Abstract
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 differentiates specialized cells for nitrogen fixation called heterocysts upon limitation of combined nitrogen in the medium. During heterocyst differentiation, expression of approximately 500 genes is upregulated with spatiotemporal regulation. In the present study, we investigated the functions of sigma factors of RNA polymerase in the regulation of heterocyst differentiation. The transcript levels of sigC, sigE, and sigG were increased during heterocyst differentiation, while expression of sigJ was downregulated. We carried out DNA microarray analysis to identify genes regulated by SigC, SigE, and SigG. It was indicated that SigC regulated the expression of genes involved in heterocyst differentiation and functions. Moreover, genes regulated by SigC partially overlapped with those regulated by SigE, and deficiency of SigC was likely to be compensated by SigE.
Keywords: sigma factor, transcriptional regulation, heterocyst differentiation, Anabaena
1. Introduction
Heterocysts are terminally differentiated cells of filamentous cyanobacteria that are specialized for the fixation of atmospheric nitrogen. Upon limitation of combined nitrogen in the medium, particular vegetative cells differentiate into heterocysts with a semi-regular spacing of 10–15 cells [1,2]. In the filamentous cyanobacterium Anabaena sp. strain PCC 7120 (hereafter referred to as Anabaena PCC 7120), about 500 genes are upregulated with spatiotemporal regulation during the process of heterocyst development [3,4,5,6]. Two genes, ntcA and hetR, are necessary for heterocyst differentiation, and their induction by nitrogen deprivation is mutually dependent. NtcA, a transcriptional regulator that globally controls the nitrogen response in cyanobacteria, is activated by binding of 2-oxoglutarate, which is the central signaling molecule reflecting the cellular nitrogen status [7,8]. NtcA activates transcription of the nrrA gene, whose product in turn activates hetR expression [9,10]. hetR is a master gene of heterocyst differentiation [11,12]. Mutation in hetR blocks early steps in differentiation, whereas ectopic expression of hetR induces the formation of heterocysts irrespective of cellular nitrogen status. HetR is a DNA-binding protein [13], and its target genes were extensively determined by genomic searches of the HetR-binding sequence and deep sequencing of HetR-bound DNA in vivo [14,15]. The hetR gene is also required for upregulation of ntcA after nitrogen deprivation, although the molecular basis of hetR-dependent regulation of ntcA remains unknown [16,17].
The sigma factor of RNA polymerase is responsible for promoter recognition and determines the specificity of transcriptional initiation [18]. Bacteria respond to environmental changes by expressing specific sigma factors to induce particular sets of genes. In Anabaena PCC 7120, there are 12 genes encoding sigma factors, three of which (sigB (all7615), sigB3 (all7608), and sigB4 (all7179)) are carried on plasmids, and the remaining nine genes (sigA (all5263), sigB2 (alr3800), sigC (all1692), sigD (alr3810), sigE (alr4249), sigF (all3853), sigG (alr3280), sigI (all2193), and sigJ (alr0277)) are carried on the chromosome [19]. We adopted the sigma factor nomenclature revised by Yoshimura et al. [20] in this study. Northern blot analysis showed that the transcript levels of sigB, sigC, and sigE (previously sigF) are increased by nitrogen deprivation [21,22]. In addition, it was indicated that transcription from the promoters of sigC, sigE, and sigG is activated in differentiating heterocysts [23]. Although the genes of group 2 sigma factors, namely, sigB, sigB2 (previously sigE), sigC, sigD, and sigE, were inactivated, none of them were individually required for heterocyst differentiation [21,24]. However, heterocyst development in sigB and sigC mutants is delayed, and some double mutants of group 2 sigma factors take a longer time to establish diazotrophic growth. It is also indicated that the sigE gene is required for normal expression of some heterocyst-specific genes, such as nifH, fdxH, and hglE2 [22]. Overexpression of sigE causes accelerated heterocyst development and an increased heterocyst frequency. These observations suggest that sigma factors are involved in the regulation of gene expression during heterocyst development.
In the present study, we identified genes regulated by SigC, SigE, and SigG of Anabaena PCC 7120, whose expression was increased after nitrogen deprivation in a hetR-dependent manner, using a DNA microarray. Inactivation of sigC resulted in downregulation of 58 genes at 8 h after nitrogen deprivation, including many genes involved in heterocyst differentiation, and retardation of heterocyst differentiation. The transcript levels of 68 genes were lower in the sigE disruptant than in the wild-type (WT) strain. It was indicated that many genes involved in heterocyst differentiation and function were regulated by both SigC and SigE. These results support the conclusion that SigC is involved in the expression of genes required for heterocyst differentiation, and that SigC and SigE have at least partially overlapping promoter specificities.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Anabaena PCC 7120 and its derivatives were grown in BG-11 medium containing NaNO3 as a nitrogen source as described previously [6]. The mutant strains DRsigEK [25] and DRhetRS [26] were used as the sigE and hetR disruptants in this study. Liquid cultures were bubbled with air containing 1.0% (v/v) CO2. For nitrogen deprivation experiments, filaments grown in BG-11 medium until they reached an OD750 of 0.4–0.5 were washed twice with sterile water and then resuspended in nitrogen-free BG-11 medium (BG-110). Spectinomycin and neomycin were added to the medium at a final concentration of 10 and 30 µg mL−1, respectively, when required.
2.2. Mutant Construction
All primers used in this study (Table 1) were designed based on genome data from CyanoBase (Fujisawa et al., 2014). DNA fragments upstream and downstream of the sigC gene were amplified by PCR using the primer pair sigC-5F and sigC-5R, and the primer pair sigC-3F and sigC-3R (Table 1), respectively. The upstream fragment was cloned between the SacI and BamHI sites of pBluescript II KS+ (Agilent Technologies), and then the downstream fragment was cloned between the BamHI and XhoI sites. A spectinomycin resistance cassette excised by digestion with BamHI from the plasmid pDW9 [27] was inserted into the BamHI site between the upstream and downstream fragments. The SacI-XhoI fragment was excised from the resultant plasmid and cloned between the SacI and XhoI sites of pRL271 [28] to construct pRsigCS. The pRsigGS plasmid was constructed as described for pRsigCS using the primer pair sigG-5F and sigG-5R, and the primer pair sigG-3F and sigG-3R (Table 1). pRsigCS and pRsigGS were transferred into Anabaena PCC 7120 by conjugation according to the method of Elhai et al. [29] to construct the deletion mutants of sigC, DRsigCS and sigG, DRsigGS, respectively.
Table 1.
