Abstract
Using isobaric-isothermal replica-exchange molecular dynamics and the all-atom explicit-solvent model, we studied the equilibrium binding of Aβ monomers to a zwitterionic dimyristoylphosphatidylcholine (DMPC) bilayer coincubated with calcium ions. Using our previous replica-exchange molecular dynamics calcium-free simulations as a control, we reached three conclusions. First, calcium ions change the tertiary structure of the bound Aβ monomer by destabilizing several long-range intrapeptide interactions, particularly the salt bridge Asp23-Lys28. Second, calcium strengthens Aβ peptide binding to the DMPC bilayer by enhancing electrostatic interactions between charged amino acids and lipid polar headgroups. As a result, Aβ monomer penetrates deeper into the bilayer, making disorder in proximal lipids and bilayer thinning more pronounced. Third, because calcium ions demonstrate strong affinity to negatively charged amino acids, a considerable influx of calcium into the area proximal to the bound Aβ monomer is observed. Consequently, the localizations of negatively charged amino acids and calcium ions in the Aβ binding footprint overlap. Based on our data, we propose a mechanism by which calcium ions strengthen Aβ-bilayer interactions. This mechanism involves two factors: 1) calcium ions make the DMPC bilayer partially cationic and thus attractive to the anionic Aβ peptide; and 2) destabilization of the Asp23-Lys28 salt bridge makes Lys28 available for interactions with the bilayer. Finally, we conclude that a single Aβ monomer does not promote permeation of calcium ions through the zwitterionic bilayer.
Introduction
Normal cellular proteolysis cleaves Aβ peptides from the amyloid precursor protein (APP), in which the segment corresponding to Aβ is partly embedded in the cellular membrane. Although there are many Aβ alloforms, the most abundant is the 40-residue Aβ1–40 peptide, which represents ∼90% of all Aβ species in cerebrospinal fluid (1). Extensive experimental and genetic evidence links Aβ peptides to the development of Alzheimer’s disease (2). Accordingly, experiments have established that Aβ peptides, especially in aggregated forms, are cytotoxic, causing degeneration of neuronal cells (3,4). One of the possible mechanisms of cytotoxicity involves perturbation of the bilayer structure induced by binding Aβ species, which in turn increases membrane permeability for ions, particularly Ca2+ (5). Consequently, interactions of Aβ peptides with cellular membranes may adversely affect ion homeostasis.
Interactions of Aβ peptides with cellular membranes or model lipid bilayers have been the subject of numerous experimental studies (6–13). According to these investigations, the type of Aβ species interacting with lipid bilayers depends on Aβ concentration. Specifically, binding of Aβ monomers is observed at low peptide concentrations ( 150 nM), whereas at elevated concentrations, Aβ oligomeric species interact with the bilayers (10,11). It appears that at least in some cases, interaction of Aβ peptides with lipid bilayers leads to their penetration into the bilayer hydrophobic core, as suggested by the analysis of electron-density profiles (7). Consequently, one may expect that binding of Aβ peptides affects the structure and stability of lipid bilayers. Indeed, experiments have discovered a spectrum of Aβ impact, from complete disintegration of the dimyristoylphosphatidylcholine (DMPC) bilayer phase (14) to modest perturbations in bilayer structure and enhanced permeability of ions (12). In general, the disordering effect induced by the bound Aβ peptide becomes stronger with deeper insertion of the peptide into the bilayer (6,13). Our recent studies employing isobaric-isothermal replica-exchange molecular dynamics (REMD) have provided a complementary molecular-level description for the interactions between Aβ10–40 monomers and zwitterionic DMPC bilayers (15,16). We have established that the central hydrophobic cluster, and particularly the C-terminus, governs Aβ binding to the bilayer by penetrating into the bilayer core. As a result Aβ monomer reduces the density of lipids within and underneath its binding footprint and causes noticeable local thinning of the DMPC bilayer. From our simulations, we further concluded that Aβ peptide disorders the orientation of lipid tails and causes significant dehydration of the lipid-water interface. Using peptide conformational ensemble in water as a control we found that Aβ monomer binding to the DMPC bilayer experiences a dramatic structural transition manifested in the appearance of stable helix structure in the C-terminus.
Calcium homeostasis is critical for cell vitality, and significant effort has been concentrated on probing Ca2+ interactions with cellular membranes. Experimental studies have firmly established that Ca2+ ions strongly interact with zwitterionic phosphatidylcholine lipid bilayers (17,18) and reorganize the distribution of lipid molecules in the bilayer (19). Furthermore, atomic force microscopy studies suggest that ions increase the mechanical strength of zwitterionic DMPC bilayers against rupture (20). Consistent findings have been reported in MD studies. It has been observed that cations bound to zwitterionic bilayer reduce the area per lipid (21) and form stable complexes consisting of a single center ion coordinating several lipid molecules (22–24). MD simulations also reveal that among mono- and divalent ions, calcium demonstrates one of the strongest binding affinities with respect to lipid bilayers (22). Although details of ion-lipid interactions may depend on the force field employed in MD, most computational studies agree that when bound to the bilayer, Ca2+ predominantly interacts with phosphate and glycerol groups (21,22).
Because experimental and computational studies implicate strong interactions of zwitterionic lipids with calcium and ensuing reorganization in the bilayer structure, one may expect that Ca2+ ions influence binding of Aβ peptides to lipid bilayers. Furthermore, it is also conceivable that Aβ peptides embedded in the bilayer affect the permeation of calcium through the lipid bilayer. Motivated by these considerations, we report in this article the REMD simulations of Aβ monomers binding to the zwitterionic DMPC bilayer in the presence of calcium ions. Our main results are as follows. First, although Ca2+ ions produce minor impact on Aβ secondary structure, they change the tertiary structure of the peptide by disrupting several intramolecular long-range interactions, particularly the salt bridge Asp23-Lys28. Second, calcium enhances Aβ-lipid interactions, causing deeper penetration of Aβ into the bilayer. This phenomenon is mostly driven by strong electrostatic interactions between charged amino acids and polar lipid headgroups. As a result, calcium ions not only further promote bilayer thinning caused by the bound Aβ monomer, they also enhance lipid disorder within the Aβ binding footprint. Third, because calcium demonstrates strong binding affinity to negatively charged amino acids, an influx of calcium into the area proximal to the bound Aβ monomer is observed to elevate the ion local concentration by >25%. Based on our data, we propose a mechanism by which calcium might strengthen Aβ-bilayer interactions. In conclusion, we argue that despite bilayer perturbation, bound Aβ monomer does not enhance the permeation of calcium through the DMPC bilayer.
Materials and Methods
All-atom explicit-solvent model
To perform REMD simulations, we used all-atom explicit-solvent CHARMM22 protein force field with CMAP corrections (25) and CHARMM36 lipid force field (26). CMAP corrections to the protein force field are necessary to improve the agreement between experimental and in silico structures in disordered regions (25). Water was represented using the TIP3 model. The simulation system consisted of two noninteracting Aβ10–40 monomers coincubated with 98 zwitterionic DMPC lipids forming a bilayer in a mixture of 3319 water molecules and 20 Ca2+ ions (Fig. 1). Chloride ions were added to neutralize the system. In total, the system contained 22,531 atoms. Each bilayer leaflet was composed of 49 lipids arranged in a 7 × 7 square shape. Aβ peptide and an equal number of calcium ions (10) were placed on each side of the lipid bilayer. The average size of the simulation box was 57.1 Å × 57.1 Å × 68.0 Å, which leads to an approximate concentration of Ca2+ of 150 mM.
Figure 1.

