Abstract
Background
Two of the more common methods of pain management after TKA are peripheral nerve blocks and intraarticular/periarticular injections. However, we are not aware of any study directly comparing the commonly used combination of a continuous femoral block given with a single-shot sciatic block with that of a periarticular injection after TKA.
Questions/purposes
This randomized clinical trial compared a combined femoral and sciatic nerve block with periarticular injection as part of a multimodal pain protocol after total knee arthroplasty with respect to (1) pain; (2) narcotic use; (3) quadriceps function and length of stay; and (4) peripheral nerve complications.
Methods
One hundred sixty patients completed randomization into two treatment arms: (1) peripheral nerve blocks (PNB; n = 79) with an indwelling femoral nerve catheter and a single shot sciatic block; or (2) periarticular injection (PAI; n = 81) using ropivacaine, epinephrine, ketorolac, and morphine. All patients received standardized general anesthesia and oral medications. The primary outcome was postoperative pain, on a 0 to 10 scale, measured on the afternoon of postoperative day 1 (POD 1). Secondary outcomes were narcotic use, quadriceps function, length of stay, and peripheral nerve complications.
Results
Mean pain scores on the afternoon of POD 1 were not different between groups (PNB group: 2.9 [SD 2.4]; PAI group: 3.0 [SD 2.2]; 95% confidence interval, −0.8 to 0.6; p = 0.76). Mean pain scores taken at three times points on POD 1 were also similar between groups. Hospital length of stay was shorter for the PAI group (2.44 days [SD 0.65] versus 2.84 days [SD 1.34] for the PNB group; p = 0.02). Narcotic consumption was higher the day of surgery for the PAI group (PAI group: 11.7 mg morphine equivalents [SD 13.1]; PNB group: 4.6 mg [SD 9.1]; p < 0.001), but thereafter, there was no difference. More patients in the PNB group had sequelae of peripheral nerve injury (mainly dysesthesia) at 6-week followup (nine [12%] versus one [1%]; p = 0.009).
Conclusions
Patients receiving periarticular injections had similar pain scores, shorter lengths of stay, less likelihood of peripheral nerve dysesthesia, but greater narcotic use on the day of surgery compared with patients receiving peripheral nerve blocks. Periarticular injections provide adequate pain relief, are simple to use, and avoid the potential complications associated with nerve blocks.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Advances in postoperative pain management after knee arthroplasty have improved short-term pain relief, earlier return of ROM, and in some cases earlier discharge from the hospital [12, 13, 25, 35]. Over the last decade multimodal pain management techniques have become an increasingly common alternative to opioids alone in the management of perioperative pain after knee arthroplasty.
Numerous methods of adjuvant postoperative pain management have been reported including standardized oral medications; periarticular injections of local anesthetic and analgesic medications; regional anesthesia using neuraxial blockade (spinal or epidural anesthetic); and peripheral nerve blocks (eg, femoral, sciatic, or psoas block), or a combination these (eg, spinal and a continuous femoral block) [7, 26, 28]. Two of the more common methods of pain management include peripheral nerve blocks (femoral with or without sciatic nerve block) and intraarticular/periarticular injections. The perceived advantage of peripheral nerve blocks is effective pain management; disadvantages include slower recovery of lower extremity function [9], time required for the procedure, complications related to the block [14, 21, 29], and added cost. Because of the limited duration of action of the local anesthetic, there is the perception that single periarticular injections may not provide good pain relief beyond the first day [20]. However, they are simpler to administer, provide for earlier return of lower extremity function [9], may have a lower risk of complications, and are less costly [2]. Many authors have shown that periarticular injections provide improved pain relief compared with no injection [6, 22, 36]. Additionally, numerous studies have compared the efficacy of various types of neuraxial anesthetics and peripheral blocks [1, 7, 23, 28, 31, 37, 38]. The addition of a sciatic block to a femoral block provides better pain relief than femoral block alone, and various combinations have been studied [4, 7, 10, 17, 37]. However, we were unable to find any study directly comparing this commonly used combination of a continuous femoral block given with a single-shot sciatic block with that of a periarticular injection.
The purpose of this study was to compare two modalities of postoperative pain management as part of a multimodal pain protocol after knee arthroplasty. We compared the use of a periarticular injection (ropivacaine, epinephrine, ketorolac, and morphine sulphate) with that of a peripheral nerve block (continuous femoral block with indwelling catheter and a single-shot sciatic block using 0.5% ropivacaine). Our primary outcome was the patient’s pain score on the afternoon of postoperative day 1. Our secondary outcomes included narcotic use, quadriceps function and length of stay, and peripheral nerve complications.
Patients and Methods
The trial was approved by the Mayo Clinic Institutional review board (10-003312) and registered on ClinicalTrials.gov (NCT01163214). Patients were recruited sequentially and assigned to receive a periarticular injection (PAI) or a peripheral nerve block (PNB) at random in a one-to-one ratio. Allocation was concealed by storing the treatment allocation schedule on a randomization web site that indicated the treatment assignment after recording the subject’s identifier.
All patients between the ages of 18 and 79 years and with weight between 50 and 125 kg who presented for unilateral TKA were eligible for inclusion in the study. Patients who had prior knee surgery using a peripheral nerve block or periarticular injection were excluded from the study because experience with one of the techniques could bias the patient’s reporting of pain and satisfaction. Additional exclusions included patients with an allergy to any of the medications used; renal insufficiency (creatinine > 1.5 mg/dL); or those taking regular narcotic medications before surgery (≥ 20 mg/day of morphine equivalent for > 7 days).
