Abstract
Background
Although malnutrition has been hypothesized to increase the risk of periprosthetic joint infection (PJI), strong evidence linking the two is lacking.
Questions/purposes
The purposes of this study were to determine (1) if one or more laboratory values suggestive of malnutrition is independently associated with being revised for an infected joint arthroplasty as opposed to for an aseptic failure; (2) the relationship between laboratory parameters suggestive of malnutrition and obesity; and (3) if one or more laboratory parameters suggestive of malnutrition is independently associated with acute PJI complicating an aseptic revision procedure.
Methods
Between 2002 and 2010, one surgeon performed 600 revision total joint arthroplasties in 547 patients; during that time, nutritional parameters (including serum albumin, total lymphocyte count, and transferrin) were routinely obtained preoperatively; complete data sets were available on 454 patients (501 procedures [84%]). We compared the frequency of having one or more laboratory parameters suggestive of malnutrition between patients undergoing a revision for septic reasons and aseptic reasons as well as between obese and nonobese patients. The 375 aseptic revisions were then assessed for the incidence of acute postoperative infection (within 90 days, diagnosed with Musculoskeletal Infection Society criteria). Multivariate logistic regression modeling was used to evaluate factors independently associated with (1) a septic as opposed to an aseptic mode of failure; and (2) acute postoperative infection after an aseptic revision.
Results
Patients in 67 of 126 (53%) revisions for PJI had one or more laboratory parameters suggestive of malnutrition compared with 123 of 375 (33%) undergoing revision for a noninfectious etiology (odds ratio [OR], 2.3 [95% confidence interval, 1.5–3.5]; p < 0.001). Patients who were of normal weight at the time of revision had the highest frequency of laboratory parameters suggestive of malnutrition (42 of 82 [51%]), although this was common in obese patients as well (76 of 238 [32%]) (p = 0.002). Among the 375 aseptic revisions, 12 developed an acute postoperative infection (3%). The frequency of infection was nine of 123 in the group having one or more laboratory parameters suggestive of malnutrition and three of 252 in the group not having such laboratory parameters (7% versus 1%; p = 0.003). Multivariate regression revealed that having laboratory parameters suggestive of malnutrition is independently associated with both chronic PJI (p = 0.003; OR, 2.1) and an acute postoperative infection complicating an aseptic revision arthroplasty (p = 0.02; OR, 5.9).
Conclusions
Having one or more laboratory parameters suggestive of malnutrition is common among patients undergoing revision arthroplasty and is independently associated with both chronic septic failure and acute postoperative infection complicating a revision performed for a noninfectious etiology. Future studies should assess the impact of a standardized screening protocol with subsequent correction of abnormal laboratory parameters suggestive of malnutrition on the risk of PJI to determine a potential causal relationship between the two.
Level of Evidence
Level III, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Despite advances in interventions to reduce the risk of surgical site infections [1, 7, 15, 27, 40], periprosthetic joint infection (PJI) remains a devastating problem, one that causes a severe burden to patients, the healthcare system, and the economy [2, 3, 8, 25, 26]. In fact, PJI accounts for approximately 15% of all revision THAs [5] and 25% of all revision TKAs [4] with an absolute number of revisions related to PJI that is projected to increase severalfold over the next two decades [25]. Given the tremendous impact of PJI on patients and society alike, optimizing patient-related risk factors for PJI is of paramount importance.
Malnutrition, as defined by abnormal laboratory parameters or anthropometric measurements, has been linked in the orthopaedic literature to postoperative complications after hip and knee arthroplasty, ranging from increased length of stay to impaired wound healing [10, 11, 13, 14, 18, 19, 21, 32, 33]. Furthermore, the reported prevalence of malnutrition among patients undergoing total joint arthroplasty (TJA) has been reported to be as high as 50% [22, 34]. As the population continues to grow increasingly obese, malnutrition may be of even greater importance for patients undergoing TJA and the surgeons who take care of them, because obese patients can be paradoxically malnourished [23, 24, 36] secondary to the consumption of calorie-dense but protein- and nutrient-poor foods [36]. Although malnutrition has been identified as a risk factor for the development of PJI after irrigation and débridement (I&D) to treat persistent postoperative wound drainage [19], previous studies suggesting a link between malnutrition and PJI after a primary or revision TJA [12, 18, 21, 32] have been limited by small sample sizes [12, 21], heterogeneous study populations consisting of both primary and revision TJAs [18, 32], and vague definitions of malnutrition [32]; thus, strong evidence linking PJI after revision TJA remains lacking.
