17 (p<0.001). The two groups had comparable postoperative pain levels: 1.6 ± 1.8 versus 1.8 ± 2.0 (p = 0.607). Further studies are planned by the authors but have not yet been published (7).
Despite increasing acceptance, total ankle replacement remains a technically demanding procedure with a flat learning curve. Intraoperative complications are not uncommon; they include fractures of the medial and/or lateral malleolus in 0 to 23% of cases and tendon injuries (posterior tibial tendon, flexor hallucis longus, flexor digitorum longus) and nerve injuries (superficial/deep peroneal nerve) in 0 to 10% of cases (31, e62–e66). Difficult steps during surgery include correct component positioning, particularly of talar components (e62, e66). Incorrect tibial component positioning can be found in 0 to 16% of all cases, and incorrect talar component positioning in 0 to 36% of all cases (e62). Numerous in vitro biomechanical studies have shown that incorrect positioning of implant components has adverse biomechanical consequences such as reduced ankle mobility, pathological tension of the periarticular ligaments, and unfavorable intra-articular pressure distribution (e67– e70). We have shown in a clinical study that patients with suboptimal positioning of talar components have a higher rate of persisting pain and worse ankle mobility (32).
Postoperative outcomes following total ankle replacement are steadily improving (Table 2; eTable 4)) but lag behind those of total knee and hip replacements (Table 3). Labek et al. (33) investigated cumulative outcomes on the basis of worldwide joint replacement registers. Outcomes following total hip and knee replacements were comparable, with 1.29 and 1.26 revisions per 100 component years. This means that after 10 years 13 out of every 100 patients need to undergo revision surgery. The outcomes following medial partial replacement were somewhat worse, with 1.53 revisions per 100 component years. Total ankle replacement was associated with the worst outcomes, however, with 3.29 revisions per 100 component years, resulting in revision surgery for 33 out of every 100 patients within 10 years (33). The causes and frequency of failure of total ankle replacement are different from those of hip and knee replacements: the main causes of failure are aseptic loosening of tibial and/or talar components, persisting pain, and septic loosening (Table 3) (34).
eTable 4. Clinical outcomes following total ankle replacement: probability of survival of implant components and postoperative range of ankle motion*.
| Study | Study type | Implant type | No. of implants | Probability of survival of implant components | Mean follow-up time | Postoperative range of motion |
|---|---|---|---|---|---|---|
| (e71) | RS, SC | Buechel–Pappas | 35 | 97% after 5 years | 5 years (3 to 150 months) |
N/A |
| (e72) | RS, SC | Agility | 207 | 76% after 9 years | N/A | N/A |
| (e73) | RS, SC | AES | 93 | 90% after 5 years | 42 months (13 to 73 months) |
N/A |
| (e6) | RS, SC | STAR | 51 | 70% after 5 years | 52 months (36 to 97 months) |
Total 28° (10 to 55°) |
| (22) | RS, SC | HINTEGRA | 123 | 93% after 6 years | 67.7±27.0 months (29 to 126 months) |
Total 35.3°± 8.1° |
| (e34) | PS, SC | HINTEGRA | 52 | 91% after 5 years, 78% after 8 years |
5 years (2 to 10 years) |
Total 38°± 9° |
| (6) | PS, SC | HINTEGRA | 722 | 94% after 5 years, 84% after 10 years |
6.3±2.9 years (2 to 12.2 years) |
N/A |
| (e74) | RS, MC | Salto (388), AES (173), HINTEGRA (22), STAR (9) |
592 | 88% after 71 months | Min. 1 year | N/A |
| (e75) | PS, SC | BOX | 62 | 91.9% after 42.5 months | 42.5 months (24 to 71 months) |
DF 8.4°±4.8° (0 to 20°); PF 17.1°±8.3° (0 to 30°) |
| (e76) | RS, SC | Salto | 98 | 98% after 5 years | 35 months (24 to 68 months) |
Total 28.3°±7° |
| (e77) | RS, SC | Salto | 98 | 85% after 10 years | 8.9 years (6.8 to 11 years) |
DF 8.6°±5.3° (−5 to 20°); PF 18.1°±7.8° (5 to 40°) |
| (e78) | PS, SC | STAR | 77 | 70.7 after 10 years, 45.6% after 14 years |
12.4 years (10.8 to 14.9 years) |
Total 22.8°±3.5° |
| (e8) | PS, SC | Buechel–Pappas | 50 | 93.5% after 10 years | 5 years (2 to 10 years) |
Total 28° (12 to 46°) |
| (e79) | PS, SC | Buechel–Pappas (normal sulcus 40; deep sulcus 75) |
115 | 74.2% (normal sulcus) after 20 years, 92% (deep Sulkus) after 12 years |
12 years 2 to 10 years, normal sulcus), 5 years (2 to 12 years, deep sulcus) |
Total 25° (10 to 47°, normal sulcus), total 29° (10 to 50°, deep sulcus) |
| (e80) | PS, SC | BOX | 20 | N/A | 12 months (7 to 14 months) |
Total 28.8°±11.3° (10 to 50°) |
| (e81) | RS, SC | Agility | 42 | 62% after 9 years | 8 years (0.5 to 11 years) | |
| (e82) | RS, SC | Buechel–Pappas | 30 | 87.6% after 5 years | 5.1±4 years (1 to 13 years) |
DF 5°; PF 30° |
| (e83) | PS, SC | LCS (19), Buechel–Pappas (74) | 93 | 84% after 8 years | 7.2 years (0.4 to 16.3 years) |
DF 7.1° (5.8 to 8.4°); PF 24.8° (22.6 to 27.2°) |
| (e9) | PS, MC | STAR (216), TPR (32), HINTEGRA (6), AES (3) |
257 | 89% after 5 years, 76% after 10 years |
4 years (5 days to 12 years) |
N/A |
| (e84) | PS, MC | BOX | 51 | 97.2% after 3 years | 30 months (24 to 48 months) |
Total 27.4° (16 to 53°) |
| (e85) | PS, MC | BOX | 158 | 96.1% after 4 years | 17 months (6 to 48 months) |
Total 26.5° (14 to 53°) |
| (e86) | PS, MC | STAR (318), Buechel–Pappas (92), AES (69), HINTEGRA (29), Mobility (23) |
531 | 78% after 5 years, 62% after 10 years |
1 to 11 years | N/A |
| (e87) | PS, MC | STAR (322), Mobility (132), AES (115), Buechel–Pappas (109), CCI (66), HINTEGRA (36) |
780 | 81% after 5 years, 69% after 10 years |
10 years | N/A |
| (e11) | RS, SC | STAR | 123 | 86% (patients with preoperative deformity up to 10°) and 75% (patients with preoperative deformity 10 to 30°) after 5 years |
4 years 2 to 8 years) |
N/A |
| (e88) | PS, MC | Agility (117), STAR (45), Mobility (29), Ramses (11) |
202 | 86% after 5 years | 28 to 75 months | N/A |
| (e12) | RS, SC | Agility | 65 | 91% after 1 year, 70% after 3 years, 67% after 5 years |
3.3 years (2 to 5.9 years) |
N/A |
| (e89) | RS, SC | TPR | 33 | 85% after 10 years | 10 to 23 years | N/A |
| ((e13) | RS, SC | STAR | 52 | 90% after 5 years, 84% after 8 years |
80 months (60 to 110 months) |
Total 23°±12° (0 to 55°) |
| ((e90) | RS, SC | Mayo | 204 | 79% after 5 years, 65% after 10 years, 61% after 15 years |
9 years 2 to 17 years) |
k. A. |
| (e14) | RS, SC | Agility | 132 | 86% after 9 years, 63% after 11 years |
9 years | DF 0° (−24 to 16°); PF 19° (−1 to 36°) |
| (e58) | PS, SC | STAR | 28 | 70% after 12 years | 1 to 12 years | N/A |