Primers used in this study.
| Primer | Sequence (5'- 3') |
|---|---|
| sigC-5F | TAGAGCTCGCGGACTCACAGAAATGGTT |
| sigC-5R | TAGGATCCAATGGCGATATCAGGGTCT |
| sigC-3F | TAGGATCCTCGCAACCTTATCCGTGACT |
| sigC-3R | ATCTCGAGTTTGGCAGTCCAGTAGGTGA |
| sigG-5F | AGGAGCTCACGTCCATGATCAAACCAA |
| sigG-5R | TAGGATCCGTGGTTCGAGAGTTTGTCA |
| sigG-3F | ACGGATCCTTGCCGAAATCACAGGTGTA |
| sigG-3R | ATCTCGAGGGCGTGGGTATATTTGATG |
| RTrrn16SF2 | GCAAGTCGAACGGTCTCTTC |
| RTrrn16SR2 | GGTATTAGCCACCGTTTCCA |
| RTsigA-F | TTGTTGCTCGCTGATGATGG |
| RTsigA-R | TTCTTGCTTTGTGTCCGACG |
| RTsigB2-F | ACACCCACACAGAAGACACA |
| RTsigB2-R | TCTTTAGCGTCAATCAGCGAC |
| RTsigC-F | ACCTGGAGCCATAGAGACGA |
| RTsigC-R | CATCCACCGACAAATCACTG |
| RTsigD-F | AGCGTAGAAGAGTGGGCAAA |
| RTsigD-R | GGATACCACTAGCCGCAAGT |
| RTsigE-F | TGGCACGTTATCCACTGCTA |
| RTsigE-R | GTCGGATGTTGCCCTATTTG |
| RTsigF-F | TTGCGGGAACAATACAACCG |
| RTsigF-R | CCATCTTGCACGGGTACATC |
| RTsigG-F | TTGATGCGAGGTGTCCAGAA |
| RTsigG-R | GGTTTGATAGCGGCGCAATA |
| RTsigI-F | GATTCGGCGGCATTAAGTGT |
| RTsigI-R | GCTTCTTGGGAATCAGCCAG |
| RTsigJ-F | GGCAGCAAGTGAGTCCTCTA |
| RTsigJ-R | GCCGGTGTGTAATTGAACCA |
| RTfbp-F | CAACCTTATCCCGTCACGTC |
| RTfbp-R | GCGACGGGCAACTAATTTAC |
| RTtalB-F | GACCACCAATCCCTCTCTGA |
| RTtalB-R | AAGCGAGGGAGACAATTTGA |
| RTcoxB3-F | AAGGGCCGACAGCATTAGTA |
| RTcoxB3-R | ATACCCACGCCCATTGTTTA |
| RTcoxA2-F | CAACGCATTCATGACCAATC |
| RTcoxA2-R | AAGGTGGGTAAGCAGTCCAA |
| RThepA-F | CAGGAATTAGCTGGGTTGACA |
| RThepA-R | ATTGAAGGTAGCACGCATCC |
| RThepB-F | AAATTTATCGCGCCAACAAG |
| RThepB-R | CTCCGACACGATGCACTAAA |
| RTntcA-F | CAAGATAAGGCCCTAGCAAATG |
| RTntcA-R | TCCGACTTGTTTCCTGTCAAC |
| RT4160-F | TCATGACTAGCCAACCCACA |
| RT4160-R | TACTGCTTCCAGCACGCTTA |
2.3. DNA Microarray Analysis
Cells used for RNA extraction were collected by centrifugation at 4 °C. Before centrifugation, the culture medium was rapidly chilled by adding crushed ice to eliminate additional effects during centrifugation. Collected cells were immediately frozen in liquid nitrogen and stored at –80 °C until use. Total RNA was extracted from whole filaments as described by Pinto et al. [30] and treated with DNase I (Takara Bio, Shiga, Japan). Global gene expression was analyzed using the Anabaena oligonucleotide microarray as described previously [6]. Microarray analyses were carried out with three sets of RNA samples (DRsigCS and DRsigEK) or two sets of RNA samples (DRsigGS) isolated from independently grown cultures. Two hybridization reactions were performed with different combinations of Cy-dyes for each set of RNA samples. Genes whose transcript levels significantly differed (p < 0.05; Student’s t-test) by at least 2-fold were determined. The microarray data were deposited in the KEGG Expression Database (accession numbers ex0001955 to ex0001970).
2.4. Quantitative Reverse Transcription PCR (qRT-PCR)
cDNA was synthesized from 1 µg of total RNA with random hexamer primers using a PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). qRT-PCR was performed with a Thermal Cycler Dice Real-time System (TP900; Takara Bio, Shiga, Japan) in a 20 µL reaction mixture containing 10 µL of THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan), 0.2 µM of each gene-specific forward and reverse primer (Table 1), and cDNA. Relative ratios were normalized against the value for 16S rRNA and are represented as means of triplicate experiments.
2.5. Acetylene Reduction Assays
The acetylene reduction assays were performed as described previously [25]. The concentration of ethylene was measured by GC-2014 gas chromatography (Shimadzu, Kyoto, Japan). The chlorophyll a content of cultures was determined by the method of Mackinney [31], and the acetylene reduction rates were normalized to the chlorophyll a content.