The purposes of our study were to determine (1) if one or more laboratory values suggestive of malnutrition is independently associated with being revised for infected joint arthroplasty failure as opposed to being revised for aseptic failure; (2) the relationship between laboratory parameters suggestive of malnutrition and obesity; and (3) if laboratory parameters suggestive of malnutrition are independently associated with acute PJI complicating an aseptic revision procedure.
Patients and Methods
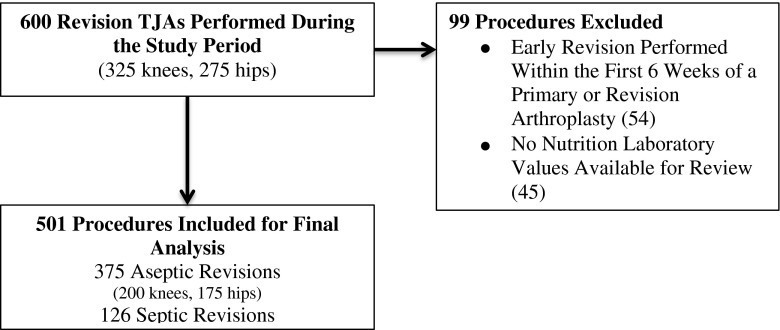
After institutional review board approval, we retrospectively reviewed data on all nonsecond-stage reimplantation revision procedures (600 total in 547 patients; 325 knees and 275 hips) performed by one fellowship-trained orthopaedic surgeon (CJDV) between November 2002 and December 2010 for potential study inclusion. During the study period, patients had a total lymphocyte count, serum albumin, and serum transferrin performed as part of their routine preoperative care. Laboratory parameters suggestive of malnutrition were defined as total lymphocyte count < 1500/mm3, serum albumin < 3.5 g/dL, or serum transferring < 200 mg/dL [10, 19]. Although less robust and precise than that suggested by the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition [38], the most commonly used definition of malnutrition in the orthopaedic literature has been at least one of three abnormally low laboratory values (serum albumin, transferring, or total lymphocyte count) [10]; therefore, for the purposes of this study, we use this same definition for potential malnutrition. Patients were excluded from our analysis if they had an early revision performed within the first 6 weeks after a primary or revision arthroplasty (in the early postoperative period, the laboratory values used to diagnose malnutrition are decreased secondary to postoperative stress and are therefore unreliable for nutritional status assessment) [14, 21, 22] or if no nutrition laboratory values were available for review.
After excluding 99 revision procedures performed in 84 patients, 501 of the original 600 revision procedures (84%) performed in 463 patients were included in our final analysis (Fig. 1): 375 failures were secondary to an aseptic mode of failure (200 knees and 175 hips) and 126 failures were secondary to a deep periprosthetic joint infection (70 knees and 56 hips). The most common reason for revision of the 375 aseptic revisions was aseptic loosening of components (Table 1). The mean age at the time of revision surgery was 65 ± 12 years (range, 31–95 years), and in the study group, 310 revisions were performed in women (62%) and 191 in men (38%).
Fig. 1.
A flowchart shows patients who underwent revision TJA included in the study.
Table 1.