(a) Sequence of Aβ10–40 monomer with color-coded regions S1–S4. (b) DMPC lipid molecule divided into atom groups of choline (G1), phosphate (G2), glycerol (G3), and two fatty acid tails (G4 and G5). (c) Simulation system including the DMPC bilayer (orange), two Aβ monomers (gray), Ca2+ ions (green), and water (thin blue lines). Cl− ions are excluded, for clarity. Phosphorus atoms with their centers of mass located at ≈±zP are purple. Most of the calcium ions near the peptide (proximal region) are bound to negatively charged amino acids, with only a few bound to G2. In the distant region, calcium ions typically coordinate G2 groups. To see this figure in color, go online.
Following the methodology from our previous studies (15,16,27,28), we used amino-truncated Aβ10–40 peptide. The selection of Aβ10–40 can be rationalized by taking into account that the N-terminus of Aβ1–40 in APP is solvated outside the cellular membrane and that Aβ10–40 peptides themselves are naturally occurring cytotoxic species (29,30). Using Aβ10–40 also affords direct comparisons with our previous simulations. We chose the DMPC lipid bilayer, because its structural and physicochemical properties are well known (31) and because it was used in our previous simulation studies of Aβ binding to the lipid bilayer in a Ca2+-free environment (15,16).
Replica-exchange simulations
REMD simulations were performed in the isobaric-isothermal (NPT) ensemble (15,16,32) using the program NAMD (33) and in-house scripts. Simulations were conducted using computational resources at George Mason University and XSEDE (34). Because specific details of REMD protocol can be found in our previous studies (15,16), we provide here only a brief overview. Temperature was controlled by underdamped Langevin dynamics, whereas pressure was controlled by the Nosé-Hoover Langevin piston method. Pressure was applied using a semiisotropic scheme, which couples x and y box dimensions (in the bilayer plane) while allowing the z dimension to adjust independently. An integration step of 1 fs was used. The system was simulated using periodic boundary conditions. Electrostatic interactions were computed using Ewald summation, whereas van der Waals interactions were smoothly switched off in the interval from 8 to 12 Å. Covalent bonds were constrained by the SHAKE algorithm.
We distributed R = 40 replicas exponentially in the temperature range 320–430 K. All replicas were simulated at the same pressure of 1 atm. Exchanges were attempted every 2 ps between all neighboring replicas along the temperature scale, generating an average acceptance rate of 27%. We produced six independent REMD trajectories, resulting in a simulation time of 4.8 μs accumulated in all replicas and trajectories. Therefore, cumulatively the system was simulated for 120 ns at each temperature. Initial unequilibrated portions of REMD trajectories were discarded, which reduced the cumulative equilibrium simulation time to 3.9 μs. REMD trajectories were started with random unbound structures of Aβ monomers and a preequilibrated DMPC bilayer. Analysis of REMD convergence based on several independent measures is presented in the Supporting Material. This analysis, together with small sampling errors, indicates approximate convergence of REMD simulations.
To prevent bilayer disintegration at high REMD temperatures, we applied harmonic constraints with force constant k = 6.5 kcal/(molÅ2) to the centers of mass of phosphorus (P) atoms in each leaflet (Fig. 1 c). These constraints acting independently on the P centers of mass in each leaflet approximately fix them at the distance = 17.35 Å from the bilayer midplane. Constraining the center of mass allows individual P atoms to fluctuate, preserving bilayer flexibility. We have shown that introduction of bilayer constraints does not affect bilayer properties at 330 K (15,16). The second set of constraints, also introduced in our earlier studies (15,16), blocks Aβ peptides and ions from crossing the periodic boundaries along the z dimension. These boundary constraints were implemented as a pair of repulsive harmonic potentials with force constant k = 10 kcal/(mol Å2), which act on peptide atoms or ions when their z positions are within 4 Å of a z periodic boundary. Lipid and water atoms were not affected. These constraints were designed to prevent peptide aggregation. To assess their impact, we computed the probability of a peptide atom (or ion) interacting with the boundary constraints. At 330 K, the corresponding probability is <0.002.
Control simulations
For brevity, we designate the REMD simulations described above as Aβ+BL+Ca. As a control, we performed MD simulations of the DMPC bilayer with calcium but without Aβ peptides (referred to as BL+Ca). Except for the absence of replica exchange, the design of control simulations performed at 330 K followed that used in Aβ+BL+Ca simulations, including the application of constraints. As further controls, we resorted to our previous NPT-REMD simulations of Aβ monomers interacting with the DMPC bilayer in calcium-free water (15,16) (referred to as Aβ+BL) and to REMD simulations of Aβ monomer in water (27) (Aβ+water). In those simulations, the systems were neutralized by adding two or one sodium ions.
Computation of structural probes
To describe interactions between Aβ and the lipid bilayer, we used the following approach. Aβ peptide was represented by the centers of mass of side chains, whereas each DMPC lipid was divided into five atom groups: choline (G1), phosphate (G2), glycerol (G3), and two fatty acid tails (G4 and G5) (see Fig. 1 b). A contact between side chain and lipid group occurs if their centers of mass are within 6.5 Å of each other. An amino acid is bound to the bilayer if its side chain forms a contact with any group of any lipid molecule. Aβ peptide is considered bound if it has at least one bound amino acid. A calcium ion is bound to an amino acid if the distance between the ion and side-chain centers of mass is <6.5 Å. The latter definition applies also to binding of calcium to lipid groups. To study coordination of phosphate groups by Ca2+, we assumed that Ca2+ is interacting with G2 when they are ≤4.5 Å apart. This lower cutoff reflects the small size of the G2 group.
Aβ secondary structure was computed using STRIDE (35). Amino acids were considered to be in a helical state if they sampled α-, 310-, or π-helices. To explore properties of Aβ sequence, we divided it into four segments: the hydrophilic N-terminus (S1, residues 10–16), the central hydrophobic cluster (S2, residues 17–21), the hydrophilic turn (S3, residues 22–28), and the hydrophobic C-terminus (S4, residues 29–40).
To describe lipid bilayers, we defined several measures. The first is the number density of lipid heavy atoms, , computed as a function of the distance to the peptide center of mass, r, and the distance to the bilayer midplane, z. (Note that r is computed in the bilayer plane.) A similar quantity, , reports the distribution of calcium ions in the bilayer. The third measure, the lipid carbon-deuterium order parameter, SCD, probes the ordering of the fatty acid tails, , with respect to the bilayer normal. SCD is defined as
| (1) |
where θ is the angle between the bilayer normal and the C-H bond. When describing lipids, we separate them into those that interact with Aβ and those that do not. Consequently, we defined the distance from the Aβ center of mass along the bilayer plane, Rc = 14 Å, at which the number of peptide-lipid contacts reaches the maximum. If a lipid center of mass is within Rc from the peptide, the lipid is referred to as proximal, whereas lipids outside this radius are classified as distant.
Thermodynamic averages of structural quantities (denoted by angled brackets) were computed using the multiple-histogram method (36) adapted for NPT simulations (37,38). Structural quantities related to Aβ represent the averages over two peptides and include the data from all six REMD trajectories. All results are reported at 330 K.