| (e91) | PS, SC | STAR | 52 | 72.7% (primary osteoarthritis) and 75.5% (rheumatoid arthritis) after 14 years |
9 years (6 to 14 years) |
N/A |
| (e92) | PS, SC | STAR | 100 | 85.7% (patients under 50) and 91.6% (patients over 50) after 5 years, 75% (patients under 50) and 80.6% (patients over 50) after 10 years |
6.8 years (1 to 15 years) |
N/A |
| (e93) | PS, SC | STAR (33 cemented, 25 uncemented) |
58 | 70% (cemented) and 95.4% (uncemented) after 12 years |
9.4±2.7 years | N/A |
| (e94) | PS, SC | AES | 38 | 79% after 2 years | 28 months (2 to 70 months) |
N/A |
| (e95) | PS, SC | LCS (19), Buechel–Pappas (74) |
93 | 80% after 15 years | 14.8 years (10.7 to 22.8 years) |
N/A |
| (e96) | PS, MC | BOX | 189 | 97% after 4 years | 21 months | Total 14 to 53° |
| (e16) | PS, SC | INBONE (211), STAR (122), Salto-Talaris (71) |
404 | 90% and 97.6% after 3.2 years with and without arthrodesis of the hindfoot |
3.2 years (2 to 6 years) |
N/A |
| (e17) | PS, SC | STAR | 84 | 96% after 5 years, 90% after 10 years |
9.1 years (2.6 to 11 years) |
DF 4.5°; PF 34.7° |
| (e97) | RS, SC | AES | 38 | 94.7% after 6 years | 57.8 months (48 to 80 months) |
N/A |
| (e98) | RS, SC | TNK | 27 | 77% after 14.1 years | 72 months (15 to 169 months) |
DF 7.5° (0 to 20°); PF 8.5° (−10 to 20°) |
| (e99) | RS, SC | Salto | 75 | 98% after 3.6 years | 43 months (27 to 73 months) |
DF 8.7°±5.6°; PF 29°±7° |
| (21) | RS, MC | Salto (91), HINTEGRA (39), AES (20), Coppelia (17), STAR (11), Ramses (4), Akile (1) |
183 | 86% (high-volume sites 88.4%; low-volume sites 84.9%) after 5 years |
39±29 months (6 to 132 months) |
N/A |
| (e100) | RS, MC | STAR | 59 | 88% after 3 years | 36 months (12 to 65 months) |
DF 10.2°±6.3°; PF 11.3°±7.9° |
| (e22) | PS, SC | Mobility | 240 | 97.7% after 4 years | 32.8±15.3 months (12 to 63 months) |
DF 8.3°±5.3°; PF 13.6°±6.4° |
| (e101) | RS, SC | Buechel–Pappas | 28 | 93% after 8.3 years | 8.3 years (5 to 12.2 years) |
Total 23° (8 to 40°) |
| (e102) | RS, SC | Salto | 401 | 86.6% (all patients), 85.1% (posttraumatic osteoarthritis), 95.6% (rheumatoid arthritis), 87.9% (patients under 55) after 5 years |
29 months (1 to 84 months) |
Total 33.1°±13.6° |
| (e103) | PS, SC | TPR (20), STAR (19) |
39 | 87% (TPR) after 12 years, 94.3% (STAR) after 6 years |
8.6 years (TPR: 3 to 13 years), 3.1 years (STAR: 1 to 6 years) |
Total (TPR) 37°. Total (STAR) 33.5° |
| ((e104) | PS, SC | Salto Talaris | 75 | 96% after 2.8 years | 2.8 years (2 to 4.5 years) |
N/A |
| (e25) | PS, MC | AES (298), STAR (217) |
515 | 83% after 5 years | 3.2 years (0.1 to 9.6 years) |
N/A |
| (e26) | RS, SC | Agility | 306 | 80% (89% in patients over 54) after 5 years |
33 ± 18 months (4 to 75 months) |
N/A |
| (e105) | PS, MC | Mobility | 88 | 89.6% after 3 years, 88.4% after 4 years |
40 months (30 to 60 months) |
N/A |
| (e106) | RS, SC | Mobility | 58 | 84% after 4 years | 32 months (14 to 49 months) |
N/A |
| (e107) | RS, SC | AES (16), Salto (4), New-Jersey (1) |
21 | 91% after 3 years, 57% after 5 years |
38±26 months | N/A |
| (e108) | PS, SC | STAR | 200 | 92.7% after 5 years | 46 months (24 to 101 months) |
N/A |
| (e109) | RS, MC | Salto | 109 | 97.5% after 2 years | 21.7 months (12 to 65 months) |
Total 32° |
| (e110) | RS, SC | HINTEGRA | 16 | 66.7% after 5 years | 61.8 months (7 to 116 months) |
Total 23.7° (12.0 to 47.5°) |
| (e111) | PS, SC | STAR | 200 | 93.3% after 5 years, 80.3% after 10 years |
88 months (60 to 156 months) |
N/A |
| (e112) | PS, SC | Buechel–Pappas (100), STAR (100) | 200 | 79% (Buechel–Pappas) and 95% (STAR) after 6 years |
Min. 36 months | N/A |
| (e30) | PS, SC | Mobility | 100 | 97% after 3 years, 93.6% after 4 years |
43 months (4 to 63 months) |
DF 7.5° (−5 to 22°), PF 14° (1 to 41°) |
*All available clinical studies (total ankle replacement) were included.
AES: Ankle Evolutive System; BOX: Bologna–Oxford; DF: dorsiflexion; N/A: information not available; LCS: low-contact stress; MC: multicenter; PF: plantar flexion; PS: prospective;
RS: retrospective; STAR: Scandinavian Total Ankle Replacement; TPR: Thomson, Prichard and Richard; SC: single-center
Table 3. Most common causes of failure of total hip, knee, and ankle replacement (given in % according to Sadoghi et al. [34]).
| Cause of failure | Total hip replacement | Total knee replacement | Total ankle replacement |
|---|---|---|---|
| Aseptic loosening | 55.2 | 29.8 | 38 |
| Luxation/instability | 11.8 | 6.2 | 8.5 |
| Septic loosening | 7.5 | 14.8 | 9.8 |
| Periprosthetic fracture | 6 | 3 | 2 |
| Pathological wear | 4.2 | 8.2 | 8 |
| Persistent pain | 3.7 | 9.5 | 12 |
| Implant failure | 2.5 | 4.7 | 5.3 |
| Technical error | 3.8 | 4.6 | 4.6 |
In 2010, Gougoulias et al. (35) performed a systematic review of the literature including 13 level IV studies with a total of 1105 ankle replacements. Seven different implant types were used. The mean failure rate (defined as replacement of one or both implant components or implant removal and conversion to arthrodesis of the ankle) five years after implantation was 10%, but there was great variation in failure rates between different centers, ranging from 0% to 32%. The percentage of patients in the included studies with persisting complaints was between 27 and 60%. Postoperative improvement in ankle mobility was relatively low, with values between 0° and 14° (35). Zaidi et al. (36) published a systematic review of the literature and meta-analysis of 58 publications with a total of 7942 ankle replacements. The success rate after 10 years was 89%, with an annual failure rate of 1.2% (95% confidence interval [CI]: 0.7 to 1.6). The mean AOFAS Ankle–Hindfoot Score rose from 40 (95% CI: 36 to 43) preoperatively to 80 (95% CI: 76 to 84) postoperatively. The range of motion of the ankle on which surgery was performed improved from a mean of 23° (95% CI: 19 to 26°) preoperatively to 34° (95% CI: 26 to 41°) postoperatively (36).
We performed a survivorship analysis of implant components in 684 patients who received a total of 722 ankle replacements (6). The mean follow-up time in this prospective study was 6.3 ± 2.9 years. The probability of success of the implant components was 94% after five years and 84% after 10 years. These results are comparable with those of current clinical studies (Table 2, eTable 4). The following factors were identified as independent risk factors for ankle replacement failure:
Age under 70 years (odds ratio [OR]: 3.84)
Etiology of ankle osteoarthritis (OR for primary osteoarthritis: 7.19; OR for posttraumatic osteoarthritis: 6.20)
Implant generation (OR for single hydroxyapatite coating: OR: 15.04) (6).