In the preoperative holding area, all patients received the same combination of oral medication: 10 mg oxycodone controlled release, 300 mg gabapentin, 1000 mg acetaminophen, and 75 mg diclofenac/200 μg misoprostol.
All patients randomized to the PNB group received a femoral nerve block with an indwelling catheter and a single-shot sciatic nerve block. Briefly the technique includes preprocedure sedation with midazolam and fentanyl, identification of the femoral nerve by landmarks or ultrasound at the discretion of the anesthesiologist, further localization by a nerve stimulator, and subsequent infiltration of 30 mL 0.5% ropivacaine without epinephrine along the lateral aspect of the nerve. Finally, a femoral catheter, through the 18-g insulating block needle, was threaded approximately 3 to 5 cm past the depth at which the quadriceps twitch response was elicited. The sciatic nerve was approached through landmarks and identified by a nerve stimulator. Once the appropriate twitch was achieved, 10 mL 0.5% ropivacaine without epinephrine was injected. The minimum threshold for stimulation for both femoral and sciatic was 0.5 mA; the maximum threshold stimulation was 0.2 mA. After the knee arthroplasty, a 0.2% plain ropivacaine infusion was started through the femoral catheter. The infusion range was 6 mL to 12 mL/hour, typically starting at 6 at 8 mL/hour and increased or decreased by 1 mL every hour as needed. The infusion was discontinued on the morning of postoperative day 2.
All surgical procedures were performed in a standardized manner with all patients receiving a cemented posterior-stabilized TKA done through a medial parapatellar approach under general anesthesia. All procedures were done under tourniquet, which was deflated either just before or after insertion of the final tibial insert after the cement was hard and before wound closure. A reinfusion drain (Constavac; Stryker, Kalamazoo, MI, USA) was used in all patients.
Patients in the PAI group received an injection cocktail, based on three weight categories, of ropivacaine, epinephrine, ketorolac, and morphine sulphate with normal saline added to bring the volume to 120 mL (Table 1). Before cementation, the posterior capsule was injected with approximately 30 mL followed by 50 mL into the medial and lateral retinaculum with the knee reduced while the cement was hardening and 40 mL into the quadriceps tendon and subcutaneous tissue just before closure.
Table 1.
Periarticular injection concentrations
| Weight (kg) | 50–74.9 | 75–99.9 | 100–125 |
|---|---|---|---|
| Ropivacaine | 200 mg | 300 mg | 400 mg |
| Epinephrine | 100 μg | 200 μg | 300 μg |
| Ketorolac | 30 mg | 30 mg | 30 mg |
| Morphine sulphate | 5 mg | 5 mg | 5 mg |
Normal saline added to bring volume to 120 mL.
Postoperatively all patients were prescribed scheduled analgesic medications as well as narcotics for breakthrough pain. Medications consisted of 1000 mg acetaminophen every 8 hours, 75 mg diclofenac/200 μg misoprostal twice a day, and 300 mg gabapentin every 8 hours. Oral narcotics were preferred for breakthrough pain but intravenous narcotics were available in the event that patients were unable to take oral medications. Narcotics were standardized to 5 mg oxycodone immediate release every 4 hours as needed, 2 mg morphine intravenously every 2 hours as needed, or 0.5 mg hydromorphone intravenously every 2 hours as needed.
The primary outcome measure was a pain score measured at rest on a linear analog scale from 0 to 10 points before the patient’s afternoon physical therapy session on the day after surgery (postoperative day 1) Secondary outcome measures were (1) pain scores measured at rest at standardized times during hospitalization (recovery room, arrival in the patient’s room, and three times daily: before morning physical therapy, before afternoon physical therapy, and at 10 pm); (2) narcotic use; (3) self-reported feeling of nausea (scale from 0 to 10 with 0 = no nausea and 10 = extreme nausea); (4) return of quadriceps function (assessed for quadriceps extensor lag and ability to straight-leg raise); (5) length of hospital stay; and (6) neurological sequelae related to either the peripheral nerve block or injection assessed at 6-week followup.
Patients were included in the primary analysis on the basis of intention to treat. The study was designed to test whether the mean pain score on the afternoon of postoperative day 1 after PNB was superior to PAI. Mean pain for the PAI group was compared with that of the PNB group by using the two-sample t-test. There were no interim analyses. Secondary outcomes were assessed by using the two-sample t-test, Pearson’s chi-square test, or Wilcoxon rank-sum test. Fisher’s exact test was used instead of Pearson’s chi-square test if the minimum expected data count was < 5. The Wilcoxon rank-sum test was used to compare the distributions of ordered categories. A sample of 75 patients per group was planned to have 80% power (α = 0.05) to detect a difference of 1 point (0.5 SD) on the primary outcome measure. The SD was estimated from Browne et al. [5] and Busch et al. [6]. Data were recorded using REDCap electronic data capture [16]. Computations were performed using SAS software Version 9.2 (SAS Institute Inc, Cary, NC, USA).