Results
Aβ conformational ensemble
Isobaric-isothermal REMD simulations allow us to explore binding of Aβ monomers to a DMPC bilayer coincubated with Ca2+ ions. As a control, we use the REMD simulations of Aβ monomers bound to the DMPC bilayer in a Ca2+-free environment (the Aβ+BL system) (15). Similar to the calcium-free system, Aβ is bound to the DMPC bilayer with a probability of ≈1.0 at 330 K. We then focus on the structure of bound Aβ peptides. Fig. 2 presents the distribution of helix structure, <H(i)>, for Aβ amino acids i (the distributions of turn, <T(i)>, and random coil, <RC(i)>, are not shown). These probabilities, when averaged over the entire peptide, yield values of <H>, <T>, and <RC> equal to 0.34 ± 0.03, 0.36 ± 0.01, and 0.28 ± 0.02, respectively. The contribution of other secondary structures (e.g., β-sheet) is negligible (0.01 ± 0.01). These data suggest that Aβ secondary structure approximately equally partitions into helix, turn, and random coil. Next, we characterize secondary structure in Aβ segments (Table 1). The table indicates that the turn is predominant in S1 and S3, that both turn and coil are well represented in S2, and that the C-terminus segment, S4, features stable helix, in which <H(i)> > 0.5 for sequence positions 30–36 (Fig. 2). Overall, the distribution of secondary structure in Aβ bound to the DMPC bilayer is not significantly affected by calcium (Table 1 and Fig. 2). For example, the average fractions of helix, turn, and random coil in the Ca2+-free system are 0.39, 0.30, and 0.31 (15). According to Table 1, in both environments, helix dominates the conformational ensemble in the C-terminus (S4).
Figure 2.

Distribution of helical structure, <H(i)>, in Aβ monomer with respect to sequence position i. Data for Aβ+BL+Ca system are in black, whereas data for the calcium-free Aβ+BL and the Aβ+water systems are in red and blue, respectively. Simulation errors are given by vertical bars. Color codes for regions S1–S4 are as in Fig. 1a. The plot shows that calcium ions have a fairly small impact on the formation of helix in the bilayer-bound Aβ monomer. To see this figure in color, go online.
Table 1.
Secondary structure of Aβ peptide in different environments
| Environment | Secondary structure | S1a | S2a | S3a | S4a |
|---|---|---|---|---|---|
| Aβ+BL+Ca | <H> | 0.14 ± 0.03 | 0.11 ± 0.03 | 0.30 ± 0.03 | 0.59 ± 0.08 |
| <T> | 0.48 ± 0.03 | 0.46 ± 0.04 | 0.48 ± 0.05 | 0.18 ± 0.05 | |
| <RC> | 0.38 ± 0.03 | 0.40 ± 0.05 | 0.22 ± 0.03 | 0.22 ± 0.02 | |
| Aβ+BLb | <H> | 0.14 ± 0.08 | 0.10 ± 0.03 | 0.39 ± 0.05 | 0.65 ± 0.05 |
| <T> | 0.46 ± 0.06 | 0.39 ± 0.03 | 0.41 ± 0.06 | 0.10 ± 0.05 | |
| <RC> | 0.40 ± 0.04 | 0.50 ± 0.01 | 0.20 ± 0.02 | 0.26 ± 0.01 | |
| Aβ+waterc | <H> | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.16 ± 0.01 | 0.17 ± 0.02 |
| <T> | 0.41 ± 0.02 | 0.59 ± 0.01 | 0.65 ± 0.02 | 0.42 ± 0.02 | |
| <RC> | 0.55 ± 0.01 | 0.38 ± 0.01 | 0.19 ± 0.01 | 0.40 ± 0.01 |
The impact of calcium on Aβ tertiary structure was assessed by computing the difference contact map, , which reports changes in the equilibrium probabilities of forming contacts between amino acids i and j in systems Aβ+BL+Ca and Aβ+BL. Fig. 3 a demonstrates that there are six intrapeptide contacts , for which , including Asn27-Ala30 (0.33), Asp23-Lys28 (−0.31), Gly29-Ile32 (0.24), Asn27-Ile32 (0.24), Gly29-Gly33 (0.21), and Phe19-Ile31 (−0.20). Three of these contacts (Asn27-Ala30, Gly29-Ile32, and Gly29-Gly33) are short-range (j − i < 5) and responsible for moderate enhancement of helix at sequence positions 27–30 (Fig. 2). The remaining three contacts (Asp23-Lys28, Asn27-Ile32, and Phe19-Ile31) are long-range interactions (j − i ≥ 5), of which the salt bridge Asp23-Lys28 is of particular interest. First, this contact is the only stable long-range interaction observed in the Aβ+BL system and is destabilized by calcium more than any other intrapeptide interaction. Second, the Asp23-Lys28 salt bridge involves an anionic amino acid, which is likely to be directly affected by calcium. (Note that although other intramolecular salt bridges can form in Aβ10–40, they have a low probability of occurrence or the involved amino acids contribute little to the interactions with the bilayer.) Consequently, the Asp23-Lys28 interaction was further analyzed by computing the probability distribution, P(rDK), of distances between the side chains of Asp23 and Lys28, rDK (Fig. 3 b). In striking contrast to the Aβ+BL system, P(rDK) in Aβ+BL+Ca is bimodal. Therefore, Ca2+ ions induce the formation of two structural states in the bound Aβ, in which the salt bridge Asp23-Lys28 is either formed (ON, with rDK < 6.5 Å) or disrupted (OFF). The probabilities of ON and OFF states are 0.48 ± 0.03 and 0.52 ± 0.03, respectively, indicating that both are about equally probable. For comparison, in the Aβ+BL system, the salt bridge is formed with a probability of 0.79 (15).
Figure 3.

(a) Difference contact map, , showing changes in the probabilities of contacts being formed between amino acids i and j in systems Aβ+BL+Ca and Aβ+BL. is defined as <ΔC(i,j)>1 − <ΔC(i,j)>2, where indices 1 and 2 stand for Aβ+BL+Ca and Aβ+BL, respectively. Color codes for the regions S1–S4 are as in Fig. 1a. (b) Probability distributions, , for the distance between the centers of mass of Asp23 and Lys28 side chains, . Data for the Aβ+BL+Ca and Aβ+BL systems are in black and red, respectively. The figure demonstrates destabilization of the Asp23-Lys28 salt bridge by calcium. To see this figure in color, go online.
In the Aβ+BL+Ca system, the Asp23-Lys28 salt bridge acts as a switch that modulates the formation of other long-range contacts. For instance, the probability of the Phe19-Ile31 contact is 0.12 when the salt bridge is OFF, but it increases to 0.45 when the salt bridge is ON. Note that the Aβ+BL system with the stable Asp23-Lys28 salt bridge has nearly the same equilibrium probability for Phe19-Ile31 contact (0.48). Similar conclusions can be made for the long-range contact Asn27-Ile32, which has a formation probability of 0.16 when the salt bridge is OFF but 0.58 when the salt bridge is ON. The relation between the disruption of the Asp23-Lys28 salt bridge and the binding of calcium is discussed below.
Aβ-bilayer interactions
As stated above, the probability of Aβ binding to the DMPC bilayer is ≈1.0 at 330 K. Fig. 4 describes the probability of amino acid i occurring at distance z from the bilayer midplane, P(z;i), providing further insight into Aβ-bilayer interactions. It can be seen that most amino acids from the central hydrophobic cluster and C-terminus (segments S2 and S4) are inserted into the bilayer below phosphorus atoms, i.e., their average positions are <z(i)> < zP = 17.35 Å. In contrast, the N-terminus and the turn (segments S1 and S3) generally prefer to remain near the bilayer surface (<z(i)> > zP). These observations are supported by the average z positions, <z>, of S1–S4 (Table 2). For S2 and S4, <z> < zP, suggesting that they have inserted into the bilayer core. For surface-bound S1 and S3, <z> > zP. Table 2 evaluates the impact of calcium on Aβ insertion into the bilayer, showing the change in segment <z> between Aβ+BL+Ca and Aβ+BL systems, <Δz>. Negative values of <Δz> indicate that calcium promotes deeper penetration into the bilayer, whereas positive values signify more shallow insertion. According to Table 2 and Fig. 4, all segments penetrate deeper into the bilayer in the presence of calcium, with the deepest insertion observed for the polar segments S1 (−3.3 Å) and S3 (−2.3 Å).
Figure 4.