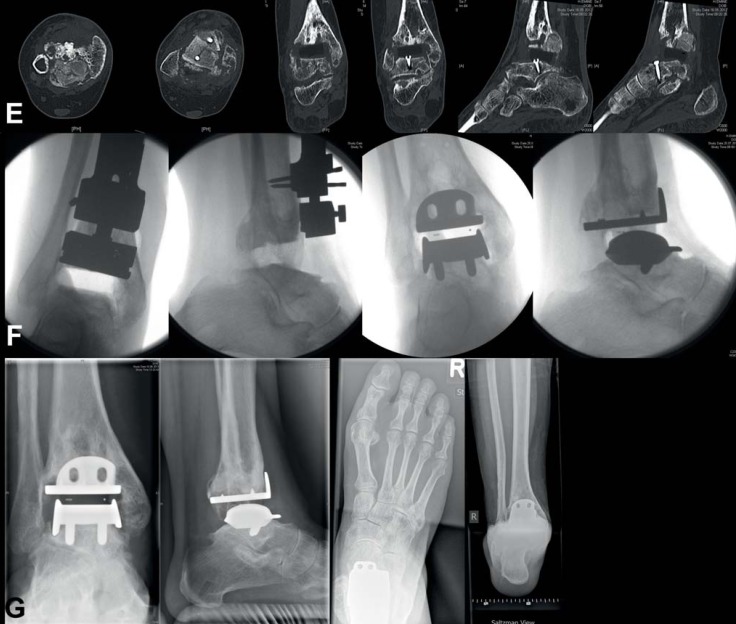
For a long time a change of approach—removal of the implant components followed by arthrodesis—was the standard procedure in cases of ankle replacement failure. The current literature describes various surgical techniques and fixation methods for such arthrodesis after prosthesis removal: bone allografts, autografts, or replacement materials (e.g. porous metals such as Trabecular Metal™) can be used to bridge the defect (37, 38, e113–e118). The alternative to converting to ankle arthrodesis is revision ankle arthroplasty (16, 17, e35, e119– e121). If possible, an implant type for which special revision components are available, e.g. a thicker metal plate for tibial components and larger weightbearing area and improved fixation for talar components, should be used. Revision surgery can be performed as one-stage or two-stage procedure. In the two-stage procedure, the goal of the first surgery is to address the bone defect. After bone integration of the autograft is achieved, the revision components can be implanted in a second surgery (eFigure 2).
eFigure 2.
Aseptic loosening of total ankle replacement 8 years after initial surgery: a) conventional X-rays in standing position show borders of loosening around both implant components, tibial and talar; b) SPECT-CT shows high metabolic activity in the areas around both implant components; c) implant components have been removed, on talar side osseous reconstruction was performed using autograft taken from iliac crest; d) and e) conventional X-rays and CT show good bone consolidation of autograft from iliac crest on the talar side 4 months postoperatively; f) fixation screws on talar side have been removed, HINTEGRA implant (revision components on talar side) were implanted; g) conventional X-rays in standing position show good osseointegration of implant components 6 months postoperatively
Conclusion
There is no gold standard treatment for advanced ankle osteoarthritis. Both, ankle arthrodesis and total ankle replacement are important treatment options in patients with end-stage ankle osteoarthritis. Attaining satisfactory intermediate-term and long-term postoperative outcomes in patients who have undergone total ankle replacement requires thorough preoperative examination and planning, taking careful account of all relative and absolute contraindications, with corresponding patient selection. If modern ankle implant designs are used, 10-year success rates of between 70 and 90% can be achieved.
Tabelle 2. Clinical outcomes following total ankle replacement: probability of survival of implant components*.
| Study | Study type | Implant type | No. of implants | Probability of survival of implant components |
|---|---|---|---|---|
| (e72) | RS, SC | Agility | 207 | 76% after 9 years |
| (22) | RS, SC | HINTEGRA | 123 | 93% after 6 years |
| (6) | PS, SC | HINTEGRA | 722 | 94% after 5 years, 84% after 10 years |
| (e74) | RS, MC | Salto (388), AES (173), HINTEGRA (22), STAR (9) |
592 | 88% after 71 months |
| (e79) | PS, SC | Buechel–Pappas (normal sulcus 40; deep sulcus 75) |
115 | 74.2% (normal sulcus) after 20 years, 92% (deep sulcus) after 12 years |
| (e9) | PS, MC | STAR (216), TPR (32), HINTEGRA (6), AES (3) | 257 | 89% after 5 years, 76% after 10 years |
| (e85) | PS, MC | BOX | 158 | 96.1% after 4 years |
| (e86) | PS, MC | STAR (318), Buechel-Pappas (92), AES (69), HINTEGRA (29), Mobility (23) |
531 | 78% after 5 years, 62% after 10 years |
| (e87) | PS, MC | STAR (322), Mobility (132), AES (115), Buechel-Pappas (109), CCI (66), HINTEGRA (36) |
780 | 81% after 5 years, 69% after 10 years |
| (e11) | RS, SC | STAR | 123 | 86% (patients with preoperative deformity up to 10°), 75% (patients with preoperative deformity 10 to 30°) after 5 years |
| (e88) | PS, MC | Agility (117), STAR (45), Mobility (29), Ramses (11) | 202 | 86% after 5 years |
| (e90) | RS, SC | Mayo | 204 | 79% after 5 years, 65% after 10 years, 61% after 15 years |
| (e14) | RS, SC | Agility | 132 | 86% after 9 years, 63% after 11 years |
| (e92) | PS, SC | STAR | 100 | 85.7% (patients under 50) and 91.6% (patients over 50) after 5 years, 75% (patients under 50) and 80.6% (patients over 50) after 10 years |
| (e96) | PS, MC | BOX | 189 | 97% after 4 years |
| (e16) | PS, SC | INBONE (211), STAR (122), Salto-Talaris (71) | 404 | 90% and 97.6% after 3.2 years with and without arthrodesis of the hindfoot |
| (21) | RS, MC | Salto (91), HINTEGRA (39), AES (20), Coppelia (17), STAR (11), Ramses (4), Akile (1) |
183 | 86% (88.4% high-volume centers; 84.9% low-volume centers) after 5 years |
| (e22) | PS, SC | Mobility | 240 | 97.7% after 4 years |
| (e102) | RS, SC | Salto | 401 | 86.6% (all patients), 85.1% (posttraumatic osteoarthritis), 95.6% (rheumatoid arthritis), 87.9% (patients under 55) after 5 years |
| (e25) | PS, MC | AES (298), STAR (217) | 515 | 83% after 5 years |
| (e26) | RS, SC | Agility | 306 | 80% (89% in patients over 54) after 5 years |
| (e108) | PS, SC | STAR | 200 | 92.7% after 5 years |
| (e109) | RS, MC | Salto | 109 | 97.5% after 2 years |
| (e111) | PS, SC | STAR | 200 | 93.3% after 5 years, 80.3% after 10 years |
| (e112) | PS, SC | Buechel-Pappas (100), STAR (100) | 200 | 79% (Buechel-Pappas) and 95% (STAR) after 6 years |
| (e30) | PS, SC | Mobility | 100 | 97% after 3 years, 93.6% after 4 years |
*Clinical studies (total ankle replacement) with at least 100 patients were included.