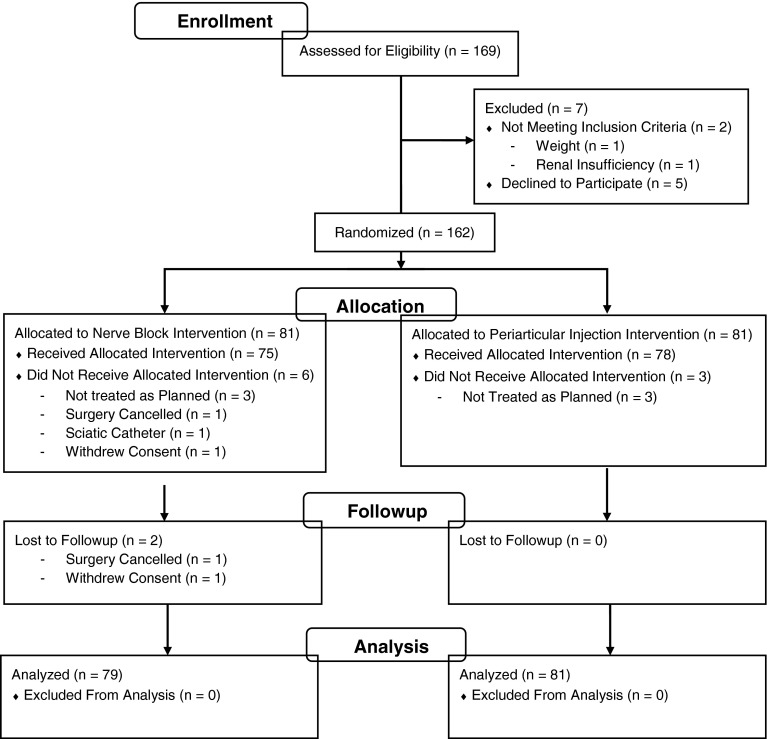
Patients were recruited from September 2010 to February 2013. Patient enrollment and subsequent randomization and analysis are presented (Fig. 1). One hundred sixty-nine patients were assessed for eligibility, and 162 were randomized with 81 patients to each group. Two patients in the PNB group were not analyzed (one did not proceed with surgery and one withdrew consent). Thus, the primary analysis based on the intention to treat included 160 patients: 79 in the PNB group and 81 in the PAI group. A subgroup analysis was also performed on patients who received the allocated treatment as per the planned protocol. This final group included 74 patients in the PNB group and 77 in the PAI group. For the PNB group, an additional five patients were excluded from the 79 used in the primary analysis (three were not treated as planned, one received a sciatic catheter instead of single injection, and one subject had previously received a nerve block procedure). For the PAI group, an additional four patients were excluded leaving 77 for the per-protocol analysis (three patients did not receive the planned treatment and one subject had a weight of 139 kg, which was greater than the 125-kg limit for inclusion). Eighty-nine patients (56%) were women. Age ranged from 42 to 79 years with a mean of 68 years (Table 2). Body mass index ranged from 20 to 49 kg/m2 with a mean of 31 kg/m2. Baseline characteristics did not differ substantially between the two treatment groups.
Fig. 1.
Patient enrollment, allocation, and analysis breakdown are detailed.
Table 2.
Baseline
| Demographic data | Nerve block (n = 79) | Periarticular injection (n = 81) |
|---|---|---|
| Female | 41 (52%) | 48 (59%) |
| Age (years); mean (SD) | 67.8 (7.9) | 67.7 (7.2) |
| Body mass index (kg/m2); mean (SD) | 30.3 (4.9) | 31.7 (5.2) |
| Weight (kg) | ||
| 50–74.9 | 14 (18%) | 11 (14%) |
| 75–99.9 | 45 (57%) | 47 (58%) |
| 100–125 | 20 (25%) | 22 (27%) |
| > 125 | 0 (0%) | 1 (1%) |
Results
The mean pain score on the afternoon of the day after surgery did not differ between the two groups (Table 3). The mean score (on a scale from 0 to 10 points) was 2.9 for the PNB group versus 3.0 for the PAI group. The difference between groups in the per-protocol subset of patients who received the allocated treatment was the same as in the full data set of the primary intention-to-treat analysis (Table 4; Δ −0.1, 95% confidence interval [CI], −0.8 to 0.6). On the day of surgery, significantly fewer patients reported pain in the PNB group than in the PAI group on arrival in the patient’s room and on the evening of the day of surgery. However, all other recorded times had no significant differences in pain scores between the two groups.
Table 3.