3. Results and Discussion
3.1. Nitrogen-Regulated Expression of Genes Encoding Sigma Factors
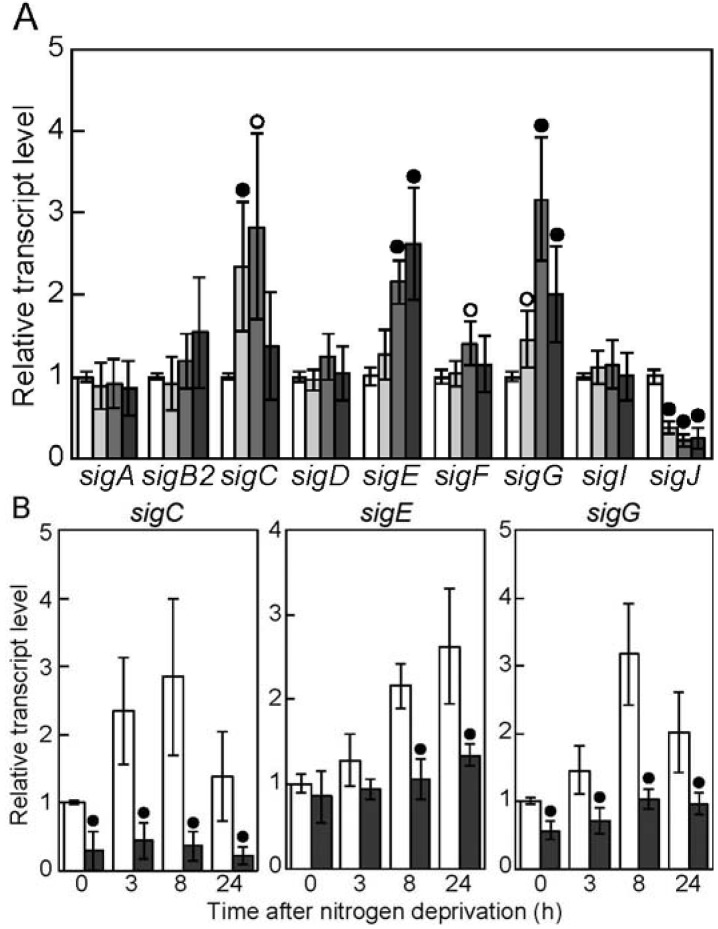
Changes in the expression of sigma factor genes on the chromosome of Anabaena PCC 7120 during heterocyst differentiation were determined by qRT-PCR (Figure 1A). The transcript levels of sigC, sigE, and sigG increased during heterocyst differentiation, while sigJ was downregulated. Expression of sigA, sigB2, sigD, sigF, and sigI did not respond to nitrogen deprivation. The sigC transcript level was increased within 3 h after nitrogen deprivation and remained high until 24 h. The sigE and sigG transcript levels were increased at 8 h after nitrogen deprivation. These results are consistent with observations using GFP reporter strains, in which the gfp gene on the multi-copy plasmid was expressed from each promoter of sigma factor genes [23], although the transcript level of sigE increased earlier than the fluorescence level of GFP expressed from the sigE promoter. The sigC, sigE, and sigG genes are specifically upregulated in differentiating heterocysts [23], and the sigE transcript level is higher in mature heterocysts than in vegetative cells [32]. To determine the correlation between expression of sigC, sigE, and sigG and heterocyst differentiation, expression of these genes was determined in the hetR disruptant DRhetRS (Figure 1B). Upregulation of these genes by nitrogen deprivation was abolished in DRhetRS, indicating that expression of sigC, sigE, and sigG is developmentally regulated and that hetR is necessary for their upregulation. In the unicellular cyanobacterium Synechocystis sp. PCC 6803, sigE is also induced by nitrogen starvation under the control of NtcA, while no induction of sigC and sigG is observed [33,34]. In Anabaena PCC 7120, NtcA and NrrA, which are highly expressed in differentiating heterocysts [6,35], bind to the promoter regions of sigE and are involved in the regulation of sigE expression [22,25]. In response to nitrogen deprivation, hetR is necessary for upregulation of ntcA, and NtcA induces the expression of nrrA [9,17]. The hetR gene is likely to affect the expression of sigE via NtcA and NrrA, although it remains to be revealed how hetR regulates expression of ntcA. A genomic survey of the HetR-binding sequence and deep sequencing of HetR-bound DNA in vivo did not show an interaction between HetR and the promoters of sigC and sigG [14,15]. Thus, the mechanisms by which hetR regulates sigC and sigG remain unknown. We constructed mutants of group 3 (sigF and sigJ) and ECF (sigG and sigI) sigma factors; however, all of these were able to form heterocysts and grow diazotrophically (unpublished data). Combined with the results of inactivation of group 2 sigma factors [21,24], no group 2, group 3, or ECF sigma factor on the chromosome of Anabaena PCC 7120 is individually required for heterocyst differentiation.
Figure 1.
Changes in the transcript levels of genes encoding sigma factors after nitrogen deprivation. (A) The relative transcript levels of sigma factor genes before (white bars) and 3 h (light gray bars), 8 h (gray bars), and 24 h (dark gray bars) after nitrogen deprivation were determined by quantitative reverse transcription PCR. The transcript levels at 0 h were set to 1 for each gene. The t-test was used to compare 0 h and each time point, and data that represent a significant difference (p < 0.01 or 0.05) are marked with black or white circles; (B) the transcript levels of sigC, sigE, and sigG were determined in the wild-type (WT) strain (white bars) and the hetR disruptant (gray bars). The transcript levels at 0 h in the WT strain were set to 1. The t-test was used to compare the WT strain and the hetR disruptant, and data that represent a significant difference (p < 0.01) are marked with black circles.
3.2. The sigC Gene Is Required for Normal Induction of Genes Involved in Heterocyst Differentiation
DNA microarray analysis was carried out to identify genes regulated by SigC and SigG using the sigC disruptant DRsigCS and the sigG disruptant DRsigGS. Gene expression during heterocyst differentiation drastically changed after 8 h of nitrogen deprivation [5]; therefore, gene expression patterns of the WT strain and DRsigCS or DRsigGS after 8 h of nitrogen deprivation were compared.
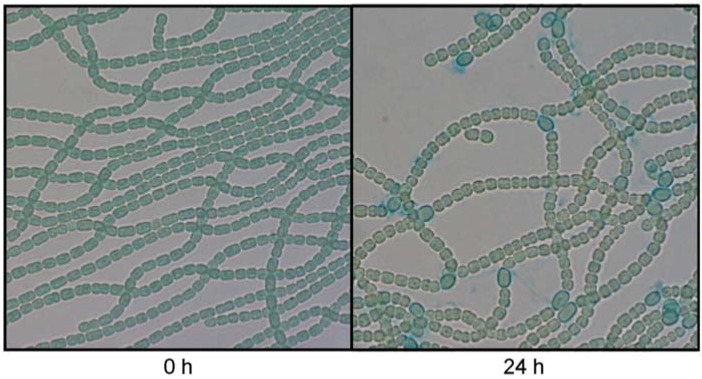
The transcript levels of 58 genes were lower in DRsigCS than in the WT strain (Table 2). Downregulated genes included genes encoding enzymes of the oxidative pentose phosphate (OPP) pathway (talB, talA, and fbp), those encoding terminal respiratory oxidases (coxB2, coxA2, and coxB3), and those involved in the synthesis of heterocyst envelope polysaccharide (HEP). Moreover, the transcript level of the ntcA gene was decreased by sigC disruption. The OPP pathway is required for nitrogen fixation in heterocysts [36], and genes involved in the OPP pathway are upregulated in heterocysts [32]. The coxBAC2 operon encodes an aa3-type cytochrome c oxidase, and the coxBAC3 operon encodes the alternative respiratory terminal oxidase [37]. Both operons are specifically expressed in heterocysts, and either oxidase is necessary for aerobic nitrogen fixation [37,38]. Synthesis of HEP depends on a cluster of genes, the HEP island (alr2825 to alr2841), and some genes, such as hepB and all4160, that are distant from the HEP island in the genome [5,13,39]. All genes in the HEP island (the relative ratio of the alr2840 transcript level in DRsigCS to that in the WT strain was −0.96 with a p value of 0.0015), hepB (the relative ratio was −0.96 with a p value of 0.0034), and all4160 were downregulated by sigC disruption (Table 2). It was indicated that SigC regulates genes involved in heterocyst differentiation and functions, which are highly expressed in heterocysts. In DRsigCS, heterocysts were observed after 24 h of nitrogen deprivation (Figure 2). However, the nitrogenase activity in DRsigCS at 24 h was reduced to about 30% of that in the WT strain (Table 3). The nitrogenase activity in DRsigCS increased to the level in the WT strain after 48 h of nitrogen deprivation (Table 3), indicating that sigC disruption did not block heterocyst differentiation, but delayed heterocyst development as previously reported [21,24].