AES: Ankle Evolutive System; BOX: Bologna–Oxford; MC: multicenter; PS: prospective; RS: retrospective; STAR: Scandinavian Total Ankle Replacement; TPR: Thomson, Prichard and Richard; SC: single-center
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Barg A, Pagenstert GI, Hugle T, et al. Ankle osteoarthritis: etiology, diagnostics, and classification. Foot Ankle Clin. 2013;18:411–426. doi: 10.1016/j.fcl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90:499–505. doi: 10.2106/JBJS.F.01299. [DOI] [PubMed] [Google Scholar]
- 3.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Goost H, Wimmer MD, Barg A, Kabir K, Valderrabano V, Burger C. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377–388. doi: 10.3238/arztebl.2014.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34:612–620. doi: 10.1177/0363546505281813. [DOI] [PubMed] [Google Scholar]
- 6.Barg A, Zwicky L, Knupp M, Henninger HB, Hintermann B. HINTEGRA total ankle replacement: Survivorship analysis in 684 patients. J Bone Joint Surg Am. 2013;95:1175–1183. doi: 10.2106/JBJS.L.01234. [DOI] [PubMed] [Google Scholar]
- 7.Saltzman CL, Mann RA, Ahrens JE, et al. Prospective controlled trial of STAR total ankle replacement versus ankle fusion: initial results. Foot Ankle Int. 2009;30:579–596. doi: 10.3113/FAI.2009.0579. [DOI] [PubMed] [Google Scholar]
- 8.Barg A, Saltzman CL. Ankle replacement. Mann’s surgery of the foot and ankle. In: Coughlin MJ, Saltzman CL, Anderson RB, editors. 9th ed. Philadelphia: Elsevier Saunders; 2014. pp. 1078–1162. [Google Scholar]
- 9.Phisitkul P, Chaichankul C, Sripongsai R, Prasitdamrong I, Tengtrakulcharoen P, Suarchawaratana S. Accuracy of anterolateral drawer test in lateral ankle instability: a cadaveric study. Foot Ankle Int. 2009;30:690–695. doi: 10.3113/FAI.2009.0690. [DOI] [PubMed] [Google Scholar]
- 10.Lindsjo U, Danckwardt-Lilliestrom G, Sahlstedt B. Measurement of the motion range in the loaded ankle. Clin Orthop Relat Res. 1985;199:68–71. [PubMed] [Google Scholar]
- 11.Saltzman CL, el Khoury GY. The hindfoot alignment view. Foot Ankle Int. 1995;16:572–576. doi: 10.1177/107110079501600911. [DOI] [PubMed] [Google Scholar]
- 12.Barg A, Harris MD, Henninger HB, et al. Medial distal tibial angle: comparison between weightbearing mortise view and hindfoot alignment view. Foot Ankle Int. 2012;33:655–661. doi: 10.3113/FAI.2012.0655. [DOI] [PubMed] [Google Scholar]
- 13.Schmid T, Krause FG. Conservative treatment of asymmetric ankle osteoarthritis. Foot Ankle Clin. 2013;18:437–448. doi: 10.1016/j.fcl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Barg A, Smirnov E, Paul J, Pagenstert G, Valderrabano V. Management der Sprunggelenksarthrose. Orthopadie Rheuma. 2013;16:44–50. [Google Scholar]
- 15.Barg A, Knupp M, Hintermann B. Simultaneous bilateral versus unilateral total ankle replacement: A patient-based comparison of pain relief, quality of life and functional outcome. J Bone Joint Surg Br. 2010;92:1659–1663. doi: 10.1302/0301-620X.92B12.25204. [DOI] [PubMed] [Google Scholar]
- 16.Hintermann B, Barg A, Knupp M. Revisionsarthroplastik des oberen Sprunggelenks. Orthopade. 2011;40:1000–1007. doi: 10.1007/s00132-011-1829-z. [DOI] [PubMed] [Google Scholar]
- 17.Hintermann B, Zwicky L, Knupp M, Henninger HB, Barg A. HINTEGRA revision arthroplasty for failed total ankle prostheses. J Bone Joint Surg Am. 2013;95:1166–1174. doi: 10.2106/JBJS.L.00538. [DOI] [PubMed] [Google Scholar]
- 18.Hintermann B, Barg A, Knupp M, Valderrabano V. Conversion of painful ankle arthrodesis to total ankle arthroplasty. J Bone Joint Surg Am. 2009;91:850–858. doi: 10.2106/JBJS.H.00229. [DOI] [PubMed] [Google Scholar]
- 19.Barg A, Knupp M, Henninger HB, Zwicky L, Hintermann B. Total ankle replacement using HINTEGRA, an unconstrained, three-component system: Surgical technique and pitfalls. Foot Ankle Clin. 2012;17:607–635. doi: 10.1016/j.fcl.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Ajis A, Henriquez H, Myerson M. Postoperative range of motion trends following total ankle arthroplasty. Foot Ankle Int. 2013;34:645–656. doi: 10.1177/1071100713481433. [DOI] [PubMed] [Google Scholar]
- 21.Pinar N, Vernet E, Bizot P, Brilhault J. Total ankle arthroplasty - total ankle arthroplasty in Western France: Influence of volume on complications and clinical outcome. Orthop Traumatol Surg Res. 2012;98:26–30. doi: 10.1016/j.otsr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Barg A, Knupp M, Anderson AE, Hintermann B. Total ankle replacement in obese patients: component stability, weight change, and functional outcome in 118 consecutive patients. Foot Ankle Int. 2011;32:925–932. doi: 10.3113/FAI.2011.0925. [DOI] [PubMed] [Google Scholar]
- 23.Naal FD, Impellizzeri FM, Loibl M, Huber M, Rippstein PF. Habitual physical activity and sports participation after total ankle arthroplasty. Am J Sports Med. 2009;37:95–102. doi: 10.1177/0363546508323253. [DOI] [PubMed] [Google Scholar]
- 24.Valderrabano V, Pagenstert G, Horisberger M, Knupp M, Hintermann B. Sports and recreation activity of ankle arthritis patients before and after total ankle replacement. Am J Sports Med. 2006;34:993–999. doi: 10.1177/0363546505284189. [DOI] [PubMed] [Google Scholar]
- 25.Valderrabano V, Frigg A, Leumann A, Horisberger M. Sprunggelenkprothese bei Valgusarthrose. Orthopade. 2011;40:971–977. doi: 10.1007/s00132-011-1825-3. [DOI] [PubMed] [Google Scholar]
- 26.Knupp M, Bolliger L, Barg A, Hintermann B. Sprunggelenkprothese bei Varusarthrose. Orthopade. 2011;40:964–970. doi: 10.1007/s00132-011-1824-4. [DOI] [PubMed] [Google Scholar]
- 27.Barg A, Henninger HB, Hintermann B. Risk factors for symptomatic deep-vein thrombosis in patients after total ankle replacement who received routine chemical thromboprophylaxis. J Bone Joint Surg Br. 2011;93:921–927. doi: 10.1302/0301-620X.93B7.26257. [DOI] [PubMed] [Google Scholar]
- 28.Naal FD, Impellizzeri FM, Rippstein PF. Which are the most frequently used outcome instruments in studies on total ankle arthroplasty? Clin Orthop Relat Res. 2010;468:815–826. doi: 10.1007/s11999-009-1036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 30.Valderrabano V, Pagenstert GI, Muller AM, Paul J, Henninger HB, Barg A. Mobile- and fixed-bearing total ankle prostheses: is there really a difference? Foot Ankle Clin. 2012;17:565–585. doi: 10.1016/j.fcl.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Saltzman CL, Amendola A, Anderson R, et al. Surgeon training and complications in total ankle arthroplasty. Foot Ankle Int. 2003;24:514–518. doi: 10.1177/107110070302400612. [DOI] [PubMed] [Google Scholar]
- 32.Barg A, Elsner A, Anderson AE, Hintermann B. The effect of three-component total ankle replacement malalignment on clinical outcome: pain relief and functional outcome in 317 consecutive patients. J Bone Joint Surg Am. 2011;93:1969–1978. doi: 10.2106/JBJS.J.01415. [DOI] [PubMed] [Google Scholar]