Pain scores and narcotic consumption
| Pain score and narcotic use | Nerve block (n = 79) | Periarticular injection (n = 81) | Δ | 95% confidence interval | p value |
|---|---|---|---|---|---|
| Pain (0 none to 10 worst); mean (SD) | |||||
| Recovery room arrival | 2.3 (2.6) | 2.6 (2.3) | −0.3 (2.5) | −1.0 to 0.5 | 0.51 |
| Patient room arrival | 1.3 (1.9) | 2.0 (1.9) | −0.7 (1.9) | −1.3 to −0.1 | 0.02 |
| Day of surgery evening | 1.6 (2.0) | 2.3 (1.9) | −0.7 (1.9) | −1.3 to −0.1 | 0.02 |
| Day 1 morning | 2.0 (2.1) | 2.5 (2.0) | −0.5 (2.0) | −1.1 to 0.2 | 0.14 |
| Day 1 afternoon* | 2.9 (2.4) | 3.0 (2.2) | −0.1 (2.3) | −0.8 to 0.6 | 0.76† |
| Day 1 evening | 2.3 (1.9) | 2.9 (2.3) | −0.6 (2.1) | −1.3 to 0.0 | 0.07 |
| Day 1 mean of morning, afternoon, and evening | 2.4 (1.6) | 2.8 (1.8) | −0.4 (1.7) | −0.9 to 0.1 | 0.15 |
| Day 2 mean of morning, afternoon, and evening | 1.7 (1.5) | 1.5 (1.4) | 0.1 (1.5) | −0.3 to 0.6 | 0.52 |
| Pain Day 1 afternoon > 3 | 27 (34%) | 29 (36%) | −0.02 | −0.16 to 0.13 | 0.83 |
| Narcotic morphine equivalents (mg); mean (SD) | |||||
| Intraoperative | 17.4 (10.1) | 23.6 (8.5) | −6.2 | −9.1 to −3.3 | < 0.001 |
| Day of surgery as needed | 4.6 (9.1) | 11.7 (13.1) | −7.3 (11.3) | −10.6 to −3.6 | < 0.001 |
| Day 1 as needed | 43 (29) | 49 (29) | −6 (29) | −15 to 3 | 0.17 |
| Day 2 as needed | 33 (28) | 30 (29) | 3 (28) | −6 to 12 | 0.51 |
| Number of patients using narcotic morphine equivalents > 0 mg | |||||
| Recovery room | 31 (39%) | 47 (58%) | −0.19 | −0.34 to −0.04 | 0.02 |
| Day of surgery as needed | 24 (30%) | 56 (69%) | −0.39 | −0.53 to −0.24 | < 0.001 |
| Day 1 as needed | 71 (90%) | 76 (94%) | −0.04 | −0.12 to 0.05 | 0.36 |
| Day 2 as needed | 72 (91%) | 68 (84%) | 0.07 | −0.03 to 0.17 | 0.17 |
* Primary outcome measure; †Wilcoxon rank-sum test p = 0.59.
Table 4.
Pain scores in the per-protocol subset (patient who received the allocated treatment)
| Pain scores | Nerve block (n = 74) | Periarticular injection (n = 77) | Δ | 95% confidence interval | p value |
|---|---|---|---|---|---|
| Pain Day 1 afternoon (0 none to 10 worst); mean (SD) | 2.9 (2.4) | 3.1 (2.2) | −0.1 (2.3) | −0.8 to 0.6 | 0.78* |
| Pain Day 1 afternoon > 0 | 64 (86%) | 68 (88%) | −0.02 | −0.12 to 0.09 | 0.74 |
| Pain Day 1 afternoon > 3 | 25 (34%) | 27 (35%) | −0.01 | −0.16 to 0.14 | 0.87 |
* Wilcoxon rank-sum test p = 0.58.
Narcotic consumption intraoperatively and on the day of surgery was lower in the PNB group than in the PAI group (intraoperative: PAI group: 23.6 mg morphine equivalents [SD 8.5], PNB group: 17.4 [SD 10.1], Δ −6.2; 95% CI, −9.1 to −3.3; p < 0.001); day of surgery: PAI group: 11.7 mg morphine equivalents [SD 13.1], PNB group: 4.6 mg [SD 9.1], Δ −7.3 [SD 11.3]; 95% CI, −10.6 to −3.6; p < 0.001). However, mean narcotic dose did not differ between groups after the day of surgery (Table 3). Although patients were asked to report nausea on a scale from 0 to 10, more than half of the scores were zero, making a t-test comparison invalid. Hence, any score that was greater than zero is reported (Table 5). The incidence of nausea did not differ between groups on the day of surgery or the day after surgery. The incidence of nausea was slightly higher in the PNB group than in the PAI group on the second day after surgery. There was no relationship to narcotic use and occurrence of nausea.
Table 5.
Results of nausea, quadriceps function, length of stay, and peripheral nerve symptoms
| Measure | Nerve block (n = 79) | Periarticular injection (n = 81) | Δ | 95% confidence interval | p value |
|---|---|---|---|---|---|
| Nausea > 0 | |||||
| Recovery room arrival | 13 (16%) | 10 (12%) | 0.04 | −0.07 to 0.15 | 0.46 |
| Patient room arrival | 14 (18%) [N = 78] | 12 (15%) | 0.03 | −0.08 to 0.15 | 0.59 |
| Day of surgery evening | 11 (14%) | 13 (16%) | −0.02 | −0.13 to 0.09 | 0.71 |
| Day 1 morning, afternoon, or evening | 18 (23%) [N = 78] | 19 (23%) | 0.00 | −0.14 to 0.13 | 0.95 |
| Day 2 morning, afternoon, or evening | 14 (18%) [N = 78] | 6 (8%) [N = 80] | 0.10 | 0.002–0.21 | 0.048 |
| Straight-leg raise | |||||
| Day 1 morning | 19 (24%) [N = 78] | 63 (79%) [N = 80] | −0.54 | −0.67 to −0.41 | < 0.001 |
| Day 1 afternoon | 21 (28%) [N = 76] | 63 (81%) [N = 78] | −0.53 | −0.66 to −0.40 | < 0.001 |
| Day 2 morning | 52 (67%) [N = 78] | 69 (87%) [N = 79] | −0.21 | −0.33 to −0.08 | 0.002 |
| Day 2 afternoon | 57 (83%) [N = 69] | 42 (82%) [N = 51] | 0.00 | −0.14 to 0.14 | 0.97 |
| Quadriceps lag (°); mean (SD) | |||||
| Day 1 morning | 18 (20) [N = 20] | 10 (12) [N = 64] | 8 (14) | 0.3–15 | 0.04 |
| Day 1 afternoon | 17 (16) [N = 21] | 7 (11) [N = 63] | 10 (13) | 4–17 | 0.002 |
| Day 2 morning | 7 (16) [N = 51] | 7 (11) [N = 68] | 0 (14) | −5 to 4 | 0.98 |
| Day 2 afternoon | 5.9 (8.7) [N = 55] | 5.8 (9.0) [N = 42] | 0.1 (8.8) | −3.5 to 3.7 | 0.96 |
| Length of stay (days); mean (SD) | 2.84 (1.34) [N = 74] | 2.44 (0.65) | 0.4 (1.1) | 0.1–0.7 | 0.02 |