Table 2.
Genes downregulated by sigC disruption.
| ORF No. | Gene | Product | ΔsigC/WT a | p b |
|---|---|---|---|---|
| all0406 | - | Unknown protein | −1.16 | 3.6 × 10−2 |
| all0438 | - | Serine/threonine kinase with WD-40 repeat | −1.53 | 5.1 × 10−3 |
| asl0597 | - | Hypothetical protein | −1.01 | 1.4 × 10−2 |
| all0918 | - | Unknown protein | −1.19 | 4.5 × 10−2 |
| all0919 | - | Probable glycosyltransferase | −1.96 | 5.9 × 10−4 |
| all1101 | - | Ferrichrome iron receptor | −1.01 | 3.0 × 10−2 |
| alr1112 | - | Probable transglycosylase | −1.04 | 3.4 × 10−2 |
| alr1276 | - | Putative acetyl transferase | −1.14 | 2.7 × 10−3 |
| asr1405 | - | Hypothetical protein | −1.11 | 6.3 × 10−3 |
| asr1408 | nifZ | Iron-sulfur cofactor synthesis protein | −1.29 | 2.9 × 10−3 |
| all1424 | - | Unknown protein | −1.19 | 9.5 × 10−5 |
| asl1778 | - | Unknown protein | −1.54 | 2.9 × 10−5 |
| alr2323 | htpG | Heat shock protein | −1.04 | 4.3 × 10−2 |
| alr2405 | isiB | Flavodoxin | −1.50 | 8.7 × 10−3 |
| alr2514 | coxB2 | Cytochrome c oxidase subunit II | −1.37 | 2.2 × 10−2 |
| alr2515 | coxA2 | Cytochrome c oxidase subunit I | −1.62 | 1.4 × 10−3 |
| asr2523 | - | Unknown protein | −1.24 | 2.4 × 10−3 |
| all2563 | talB | Transaldolase | −1.13 | 1.9 × 10−2 |
| alr2582 | - | Hypothetical protein | −1.41 | 9.5 × 10−3 |
| all2637 | - | Unknown protein | −1.08 | 3.5 × 10−2 |
| all2655 | - | Unknown protein | −1.16 | 1.2 × 10−2 |
| alr2730 | - | Hypothetical protein | −1.12 | 1.3 × 10−2 |
| alr2731 | coxB3 | Cytochrome c oxidase subunit II | −1.11 | 2.0 × 10−3 |
| alr2818 | hetP | Heterocyst differentiation protein | −1.39 | 1.7 × 10−3 |
| alr2822 | - | Hypothetical protein | −1.48 | 3.9 × 10−4 |
| alr2823 | - | Hypothetical protein | −1.58 | 1.3 × 10−4 |
| alr2824 | - | Hypothetical protein | −2.24 | 4.9 × 10−4 |
| alr2825 | - | Glucose-1-P cytidylyltransferase | −1.38 | 2.1 × 10−4 |
| alr2826 | - | Hypothetical protein | −1.77 | 3.8 × 10−4 |
| alr2827 | - | Putative epimerase/dehydratase | −1.32 | 4.2 × 10−4 |
| alr2828 | - | Unknown protein | −1.92 | 7.3 × 10−4 |
| alr2829 | - | Unknown protein | −1.70 | 3.1 × 10−4 |
| alr2830 | rfbC | dTDP-4-dehydrorhamnose 3,5-epimerase | −1.40 | 3.1 × 10−4 |
| alr2831 | - | Probable NAD(P)-dependent oxidoreductase | −1.54 | 1.5 × 10−3 |
| alr2832 | - | Putative glycosyltransferase | −1.16 | 9.6 × 10−5 |
| alr2833 | - | Hypothetical protein | −1.44 | 3.7 × 10−3 |
| alr2834 | hepC | Similar to glycosyltransferase | −1.65 | 2.7 × 10−5 |
| alr2835 | hepA | ATP-binding protein of ABC transporter | −1.54 | 5.1 × 10−3 |
| alr2836 | - | Glycosyltransferase | −1.38 | 3.9 × 10−5 |
| alr2837 | - | Glycosyltransferase | −1.07 | 3.2 × 10−3 |
| alr2838 | - | Unknown protein | −1.00 | 2.7 × 10−2 |
| alr2839 | - | Glycosyltransferase | −1.13 | 5.6 × 10−3 |
| alr2841 | - | Unknown protein | −1.21 | 1.9 × 10−3 |
| alr2887 | - | Hypothetical protein | −1.00 | 2.0 × 10−2 |
| all3420 | - | Carboxyl-terminal processing protease | −2.65 | 1.5 × 10−3 |
| all3780 | - | Similar to kinesin light chain | −1.08 | 3.5 × 10−2 |
| all3999 | - | Unknown protein | −1.37 | 9.7 × 10−3 |
| all4000 | - | Photosystem II CP43 protein PsbC homolog | −1.57 | 1.8 × 10−2 |
| all4001 | isiA | Photosystem II chlorophyll a-binding protein | −2.59 | 1.4 × 10−2 |
| all4002 | - | Photosystem II CP43 protein PsbC homolog | −2.18 | 1.3 × 10−2 |
| all4003 | - | Photosystem II CP43 protein PsbC homolog | −1.87 | 1.1 × 10−2 |
| all4020 | talA | Transaldolase | −1.10 | 2.4 × 10−2 |
| all4021 | fbp | Fructose 1,6-bisphosphatase | −1.35 | 9.2 × 10−3 |
| all4160 | - | Probable glycosyltransferase | −1.12 | 1.4 × 10−3 |
| asl4206 | rps17 | 30S ribosomal protein S17 | −1.10 | 4.8 × 10−2 |
| alr4392 | ntcA | Nitrogen-responsive global transcriptional regulator | −1.24 | 6.4 × 10−3 |
| alr4984 | - | Unknown protein | −1.39 | 1.3 × 10−4 |
| alr5256 | - | Biotin acetyl-CoA carboxylase ligase | −1.05 | 5.4 × 10−4 |
a Relative ratios of transcript levels in the sigC disruptant to those in the wild-type strain at 8 h after nitrogen deprivation are shown in the base 2 logarithm; b p values for the relative ratios were determined by the Student’s t-test.