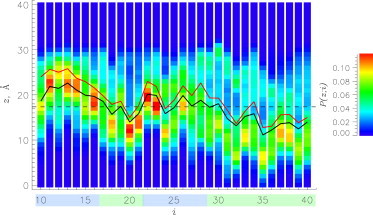
Probabilities, , of occurrence of amino acids i at distance z from the bilayer midplane (z = 0 Å). Black and red lines show the average positions of amino acids i along the z axis, , for the Aβ+BL+Ca and Aβ+BL systems, respectively. Color codes for regions S1–S4 are as in Fig. 1a. The dashed line marks the average position of the center of mass of phosphorus atoms, zP. The plot reveals that calcium promotes deeper insertion of Aβ monomer into the DMPC bilayer. To see this figure in color, go online.
Table 2.
Binding and insertion of Aβ monomer into the DMPC bilayer
| Measure | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| <z>a | 20.8 ± 0.06 | 16.3 ± 1.4 | 18.5 ± 1.5 | 14.3 ± 2.2 |
| <Δz>b | −3.3 ± 0.06 | −1.5 ± 1.4 | −2.3 ± 1.5 | −1.5 ± 2.2 |
| ΔPsb | 0.14 ± 0.02 | 0.02 ± 0.06 | 0.02 ± 0.07 | 0.11 ± 0.09 |
| ΔPib | 0.15 ± 0.04 | 0.09 ± 0.11 | 0.12 ± 0.11 | 0.03 ± 0.14 |
data for Aβ+BL+Ca.
<Δz> = <Δz>1 − <Δz>2, where indices 1 and 2 stand for Aβ+BL+Ca and Aβ+BL, respectively. and are defined in a similar way.
The results presented above are supported by our computations of the probabilities of binding to the bilayer surface, Ps(i). Specifically, Ps(i) is defined as the probability of amino acid i occurring in the interval (zP,zP + 6.5 Å). Table 2 shows the calcium-driven changes in Ps, ΔPs, for the peptide segments. The largest change occurs for S1 (0.14). As a direct measure of Aβ penetration into the bilayer, we introduce the probability of insertion, Pi(i), which is the probability of occurrence of amino acid i below zP. Table 2 reveals that S1 and S3 are characterized by the largest changes in Pi(i), ΔPi, (0.15 and 0.12, respectively). Thus, consistent with the results shown in Fig. 4, addition of calcium causes all segments, particularly S1 and S3, to penetrate deeper into the bilayer.
If calcium induces deeper insertion of Aβ into the bilayer, it is important to map the interactions responsible for this effect. To this end, we computed the average number of contacts between amino acid i and lipid group k, <Cl(i,k)>. Fig. 5 presents the thermally weighted difference <ΔCl(i,k)>, defined as the change in <Cl(i,k)> between the Aβ+BL+Ca and Aβ+BL systems. The top contacts (with <ΔCl(i,k)> > 0.30) are Lys28-G2 (0.47), Glu11-G2 (0.47), Lys28-G3 (0.43), Asp23-G2 (0.41), Glu22-G2 (0.32), Tyr10-G3 (0.32), and His13-G1 (0.31). All the residues involved occur in segments S1 or S3, and all of the five most strengthened contacts are formed by charged amino acids. Furthermore, Table 3 lists <ΔCl(i,k)> averaged across segments S1–S4 and lipid groups G1–G3 and G4,G5. The largest change occurs in the binding of S1 and S3 to lipid headgroups (0.18 and 0.07, respectively). These observations suggest that calcium mainly enhances Aβ-lipid electrostatic interactions, resulting in stronger binding of polar segments S1 and S3 (particularly, charged amino acids Glu11, Glu22, Asp23, and Lys28) to the lipid headgroups. A simple way to assess the contribution of charged amino acids to Aβ-bilayer interactions is to compute the ratio of <ΔCl> attributed to Glu11, Glu22, Asp23, Lys28 to the total <ΔCl> caused by calcium. We found that 52% of the increase in Aβ interactions with the lipid headgroups is related to these four amino acids. These conclusions are consistent with the above analysis of insertion of amino acids and with the disruption of the Asp23-Lys28 salt bridge.
Figure 5.

Difference contact map, <ΔCl(i,k)>, displaying changes in the probability of contacts forming between amino acids i and lipid groups k. <ΔCl(i,k)> is defined as <ΔCl(i,k)>1 − <ΔCl(i,k)>2, where indices 1 and 2 stand for Aβ+BL+Ca and Aβ+BL, respectively. Color codes for the regions S1–S4 are as in Fig. 1a. The plot implicates calcium ions in promoting stronger binding of polar segments S1 and S3 (particularly charged amino acids Glu11, Glu22, Asp23, and Lys28) to lipid headgroups. To see this figure in color, go online.
Table 3.
Changes in Aβ-bilayer interactions induced by calcium
| Lipid groupa | Peptide | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| G1–G3 | 0.06 ± 0.02 | 0.18 ± 0.04 | 0.01 ± 0.02 | 0.07 ± 0.03 | 0.01 ± 0.02 |
| G4,G5 | 0.04 ± 0.04 | 0.05 ± 0.01 | 0.06 ± 0.06 | 0.06 ± 0.04 | 0.03 ± 0.06 |
Changes in Aβ-bilayer interactions are quantified by <ΔCl(i,k)> = <ΔCl(i,k)1> −<ΔCl(i,k)2>, where indices 1 and 2 stand for Aβ+BL+Ca and Aβ+BL systems.
Averages are normalized by the number of lipid groups.
Impact on bilayer structure
It follows from the results above that calcium enhances interactions of Aβ with the DMPC bilayer and causes deeper peptide insertion. Therefore, one may expect that Aβ binding affects bilayer structure. Fig. 6 displays the number density of heavy lipid atoms, , at distance r from the peptide center of mass and distance z from the bilayer midplane (see Materials and Methods). The figure vividly illustrates that the bound Aβ monomer indents the lipid bilayer and depresses lipid density. As in our previous study, bilayer indentation was quantified by defining the boundary zl(r), which corresponds to an ≈50% drop in lipid density with respect to the maximum for a given r (16). For distant lipids, this boundary approximately matches the bilayer-water interface, where atom number densities of water and lipids are equal. Then, the indentation depth is taken as the difference between the average values of zl(r) for distant (r > Rc) lipids and proximal lipids located in the center of the Aβ binding footprint (r < 6 Å). In the Aβ+BL system (16), the indentation depth was found to be 6.2 Å. With the addition of calcium, the bilayer indentation depth increases to 7.5 Å. Fig. 6 also reveals a minor but noticeable indentation of the lower leaflet immediately underneath the Aβ monomer bound to the upper leaflet. Taking into account this circumstance, the total thinning of the DMPC bilayer, , is 8.5 Å in the Aβ+BL+Ca system. By comparison, in Aβ+BL is 7.2 Å, implying that calcium increases bilayer thinning induced by Aβ by 1.3 Å.
Figure 6.

Number density of lipid heavy atoms, nl(r,z), computed as a function of distance r from the peptide center of mass and distance z from the bilayer midplane at z = 0 Å. Black and red lines mark the boundaries of the lipid bilayer, zl(r), for the Aβ+BL+Ca and Aβ+BL systems, respectively. The boundaries of the proximal region are represented by dashed vertical lines. The figure shows that calcium enhances thinning of the DMPC bilayer caused by bound Aβ monomer. To see this figure in color, go online.