| Neurological changes at Week 6 | |||||
| Femoral nerve | 1 (1%) [N = 77] | 0 (0%) [N = 79] | 0.01 | −0.01 to 0.04 | 0.49 |
| Common peroneal nerve | 6 (8%) [N = 78] | 0 (0%) [N = 79] | 0.08 | 0.02–0.14 | 0.01 |
| Tibial nerve | 2 (3%) [N = 78] | 1 (1%) [N = 79] | 0.01 | −0.03 to 0.06 | 0.62 |
| Femoral, peroneal, or tibial nerve | 9 (12%) [N = 77] | 1 (1%) [N = 79] | 0.10 | 0.03–0.20 | 0.009 |
Not surprisingly, quadriceps function was lower in the PNB group than in the PAI group. Fewer patients in the PNB group were able to perform a straight-leg raise, with or without extensor lag, during the first 2 days after surgery. On the morning of day 1, 19 (24%) of the PNB group could perform a straight-leg raise versus 63 (79%) (95% CI, −0.67 to −0.41; p < 0.001; Table 4) in the PAI group. By the afternoon of postoperative day 2, for those remaining in the hospital, quadriceps function no longer differed between the two groups.
Mean length of stay was 0.4 days longer in the PNB group than in the PAI group (Table 5; 2.84 days PNB versus 2.44 PAI group; 95% CI, 0.1–0.7; p = 0.02).
Postoperative sensory changes at 6 weeks followup, noted mainly as a dysesthesia, were more common in the PNB group than in the PAI group. Twelve percent (nine of 77) of patients in the nerve block group experienced sensory change versus one (one of 79 in the injection group; p = 0.009). No motor changes were observed in either group.
The majority of adverse events were unrelated to the type of postoperative pain management and typical of a population undergoing knee arthroplasty. However, of note, three patients in the PNB group and no patient in the PAI group fell postoperatively (Table 6; p = 0.12).
Table 6.
Adverse events within 4 days
| Event | Nerve block (n = 79) | Periarticular injection (n = 81) | Δ | p value |
|---|---|---|---|---|
| Vomiting | 19 (24%) | 16 (20%) | 0.04 | 0.51 |
| Fall | 3 (4%) | 0 (0%) | 0.04 | 0.12 |
| Syncope | 3 (4%) | 1 (1%) | 0.03 | 0.36 |
| Urinary tract infection | 2 (3%) | 0 (0%) | 0.03 | 0.24 |
| Acute pulmonary embolism | 1 (1%) | 0 (0%) | 0.01 | 0.49 |
| Acute renal failure | 1 (1%) | 0 (0%) | 0.01 | 0.49 |
| Lumbar vertebral fracture after fall | 1 (1%) | 0 (0%) | 0.01 | 0.49 |
| Stroke middle cerebral artery | 1 (1%) | 0 (0%) | 0.01 | 0.49 |
| Pneumonia | 2 (2%) | 2 (2%) | 0.00 | > 0.99 |
| Cellulitis | 0 (0%) | 2 (2%) | −0.02 | 0.50 |
| Urinary retention | 26 (33%) | 33 (41%) | −0.08 | 0.30 |
Discussion
Postoperative pain management is receiving increasing emphasis in the overall surgical care of patients. Regulatory agencies mandate that patients be asked their level of pain after surgery and that pain be treated accordingly [33]. Methods of postoperative pain management have evolved from the use of intramuscular narcotics, nurse-administered intravenous narcotics, and patient-controlled analgesics to the current use of multimodal pain control, typically using a combination of preoperative oral analgesic medications, some form of regional anesthesia, and postoperative oral or intravenous narcotics for breakthrough pain. Although peripheral nerve blocks and intraarticular/periarticular injections are commonly used after TKA, we could find no study that directly compared the commonly used combination of a continuous femoral block given with a single-shot sciatic block with that of a periarticular injection after TKA. We therefore performed a randomized trial to compare them in terms of pain, analgesic use, quadriceps function and length of stay, and neurological complications.
The study had several limitations. We were unable to blind patients and staff to the treatment arms that each patient received because the presence of the femoral catheter and neurological changes expected from peripheral blocks would make it obvious as to which group the patients were allocated. Additionally, we evaluated patients’ pain at rest and not with activity. Some studies have shown higher pain levels with activity or during use of a continuous passive motion machine [7, 11]; however, one might expect that both groups would have proportionally higher pain with activity and hence no difference between the groups. Length of stay data were calculated from the medical record and not necessarily when the patient was deemed ready for discharge; however, fortunately delays in discharge for social reasons, placement, or weather-related discharge delays are rare. All patients undergoing joint replacement attend a preoperative education class where discharge planning is begun. Randomization should eliminate any bias regarding length of stay data because all patients had the same postoperative physical therapy protocol. Lastly, the study was not powered to identify certain rare events such as falls. A larger sample may have shown significance in the trend of more falls in the peripheral nerve block group.