Reasons for revision
| Reason for revision | Number of patients |
|---|---|
| Hip arthroplasties (N = 231) | |
| Aseptic loosening | 83 (36%) |
| Periprosthetic joint infection | 56 (24%) |
| Instability | 39 (17%) |
| Polyethylene wear/osteolysis | 35 (15%) |
| Periprosthetic fracture | 5 (2.2%) |
| Component fracture | 2 (0.9%) |
| Heterotopic ossification | 2 (0.9%) |
| Iliopsoas tendonitis | (0.9%) |
| Liner disassociation | 2 (0.9%) |
| Other | 5 (2.2%) |
| Knee arthroplasties (N = 270)* | |
| Aseptic loosening | 71 (26%) |
| Periprosthetic joint infection | 70 (26%) |
| Instability | 36 (13%) |
| Stiffness | 28 (10%) |
| Failed partial knee arthroplasty | 15 (6%) |
| Polyethylene wear/osteolysis | 13 (4.8%) |
| Extensor mechanism disruption | 10 (3.7%) |
| Patellar maltracking | 6 (2.2%) |
| Component malposition/malrotation | 5 (1.9%) |
| Avascular necrosis of patella | 3 (1.1%) |
| Flexion contracture | 3 (1.1%) |
| Pain | 3 (1.1%) |
| Periprosthetic fracture | 2 (0.7%) |
| Unresurfaced patella | 2 (0.7%) |
| Other | 3 (1.1%) |
* Percentages do not add to 100 as a result of rounding.
Age, sex, insurance type, race, Charlson Comorbidity Index (CCI), and body mass index (BMI) at the time of revision were also reviewed for each patient. Race was coded as “white” or “nonwhite” and insurance was coded as “private” or “nonprivate.” All data were collected by review of a prospective database and patient charts by someone other than the operating surgeon. Each patient was categorized as underweight (BMI < 18.5 kg/m2), normal weight (BMI ≥ 18.5 kg/m2 and < 25 kg/m2), overweight (BMI ≥ 25 kg/m2 and < 30 kg/m2), or obese (BMI ≥ 30 kg/m2) [39].
The patients who underwent an aseptic revision were subsequently reviewed for the presence of PJI presenting within the first 90 days postoperatively. Infection was diagnosed using Musculoskeletal Infection Society criteria [31]. All patients were followed for 90 days postoperatively. No patients were recalled specifically for this study; all data were obtained from medical records.
Statistical Analysis
The prevalence of having one or more laboratory parameters suggestive of malnutrition and demographic variables was compared between the patients who underwent the 375 aseptic revisions and the patients who underwent the 126 septic revisions and between patients who underwent aseptic revision who did and did not have an acute postoperative infection complicating their procedure using Fisher’s exact test and Student’s t-test, as appropriate. The pooled variance t-test was performed unless there was a violation of the homogeneity of variance assumption, in which case the Satterthwaite adjustment method was used. Multivariate logistic regression modeling was used to evaluate independently associated factors for (1) septic as opposed to an aseptic mode of failure; and (2) acute postoperative infection complicating an aseptic revision. Covariates of age, sex, BMI, CCI, race, and insurance type were included in the model to adjust for these effects. A power analysis was performed a priori indicating that a minimum of 36 patients per group would be required to detect a difference in prevalence of having one or more laboratory parameters suggestive of malnutrition of 35% versus 5% with an α value of 0.05 and power of 0.90. These values were chosen based on prior work [19] that showed that the prevalence of potential malnutrition (using the same definition as in our study) at the time of I&D after a THA or TKA was 35% in those who developed subsequent PJI compared with 5% in those who did not. All analysis was performed using SAS® Version 9.1.3 software (SAS Institute Inc, Cary, NC, USA).
Results
The overall prevalence of malnutrition in our study population was 38% with patients in 190 of 501 procedures having one or more laboratory parameters suggestive of malnutrition. Patients in 67 of 126 (53%) revisions for PJI had laboratory parameters suggestive of malnutrition compared with 123 of 375 (33%) undergoing revision for a noninfectious etiology (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.5–3.5; p < 0.001). Demographics of the two groups are presented (Table 2). Multivariate regression analysis revealed that having one or more laboratory parameters suggestive of malnutrition is independently associated with septic revision (p = 0.003; OR, 2.1, 95% CI, 1.294–3.512) along with younger age (p = 0.0445; OR, 0.979; 95% CI, 0.957–1.001), male sex (p = 0.0250; OR, 1.776; 95% CI, 1.075–2.936), and having nonprivate insurance (p = 0.0143; OR, 2.311; 95% CI, 1.182–4.516) (Table 3).
Table 2.