Figure 2.
Heterocyst development after nitrogen deprivation in the sigC disruptant. Micrographs were taken before and 24 h after nitrogen deprivation. The polysaccharide layer of heterocysts was stained with Alcian blue.
Table 3.
Nitrogenase activities of the wild-type (WT) strain, the sigC disruptant, and the sigE disruptant.
| Strain | Nitrogenase activity a | |
|---|---|---|
| (µmol C2H4/mg chla/h) | ||
| 24 h | 48 h | |
| WT | 19.4 ± 7.0 | 17.4 ± 2.5 |
| DRsigCS | 6.0 ± 3.0 | 19.0 ± 5.5 |
| DRsigEK | 19.3 ± 6.3 | 11.0 ± 2.4 |
a Nitrogenase activities were determined at 24 h and 48 h after nitrogen deprivation. Means ± S.D. of at least four independent experiments are shown.
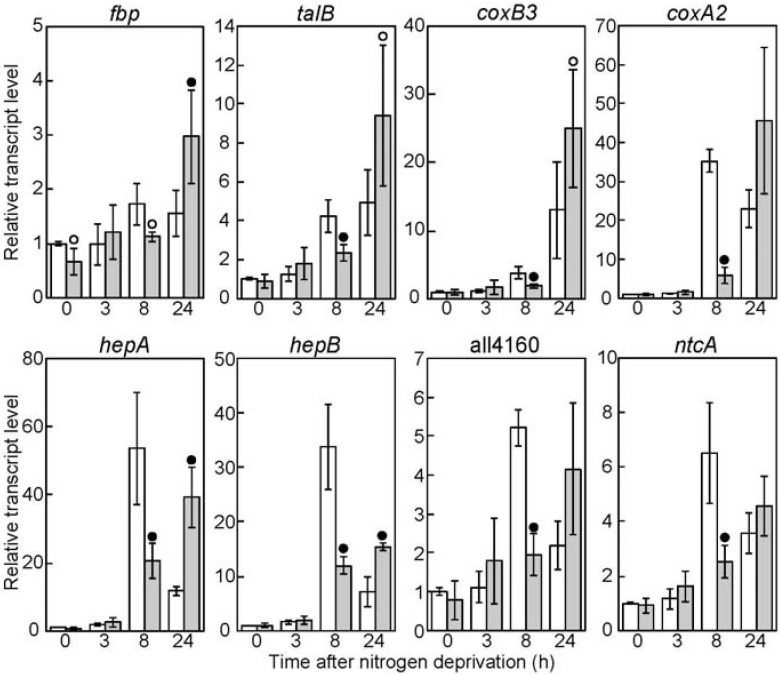
We further analyzed the effects of sigC inactivation on gene expression during a time series of 24 h of heterocyst differentiation by qRT-PCR. The transcript levels of fbp, talB, coxB3, coxA2, hepA, hepB, all4160, and ntcA in DRsigCS after 8 h of nitrogen deprivation were lower than those in the WT strain (Figure 3). In the WT strain, expression of all these genes, except for that of coxB3, was constant or decreased between 8 h and 24 h, while their expression in DRsigCS was increased, which could be due to transcription by RNA polymerase with a sigma factor other than SigC. It is indicated that SigC is required for upregulation of genes expressed in heterocysts, although another sigma factor(s) can compensate for the deficiency of SigC.
Figure 3.
Changes in the transcript levels of genes downregulated by sigC disruption during heterocyst differentiation. The transcript levels of fbp, talB, coxB3, coxA2, hepA, hepB, all4160, and ntcA were determined in the wild-type (WT) strain (white bars) and the sigC disruptant (gray bars). The transcript levels at 0 h in the WT strain were set to 1. The t-test was used to compare the WT strain and the sigC disruptant, and data that represent a significant difference (p < 0.01 or 0.05) are marked with black or white circles.
The transcript levels of 14 genes were lower in DRsigGS than in the WT strain after 8 h of nitrogen deprivation (Table 4). No genes known to be involved in heterocyst differentiation were downregulated by sigG disruption. Further detailed analyses are required to clarify the relationship between the sigG gene and heterocyst differentiation.
Table 4.
Genes downregulated by sigG disruption.
| ORF No. | Gene | Product | ΔsigG/WT a | p b |
|---|---|---|---|---|
| alr2313 | - | Unknown protein | −2.56 | 4.7 × 10−5 |
| all2564 | pyk1 | Pyruvate kinase | −1.74 | 1.3 × 10−2 |
| alr3281 | - | Hypothetical protein | −1.05 | 7.3 × 10−4 |
| alr3301 | - | Unknown protein | −1.34 | 5.6 × 10−3 |
| alr3445 | - | Hypothetical protein | −1.24 | 9.4 × 10−4 |
| alr3608 | - | Similar to endoglucanase | −1.12 | 3.0 × 10−3 |
| alr3816 | - | Unknown protein | −1.41 | 3.6 × 10−3 |
| alr3817 | - | Unknown protein | −1.09 | 6.6 × 10−3 |
| all3983 | - | Similar to surface layer protein | −1.79 | 5.7 × 10−3 |
| all4254 | - | Unknown protein | −1.07 | 1.6 × 10−4 |
| all4427 | - | Similar to phytanoyl-CoA hydroxylase | −1.03 | 6.5 × 10−4 |
| all4523 | - | Hypothetical protein | −1.32 | 2.3 × 10−3 |
| all4830 | - | Mannosyl transferase | −1.76 | 9.4 × 10−4 |
| alr5340 | - | Hypothetical protein | −1.38 | 4.1 × 10−3 |
a Relative ratios of transcript levels in the sigG disruptant to those in the wild-type strain at 8 h after nitrogen deprivation are shown in the base 2 logarithm; b p values for the relative ratios were determined by the Student’s t-test.
3.3. Genes Regulated by SigC Partially Overlap with Those Regulated by SigE
It was indicated that sigE is involved in the regulation of some late-stage heterocyst-specific genes, such as nifH and fdxH, which are expressed between 18 h and 24 h [22]. However, the transcript level of sigE was increased within 8 h after nitrogen deprivation (Figure 1). To determine the role of sigE at the earlier stage of heterocyst differentiation, gene expression patterns in the WT strain and the sigE disruptant DRsigEK after 8 h of nitrogen deprivation were compared.