- 33.Labek G, Thaler M, Janda W, Agreiter M, Stockl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. J Bone Joint Surg Br. 2011;93:293–297. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]
- 34.Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Bohler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28:1329–1332. doi: 10.1016/j.arth.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements? A systematic review of the literature. Clin Orthop Relat Res. 2010;468:199–208. doi: 10.1007/s11999-009-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaidi R, Cro S, Gurusamy K, et al. The outcome of total ankle replacement: A systematic review and meta-analysis. Bone Joint J. 2013;95:1500–1507. doi: 10.1302/0301-620X.95B11.31633. [DOI] [PubMed] [Google Scholar]
- 37.Hopgood P, Kumar R, Wood PL. Ankle arthrodesis for failed total ankle replacement. J Bone Joint Surg Br. 2006;88:1032–1038. doi: 10.1302/0301-620X.88B8.17627. [DOI] [PubMed] [Google Scholar]
- 38.Culpan P, Le Strat V, Piriou P, Judet T. Arthrodesis after failed total ankle replacement. J Bone Joint Surg Br. 2007;89:1178–1183. doi: 10.1302/0301-620X.89B9.19108. [DOI] [PubMed] [Google Scholar]
- e1.Egloff C, Gloyer M, Barg K, et al. Arthrose des oberen Sprunggelenks - Ätiologie und Biomechanik. Fuss Sprungg. 2013;11:179–185. [Google Scholar]
- e2.Egloff C, Hugle T, Valderrabano V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13583. 0. [DOI] [PubMed] [Google Scholar]
- e3.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Wang B, Saltzman CL, Chalayon O, Barg A. Does the subtalar joint compensate for ankle malalignment in end-stage ankle arthritis? Clin Orthop Relat Res. 2014;473:318–325. doi: 10.1007/s11999-014-3960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Horisberger M, Valderrabano V, Hintermann B. Posttraumatic ankle osteoarthritis after ankle-related fractures. J Orthop Trauma. 2009;23:60–67. doi: 10.1097/BOT.0b013e31818915d9. [DOI] [PubMed] [Google Scholar]
- e6.Anderson T, Montgomery F, Carlsson A. Uncemented STAR total ankle prostheses. Three to eight-year follow-up of fifty-one consecutive ankles. J Bone Joint Surg Am. 2003;85:1321–1329. [PubMed] [Google Scholar]
- e7.Besse JL, Brito N, Lienhart C. Clinical evaluation and radiographic assessment of bone lysis of the AES total ankle replacement. Foot Ankle Int. 2009;30:964–975. doi: 10.3113/FAI.2009.0964. [DOI] [PubMed] [Google Scholar]
- e8.Buechel FF, Sr., Buechel FF, Jr., Pappas MJ. Ten-year evaluation of cementless Buechel-Pappas meniscal bearing total ankle replacement. Foot Ankle Int. 2003;24:462–272. doi: 10.1177/107110070302400603. [DOI] [PubMed] [Google Scholar]
- e9.Fevang BT, Lie SA, Havelin LI, Brun JG, Skredderstuen A, Furnes O. 257 ankle arthroplasties performed in Norway between 1994 and 2005. Acta Orthop. 2007;78:575–583. doi: 10.1080/17453670710014257. [DOI] [PubMed] [Google Scholar]
- e10.Hagena FW, Christ R, Kettrukat M. Die Endoprothese am oberen Sprunggelenk. Fuss Sprungg. 2003;1:48–55. [Google Scholar]
- e11.Hobson SA, Karantana A, Dhar S. Total ankle replacement in patients with significant pre-operative deformity of the hindfoot. J Bone Joint Surg Br. 2009;91:481–486. doi: 10.1302/0301-620X.91B4.20855. [DOI] [PubMed] [Google Scholar]
- e12.Hurowitz EJ, Gould JS, Fleisig GS, Fowler R. Outcome analysis of agility total ankle replacement with prior adjunctive procedures: two to six year followup. Foot Ankle Int. 2007;28:308–312. doi: 10.3113/FAI.2007.0308. [DOI] [PubMed] [Google Scholar]
- e13.Karantana A, Hobson S, Dhar S. The Scandinavian total ankle replacement: Survivorship at 5 and 8 years comparable to other series. Clin Orthop Relat Res. 2010;468:951–957. doi: 10.1007/s11999-009-0971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Knecht SI, Estin M, Callaghan JJ, et al. The agility total ankle arthroplasty. Seven to sixteen-year follow-up. J Bone Joint Surg Am. 2004;86:1161–1171. [PubMed] [Google Scholar]
- e15.Kumar A, Dhar S. Total ankle replacement: Early results during learning perios. Foot Ankle Surg. 2007;13:19–23. [Google Scholar]
- e16.Lewis JS, Jr., Adams SB, Jr., Queen RM, Deorio JK, Nunley JA, Easley ME. Outcomes after total ankle replacement in association with ipsilateral hindfoot arthrodesis. Foot Ankle Int. 2014;35:535–542. doi: 10.1177/1071100714528495. [DOI] [PubMed] [Google Scholar]
- e17.Mann JA, Mann RA, Horton E. STAR ankle: Long-term results. Foot Ankle Int. 2011;32:473–484. doi: 10.3113/FAI.2011.0473. [DOI] [PubMed] [Google Scholar]
- e18.Nunley JA, Caputo AM, Easley ME, Cook C. Intermediate to long-term outcomes of the STAR total ankle replacement: The patient perspective. J Bone Joint Surg Am. 2012;94:43–48. doi: 10.2106/JBJS.J.01613. [DOI] [PubMed] [Google Scholar]
- e19.Pyevich MT, Saltzman CL, Callaghan JJ, Alvine FG. Total ankle arthroplasty: A unique design. Two to twelve-year follow-up. J Bone Joint Surg Am. 1998;80:1410–1420. [PubMed] [Google Scholar]
- e20.Queen RM, Grier AJ, Butler RJ, et al. The influence of concomitant triceps surae lengthening at the time of total ankle arthroplasty on postoperative outcomes. Foot Ankle Int. 2014;35:863–870. doi: 10.1177/1071100714539662. [DOI] [PubMed] [Google Scholar]
- e21.Ramaskandhan JR, Kakwani R, Kometa S, Bettinson K, Siddique MS. Two-year outcomes of MOBILITY Total Ankle Replacement. J Bone Joint Surg Am. 2014;96 doi: 10.2106/JBJS.L.00536. [DOI] [PubMed] [Google Scholar]
- e22.Rippstein PF, Huber M, Coetzee JC, Naal FD. Total ankle replacement with use of a new three-component implant. J Bone Joint Surg Am. 2011;93:1426–1435. doi: 10.2106/JBJS.J.00913. [DOI] [PubMed] [Google Scholar]
- e23.Schimmel JJ, Walschot LH, Louwerens JW. Comparison of the short-term results of the first and last 50 Scandinavian total ankle replacements: Assessment of the learning curve in a consecutive series. Foot Ankle Int. 2014;35:326–333. doi: 10.1177/1071100713518187. [DOI] [PubMed] [Google Scholar]
- e24.Schuberth JM, Babu NS, Richey JM, Christensen JC. Gutter impingement after total ankle arthroplasty. Foot Ankle Int. 2013;34:329–337. doi: 10.1177/1071100712466850. [DOI] [PubMed] [Google Scholar]
- e25.Skytta ET, Koivu H, Eskelinen A, Ikavalko M, Paavolainen P, Remes V. Total ankle replacement: A population-based study of 515 cases from the Finnish arthroplasty register. Acta Orthop. 2010;81:114–118. doi: 10.3109/17453671003685459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Spirt AA, Assal M, Hansen ST., Jr. Complications and failure after total ankle arthroplasty. J Bone Joint Surg Am. 2004;86:1172–1178. doi: 10.2106/00004623-200406000-00008. [DOI] [PubMed] [Google Scholar]
- e27.Sung KS, Ahn J, Lee KH, Chun TH. Short-term results of total ankle arthroplasty for end-stage ankle arthritis with severe varus deformity. Foot Ankle Int. 2014;35:225–231. doi: 10.1177/1071100713517102. [DOI] [PubMed] [Google Scholar]