Thinning of the DMPC bilayer is consistent with the analysis of the order parameter −<SCD>, which probes the orientation of C-H bonds in fatty tails. Fig. 7 shows −<SCD(i)> computed for proximal and distant lipids. All fatty acid carbons i in proximal lipids feature smaller −<SCD(i)> compared to those in distant lipids. Indeed, the average −<SCD> values for proximal and distant lipids are 0.12 ± 0.01 and 0.17 ± 0.00, respectively. This finding is consistent with that obtained for the Aβ+BL system, where the corresponding quantities were 0.13 ± 0.00 and 0.16 ± 0.00, respectively (16). The decrease in −<SCD> for proximal lipids can be attributed to disordering of fatty acid tails by Aβ. Furthermore, in the Aβ+BL+Ca system, as compared to Aβ+BL, there is a statistically significant decrease in −<SCD(i)> for six carbons in the proximal chains. Thus, Ca2+ ions not only increase bilayer thinning, but also enhance lipid disorder within the Aβ binding footprint.
Figure 7.
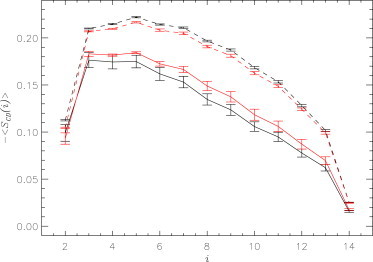
The lipid carbon-deuterium order parameter, −<SCD(i)>, computed for carbon atoms i in the fatty acid chains, . Solid and dashed lines represent proximal and distant lipids, respectively, whereas data represented in black and red are for the Aβ+BL+Ca and Aβ+BL systems, respectively. Errors are shown by vertical bars. The plot reveals that calcium ions slightly enhance the disorder in proximal lipid tails. To see this figure in color, go online.
It is worth noting that calcium impacts the bilayer structure outside of the Aβ binding footprint. For example, calcium leads to a minor but systemic increase in −<SCD> for all carbons in distant lipids (from 0.16 in Aβ+BL to 0.17 in Aβ+BL+Ca). The effect of calcium on distant lipids is opposite to that observed for proximal lipids, for which it decreases −<SCD>. It is likely that these conflicting effects of calcium on fatty acid structure are due to enhancement of Aβ insertion into the bilayer caused by calcium. As a result, Aβ impact on lipid structure overrides the direct influence of calcium on lipids (see Discussion).
Calcium distribution in the DMPC bilayer and interactions with Aβ
To understand the impact of calcium ions on Aβ binding to the DMPC bilayer, it is important to probe the interactions between the peptide and Ca2+. We measure calcium affinity to Aβ by computing the number of contacts Ca2+ ions form with amino acid i, <Ci(i)>. Fig. 8 indicates that all three negatively charged amino acids, Glu11, Glu22, and Asp23, form strong interactions with calcium. On average, the number of contacts formed by these amino acids with Ca2+ is 1.0 ± 0.1, i.e., there is about one calcium ion bound to each of these amino acids. For purposes of comparison, the average number of contacts formed by other residues is >10-fold smaller at just 0.04 ± 0.01. The average number of all calcium ions bound to Aβ monomer, <Ni>, per leaflet is 2.4 ± 0.1. However, if the computation is restricted to those ions bound to negatively charged amino acids, <Ni> is barely reduced to 2.2 ± 0.2. Therefore, ∼90% of all calcium ions interacting with the peptide are bound to anionic amino acids. These findings highlight the importance of electrostatic interactions in binding of calcium to Aβ.
Figure 8.

Average numbers of Ca2+ ions interacting with amino acids i, <Ci(i)>, (black). Light green and orange lines represent <Ci(i)> computed with the Asp23-Lys28 salt bridge formed and disrupted, respectively. Color codes for regions S1–S4 are as in Fig. 1a. Errors are shown by vertical bars. The plot underscores the strong affinity of calcium ions for negatively charged amino acids and the effect of salt bridge disruption on Ca2+ binding to Glu22 and Asp23. To see this figure in color, go online.
To explore the interactions between Ca2+ and lipids we computed the number of calcium contacts with lipid groups G1–G5, . We found that values for G1, G2, G3, and G4/G5 are 0.42 ± 0.02, 0.49 ± 0.02, 0.39 ± 0.01, and 0.00 ± 0.00, respectively. Qualitatively, this distribution of ion-lipid interactions applies to distant as well as proximal lipids. Thus, calcium binds exclusively to the polar headgroups, and there are virtually no calcium ions in the bilayer hydrophobic core.
Spatial distribution of calcium ions in the DMPC bilayer can be directly deduced from , the number density of calcium at distance r from the peptide center of mass and distance z from the bilayer midplane (Fig. 9). This plot shows that in the distant region (r > Rc), the maximum density of calcium occurs at z ≈ 17.4 Å, which is consistent with Ca2+ binding to the phosphate group. In the center of the binding footprint (r < 6 Å), the maximal occurs at a larger distance from the bilayer midplane, z ≈ 20.8 Å. As an additional measure, we analyzed the calcium surface number density, ns. (Note that ns refers to ions that are bound to either Aβ or lipids.) In the proximal region, ns = 3.4 × 10−3 Å−2, whereas in the distant region, it is reduced to 2.7 × 10−3 Å−2. Using surface number densities is a straightforward way to determine the total numbers of Ca2+ ions located in the proximal and distant regions (per leaflet), which are 2.1 and 7.1, respectively.
Figure 9.

Number density of calcium ions in the DMPC bilayer, ni(r,z), computed as a function of distance r from the peptide center of mass and distance z from the bilayer midplane at z = 0 Å. The boundaries of the proximal region are represented by dashed vertical lines. The figure illustrates the influx of calcium into the Aβ binding footprint driven by the affinity of the ions for negatively charged amino acids. To see this figure in color, go online.
Because calcium ions demonstrate strong affinity to phosphate groups (G2) and negatively charged amino acids, we investigate the coordination of G2 by Ca2+ and the impact on this coordination produced by the Aβ peptide. Fig. 10 shows the probability distributions, P(Cn), of the number of G2 groups coordinated by a calcium ion, Cn. According to the bar graph, P(Cn) reaches its maximum at Cn = 3 or 4 in the distant region. Indeed, the average number of phosphate groups, <Cn>, coordinated by Ca2+ in this region is 2.7. In contrast, for calcium ions bound to negatively charged amino acids in the proximal region, P(Cn) peaks at Cn = 0, implying that ∼75% of such calcium ions do not interact with lipid G2. As a result, the average coordination number, <Cn>, drops to 0.4. Interestingly, for calcium ions in the proximal region that are not bound to negatively charged amino acids, <Cn> ≈ 3.0, which is similar to the corresponding value in the distant region. These computations suggest that coordination of phosphate groups by Ca2+ is weakened when ions are bound to anionic amino acids.
Figure 10.

Probability distributions, P(Cn), for the number of phosphate groups coordinated by a calcium ion, Cn. Gray and dashed bars correspond to P(Cn) values computed for distant ions and for proximal ions bound to Aβ negatively charged amino acids. It follows from the plot that binding to Aβ compromises the ability of Ca2+ to coordinate phosphate groups.
It is also important to directly evaluate the ability of calcium ions in the proximal region to coordinate negatively charged amino acids with phosphate G2. Consistent with the computations above, we found the average number of such Ca2+ ions to be 0.7, or ∼17% of proximal ions. Therefore, there are relatively few calcium ions coordinating negatively charged amino acids and phosphate groups. However, we show in the Discussion that this apparently small number of coordinating Ca2+ ions is sufficient to enhance interactions between Aβ peptides and the bilayer.
Discussion
REMD simulations in the isobaric-isothermal ensemble allowed us to compute the equilibrium properties of Aβ monomer interacting with the DMPC bilayer coincubated with calcium. Our results are summarized in this section.