The transcript levels of 68 genes were lower in DRsigEK than in the WT strain, including the xfp operon whose expression is activated by sigE [25] (Table 5). In addition to nif genes (nifV1, nifZ, and nifU), genes required for the synthesis of the glycolipid layer of the heterocyst envelop, namely, devBC [40] and hglT [41], and the hetN gene, which suppresses heterocyst differentiation [42], were downregulated by disruption of sigE. These genes were not identified as being downregulated in DRsigCS, while coxBAC2, talB, and genes involved in the synthesis of HEP were downregulated in DRsigEK as well as in DRsigCS. It is indicated that sigE is also involved in the regulation of genes that are induced within 8 h after nitrogen deprivation and that genes regulated by SigC and SigE partially overlap. To ascertain the role of sigE in heterocyst development, nitrogenase activities were determined. Nitrogenase activity in DRsigEK after 24 h of nitrogen deprivation was comparable with that in the WT strain, while nitrogenase activity in DRsigEK was reduced to 60% of that in the WT strain after 48 h (Table 3). Thus, single disruption of sigE had little effect on heterocyst development. However, a previous study found that heterocyst formation takes a longer time in the double mutant of sigC and sigE than in the single mutants of sigC or sigE [24]. These results support the idea that SigC and SigE have at least partially overlapping promoter specificities.
Table 5.
Genes downregulated by sigE disruption.
| ORF No. | Gene | Product | ΔsigE/WT a | p b |
|---|---|---|---|---|
| asl0046 | - | Hypothetical protein | −1.59 | 1.2 × 10−3 |
| all0178 | - | Flavoprotein | −1.00 | 1.5 × 10−3 |
| all0349 | - | Unknown protein | −1.24 | 2.0 × 10−3 |
| all0438 | - | Serine/threonine kinase with WD-40 repeat | −1.24 | 6.7 × 10−3 |
| all0916 | - | ABC transporter ATP-binding subunit | −1.05 | 4.0 × 10−2 |
| all0917 | - | ABC transporter permease protein | −1.22 | 6.6 × 10−4 |
| all0918 | - | Unknown protein | −1.12 | 6.2 × 10−4 |
| all0919 | - | Probable glycosyltransferase | −1.21 | 2.5 × 10−5 |
| alr1407 | nifV1 | Homocitrate synthase | −1.41 | 2.2 × 10−4 |
| asr1408 | nifZ | Iron-sulfur cofactor synthesis protein | −1.06 | 5.3 × 10−3 |
| all1425 | - | Unknown protein | −1.07 | 4.9 × 10−2 |
| all1456 | nifU | Nitrogen fixation protein | −1.00 | 4.5 × 10−2 |
| all1814 | - | Unknown protein | −1.03 | 1.3 × 10−2 |
| alr2514 | coxB2 | Cytochrome c oxidase subunit II | −1.40 | 4.4 × 10−2 |
| alr2515 | coxA2 | Cytochrome c oxidase subunit I | −1.62 | 2.8 × 10−2 |
| alr2516 | coxC2 | Cytochrome c oxidase subunit III | −1.59 | 2.4 × 10−2 |
| alr2517 | - | Hypothetical protein | −1.13 | 7.1 × 10−3 |
| asr2523 | - | Unknown protein | −1.36 | 1.8 × 10−4 |
| alr2524 | - | Unknown protein | −2.04 | 2.5 × 10−3 |
| all2563 | talB | Transaldolase | −1.23 | 1.1 × 10−4 |
| all2564 | pyk1 | Pyruvate kinase | −1.45 | 2.7 × 10−3 |
| all2566 | gap1 | Glyceraldehyde-3-phosphate dehydrogenase | −1.23 | 6.8 × 10−6 |
| all2567 | xfp | Phosphoketolase | −1.33 | 7.3 × 10−5 |
| all2571 | - | Unknown protein | −1.37 | 6.8 × 10−4 |
| alr2582 | - | Hypothetical protein | −1.73 | 1.3 × 10−3 |
| all2635 | - | Polyketide synthase type I | −1.19 | 4.0 × 10−2 |
| all2647 | - | Microcystin synthetase B | −1.16 | 2.6 × 10−2 |
| all2650 | - | ABC transporter ATP-binding protein | −1.39 | 3.4 × 10−2 |
| all2652 | - | Hypothetical protein | −1.03 | 2.8 × 10−2 |
| all2655 | - | Unknown protein | −1.45 | 3.7 × 10−2 |
| alr2729 | - | Hypothetical protein | −1.33 | 3.5 × 10−4 |
| alr2730 | - | Hypothetical protein | −1.12 | 4.5 × 10−5 |
| alr2818 | hetP | Heterocyst differentiation protein | −1.34 | 1.2 × 10−4 |
| alr2822 | - | Hypothetical protein | −1.42 | 3.6 × 10−3 |
| alr2823 | - | Hypothetical protein | −1.60 | 3.6 × 10−3 |
| alr2824 | - | Hypothetical protein | −1.93 | 1.2 × 10−4 |
| alr2825 | - | Glucose-1-P cytidylyltransferase | −1.49 | 3.0 × 10−3 |
| alr2826 | - | Hypothetical protein | −1.75 | 4.7 × 10−6 |
| alr2827 | - | Putative epimerase/dehydratase | −1.42 | 1.0 × 10−4 |
| alr2828 | - | Unknown protein | −1.87 | 4.4 × 10−5 |
| alr2829 | - | Unknown protein | −1.92 | 3.1 × 10−5 |
| alr2830 | rfbC | dTDP-4-dehydrorhamnose 3,5-epimerase | −1.69 | 3.9 × 10−5 |
| alr2831 | - | Probable NAD(P)-dependent oxidoreductase | −1.19 | 4.0 × 10−2 |
| alr2832 | - | Putative glycosyltransferase | −1.69 | 1.0 × 10−6 |
| alr2833 | - | Hypothetical protein | −1.61 | 1.2 × 10−5 |
| alr2834 | hepC | Similar to glycosyltransferase | −1.94 | 1.3 × 10−4 |
| alr2835 | hepA | ATP-binding protein of ABC transporter | −1.74 | 1.4 × 10−5 |
| alr2836 | - | Glycosyltransferase | −1.57 | 2.2 × 10−4 |
| alr2838 | - | Unknown protein | −1.18 | 9.3 × 10−4 |
| alr2839 | - | Glycosyltransferase | −1.17 | 4.9 × 10−4 |
| alr2840 | - | Glycosyltransferase | −1.38 | 1.9 × 10−4 |
| alr2841 | - | Unknown protein | −1.32 | 6.8 × 10−5 |
| alr2857 | - | Unknown protein | −1.22 | 2.6 × 10−3 |
| alr3195 | - | Probable glutathione S-transferase | −1.04 | 1.5 × 10−4 |
| alr3301 | - | Unknown protein | −1.05 | 1.8 × 10−4 |
| all3420 | - | Carboxyl-terminal processing protease | −1.40 | 3.0 × 10−3 |
| alr3698 | hepB | Heterocyst envelope polysaccharide synthesis protein | −1.35 | 4.5 × 10−4 |
| alr3699 | - | Similar to glycosyltransferase | −1.00 | 7.7 × 10−4 |
| alr3710 | devB | Membrane fusion protein of ABC transporter | −1.92 | 1.9 × 10−4 |
| alr3711 | devC | Substrate-binding protein of ABC transporter | −1.71 | 9.3 × 10−6 |
| all3773 | - | Serine/threonine kinase | −1.47 | 5.9 × 10−3 |
| all3780 | - | Similar to kinesin light chain | −1.80 | 1.4 × 10−3 |
| all3793 | - | Unknown protein | −1.25 | 3.9 × 10−3 |
| all4160 | - | Probable glycosyltransferase | −1.27 | 2.3 × 10−3 |
| alr4984 | - | Unknown protein | −1.60 | 1.4 × 10−3 |
| all4991 | desC | Delta-9 desaturase | −1.06 | 2.2 × 10−2 |
| all5341 | hglT | Heterocyst-specific glycolipid synthase | −1.65 | 6.0 × 10−3 |
| alr5358 | hetN | Ketoacyl reductase | −1.34 | 1.9 × 10−3 |
a Relative ratios of transcript levels in the sigE disruptant to those in the wild-type strain at 8 h after nitrogen deprivation are shown in the base 2 logarithm; b p values for the relative ratios were determined by the Student’s t-test.