- e28.Valderrabano V, Hintermann B, Dick W. Scandinavian total ankle replacement: A 37 -year average followup of 65 patients. Clin Orthop Relat Res. 2004;424:47–56. [PubMed] [Google Scholar]
- e29.Vienne P, Nothdurft P. OSG-Totalendoprothese Agility: Indikationen, Operationstechnik und Ergebnisse. Fuss Sprungg. 2004;2:17–28. [Google Scholar]
- e30.Wood PL, Karski MT, Watmough P. Total ankle replacement: The results of 100 mobility total ankle replacements. J Bone Joint Surg Br. 2010;92:958–962. doi: 10.1302/0301-620X.92B7.23852. [DOI] [PubMed] [Google Scholar]
- e31.Yoon HS, Lee J, Choi WJ, Lee JW. Periprosthetic osteolysis after total ankle arthroplasty. Foot Ankle Int. 2014;35:14–21. doi: 10.1177/1071100713509247. [DOI] [PubMed] [Google Scholar]
- e32.Taylor KF, Bojescul JA, Howard RS, Mizel MS, McHale KA. Measurement of isolated subtalar range of motion: a cadaver study. Foot Ankle Int. 2001;22:426–432. doi: 10.1177/107110070102200512. [DOI] [PubMed] [Google Scholar]
- e33.Stufkens SA, Barg A, Bolliger L, Stucinskas J, Knupp M, Hintermann B. Measurement of the medial distal tibial angle. Foot Ankle Int. 2011;32:288–293. doi: 10.3113/FAI.2011.0288. [DOI] [PubMed] [Google Scholar]
- e34.Barg A, Henninger HB, Knupp M, Hintermann B. Simultaneous bilateral total ankle replacement using a 3-component prosthesis: Outcome in 26 patients followed for 2-10 years. Acta Orthop. 2011;82:704–710. doi: 10.3109/17453674.2011.623570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e35.Ellington JK, Gupta S, Myerson MS. Management of failures of total ankle replacement with the agility total ankle arthroplasty. J Bone Joint Surg Am. 2013;95:2112–2118. doi: 10.2106/JBJS.K.00920. [DOI] [PubMed] [Google Scholar]
- e36.Hintermann B, Barg A, Knupp M, Valderrabano V. Conversion of painful ankle arthrodesis to total ankle arthroplasty. Surgical technique. J Bone Joint Surg Am. 2010;92:55–66. doi: 10.2106/JBJS.I.01301. [DOI] [PubMed] [Google Scholar]
- e37.Barg A, Hintermann B. Takedown of painful ankle fusion and total ankle replacement using a 3-component ankle prosthesis. Tech Foot & Ankle. 2010;9:190–198. [Google Scholar]
- e38.Amin A, Mahoney J, Daniels TR. Anteromedial approach for ankle arthoplasty and arthrodesis: technique tip. Foot Ankle Int. 2012;33:1011–1014. doi: 10.3113/FAI.2012.1011. [DOI] [PubMed] [Google Scholar]
- e39.Bibbo C. A modified anterior approach to the ankle. J Foot Ankle Surg. 2013;52:136–137. doi: 10.1053/j.jfas.2012.10.010. [DOI] [PubMed] [Google Scholar]
- e40.Solomon LB, Ferris L, Henneberg M. Anatomical study of the ankle with view to the anterior arthroscopic portals. ANZ J Surg. 2006;76:932–936. doi: 10.1111/j.1445-2197.2006.03909.x. [DOI] [PubMed] [Google Scholar]
- e41.Easley ME. Surgical treatment of the arthritic varus ankle. Foot Ankle Clin. 2012;17:665–686. doi: 10.1016/j.fcl.2012.09.002. [DOI] [PubMed] [Google Scholar]
- e42.Barg A, Pagenstert GI, Leumann AG, Muller AM, Henninger HB, Valderrabano V. Treatment of the arthritic valgus ankle. Foot Ankle Clin. 2012;17:647–663. doi: 10.1016/j.fcl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- e43.Pagenstert G, Knupp M, Valderrabano V, Hintermann B. Realignment surgery for valgus ankle osteoarthritis. Oper Orthop Traumatol. 2009;21:77–87. doi: 10.1007/s00064-009-1607-9. [DOI] [PubMed] [Google Scholar]
- e44.Barg A, Saltzman CL. Single-stage supramalleolar osteotomy for coronal plane deformity. Curr Rev Musculoskelet Med. 2014;7:277–291. doi: 10.1007/s12178-014-9231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e45.Barg A, Pagenstert GI, Horisberger M, et al. Supramalleolar osteotomies for degenerative joint disease of the ankle joint: indication, technique and results. Int Orthop. 2013;37:1683–1695. doi: 10.1007/s00264-013-2030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e46.Stufkens SA, Knupp M, Hintermann B. Medial displacement calcaneal osteotomy. Tech Foot & Ankle. 2009;8:85–90. [Google Scholar]
- e47.Hintermann B, Valderrabano V, Kundert HP. Lengthening of the lateral column and reconstruction of the medial soft tissue for treatment of acquired flatfoot deformity associated with insufficiency of the posterior tibial tendon. Foot Ankle Int. 1999;20:622–629. doi: 10.1177/107110079902001002. [DOI] [PubMed] [Google Scholar]
- e48.Backus JD, McCormick JJ. Tendon transfers in the treatment of the adult flatfoot. Foot Ankle Clin. 2014;19:29–48. doi: 10.1016/j.fcl.2013.11.002. [DOI] [PubMed] [Google Scholar]
- e49.Davies MB, Rosenfeld PF, Stavrou P, Saxby TS. A comprehensive review of subtalar arthrodesis. Foot Ankle Int. 2007;28:295–297. doi: 10.3113/FAI.2007.0295. [DOI] [PubMed] [Google Scholar]
- e50.Knupp M, Stufkens SA, Hintermann B. Triple arthrodesis. Foot Ankle Clin. 2011;16:61–67. doi: 10.1016/j.fcl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- e51.Hintermann B, Knupp M, Pagenstert GI. Deltoid ligament injuries: Diagnosis and management. Foot Ankle Clin. 2006;11:625–637. doi: 10.1016/j.fcl.2006.08.001. [DOI] [PubMed] [Google Scholar]
- e52.Wimmer MD, Vavken P, Barg A, Valderrabano V, Pagenstert GI. Anatomic bundle reconstruction of the deltoid ligament. Sport Orthop Traumatol. 2013;29:214–218. [Google Scholar]
- e53.Knupp M, Pagenstert G, Valderrabano V, Hintermann B. Osteotomien zur Entlastung der Varusarthrose im oberen Sprunggelenk. Oper Orthop Traumatol. 2008;20:262–273. doi: 10.1007/s00064-008-1308-9. [DOI] [PubMed] [Google Scholar]
- e54.Weseley MS, Barenfeld PA. Mechanism of the Dwyer calcaneal osteotomy. Clin Orthop Relat Res. 1970;70:137–140. [PubMed] [Google Scholar]
- e55.Knupp M, Horisberger M, Hintermann B. A new z-shaped calcaneal osteotomy for 3-plane correction of severe varus deformity of the hindfoot. Tech Foot & Ankle. 2008;7:90–95. [Google Scholar]
- e56.Valderrabano V, Wiewiorski M, Frigg A, Hintermann B, Leumann A. Direkte anatomische Rekonstruktion des lateralen Bandapparates bei chronischer lateraler Instabilität des oberen Sprunggelenks. Unfallchirurg. 2007;110:701–704. doi: 10.1007/s00113-007-1314-7. [DOI] [PubMed] [Google Scholar]
- e57.Pagenstert GI, Hintermann B, Knupp M. Operative management of chronic ankle instability: Plantaris graft. Foot Ankle Clin. 2006;11:567–583. doi: 10.1016/j.fcl.2006.05.002. [DOI] [PubMed] [Google Scholar]
- e58.Kofoed H. Cylindrical cemented ankle arthroplasty: A prospective series with long-term follow-up. Foot Ankle Int. 1995;16:474–479. doi: 10.1177/107110079501600803. [DOI] [PubMed] [Google Scholar]
- e59.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- e60.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- e61.Buechel FF, Pappas MJ, Iorio LJ. New Jersey low contact stress total ankle replacement: Biomechanical rationale and review of 23 cementless cases. Foot Ankle. 1988;8:279–290. doi: 10.1177/107110078800800603. [DOI] [PubMed] [Google Scholar]
- e62.Lee KB, Cho SG, Hur CI, Yoon TR. Perioperative complications of HINTEGRA total ankle replacement: Our initial 50 cases. Foot Ankle Int. 2008;29:978–984. doi: 10.3113/FAI.2008.0978. [DOI] [PubMed] [Google Scholar]