The primary outcome of the pain score on the afternoon of postoperative day 1 was chosen because it was felt that the periarticular injection would have lost all or the majority of its effect by this time and therefore this time point would reflect a “truer” evaluation of differences between the two methods. We found that pain scores did not differ between the two groups for our primary outcome, although pain scores were statistically higher the day of surgery in the PAI group; however, the differences between the two groups at those time points were less than 1 point, thus unlikely to be clinically relevant. Although no other study used the same pain management protocols, similar studies comparing periarticular injection with nerve blocks showed results close to our study. In a recent clinical trial, Chaumeron et al. [9] used a single-strength periarticular injection, which was redosed through an intraarticular catheter on postoperative day 1 and compared this with an indwelling femoral nerve catheter (no sciatic block) used for 48 to 72 hours. This study found lower pain scores for the first 8 hours after surgery in the periarticular injection group and no difference thereafter [9]. Other similar studies found opposing results: Carli et al. [8] found no differences in pain scores and Toftdahl et al. [34] and Affas et al. [2] found lower scores in patients receiving periarticular injection compared with those receiving isolated femoral nerve blocks during the first postoperative day [2, 8, 34]. The use of combined sciatic and femoral nerve blocks over femoral nerve block alone has been shown to have improved pain scores and seems more beneficial for pain management [1, 10, 17]. When comparing intraoperative periarticular injections with intraarticular injections given after closure of the capsule, better pain scores are generally found in studies using periarticular injections [12, 15, 30, 36]. With the exception of a few studies showing minimal improved pain [24, 32], the majority of studies using intraarticular injections showed no improvement in pain scores postoperatively [3, 5, 15, 19, 27]. Hence, most studies on this topic indicate that there is an advantage to direct injection of the soft tissues over simple intraarticular injection.
Despite similar pain scores, narcotic use intraoperatively and on the day of surgery was greater in the PAI group. The increased intraoperative use in this group of patients may relate to the lack of local anesthetic blockade during the procedure and the anesthesiologist’s desire to preemptively prevent pain postoperatively. Administering the periarticular injection at the start of the procedure, during the exposure, may reduce the intraoperative need for narcotics. However, patients receiving the PAI also had higher narcotic consumption on the day of surgery, which is unlikely to be the result of the anesthesiologist bias to give narcotics in this group, but rather a result of true increased narcotic demand in this group of patients. The increased narcotic use on the day of surgery found in our study is in distinction to that of other authors [9, 34]. Like with the pain scores in their study, Chaumeron et al. [9] found lower narcotic consumption in the first 8 hours postoperatively but no difference thereafter. The studies by Carli et al. [8] and Toftdahl et al. [34] found contrasting results regarding narcotic consumption. Toftdahl et al. [34] found lower narcotic use from the end of surgery to the end of postoperative day 1 in the injection groups, whereas Carli et al. [8] found less morphine use in the femoral block group to the end of postoperative day 2. A notable difference between these two studies was the intraoperative infiltration of the posterior capsule in both the injection and nerve block groups in the study by Carli et al. [8]. When comparing studies using intraarticular injection after closure of the capsule with intraoperative periarticular injection, unlike pain scores that were generally more favorable with the periarticular injections, narcotic consumption was usually lower in both intraarticular and periarticular injection studies, although the difference tended to be greater for periarticular injections [5, 6, 12, 19, 27, 36].
Not surprisingly, the return of quadriceps function was slower in the PNB group. The femoral catheter was removed the morning of postoperative day 2 and by the afternoon, for those patients not yet discharged, quadriceps function became equal between the groups. The earlier return of quadriceps function in the PAI group allowed patients to be mobilized faster and resulted in nearly a half day less length of stay. The finding of earlier return in quadriceps function was also seen in a recent publication by Chaumeron et al. [9]. In their study, the authors found a greater motor blockade postoperatively and slower return in quadriceps function up to postoperative day 3 in the nerve block group. Additionally, the patients undergoing a nerve block also had shorter walking distances on days 0, 2, and 3. However, unlike our study, they found no difference in length of stay, but all their patients were kept in the hospital for a minimum of 5 days after surgery. In the similar study by Toftdahl et al. [34], comparing femoral nerve block with periarticular and postoperative intraarticular injection, the authors found improved quadriceps function and walking distance in the injection group on postoperative days 1 and 2. Median length hospital stay was 6 days for the nerve block group and 5 days for the injection group [34]. Lastly, a study on bilateral TKAs comparing only infiltration in one knee with no infiltration in the other knee also found improved quadriceps function up to 2 weeks after surgery [25]. Although we found a statistically shorter length of stay (almost a half day less) in the PAI group, this may not translate into significant savings because the majority of cost is incurred early in the hospital stay for joint replacement.