Demographics of patients undergoing revision arthroplasty
| Demographic | Aseptic revision (N = 375) | Septic revision (N = 126) | p value |
|---|---|---|---|
| Age (years)* | 65.3 ± 11.8 (34–87) | 63.8 ± 12.5 (31–95) | 0.2046 |
| Sex (number of patients) | |||
| Male | 132 (35%) | 59 (47%) | 0.0258 |
| Female | 243 (65%) | 67 (53%) | |
| Insurance† | |||
| Nonprivate | 259 (74%) | 97 (84%)†† | 0.0302 |
| Private | 89 (26%) | 18 (16%)†† | |
| Race‡ | |||
| White | 255 (69%) | 95 (77%) | 0.1366 |
| Nonwhite | 113 (31%) | 29 (23%) | |
| CCI*,§ | 1.0 ± 1.5 (0–9) | 1.3 ± 1.7 (0–9) | 0.1468¶ |
| BMI*,|| | 32.3 ± 8.2 (18–51) | 33.6 ± 8.7 (20–64) | 0.2021 |
| Nutrition status | |||
| Laboratory parameters suggestive of malnutrition | 123 (33%) | 67 (53%) | < 0.001 |
| Normal nutrition laboratory values | 252 (67%) | 59 (47%) | |
| Paradoxical “malnutrition”** | |||
| Yes | 52 (29%) | 24 (40%) | 0.1494 |
| No | 126 (71%) | 36 (60%) | |
* Values are expressed as mean ± SD with range in parentheses; †data missing for 38 patients; ‡data missing for nine patients; §data missing for four patients; ||data missing for 80 patients; ¶required Satterthwaite approximation; **the presence of one or more laboratory parameters suggestive of malnutrition among those who were obese; ††percentages do not add to 100 as a result of rounding; CCI = Charlson Comorbidity Index; BMI = body mass index.
Table 3.
Potentially associated factors in multivariate analysis for chronic septic failure
| Variable | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age (younger age worse) | 0.979 | 0.957–1.001 | 0.0445 |
| Sex (male worse) | 1.776 | 1.075–2.936 | 0.025 |
| Body mass index | 1.021 | 0.992–1.051 | 0.1547 |
| Charlson Comorbidity Index | 0.938 | 0.793–1.110 | 0.4554 |
| Insurance type (nonprivate worse) | 2.311 | 1.182–4.516 | 0.0143 |
| Race (white versus nonwhite) | 1.563 | 0.872–2.800 | 0.1335 |
| Laboratory parameters suggestive of malnutrition (yes versus no) | 2.131 | 1.294–3.512 | 0.003 |
The presence of one or more laboratory parameters suggestive of malnutrition was most common among patients of normal weight at the time of revision compared with overweight patients (p = 0.012; OR, 2.8; 95% CI, 1.5–5.2) and obese patients (p = 0.0023; OR, 2.2; 95% CI, 1.3–3.7) (Table 4). Paradoxical “malnutrition” (obese but having laboratory parameters suggestive of malnutrition) was common in both the aseptic and septic failure groups (41% and 29%; p = 0.1494). Demographic characteristics of patients with laboratory parameters suggestive of malnutrition are presented (Table 5).
Table 4.
Laboratory parameters suggestive of malnutrition status by body mass index
| Variable of interest | Underweight | Normal weight | Overweight | Obese |
|---|---|---|---|---|
| Total number of patients* | 2 | 82 | 99 | 238 |
| Number with laboratory parameters suggestive of malnutrition | 1 (50%) | 42 (51%) | 27 (27%) | 76 (32%) |
| p value (normal weight versus others) | 1 | – | 0.0012 | 0.0023 |
* Data were missing for 80 patients; underweight = body mass index (BMI) ≤ 18.5 kg/m2; normal weight = BMI ≥ 18.5 kg/m2 and ≤ 25 kg/m2; overweight = BMI ≥ 25 kg/m2 and ≤ 30 kg/m2; obese = BMI ≥ 30 kg/m2.
Table 5.