- e63.Lee KT, Lee YK, Young KW, et al. Perioperative complications of the MOBILITY total ankle system: Comparison with the HINTEGRA total ankle system. J Orthop Sci. 2010;15:317–322. doi: 10.1007/s00776-010-1456-2. [DOI] [PubMed] [Google Scholar]
- e64.Lee KT, Lee YK, Young KW, Kim JB, Seo YS. Perioperative complications and learning curve of the mobility total ankle system. Foot Ankle Int. 2013;34:210–214. doi: 10.1177/1071100712467430. [DOI] [PubMed] [Google Scholar]
- e65.Myerson MS, Mroczek K. Perioperative complications of total ankle arthroplasty. Foot Ankle Int. 2003;24:17–21. doi: 10.1177/107110070302400102. [DOI] [PubMed] [Google Scholar]
- e66.Schuberth JM, Patel S, Zarutsky E. Perioperative complications of the Agility total ankle replacement in 50 initial, consecutive cases. J Foot Ankle Surg. 2006;45:139–146. doi: 10.1053/j.jfas.2006.02.013. [DOI] [PubMed] [Google Scholar]
- e67.Espinosa N, Walti M, Favre P, Snedeker JG. Misalignment of total ankle components can induce high joint contact pressures. J Bone Joint Surg Am. 2010;92:1179–1187. doi: 10.2106/JBJS.I.00287. [DOI] [PubMed] [Google Scholar]
- e68.Fukuda T, Haddad SL, Ren Y, Zhang LQ. Impact of talar component rotation on contact pressure after total ankle arthroplasty: A cadaveric study. Foot Ankle Int. 2010;31:404–411. doi: 10.3113/FAI.2010.0404. [DOI] [PubMed] [Google Scholar]
- e69.Saltzman CL, Tochigi Y, Rudert MJ, McIff TE, Brown TD. The effect of agility ankle prosthesis misalignment on the peri-ankle ligaments. Clin Orthop Relat Res. 2004;424:137–142. doi: 10.1097/01.blo.0000132463.80467.65. [DOI] [PubMed] [Google Scholar]
- e70.Tochigi Y, Rudert MJ, Brown TD, McIff TE, Saltzman CL. The effect of accuracy of implantation on range of movement of the Scandinavian total ankle replacement. J Bone Joint Surg Br. 2005;87:736–740. doi: 10.1302/0301-620X.87B5.14872. [DOI] [PubMed] [Google Scholar]
- e71.Ali MS, Higgins GA, Mohamed M. Intermediate results of Buechel Pappas unconstrained uncemented total ankle replacement for osteoarthritis. J Foot Ankle Surg. 2007;46:16–20. doi: 10.1053/j.jfas.2006.10.005. [DOI] [PubMed] [Google Scholar]
- e72.Alvine FG. The agility ankle replacement: The good and the bad. Foot Ankle Clin. 2002;7:737–753. doi: 10.1016/s1083-7515(02)00060-8. [DOI] [PubMed] [Google Scholar]
- e73.Anders H, Kaj K, Johan J, Urban R. The AES total ankle replacement: A mid-term analysis of 93 cases. Foot Ankle Surg. 2010;16:61–64. doi: 10.1016/j.fas.2009.06.001. [DOI] [PubMed] [Google Scholar]
- e74.Besse JL, Colombier JA, Asencio J, et al. Total ankle arthroplasty in France. Orthop Traumatol Surg Res. 2010;96:291–303. doi: 10.1016/j.otsr.2010.03.002. [DOI] [PubMed] [Google Scholar]
- e75.Bianchi A, Martinelli N, Sartorelli E, Malerba F. The Bologna-Oxford total ankle replacement: A mid-term follow-up study. J Bone Joint Surg Br. 2012;94:793–798. doi: 10.1302/0301-620X.94B6.28283. [DOI] [PubMed] [Google Scholar]
- e76.Bonnin M, Judet T, Colombier JA, Buscayret F, Graveleau N, Piriou P. Midterm results of the Salto Total Ankle Prosthesis. Clin Orthop Relat Res. 2004;424:6–18. doi: 10.1097/01.blo.0000132407.75881.a0. [DOI] [PubMed] [Google Scholar]
- e77.Bonnin M, Gaudot F, Laurent JR, Ellis S, Colombier JA, Judet T. The salto total ankle arthroplasty: survivorship and analysis of failures at 7 to 11 years. Clin Orthop Relat Res. 2011;469:225–236. doi: 10.1007/s11999-010-1453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e78.Brunner S, Barg A, Knupp M, et al. The Scandinavian total ankle replacement: Long-term, eleven to fifteen-year, survivorship analysis of the prosthesis in seventy-two consecutive patients. J Bone Joint Surg Am. 2013;95:711–718. doi: 10.2106/JBJS.K.01580. [DOI] [PubMed] [Google Scholar]
- e79.Buechel FF, Sr., Buechel FF, Jr., Pappas MJ. Twenty-year evaluation of cementless mobile-bearing total ankle replacements. Clin Orthop Relat Res. 2004;424:19–26. doi: 10.1097/01.blo.0000132243.41419.59. [DOI] [PubMed] [Google Scholar]
- e80.Cenni F, Leardini A, Pieri M, et al. Functional performance of a total ankle replacement: thorough assessment by combining gait and fluoroscopic analyses. Clin Biomech (Bristol, Avon) 2013;28:79–87. doi: 10.1016/j.clinbiomech.2012.10.008. [DOI] [PubMed] [Google Scholar]
- e81.Criswell BJ, Douglas K, Naik R, Thomson AB. High revision and reoperation rates using the Agility Total Ankle System. Clin Orthop Relat Res. 2012;470:1980–1986. doi: 10.1007/s11999-012-2242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e82.Dhawan R, Turner J, Sharma V, Nayak RK. Tri-Component, mobile bearing, total ankle replacement: Mid-term functional outcome and survival. J Foot Ankle Surg. 2012;51:566–569. doi: 10.1053/j.jfas.2012.05.002. [DOI] [PubMed] [Google Scholar]
- e83.Doets HC, Brand R, Nelissen RG. Total ankle arthroplasty in inflammatory joint disease with use of two mobile-bearing designs. J Bone Joint Surg Am. 2006;88:1272–1284. doi: 10.2106/JBJS.E.00414. [DOI] [PubMed] [Google Scholar]
- e84.Giannini S, Romagnoli M, O’Connor JJ, Malerba F, Leardini A. Total ankle replacement compatible with ligament function produces mobility, good clinical scores, and low complication rates: An early clinical assessment. Clin Orthop Relat Res. 2010;468:2746–2753. doi: 10.1007/s11999-010-1432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e85.Giannini S, Romagnoli M, O’Connor JJ, et al. Early clinical results of the BOX ankle replacement are satisfactory: A multicenter feasibility study of 158 ankles. J Foot Ankle Surg. 2011;50:641–647. doi: 10.1053/j.jfas.2011.06.003. [DOI] [PubMed] [Google Scholar]
- e86.Henricson A, Skoog A, Carlsson A. The Swedish ankle arthroplasty register: An analysis of 531 arthroplasties between 1993 and 2005. Acta Orthop. 2007;78:569–574. doi: 10.1080/17453670710014248. [DOI] [PubMed] [Google Scholar]
- e87.Henricson A, Nilsson JA, Carlsson A. 10-year survival of total ankle arthroplasties: A report on 780 cases from the Swedish ankle register. Acta Orthop. 2011;82:655–659. doi: 10.3109/17453674.2011.636678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e88.Hosman AH, Mason RB, Hobbs T, Rothwell AG. A New Zealand national joint registry review of 202 total ankle replacements followed for up to 6 years. Acta Orthop. 2007;78:584–591. doi: 10.1080/17453670710014266. [DOI] [PubMed] [Google Scholar]
- e89.Jensen NC, Kroner K. Total ankle joint replacement: A clinical follow up. Orthopedics. 1992;15:236–239. doi: 10.3928/0147-7447-19920201-21. [DOI] [PubMed] [Google Scholar]
- e90.Kitaoka HB, Patzer GL, Ilstrup DM, Wallrichs SL. Survivorship analysis of the Mayo total ankle arthroplasty. J Bone Joint Surg Am. 1994;76:974–979. doi: 10.2106/00004623-199407000-00003. [DOI] [PubMed] [Google Scholar]