A number of studies have described complications related to nerve blocks [14, 21, 29]. Feibel et al. [14] reported on two groups of patients undergoing TKA with both groups receiving femoral nerve blocks but with different durations. The first group of 469 patients had the block running for 2 to 3 days, whereas the second group of 721 patients had the block discontinued after 12 hours. They noted eight falls with four in each group. Additionally, they also reported nine (0.8%) femoral nerve palsies, two of which were permanent. Sharma et al. [29] compared 757 knees that received a continuous femoral nerve block with 261 knees with no block. They noted 12 (1.6%) falls in the block group versus one (0.4%) in the group without blocks. Six patients experienced femoral neuropathy/neuritis, five in the block group and one in the nonblock group. The type of anesthetic used was not reported. Complications related to the periarticular injection have not been reported in numerous series using injections [9, 18, 22, 31]. Theoretically, intravascular or intraneural injection could occur when the posterior capsule is injected. We noted complications typical of a population undergoing knee arthroplasty and did not find any statistical difference between the two groups with the exception of neuritis. Nine patients (12%) in the PNB group versus one (1%) in the PAI group reported sensory changes at 6 weeks postoperatively. These changes were typically described as a dysesthesia in the distribution of the nerve. Additionally, although not statically significant, we noted three falls in the PNB group and none in the PAI group. One patient who fell also sustained a lumber compression fracture.
The strengths of this study include its randomized design and its consistent approaches to standardized patient care including preoperative and postoperative medications, surgical procedure, and postoperative therapy protocols. It also was adequately powered and relatively homogeneous; we excluded patients who previously had one of the techniques used with prior knee surgery (which excluded a surprisingly large number of patients) and also excluded patients who were narcotic-tolerant. Additionally, we evaluated patients beyond the hospitalization for any neurologic complications related to the procedure, something many prior studies have not done [2, 9, 34, 36]. Before the initiation of this study, our routine pain management technique after knee arthroplasty was the use of peripheral nerve blocks together with multimodal medications. Based on the result of this study, we now use periarticular injections in combination with scheduled analgesic medications for routine primary knee arthroplasties, although we still use peripheral nerve blocks in revision situations or unique situations of specific patient request. We conclude that periarticular injections provide comparable pain management to peripheral nerve blocks, are simpler to administer, avoid the sensory dysesthesia seen in a small number of patients with nerve blocks, and result in a slightly reduced length of stay.
Acknowledgments
We thank Debra Ryan, our main study coordinator for this project, and Charles (“Scott”) Clarke and all the physical therapists involved in helping our patients postoperatively and collecting data on quadriceps function. Additionally, we also thank Sundeep Khosla MD.
Footnotes
Electronic data capture was supported by grant UL1 TR000135 (SK).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Mayo Clinic Arizona, Phoenix, AZ, USA.
References
- 1.Abdallah FW, Brull R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A systematic review. Reg Anesth Pain Med. 2011;36:493–498. doi: 10.1097/AAP.0b013e318228d5d4. [DOI] [PubMed] [Google Scholar]
- 2.Affas F, Nygards E-B, Stiller C-O, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop. 2011;82:441–447. doi: 10.3109/17453674.2011.581264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badner NH, Bourne RB, Rorabeck CH, MacDonald SJ, Doyle JA. Intra-articular injection of bupivacaine in knee-replacement operations. Results of use for analgesia and for preemptive blockade. J Bone Joint Surg Am. 1996;78:734–738. doi: 10.2106/00004623-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David B, Schmalenberger K, Chelly JE. Analgesia after total knee arthroplasty: is continuous sciatic blockade needed in addition to continuous femoral blockade? Anesth Analg. 2004;98:747–749. doi: 10.1213/01.ANE.0000096186.89230.56. [DOI] [PubMed] [Google Scholar]
- 5.Browne C, Copp S, Reden L, Pulido P, Colwell C., Jr Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19:377–380. doi: 10.1016/j.arth.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88:959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 7.Cappelleri G, Ghisi D, Fanelli A, Albertin A, Somalvico F, Aldegheri G. Does continuous sciatic nerve block improve postoperative analgesia and early rehabilitation after total knee arthroplasty? A prospective, randomized, double-blinded study. Reg Anesth Pain Med. 2011;36:489–492. doi: 10.1097/AAP.0b013e3182286a2b. [DOI] [PubMed] [Google Scholar]
- 8.Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M, Morabito A, Tanzer M. Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block. Br J Anaesth. 2010;105:185–195. doi: 10.1093/bja/aeq112. [DOI] [PubMed] [Google Scholar]
- 9.Chaumeron A, Audy D, Drolet P, Lavigne M, Vendittoli P-A. Periarticular injection in knee arthroplasty improves quadriceps function [Erratum in Clin Orthop Relat Res. 2013;471:2042] Clin Orthop Relat Res. 2013;471:2284–2295. doi: 10.1007/s11999-013-2928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook P, Stevens J, Gaudron C. Comparing the effects of femoral nerve block versus femoral and sciatic nerve block on pain and opiate consumption after total knee arthroplasty. J Arthroplasty. 2003;18:583–586. doi: 10.1016/S0883-5403(03)00198-0. [DOI] [PubMed] [Google Scholar]