Calcium changes the structure of Aβ peptide bound to the DMPC bilayer
It follows from our simulations that calcium ions change the conformational ensemble of the bilayer-bound Aβ monomer. The most pronounced change is observed in Aβ tertiary structure, in which calcium induces two equally probable states, with the salt bridge Asp23-Lys28 formed (0.48) or disrupted (0.52). For comparison, this salt bridge is formed with overwhelming probability (0.79) in the simulations without calcium (15). We have argued that the disruption of this salt bridge affects other long-range contacts (e.g., Phe19-Ile31 and Asn27-Ile32).
Fig. 8 indicates that salt bridge stability is linked to calcium binding. When the salt bridge is ON, amino acid Asp23 and its neighbor Glu22 each bind on average 0.7 calcium ions. Turning the salt bridge OFF makes these amino acids available for Ca2+, increasing the number of bound ions more than twofold, to 1.6. On average, these amino acids together interact with 1.5 calcium ions, of which 60% (or 0.9) are attributed to the ions binding simultaneously to both amino acids. Another charged amino acid, Glu11, interacts with a smaller number of calcium ions (0.7). Thus, preference of calcium to bind to Glu22 and Asp23 can be explained by their adjacent positions in the Aβ sequence, which allows a single divalent Ca2+ ion to interact with two negatively charged amino acids at once. Of more importance, electrostatic interactions of calcium ions with negatively charged amino acids interfere with the intrapeptide salt bridge Asp23-Lys28, resulting in a shift in the Aβ conformational ensemble.
In addition, calcium subtly but systemically affects Aβ secondary structure. In general, binding of Aβ monomer to the DMPC bilayer elicits dramatic structural conversion in the C-terminus (S4) from random coil and turn to stable helix (Fig. 2 and Table 1) (15). Indeed, according to Table 1 <H> of S4 is 0.17 in water, which increases fourfold, to 0.65, in the calcium-free, bilayer-bound Aβ. The addition of calcium lowers <H> in S4 to 0.59. We have explained helix stabilization in the Aβ+BL system by lipids forming cross-bridges between residues i and or i and (15). Specifically, we have shown that 60% of DMPC lipid contacts are involved in such cross-bridging of S4 residues, contributing to the stabilization of helical structure. With the addition of calcium, this percentage decreases to 55%, consistent with weaker helix propensity in the Aβ+BL+Ca system.
Calcium strengthens Aβ peptide interactions with the DMPC bilayer
We also observed that calcium increases the binding affinity of Aβ peptides for the DMPC bilayer. In particular, Aβ penetrates deeper into the bilayer compared to the calcium-free system. This conclusion follows from the data reported in Fig. 4 and Table 2, where <Δz> is negative for all Aβ segments. Specifically, calcium causes hydrophobic S2 and S4 to penetrate deeper into the bilayer by 1.5 Å, whereas polar segments S1 and S3 move 3.3 and 2.3 Å, respectively, closer to the bilayer midplane. As a result, the central hydrophobic cluster (S2) and C-terminus (S4) are positioned below phosphorus atoms in a leaflet (<z> < zP = 17.35 Å). These changes are reflected in the ΔPs and ΔPi values, which show that, compared to Aβ+BL, Aβ segments, particularly S1 and S3, demonstrate stronger binding to the bilayer surface and increased probability of insertion into the bilayer (Table 2).
Tighter binding of Aβ peptide to the DMPC bilayer is largely due to stronger electrostatic interactions of charged amino acids with polar lipid headgroups. Analyzing the data in Fig. 5, we identified seven contacts most strengthened by calcium. Among these, the five top contacts all involve charged residues (Glu11, Glu22, Asp23, Lys28) and, in all but one case, anionic phosphate group G2. Generally, these four charged amino acids deliver 52% of the increase in Aβ-lipid headgroup interactions. This result agrees with Table 3, showing that the largest change in Aβ-lipid interactions is due to stronger contacts of polar segments S1 and S3 with lipid headgroups.
Due to enhanced interactions between Aβ and the DMPC bilayer, we observed a stronger impact of peptide binding on the bilayer structure. Specifically, binding of the Aβ monomer causes bilayer thinning by ΔD = 8.5 Å (Fig. 6). By comparison, in the calcium-free system, ΔD = 7.2 Å, i.e., calcium adds an extra 1.3 Å to bilayer thinning. This enhanced thinning is reflected in a suppressed order parameter, −<SCD>, for proximal lipids, which implicates their increased disorder in the system containing calcium (Fig. 7). This observation is interesting, because calcium is known to increase ordering of lipids (22), which is also seen in our simulations for distant lipids (Fig. 7). Therefore, we surmise that Aβ impact on the lipid structure overrides the calcium ordering influence on lipids.
Strong affinity for negatively charged amino acids drives calcium redistribution in the DMPC bilayer
Our simulation results implicate a strong affinity of calcium ions for negatively charged amino acids. According to Fig. 8, the average number of Ca2+ ions bound to these amino acids is 1.0, whereas the corresponding number bound to other amino acids is >10-fold smaller (0.04). Furthermore, ∼90% of all ions bound to Aβ monomer interact with just three anionic amino acids, Glu11, Glu22, Asp23. These findings emphasize that electrostatic interaction is the dominant factor governing the binding of Ca2+ to Aβ. We have argued above that these interactions change Aβ tertiary structure by disrupting the Asp23-Lys28 salt bridge. Below, we analyze the consequences of the strong affinity of calcium for negatively charged amino acids on the distribution of ions in the bilayer.
Consistent with prior studies (21,22) Ca2+ almost exclusively interacts with polar headgroups G1–G3, showing the strongest affinity for phosphate group G2. Accordingly, in Fig. 9, the average location of calcium along the z axis in the distant region is 17.4 Å, which matches the average z position of the phosphorus center of mass (zP = 17.35 Å). However, the average position of proximal calcium along the z axis is moved 3.4 Å away from the bilayer midplane. This redistribution of calcium may be explained by its strong affinity for negatively charged amino acids, which partially extract Ca2+ from the bilayer. Another consequence of calcium affinity for negatively charged amino acids is revealed by the analysis of Ca2+ surface number density, ns. Comparing the ns values for the proximal and distant regions, we detected an influx of calcium into the proximal region that increased ns by >25%. Also, using ns and the areas of proximal and distant regions, we can estimate the probability of Ca2+ binding to the DMPC bilayer and/or the Aβ peptide. We found that the number of bound calcium ions is 18.3. Since the total number of ions is 20, the probability of Ca2+ binding to lipids or peptides is ∼0.92 in agreement with previously published reports (22). Furthermore, the binding probability for Ca2+ permits us to compute the average number of free ions, which is found to be 1.7. This number corresponds to a concentration of free calcium of 13 mM, which is close to experimental concentrations (18).
If electrostatic interactions of calcium with anionic amino acids redistribute ions in the bilayer, then the spatial distributions of these amino acids and ions should be correlated. This conjecture is confirmed in Fig. S4 in the Supporting Material using the number density of negatively charged amino acids, n−(r,z), computed as a function of distance r from the peptide center of mass and distance z from the bilayer midplane. The figure reveals that negatively charged amino acids are localized near the center of the proximal region, i.e., the Aβ binding footprint. This distribution of negatively charged amino acids matches that of Ca2+ ions in Fig. 9, in which the ion number density peaks near the Aβ center of mass at Å. Therefore, Figs. 9 and S4 vividly demonstrate that calcium ions on the surface of the DMPC bilayer migrate to the bound Aβ peptide.