- e91.Kofoed H, Sorensen TS. Ankle arthroplasty for rheumatoid arthritis and osteoarthritis: Prospective long-term study of cemented replacements. J Bone Joint Surg Br. 1998;80:328–332. doi: 10.1302/0301-620x.80b2.8243. [DOI] [PubMed] [Google Scholar]
- e92.Kofoed H, Lundberg-Jensen A. Ankle arthroplasty in patients younger and older than 50 years: A prospective series with long-term follow-up. Foot Ankle Int. 1999;20:501–506. doi: 10.1177/107110079902000807. [DOI] [PubMed] [Google Scholar]
- e93.Kofoed H. Scandinavian total ankle replacement (STAR) Clin Orthop Relat Res. 2004;424:73–79. doi: 10.1097/01.blo.0000132414.41124.06. [DOI] [PubMed] [Google Scholar]
- e94.Kokkonen A, Ikavalko M, Tiihonen R, Kautiainen H, Belt EA. High rate of osteolytic lesions in medium-term followup after the AES total ankle replacement. Foot Ankle Int. 2011;32:168–175. doi: 10.3113/FAI.2011.0168. [DOI] [PubMed] [Google Scholar]
- e95.Kraal T, van der Heide HJ, van Poppel BJ, Fiocco M, Nelissen RG, Doets HC. Long-term follow-up of mobile-bearing total ankle replacement in patients with inflammatory joint disease. Bone Joint J. 2013;95:1656–1661. doi: 10.1302/0301-620X.95B12.32146. [DOI] [PubMed] [Google Scholar]
- e96.Leardini A, O’Connor JJ, Catani F, Romagnoli M, Giannini S. Preliminary results of a biomechanics driven design of a total ankle prosthesis. J Foot Ankle Res. 2008;1 [Google Scholar]
- e97.Morgan SS, Brooke B, Harris NJ. Total ankle replacement by the ankle evolution system: Medium-term outcome. J Bone Joint Surg Br. 2010;92:61–65. doi: 10.1302/0301-620X.92B1.22360. [DOI] [PubMed] [Google Scholar]
- e98.Nishikawa M, Tomita T, Fujii M, et al. Total ankle replacement in rheumatoid arthritis. Int Orthop. 2004;28:123–126. doi: 10.1007/s00264-003-0512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e99.Nodzo SR, Miladore MP, Kaplan NB, Ritter CA. Short to midterm clinical and radiographic outcomes of the salto total ankle prosthesis. Foot Ankle Int. 2014;35:22–29. doi: 10.1177/1071100713510497. [DOI] [PubMed] [Google Scholar]
- e100.Reuver JM, Dayerizadeh N, Burger B, Elmans L, Hoelen M, Tulp N. Total ankle replacement outcome in low volume centers: Short-term followup. Foot Ankle Int. 2010;31:1064–1068. doi: 10.3113/FAI.2010.1064. [DOI] [PubMed] [Google Scholar]
- e101.San Giovanni TP, Keblish DJ, Thomas WH, Wilson MG. Eight-year results of a minimally constrained total ankle arthroplasty. Foot Ankle Int. 2006;27:418–426. doi: 10.1177/107110070602700606. [DOI] [PubMed] [Google Scholar]
- e102.Schenk K, Lieske S, John M, et al. Prospective study of a cementless, mobile-bearing, third generation total ankle prosthesis. Foot Ankle Int. 2011;32:755–763. doi: 10.3113/FAI.2011.0755. [DOI] [PubMed] [Google Scholar]
- e103.Schill S, Biehl C, Thabe H. Prothetische Versorgung des Sprunggelenks: Mittelfristige Ergebnisse nach Thompson-Richards- und STAR-Prothesen. Orthopade. 1998;27:183–187. doi: 10.1007/PL00003489. [DOI] [PubMed] [Google Scholar]
- e104.Schweitzer KM, Adams SB, Viens NA, et al. Early prospective clinical results of a modern fixed-bearing total ankle arthroplasty. J Bone Joint Surg Am. 2013;95:1002–1011. doi: 10.2106/JBJS.L.00555. [DOI] [PubMed] [Google Scholar]
- e105.Sproule JA, Chin T, Amin A, et al. Clinical and radiographic outcomes of the mobility total ankle arthroplasty system: early results from a prospective multicenter study. Foot Ankle Int. 2013;34:491–497. doi: 10.1177/1071100713477610. [DOI] [PubMed] [Google Scholar]
- e106.Summers JC, Bedi HS. Reoperation and patient satisfaction after the mobility total ankle arthroplasty. ANZ J Surg. 2012;83:371–375. doi: 10.1111/ans.12002. [DOI] [PubMed] [Google Scholar]
- e107.Trincat S, Kouyoumdjian P, Asencio G. Total ankle arthroplasty and coronal plane deformities. Orthop Traumatol Surg Res. 2012;98:75–84. doi: 10.1016/j.otsr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- e108.Wood PL, Deakin S. Total ankle replacement. The results in 200 ankles. J Bone Joint Surg Br. 2003;85:334–341. doi: 10.1302/0301-620x.85b3.13849. [DOI] [PubMed] [Google Scholar]
- e109.Weber M, Bonnin M, Columbier JA, Judet T. Erste Ergebnisse der SALTO-Sprunggelenkendoprothese: Eine französische Multizenterstudie mit 115 Implantaten. Fuss Sprungg. 2004;2:29–37. [Google Scholar]
- e110.Willegger M, Trnka HJ, Schuh R. The HINTEGRA ankle arthroplasty: Intermediate term results of 16 consecutive ankles and a review on the current literature. Clin Res Foot Ankle. 2013;2 [Google Scholar]
- e111.Wood PL, Prem H, Sutton C. Total ankle replacement: Medium-term results in 200 Scandinavian total ankle replacements. J Bone Joint Surg Br. 2008;90:605–609. doi: 10.1302/0301-620X.90B5.19677. [DOI] [PubMed] [Google Scholar]
- e112.Wood PL, Sutton C, Mishra V, Suneja R. A randomised, controlled trial of two mobile-bearing total ankle replacements. J Bone Joint Surg Br. 2009;91:69–74. doi: 10.1302/0301-620X.91B1.21346. [DOI] [PubMed] [Google Scholar]
- e113.Thomason K, Eyres KS. A technique of fusion for failed total replacement of the ankle: Tibio-allograft-calcaneal fusion with a locked retrograde intramedullary nail. J Bone Joint Surg Br. 2008;90:885–888. doi: 10.1302/0301-620X.90B7.20221. [DOI] [PubMed] [Google Scholar]
- e114.Kotnis R, Pasapula C, Anwar F, Cooke PH, Sharp RJ. The management of failed ankle replacement. J Bone Joint Surg Br. 2006;88:1039–1047. doi: 10.1302/0301-620X.88B8.16768. [DOI] [PubMed] [Google Scholar]
- e115.Espinosa N, Wirth SH. Sprunggelenkarthrodese nach gescheiterter Endoprothesenimplantation. Orthopade. 2011;40:1008–1017. doi: 10.1007/s00132-011-1830-6. [DOI] [PubMed] [Google Scholar]
- e116.Horisberger M, Paul J, Wiewiorski M, et al. Commercially available trabecular metal ankle interpositional spacer for tibiotalocalcaneal arthrodesis secondary to severe bone loss of the ankle. J Foot Ankle Surg. 2014;53:383–387. doi: 10.1053/j.jfas.2013.11.004. [DOI] [PubMed] [Google Scholar]
- e117.Wunschel M, Leichtle UG, Leichtle CI, et al. Fusion following failed total ankle replacement. Clin Podiatr Med Surg. 2013;30:187–198. doi: 10.1016/j.cpm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- e118.Donnenwerth MP, Roukis TS. Tibio-talo-calcaneal arthrodesis with retrograde compression intramedullary nail fixation for salvage of failed total ankle replacement: A systematic review. Clin Podiatr Med Surg. 2013;30:199–206. doi: 10.1016/j.cpm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- e119.McCollum G, Myerson MS. Failure of the agility total ankle replacement system and the salvage options. Clin Podiatr Med Surg. 2013;30:207–223. doi: 10.1016/j.cpm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- e120.Deorio JK. Revision INBONE total ankle replacement. Clin Podiatr Med Surg. 2013;30:225–236. doi: 10.1016/j.cpm.2012.10.003. [DOI] [PubMed] [Google Scholar]
- e121.Espinosa N, Wirth SH. Revision of the aseptic and septic total ankle replacement. Clin Podiatr Med Surg. 2013;30:171–185. doi: 10.1016/j.cpm.2012.10.004. [DOI] [PubMed] [Google Scholar]