- 11.Essving P, Axelsson K, Aberg E, Spannar H, Gupta A, Lundin A. Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: a randomized controlled trial. Anesth Analg. 2011;113:926–933. doi: 10.1213/ANE.0b013e3182288deb. [DOI] [PubMed] [Google Scholar]
- 12.Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A. Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta Orthop. 2010;81:354–360. doi: 10.3109/17453674.2010.487241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajardo M, Collins J, Landa J, Adler E, Meere P, Di Cesare PE. Effect of a perioperative intra-articular injection on pain control and early range of motion following bilateral TKA. Orthopedics. 2011;34:354. doi: 10.3928/01477447-20110317-11. [DOI] [PubMed] [Google Scholar]
- 14.Feibel RJ, Dervin GF, Kim PR, Beaule PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. J Arthroplasty. 2009;24:132–137. doi: 10.1016/j.arth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs DMR, Green TP, Esler CN. The local infiltration of analgesia following total knee replacement: a review of current literature. J Bone Joint Surg Br. 2012;94:1154–1159. doi: 10.1302/0301-620X.94B9.28611. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt KJ, Bourne MH, Mariani EM. Single-injection femoral and sciatic nerve blocks for pain control after total knee arthroplasty. J Arthroplasty. 2009;24:533–538. doi: 10.1016/j.arth.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274–1277. doi: 10.1016/j.arth.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Klasen JA, Opitz SA, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 1999;43:1021–1026. doi: 10.1034/j.1399-6576.1999.431009.x. [DOI] [PubMed] [Google Scholar]
- 20.Koh IJ, Kang YG, Chang CB, Kwon SK, Seo ES, Seong SC, Kim TK. Additional pain relieving effect of intraoperative periarticular injections after simultaneous bilateral TKA: a randomized, controlled study. Knee Surg Sports Traumatol Arthrosc. 2010;18:916–922. doi: 10.1007/s00167-010-1051-2. [DOI] [PubMed] [Google Scholar]
- 21.Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27:564–568. doi: 10.1016/j.arth.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–130. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 23.Mahadevan D, Walter RP, Minto G, Gale TC, McAllen CJ, Oldman M. Combined femoral and sciatic nerve block vs combined femoral and periarticular infiltration in total knee arthroplasty: a randomized controlled trial. J Arthroplasty. 2012;27:1806–1811. doi: 10.1016/j.arth.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;12:546–552. doi: 10.1016/S0883-5403(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 25.Mullaji A, Kanna R, Shetty GM, Chavda V, Singh DP. Efficacy of periarticular injection of bupivacaine, fentanyl, and methylprednisolone in total knee arthroplasty: a prospective, randomized trial. J Arthroplasty. 2010;25:851–857. doi: 10.1016/j.arth.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Ng F-Y, Chiu K-Y, Yan CH, Ng K-FJ. Continuous femoral nerve block versus patient-controlled analgesia following total knee arthroplasty. J Orthop Surg. 2012;20:23–26. doi: 10.1177/230949901202000105. [DOI] [PubMed] [Google Scholar]
- 27.Ritter MA, Koehler M, Keating EM, Faris PM, Meding JB. Intra-articular morphine and/or bupivacaine after total knee replacement. J Bone Joint Surg Br. 1999;81:301–303. doi: 10.1302/0301-620X.81B2.9110. [DOI] [PubMed] [Google Scholar]
- 28.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S, Iorio R, Specht LM, Davies-Lepie S, Healy WL. Complications of femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res. 2010;468:135–140. doi: 10.1007/s11999-009-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spreng UJ, Dahl V, Hjall A, Fagerland MW, Raeder J. High-volume local infiltration analgesia combined with intravenous or local ketorolac + morphine compared with epidural analgesia after total knee arthroplasty. Br J Anaesth. 2010;105:675–682. doi: 10.1093/bja/aeq232. [DOI] [PubMed] [Google Scholar]
- 31.Tammachote N, Kanitnate S, Manuwong S, Yakumpor T, Panichkul P. Is pain after TKA better with periarticular injection or intrathecal morphine? Clin Orthop Relat Res. 2013;471:1992–1999. doi: 10.1007/s11999-013-2826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishii S. The efficacy of intra-articular analgesia after total knee arthroplasty in patients with rheumatoid arthritis and in patients with osteoarthritis. J Arthroplasty. 2001;16:306–311. doi: 10.1054/arth.2001.21496. [DOI] [PubMed] [Google Scholar]
- 33.The Joint Comission. Facts about pain management. Available at: http://www.jointcommission.org/topics/pain_management.aspx. Accessed September 22, 2013.
- 34.Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop. 2007;78:172–179. doi: 10.1080/17453670710013645. [DOI] [PubMed] [Google Scholar]
- 35.Tripuraneni KR, Woolson ST, Giori NJ. Local infiltration analgesia in TKA patients reduces length of stay and postoperative pain scores. Orthopedics. 2011;34:173. doi: 10.3928/01477447-20110124-11. [DOI] [PubMed] [Google Scholar]
- 36.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 37.Wegener JT, van Ooij B, van Dijk CN, Hollmann MW, Preckel B, Stevens MF. Value of single-injection or continuous sciatic nerve block in addition to a continuous femoral nerve block in patients undergoing total knee arthroplasty: a prospective, randomized, controlled trial. Reg Anesth Pain Med. 2011;36:481–488. doi: 10.1097/AAP.0b013e318228c33a. [DOI] [PubMed] [Google Scholar]
- 38.Yadeau JT, Goytizolo EA, Padgett DE, Liu SS, Mayman DJ, Ranawat AS, Rade MC, Westrich GH. Analgesia after total knee replacement: local infiltration versus epidural combined with a femoral nerve blockade: a prospective, randomised pragmatic trial. Bone Joint J. 2013;95:629–635. doi: 10.1302/0301-620X.95B5.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]