Demographics based on nutritional status
| Demographic | Normal laboratory parameters (N = 311) | Laboratory parameters suggestive of malnutrition (N = 190) | p value |
|---|---|---|---|
| Age (years)* | 63.7 ± 11.8 (31–95) | 67.0 ± 11.9 (34–88) | 0.0025 |
| Sex (number of patients) | |||
| Male | 109 (35%) | 82 (43%) | 0.0725 |
| Female | 202 (65%) | 108 (57%) | |
| Insurance† | |||
| Nonprivate | 204 (71%) | 152 (86%) | < 0.001 |
| Private | 83 (29%) | 24 (14%) | |
| Race‡ | |||
| White | 210 (68%) | 140 (76%) | 0.1002 |
| Nonwhite | 97 (32%) | 45 (24%) | |
| CCI*,§ | 0.89 ± 1.2 (0–9) | 1.5 ± 2.0 (0–9) | < 0.001 |
| BMI*,|| | 32.9 ± 7.6 (18–55) | 32.1 ± 9.5 (18–64) | 0.4312¶ |
* Values are expressed as mean ± SD with range in parentheses; †data missing for 38 patients; ‡data missing for nine patients; §data missing for four patients; ||data missing for 80 patients; ¶required Satterthwaite approximation; CCI = Charlson Comorbidity Index; BMI = body mass index.
We found that having one or more laboratory parameters suggestive of malnutrition is independently associated with an acute postoperative infection complicating an aseptic revision arthroplasty (p = 0.02; OR, 5.9; 95% CI, 1.317–26.057) (Table 6). Of the 375 aseptic revisions, 12 developed an acute postoperative infection within the first 90 days (3%). The frequency of infection was nine of 123 in the group having laboratory parameters suggestive of malnutrition and three of 252 in the group having normal laboratory parameters (7% versus 1%; p = 0.003). Age was also independently associated with each additional year of age decreasing the odds of acute infection by 8%.
Table 6.
Potentially associated factors in multivariate analysis for acute periprosthetic joint infection complicating aseptic revision arthroplasty
| Variable | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age (younger worse) | 0.923 | 0.864–0.987 | 0.0189 |
| Sex (male versus female) | 2.351 | 0.541–10.407 | 0.26 |
| Body mass index | 0.935 | 0.846–1.033 | 0.1873 |
| Charlson Comorbidity Index | 0.610 | 0.290–1.284 | 0.1928 |
| Insurance type (nonprivate versus private) |
8.982 | 0.808–99.87 | 0.074 |
| Race (white versus nonwhite) | 5.365 | 0.567–50.793 | 0.143 |
| Laboratory parameters suggestive of malnutrition (yes versus no) | 5.858 | 1.317–26.057 | 0.0202 |
Discussion
Although having one or more laboratory parameters suggestive of malnutrition is commonly cited as increasing susceptibility to PJI after hip and knee arthroplasty, direct data confirming this link have been lacking. Hence, we sought to assess whether having laboratory parameters suggestive of malnutrition was independently associated with septic as opposed to a noninfectious mode of failure in a series of patients undergoing revision TJA, the relationship of having laboratory parameters suggestive of malnutrition to obesity, and if having laboratory parameters suggestive of malnutrition predisposed to an acute postoperative infection complicating an aseptic revision procedure. Our results suggest that the presence of one or more laboratory parameters suggestive of malnutrition is common among both obese and normal-weight patients, and in our data set, it was independently associated with both chronic PJI and the development of an acute postoperative infection after an aseptic revision.