4. Concluding Remarks
In the present study, genes regulated by SigC, SigE, and SigG of Anabaena PCC 7120 were identified. It was suggested that group 2 sigma factors of Anabaena PCC 7120 have at least partially overlapping promoter specificities by genetic analysis of sigma factor genes. In this study, comprehensive analysis of gene expression in mutants of sigma factor genes showed that genes regulated by SigC and SigE partially overlap. It was indicated that coxA2, talB, hepB, all4160, and the HEP island are regulated by both SigC and SigE. The transcript levels of these genes were lower in the sigC disruptant than in the WT strain at 8 h after nitrogen deprivation, whereas the expression of these genes was higher in the sigC disruptant than in the WT strain after 24 h, suggesting that SigE compensated for the deficiency of SigC. We cannot distinguish between direct and indirect regulation based on the data presented here. Therefore, the possibility that a transcriptional regulator whose expression depends on both SigC and SigE is responsible for the overlap between the genes regulated by SigC and SigE cannot be ruled out, although no gene encoding a transcriptional regulator was downregulated in both the sigC and sigE disruptants. Genes required for heterocyst differentiation are induced in differentiating cells. SigC and SigE are likely to act cooperatively to fully and rapidly induce these genes in differentiating cells. The expression patterns of fbp and coxB3 were similar to those of genes regulated by both sigC and sigE. However, fbp and coxB3 were not regulated by SigE, indicating that sigma factors other than SigE can also compensate for the deficiency of SigC. In Synechocystis PCC 6803, it is indicated that multiple group 2 sigma factors can recognize the same promoter as the group 1 sigma factor SigA [43]. In Anabaena PCC 7120, there are seven group 2 sigma factors. It is proposed that redundancy of transcriptional regulators is not only a selective advantage, but also permits strict regulation of gene expression [44].
Acknowledgments
This study was supported in part by the Precursory Research for Embryonic Science and Technology (PRESTO) program from the Japan Science and Technology Agency to Shigeki Ehira, a Grant-in-aid for Young Scientists (B) 26870472 from the Japan Society for the Promotion of Science to Shigeki Ehira, and the JGC-S Scholarship Foundation to Shigeki Ehira.
Author Contributions
Shigeki Ehira and Shogo Miyazaki performed the experiments. Shigeki Ehira designed the research, analyzed the data, and wrote the paper. Both authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Flores E., Herrero A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010;8:39–50. doi: 10.1038/nrmicro2242. [DOI] [PubMed] [Google Scholar]
- 2.Kumar K., Mella-Herrera R.A., Golden J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010;2:a000315. doi: 10.1101/cshperspect.a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitschke J., Vioque A., Haas F., Hess W.R., Muro-Pastor A.M. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc. Natl. Acad. Sci. USA. 2011;108:20130–20135. doi: 10.1073/pnas.1112724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty B.L., van Nieuwerburgh F., Head S.R., Golden J.W. Directional RNA deep sequencing sheds new light on the transcriptional response of Anabaena sp. strain PCC 7120 to combined-nitrogen deprivation. BMC Genomics. 2011;12 doi: 10.1186/1471-2164-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehira S., Ohmori M., Sato N. Genome-wide expression analysis of the responses to nitrogen deprivation in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2003;10:97–113. doi: 10.1093/dnares/10.3.97. [DOI] [PubMed] [Google Scholar]
- 6.Ehira S., Ohmori M. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 2006;59:1692–1703. doi: 10.1111/j.1365-2958.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 7.Herrero A., Muro-Pastor A.M., Valladares A., Flores E. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 2004;28:469–487. doi: 10.1016/j.femsre.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M.-X., Jiang Y.-L., He Y.-X., Chen Y.-F., Teng Y.-B., Chen Y., Zhang C.-C., Zhou C.-Z. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc. Natl. Acad. Sci. USA. 2010;107:12487–12492. doi: 10.1073/pnas.1001556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muro-Pastor A.M., Olmedo-Verd E., Flores E. All4312, an NtcA-regulated two-component response regulator in Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 2006;256:171–177. doi: 10.1111/j.1574-6968.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 10.Ehira S., Ohmori M. NrrA directly regulates expression of hetR during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2006;188:8520–8525. doi: 10.1128/JB.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buikema W.J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991;5:321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 12.Buikema W.J., Haselkorn R. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA. 2001;98:2729–2734. doi: 10.1073/pnas.051624898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X., Dong Y., Zhao J. HetR homodimer is a DNA-binding protein required for heterocyst differentiation, and the DNA-binding activity is inhibited by PatS. Proc. Natl. Acad. Sci. USA. 2004;101:4848–4853. doi: 10.1073/pnas.0400429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videau P., Ni S., Rivers O.S., Ushijima B., Feldmann E.A, Cozy L.M., Kennedy M.A., Callahan S.M. Expanding the direct HetR regulon in Anabaena sp. strain PCC 7120. J. Bacteriol. 2014;196:1113–1121. doi: 10.1128/JB.01372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty B.L., Johnson D., Golden J.W. Deep sequencing of HetR-bound DNA reveals novel HetR targets in Anabaena sp. strain PCC7120. BMC Microbiol. 2014;14 doi: 10.1186/s12866-014-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo S., Valladares A., Flores E., Herrero A. Transcription activation by NtcA in the absence of consensus NtcA-binding sites in an anabaena heterocyst differentiation gene promoter. J. Bacteriol. 2012;194:2939–2948. doi: 10.1128/JB.05994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro-Pastor A.M., Valladares A., Flores E., Herrero A. Mutual dependence of the expression of the cell differentiation regulatory protein HetR and the global nitrogen regulator NtcA during heterocyst development. Mol. Microbiol. 2002;44:1377–1385. doi: 10.1046/j.1365-2958.2002.02970.x. [DOI] [PubMed] [Google Scholar]