Mechanism of calcium effect on Aβ-bilayer interactions
The results discussed above raise the question as to the mechanism by which calcium increases the binding affinity of the Aβ monomer for the DMPC bilayer. To answer this question, we need to consider the balance of interactions of Ca2+ with amino acids and lipids. The average number of proximal calcium ions is 4.2 in both leaflets, of which 3.2 (or 78%) are bound to negatively charged amino acids and only 0.7 (or 17%) coordinate anionic amino acids and phosphate groups. Consistent with these findings, the data displayed in Fig. 10 indicate that ∼75% of proximal calcium bound to anionic amino acids do not interact with phosphate groups. As stated above, a strong electrostatic attraction between Ca2+ ions and negatively charged amino acids results in an influx of ions to the Aβ binding footprint. Because lipid density is reduced near bound Aβ (Fig. 6), calcium ions coordinate threefold fewer phosphate groups than in the distant Aβ-free regions (to be specific, a reduction from 2.7 to 1.0). Thus, Aβ peptide compromises the ability of Ca2+ to coordinate phosphate groups.
Nevertheless, only a few calcium ions (0.7 in both leaflets) coordinating negatively charged amino acids and phosphate groups are apparently sufficient to increase Aβ affinity to the DMPC bilayer. To confirm this assertion, we recomputed Fig. 5 first applying the requirement that amino acid i is bound by calcium and then that i is not bound by Ca2+. The results, plotted in Fig. S5, show that binding of Ca2+ to Glu22, Asp23, and Glu11 dramatically increases their affinity with respect to the bilayer, particularly the phosphate groups. Indeed, the numbers of lipid groups G1–G3 forming contacts with calcium-bound Glu11, Glu22, and Asp23 are 0.81, 1.38, and 0.86, respectively. When calcium is not bound to these amino acids, these numbers are reduced to 0.39, 0.03, and 0.02, indicating a striking loss of affinity of negatively charged amino acids with respect to the bilayer. Thus, calcium coordination explains the stronger binding of negatively charged amino acids to the bilayer seen in Fig. 5.
Fig. 5 also implicates positively charged Lys28 in stronger interactions with phosphate groups. Given that Lys28-bilayer interactions are activated in the system containing calcium, it is reasonable to suggest that destabilization of the Asp23-Lys28 salt bridge by calcium frees Lys28 to interact with the bilayer. Indeed, if the salt bridge is ON, the number of contacts between Lys28 and the phosphate group is just 0.01, but when the salt bridge is disrupted, this number increases to 0.6. Thus, Ca2+ ions enable four charged amino acids to interact with lipid headgroups, and these amino acids together account for 52% of the increase in Aβ binding affinity. In summary, we propose that the mechanism by which calcium ions strengthen Aβ-bilayer interactions is based on two factors: 1) calcium ions make the DMPC bilayer partially cationic and thus attractive to anionic Aβ; and 2) destabilization of the Asp23-Lys28 salt bridge makes Lys28 available for interactions with the bilayer. This mechanism combines a direct (first factor) and an indirect (second factor) influence of calcium on Aβ-bilayer interactions. We believe that the capacity of a few Ca2+ ions to increase the affinity of Aβ for the DMPC bilayer is partially related to the divalent nature of this ion.
Although we studied the amino-truncated version of Aβ peptide with the net charge of −1, the mechanism proposed above is likely to be applicable to full-length Aβ1–40, which is also anionic (−3) at normal physiological conditions. Indeed, Aβ1–40 contains three anionic amino acids (Asp1, Glu3, and Asp7) in the N-terminus. Based on our proposed binding mechanism, Ca2+ ions are likely to strengthen the interactions of these amino acids with the DMPC bilayer.
Comparison with previous studies and possible biomedical implications
It is important to compare our results with those of previous studies. An MD study probing calcium interactions with the zwitterionic DOPC bilayer has shown that the area per lipid, Al, decreases depending on calcium concentration (21). Specifically, when 10 calcium ions are added to the DOPC leaflet (approximately matching our stoichiometric ratio), Al decreases by 6%, from 66 to 62 Å2 at 310 K. We previously reported that the Al for the DMPC bilayer free of Aβ and calcium is 65.1 ± 0.0 Å2 at 330 K (16). In the study presented here, we used Voronoi tessellation to compute the Al in the control Aβ-free BL+Ca system and found it to be reduced by 11%, to 57.7 ± 0.2 Å2. Also in the BL+Ca system, we obtained the radial number density function for phosphorus atoms, gPP(r), where r is the distance between phosphorus atoms (Fig. S6). In the calcium- and Aβ-free DMPC bilayer, gPP(r) reaches its maximum at 6 Å (16). When calcium is added, this maximum shifts to 4.5 Å, indicating closer packing of lipids. Thus, although we use different lipids, the results show a tendency of calcium ions to induce tighter packing of lipids, just as reported earlier (21). Our findings are also consistent with experiments in which calcium ions were implicated in increasing the mechanical stability of DMPC bilayers (20). The ability of cations to coordinate multiple lipid molecules has been observed in numerous previous studies (22–24). In agreement, our simulations report that in Aβ-free regions, a single calcium ion coordinates up to three or four phosphate groups. Finally, our REMD simulations reveal that because calcium ions prefer binding to phosphate groups, the distribution of ions along the z axis peaks at the location of phosphorus atoms in the distant region. The same observation was made in a previous study (22). Thus, taken together, our results concerning calcium interactions with the lipid bilayers agree well with those of previous studies.
It has been suggested that cytotoxicity of Aβ peptides is associated with structural perturbations in the lipid bilayers induced by binding peptides (5,12). Our REMD simulations demonstrate that Aβ monomer indeed depresses lipid density near its binding footprint and causes bilayer thinning. Nevertheless, our results give no indication that binding of the Aβ monomer can promote calcium permeation through the zwitterionic lipid bilayer. For example, the data in Fig. 9 give no evidence that Ca2+ ions penetrate deeper into the bilayer near the Aβ binding footprint. In fact, anionic Aβ monomer may even impede calcium traffic through the lipid bilayer serving as attractor to the ions. These observations offer a possible explanation for the low cytotoxicity of Aβ monomers.
Conclusions
Using isobaric-isothermal REMD and the all-atom explicit-solvent model, we studied the equilibrium binding of Aβ monomers to zwitterionic DMPC bilayer coincubated with calcium. Using as a control our previous REMD calcium-free simulations, we reached several conclusions. First, calcium ions change the tertiary structure of the bound Aβ monomer by destabilizing several long-range intrapeptide interactions, particularly the salt bridge Asp23-Lys28. Second, calcium strengthens Aβ peptide binding to the DMPC bilayer by enhancing electrostatic interactions between charged amino acids and lipid polar headgroups. As a result, Aβ monomer penetrates deeper into the bilayer, making disorder in proximal lipids and bilayer thinning more pronounced. Third, because calcium ions demonstrate strong affinity with respect to negatively charged amino acids, a considerable influx of calcium into the area proximal to the bound Aβ monomer is observed. As a result, the localizations of negatively charged amino acids and calcium ions in the Aβ binding footprint overlap. Based on our data, we propose a mechanism by which calcium ions strengthen Aβ-bilayer interactions. This mechanism involves two factors: 1) calcium ions make the DMPC bilayer partially cationic and thus attractive to anionic Aβ; and 2) destabilization of Asp23-Lys28 salt bridge makes Lys28 available for interactions with the bilayer. Finally, we concluded that a sole Aβ monomer does not promote permeation of calcium ions through the zwitterionic bilayer.
Author Contributions
D.K.K. designed the research; C.L. performed the research; C.L. and D.K.K. analyzed the data; C.L. and D.K.K. wrote the article.
Acknowledgments
The authors acknowledge the XSEDE computer time award CHE-100033 for the computational resources in Keeneland.