We note several limitations of our study. First, this is a retrospective study and selection bias is a concern, because we excluded several potential patients who did not have malnutrition laboratory values available for review; additionally, several patients lacked demographic data points such as BMI and insurance type and had to be dropped in the multivariate analysis. However, because the number of patients with missing data was a small proportion of the total study population and our prevalence of having one or more laboratory parameters suggestive of malnutrition is consistent with previous estimates in patients undergoing TJA [22, 34], we believe that these missing data did not substantially bias our results. Furthermore, although a prospective study is ideal, the rarity of PJI makes prospectively studying the topic difficult. Our series was from an urban tertiary referral center that specializes in the treatment of complex and infected revisions, calling into question the generalizability of our results and raising potential bias toward infected and complex cases that are more prone to infection. However, in our multivariate analysis, we attempted to adjust for these potential confounders such as age and medical comorbidities. Fourth, our sample size is relatively small with only 12 acute infections noted in our study. Nevertheless, our sample size was large enough to identify the association. Finally, although serum biochemical markers are good indicators of nutritional status, they can be affected by chronic and acute diseases associated with inflammation and stress [12, 16, 20, 28]. Although we attempted to account for such potential confounders by multivariate analysis taking into account patient comorbidities, the possibility remains that the presence of low malnutrition laboratory values does not indicate malnutrition in all patients (serum albumin has been shown to not consistently change with caloric deprivation or weight loss [38]) and may, in fact, be more reflective of an inflammatory response than poor nutritional status [38]. Furthermore, it is a possibility that chronic PJI could theoretically cause laboratory abnormalities suggestive of malnutrition; because our study was purely observational, we are unable to comment on the existence of a causal relationship between potential malnutrition and PJI nor on the direction of such a relationship if it exists. Our definition of potential malnutrition is thus limited and should be interpreted in light of these considerations.
In our series of 501 revision TJAs, the prevalence of having one or more laboratory parameters suggestive of malnutrition was 38%, which is consistent with previous reports’ prevalence of malnutrition ranging from 8.5% to 50% for patients undergoing both primary and revision TJA [14, 18, 21, 22, 34, 35]. Our findings may be reflective of society as a whole; the general hospital and elderly populations have estimated malnutrition prevalence rates of 11% to 44% and 29% to 61%, respectively [9, 17]. Patients undergoing septic revision for chronic PJI were more likely to have laboratory parameters suggestive of malnutrition than those undergoing aseptic revision. Furthermore, after accounting for demographic variables and comorbidity index, the presence of laboratory parameters suggestive of malnutrition remained independently associated with septic revision, suggesting a potential link between the presence of laboratory parameters suggestive of malnutrition and the development of chronic PJI. In both a retrospective study of 6489 primary and revision TKAs [32] and a prospective study of 213 TKAs [12], preoperative malnutrition increased the risk of postoperative infection. However, the results of these studies are difficult to interpret, because both studies grouped deep and superficial infections as the infectious outcome variable. Furthermore, the study of 6489 TKAs [32] did not define what constituted malnutrition. The prospective study of 213 TKAs [12], on the other hand, defined malnutrition as decreased triceps skin fold, which is an indirect measure of malnutrition. Recently, a prospective study of 2161 patients undergoing elective primary or revision TJA (grouped together) found that malnutrition was more common among patients who developed PJI compared with those who did not [18]; however, in multivariate analysis, malnutrition was not shown to be an independent risk factor for PJI, suggesting that other confounding variables may have influenced their finding. We also found that patients undergoing septic revision were more likely to be male and to have nonprivate insurance, which is consistent with previous studies’ findings that male sex [6, 29] and lower socioeconomic status are risk factors for PJI [30, 37].
Interestingly, many patients were both obese and had one or more laboratory parameters suggestive of malnutrition in both the septic and aseptic groups, demonstrating a paradoxical “malnutrition” (laboratory parameters suggestive of malnutrition in an obese patient). Although it may seem logical that obese patients, by virtue of having caloric excess, would be adequately nourished (or even overnourished), our data show that the relationship between laboratory parameters suggestive of malnutrition and obesity is not so straightforward. In fact, our results suggest the opposite, that obese patients frequently have laboratory parameters suggestive of malnutrition, perhaps secondary to the consumption of calorie-dense, nutrient-poor foods [36]. This finding is consistent with previous observations that obese patients are frequently paradoxically malnourished [23, 24, 36] and confirms previous findings from a recently published study on the impact of malnutrition on TJA outcomes [18]. Nevertheless, normal-weight patients were more likely to have laboratory parameters suggestive of malnutrition than those who were overweight or obese; hence, we believe this indicates that laboratory parameters suggestive of malnutrition are a potential problem in patients of any size or weight.