- 18.Wösten M.M. Eubacterial sigma-factors. FEMS Microbiol. Rev. 1998;22:127–150. doi: 10.1016/S0168-6445(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko T., Nakamura Y., Wolk C.P., Kuritz T., Sasamoto S., Watanabe A., Iriguchi M., Ishikawa A., Kawashima K., Kimura T., et al. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001;8:205–213, 227–253. doi: 10.1093/dnares/8.5.205. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura H., Okamoto S., Tsumuraya Y., Ohmori M. Group 3 sigma factor gene, sigJ, a key regulator of desiccation tolerance, regulates the synthesis of extracellular polysaccharide in cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2007;14:13–24. doi: 10.1093/dnares/dsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahamsha B., Haselkorn R. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: Cloning, expression, and inactivation of the sigB and sigC genes. J. Bacteriol. 1992;174:7273–7282. doi: 10.1128/jb.174.22.7273-7282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mella-Herrera R.A, Neunuebel M.R., Kumar K., Saha S.K., Golden J.W. The sigE gene is required for normal expression of heterocyst-specific genes in Anabaena sp. strain PCC 7120. J. Bacteriol. 2011;193:1823–1832. doi: 10.1128/JB.01472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldea M.R., Mella-Herrera R.A, Golden J.W. Sigma factor genes sigC, sigE, and sigG are upregulated in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2007;189:8392–8396. doi: 10.1128/JB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khudyakov I.Y., Golden J.W. Identification and inactivation of three group 2 sigma factor genes in Anabaena sp. strain PCC 7120. J. Bacteriol. 2001;183:6667–6675. doi: 10.1128/JB.183.22.6667-6675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehira S., Ohmori M. NrrA, a nitrogen-regulated response regulator protein, controls glycogen catabolism in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. J. Biol. Chem. 2011;286:38109–38114. doi: 10.1074/jbc.M111.289124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehira S., Ohmori M. The pknH gene restrictively expressed in heterocysts is required for diazotrophic growth in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology. 2012;158:1437–1443. doi: 10.1099/mic.0.057729-0. [DOI] [PubMed] [Google Scholar]
- 27.Golden J.W., Wiest D.R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988;242:1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- 28.Black T.A., Cai Y., Wolk C.P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 29.Elhai J., Vepritskiy A., Muro-Pastor A.M., Flores E., Wolk C.P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1997;179:1998–2005. doi: 10.1128/jb.179.6.1998-2005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto F.L., Thapper A., Sontheim W., Lindblad P. Analysis of current and alternative phenol based RNA extraction methodologies for cyanobacteria. BMC Mol. Biol. 2009;10:79. doi: 10.1186/1471-2199-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackinney G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941;140:315–322. [Google Scholar]
- 32.Ehira S. Transcriptional Regulation of Heterocyst Differentiation in Anabaena sp. strain PCC 7120. Russ. J. Plant Physiol. 2013;60:443–452. doi: 10.1134/S1021443713040043. [DOI] [Google Scholar]
- 33.Krasikov V., Aguirre von Wobeser E., Dekker H.L., Huisman J., Matthijs H.C.P. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol. Plant. 2012;145:426–439. doi: 10.1111/j.1399-3054.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- 34.Muro-Pastor A.M., Herrero A., Flores E. Nitrogen-regulated group 2 sigma factor from Synechocystis sp. strain PCC 6803 involved in survival under nitrogen stress. J. Bacteriol. 2001;183:1090–1095. doi: 10.1128/JB.183.3.1090-1095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olmedo-Verd E., Muro-Pastor A.M., Flores E., Herrero A. Localized induction of the ntcA regulatory gene in developing heterocysts of Anabaena sp. strain PCC 7120. J. Bacteriol. 2006;188:6694–6699. doi: 10.1128/JB.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechno-Yossef S., Fan Q., Wojciuch E., Wolk C.P. Identification of ten Anabaena sp. genes that under aerobic conditions are required for growth on dinitrogen but not for growth on fixed nitrogen. J. Bacteriol. 2011;193:3482–3489. doi: 10.1128/JB.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valladares A., Herrero A., Pils D., Schmetterer G., Flores E. Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol. Microbiol. 2003;47:1239–1249. doi: 10.1046/j.1365-2958.2003.03372.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones K.M., Haselkorn R. Newly identified cytochrome c oxidase operon in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 specifically induced in heterocysts. J. Bacteriol. 2002;184:2491–2499. doi: 10.1128/JB.184.9.2491-2499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Lechno-Yossef S., Gong Y., Fan Q., Wolk C.P., Xu X. Predicted glycosyl transferase genes located outside the HEP island are required for formation of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 2007;189:5372–5378. doi: 10.1128/JB.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiedler G., Arnold M., Hannus S., Maldener I. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 1998;27:1193–1202. doi: 10.1046/j.1365-2958.1998.00762.x. [DOI] [PubMed] [Google Scholar]
- 41.Awai K., Wolk C.P. Identification of the glycosyl transferase required for synthesis of the principal glycolipid characteristic of heterocysts of Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 2007;266:98–102. doi: 10.1111/j.1574-6968.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- 42.Callahan S.M., Buikema W.J. The role of HetN in maintenance of the heterocyst pattern in Anabaena sp. PCC 7120. Mol. Microbiol. 2001;40:941–950. doi: 10.1046/j.1365-2958.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- 43.Imamura S., Tanaka K., Shirai M., Asayama M. Growth phase-dependent activation of nitrogen-related genes by a control network of group 1 and group 2 sigma factors in a cyanobacterium. J. Biol. Chem. 2006;281:2668–2675. doi: 10.1074/jbc.M509639200. [DOI] [PubMed] [Google Scholar]
- 44.Kafri R., Springer M., Pilpel Y. Genetic redundancy: New tricks for old genes. Cell. 2009;136:389–392. doi: 10.1016/j.cell.2009.01.027. [DOI] [PubMed] [Google Scholar]