Supporting Material
References
- 1.Haass C., Schlossmacher M.G., Selkoe D.J. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Williams T.L., Serpell L.C. Membrane and surface interactions of Alzheimer’s Aβ peptide—insights into the mechanism of cytotoxicity. FEBS J. 2011;278:3905–3917. doi: 10.1111/j.1742-4658.2011.08228.x. [DOI] [PubMed] [Google Scholar]
- 5.Sepulveda F.J., Parodi J., Aguayo L.G. Synaptotoxicity of Alzheimer β amyloid can be explained by its membrane perforating property. PLoS ONE. 2010;5:e11820–e11829. doi: 10.1371/journal.pone.0011820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzi E., Hölzemann G., Seelig J. Interaction of Alzheimer β-amyloid peptide1–40 with lipid membranes. Biochemistry. 1997;36:14845–14852. doi: 10.1021/bi971843e. [DOI] [PubMed] [Google Scholar]
- 7.Mason R.P., Jacob R.F., Wood W.G. Distribution and fluidizing action of soluble and aggregated amyloid β-peptide in rat synaptic plasma membranes. J. Biol. Chem. 1999;274:18801–18807. doi: 10.1074/jbc.274.26.18801. [DOI] [PubMed] [Google Scholar]
- 8.Yip C.M., McLaurin J. Amyloid-β peptide assembly: a critical step in fibrillogenesis and membrane disruption. Biophys. J. 2001;80:1359–1371. doi: 10.1016/S0006-3495(01)76109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokvist M., Lindström F., Gröbner G. Two types of Alzheimer’s β-amyloid1–40 peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J. Mol. Biol. 2004;335:1039–1049. doi: 10.1016/j.jmb.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Quist A., Doudevski I., Lal R. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nag S., Chen J., Maiti S. Measurement of the attachment and assembly of small amyloid-β oligomers on live cell membranes at physiological concentrations using single-molecule tools. Biophys. J. 2010;99:1969–1975. doi: 10.1016/j.bpj.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambroggio E.E., Kim D.H., Fidelio G.D. Surface behavior and lipid interaction of Alzheimer β-amyloid peptide 1–42: a membrane-disrupting peptide. Biophys. J. 2005;88:2706–2713. doi: 10.1529/biophysj.104.055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau T.-L., Ambroggio E.E., Separovic F. Amyloid-β peptide disruption of lipid membranes and the effect of metal ions. J. Mol. Biol. 2006;356:759–770. doi: 10.1016/j.jmb.2005.11.091. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa Y., Suzuki Y., Asakura T. The interaction of amyloid Aβ1–40 with lipid bilayers and ganglioside as studied by 31P solid-state NMR. Chem. Phys. Lipids. 2009;158:54–60. doi: 10.1016/j.chemphyslip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart C., Klimov D.K. Alzheimer’s Aβ10–40 peptide binds and penetrates DMPC bilayer: an isobaric-isothermal replica exchange molecular dynamics study. J. Phys. Chem. B. 2014;118:2638–2648. doi: 10.1021/jp412153s. [DOI] [PubMed] [Google Scholar]
- 16.Lockhart C., Klimov D.K. Binding of Aβ peptide creates lipid density depression in DMPC bilayer. Biochim. Biophys. Acta. 2014;1838:2678–2688. doi: 10.1016/j.bbamem.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Altenbach C., Seelig J. Ca2+ binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a Ca2+ complex with two phospholipid molecules. Biochemistry. 1984;23:3913–3920. doi: 10.1021/bi00312a019. [DOI] [PubMed] [Google Scholar]
- 18.Sinn C.G., Antonietti M., Dimova R. Binding of calcium to phosphatidylcholine-phosphatidylserine membranes. Colloids Surf. A Physicochem. Eng. Asp. 2006;282–283:410–419. [Google Scholar]
- 19.Picas L., Montero M.T., Hernandez-Borrell J. Calcium-induced formation of subdomains in phosphatidylethanolamine-ethanolamine-phosphatidylglycerol bilayers: a combined DSC, 31P NMR, and AFM study. J. Phys. Chem. B. 2009;133:4648–4655. doi: 10.1021/jp8102468. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Manyes S., Oncins G., Sanz F. Effect of ion-binding and chemical phospholipid structure on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophys. J. 2005;89:1812–1826. doi: 10.1529/biophysj.105.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernier P.T., Ziegler M.J., Dimova R. Calcium binding and head group dipole angle in phosphatidylserine-phosphatidylcholine bilayers. Langmuir. 2009;25:1020–1027. doi: 10.1021/la8025057. [DOI] [PubMed] [Google Scholar]
- 22.Tsai H.-H.G., Lai W.-X., Tseng W.-H. Molecular dynamics simulation of cation-phospholipid clustering in phospholipid bilayers: possible role in stalk formation during membrane fusion. Biochim. Biophys. Acta. 2012;1818:2742–2755. doi: 10.1016/j.bbamem.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Böckmann R.A., Grubmüller H. Multistep binding of divalent cations to phospholipid bilayers: a molecular dynamics study. Angew. Chem. Int. Ed. Engl. 2004;43:1021–1024. doi: 10.1002/anie.200352784. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W., Róg T., Karttunen M. Role of phosphatidylglycerols in the stability of bacterial membranes. Biochimie. 2008;90:930–938. doi: 10.1016/j.biochi.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Buck M., Bouguet-Bonnet S., MacKerell A.D., Jr. Importance of the CMAP correction to the CHARMM22 protein force field: dynamics of hen lysozyme. Biophys. J. 2006;90:L36–L38. doi: 10.1529/biophysj.105.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klauda J.B., Venable R.M., Pastor R.W. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockhart C., Kim S., Klimov D.K. Explicit solvent molecular dynamics simulations of Aβ peptide interacting with ibuprofen ligands. J. Phys. Chem. B. 2012;116:12922–12932. doi: 10.1021/jp306208n. [DOI] [PubMed] [Google Scholar]
- 28.Lockhart C., Klimov D.K. Molecular interactions of Alzheimer’s biomarker FDDNP with Aβ peptide. Biophys. J. 2012;103:2341–2351. doi: 10.1016/j.bpj.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sergeant N., Bombois S., Delacourte A. Truncated β-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J. Neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- 30.Albertini V., Bruno A., Ghidoni R. Optimization protocol for amyloid-β peptides detection in human cerebrospinal fluid using SELDI TOF MS. Proteomics Clin. Appl. 2010;4:352–357. doi: 10.1002/prca.200900166. [DOI] [PubMed] [Google Scholar]
- 31.Nagle J.F., Tristram-Nagle S. Structure of lipid bilayers. Biochim. Biophys. Acta. 2000;1469:159–195. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okabe T., Kawata M., Mikami M. Replica exchange Monte Carlo method for the isobaric-isothermal ensemble. Chem. Phys. Lett. 2001;335:435–439. [Google Scholar]
- 33.Kale L., Skeel R., Schulten K. NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 1999;151:283–312. [Google Scholar]
- 34.Towns J., Cockerill T., Wilkens-Diehr N. XSEDE: accelerating scientific discovery. Comput. Sci. Eng. 2014;16:62–74. [Google Scholar]
- 35.Frishman D., Argos P. Knowledge-based protein secondary structure assignment. Proteins. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 36.Ferrenberg A.M., Swendsen R.H. Optimized Monte Carlo data analysis. Phys. Rev. Lett. 1989;63:1195–1198. doi: 10.1103/PhysRevLett.63.1195. [DOI] [PubMed] [Google Scholar]
- 37.Chang J., Sandler S. Determination of liquid-solid transition using histogram reweighting method and expanded ensemble simulations. J. Chem. Phys. 2003;118:8390–8395. doi: 10.1063/1.1638377. [DOI] [PubMed] [Google Scholar]
- 38.Conrad P., de Pablo J. Comparison of histogram reweighting techniques for a flexible water model. Fluid Phase Equilib. 1998;150–151:51–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