The most concerning finding in our study, however, was the strong relationship between the presence of one or more laboratory parameters suggestive of malnutrition and the incidence of an acute postoperative infection complicating an aseptic revision with an OR of almost 6. In a somewhat more complex clinical scenario, one retrospective study showed that malnutrition, using the same definition as the one used in this study, is a risk factor for the failure (ie, subsequent progression to PJI) of a persistently draining wound treated with I&D [19]. This suggests that surgeons may wish to consider delaying an elective revision if potential malnutrition is identified by way of one or more laboratory parameters. However, we must again highlight that our study was observational in nature and, as such, unfortunately cannot provide insight into causality between potential malnutrition and infection risk; such a relationship remains unknown. Furthermore, whether infection may cause these laboratory abnormalities or vice versa remains indeterminate as well.
In summary, our study suggests that having one or more laboratory parameters suggestive of malnutrition is common among patients undergoing revision arthroplasty and is independently associated with both chronic septic failure and acute postoperative infection complicating revisions performed for a noninfectious etiology. Nevertheless, a direct causal effect of malnutrition on PJI remains unclear. A prospective study implementing a standardized protocol of malnutrition screening and subsequent correction as needed would be ideal to assess such a potential relationship. Encouragingly, efforts for such a study are reportedly already underway [18].
Acknowledgments
We thank Laura Quigley for her assistance in maintaining our institution’s total joint arthroplasty database.
Footnotes
One of the authors certifies that he (CJDV), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Biomet, Inc (Warsaw, IN, USA), an amount less than USD 10,000 from Convatec (Skillman, NJ, USA), an amount of USD 10,000 to USD 100,000 from DePuy (Warsaw, IN, USA), an amount of USD 10,000 to USD 100,000 from Smith & Nephew, Inc (Memphis, TN, USA), and an amount of USD 10,000 to USD 100,000 from CD Diagnostics (Wynnewood, PA, USA). One of the authors certifies that he (CJDV), or a member of his or her immediate family, has or may receive funding, during the study period, an amount USD 10,000 to USD 100,000 from Stryker (Kalamazoo, MI, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94:42–46. doi: 10.1302/0301-620X.94B11.30833. [DOI] [PubMed] [Google Scholar]
- 2.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boettner F, Cross MB, Nam D, Kluthe T, Schulte M, Goetze C. Functional and emotional results differ after aseptic vs septic revision hip arthroplasty. HSS J. 2011;7:235–238. doi: 10.1007/s11420-011-9211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Ward DT, Lau EC, Chan V, Wetters NG, Naziri Q, Odum S, Fehring TK, Mont MA, Gioe TJ, Della Valle CJ. Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a case control study. J Arthroplasty. 2014;29:154–156. doi: 10.1016/j.arth.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27:27–30. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong). 2008;16:58–65. doi: 10.1177/230949900801600115. [DOI] [PubMed] [Google Scholar]
- 9.Corish CA, Kennedy NP. Protein-energy undernutrition in hospital in-patients. Br J Nutr. 2000;83:575–591. doi: 10.1017/S000711450000074X. [DOI] [PubMed] [Google Scholar]
- 10.Cross MB, Yi PH, Thomas CF, Garcia J, Della Valle CJ. Evaluation of malnutrition in orthopaedic surgery. J Am Acad Orthop Surg. 2014;22:193–199. doi: 10.5435/JAAOS-22-03-193. [DOI] [PubMed] [Google Scholar]
- 11.Del Savio GC, Zelicof SB, Wexler LM, Byrne DW, Reddy PD, Fish D, Ende KA. Preoperative nutritional status and outcome of elective total hip replacement. Clin Orthop Relat Res. 1996;326:153–161. doi: 10.1097/00003086-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Font-Vizcarra L, Lozano L, Ríos J, Forga MT, Soriano A. Preoperative nutritional status and post-operative infection in total knee replacements: a prospective study of 213 patients. Int J Artif Organs. 2011;34:876–881. doi: 10.5301/ijao.5000025. [DOI] [PubMed] [Google Scholar]
- 13.Gherini S, Vaughn BK, Lombardi AV, Jr, Mallory TH. Delayed wound healing and nutritional deficiencies after total hip arthroplasty. Clin Orthop Relat Res. 1993;293:188–195. [PubMed] [Google Scholar]
- 14.Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6:321–325. doi: 10.1016/S0883-5403(06)80183-X. [DOI] [PubMed] [Google Scholar]
- 15.Hanssen AD, Osmon DR, Nelson CL. Prevention of deep periprosthetic joint infection. Instr Course Lect. 1997;46:555–567. [PubMed] [Google Scholar]
- 16.Hedström M, Ljungqvist O, Cederholm T. Metabolism and catabolism in hip fracture patients: nutritional and anabolic intervention—a review. Acta Orthop. 2006;77:741–747. doi: 10.1080/17453670610012926. [DOI] [PubMed] [Google Scholar]
- 17.Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82:2–8. doi: 10.1136/pgmj.2005.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang R, Greenky M, Kerr GJ, Austin MS, Parvizi J. The effect of malnutrition on patients undergoing elective joint arthroplasty. J Arthroplasty. 2013;28:21–24. doi: 10.1016/j.arth.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466:1368–1371. doi: 10.1007/s11999-008-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeejeebhoy KN. Nutritional assessment. Nutrition. 2000;16:585–590. doi: 10.1016/S0899-9007(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JE, Jensen TG, Smith TK, Johnston DA, Dudrick SJ. Nutrition in orthopaedic surgery. J Bone Joint Surg Am. 1982;64:1263–1272. [PubMed] [Google Scholar]
- 22.Jensen JE, Smith TK, Jensen TG, Dudrick SJ, Butler JE, Johnston DA. The Frank Stinchfield Award Paper. Nutritional assessment of orthopaedic patients undergoing total hip replacement surgery. Hip. 1981:123–135. [PubMed]
- 23.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 24.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008;18:1028–1034. doi: 10.1007/s11695-007-9350-5. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Kakis A, Nichols A, Ries MD, Vail TP, Bozic KJ. Targeted use of vancomycin as perioperative prophylaxis reduces periprosthetic joint infection in revision TKA. Clin Orthop Relat Res. 2014;472:227–231. doi: 10.1007/s11999-013-3029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Moriyama Y, Kariyazono H, Hamada N, Toyohira H, Taira A, Yamada K. Influence of preoperative nutritional state on inflammatory response after surgery. Nutrition. 1999;15:834–841. doi: 10.1016/S0899-9007(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 29.Namba RS, Inacio MCS, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95:775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 30.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105–109. doi: 10.1016/j.arth.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Parvizi J, Zmistowski B, Bauer TW, Berbari EF, Springer BD, Della Valle CJ, Garvin KL, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Pratt WB, Veitch JM, McRoberts RL. Nutritional status of orthopedic patients with surgical complications. Clin Orthop Relat Res. 1981;155:81–84. [PubMed] [Google Scholar]
- 34.Rai J, Gill SS, Kumar BR. The influence of preoperative nutritional status in wound healing after replacement arthroplasty. Orthopedics. 2002;25:417–421. doi: 10.3928/0147-7447-20020401-17. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzkopf R, Russell TA, Shea M, Slover JD. Correlation between nutritional status and Staphylococcus colonization in hip and knee replacement patients. Bull NYU Hosp Jt Dis. 2011;69:308–311. [PubMed] [Google Scholar]
- 36.Via M. The malnutrition of obesity: micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012;2012:103472. doi: 10.5402/2012/103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb BG, Lichtman DM, Wagner RA. Risk factors in total joint arthroplasty: comparison of infection rates in patients with different socioeconomic backgrounds. Orthopedics. 2008;31:445. doi: 10.3928/01477447-20080801-36. [DOI] [PubMed] [Google Scholar]
- 38.White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112:730–738. [DOI] [PubMed]
- 39.World Health Organization. Global Database on Body Mass Index: BMI Classification. Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed April 19, 2014.
- 40.Young SW, Zhang M, Freeman JT, Mutu-Grigg J, Pavlou P, Moore GA. The Mark Coventry Award: Higher tissue concentrations of vancomycin with low-dose intraosseous regional versus systemic prophylaxis in TKA: a randomized trial. Clin Orthop Relat Res. 2014;472:57–65. doi: 10.1007/s11999-013-3038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]