Abstract
Background
Posterior spinal fusion (PSF) is commonly performed for patients with adolescent idiopathic scoliosis (AIS). Identifying factors associated with perioperative morbidity and PSF may lead to strategies for reducing the frequency of adverse events (AEs) in patients and total hospital costs.
Questions/purposes
What is the frequency of and what factors are associated with postoperative: (1) AEs, (2) extended length of stay (LOS), and (3) readmission in patients with AIS undergoing PSF?
Patients and Methods
Patients, aged 11 to 18 years, who underwent PSF for AIS during 2012, were identified from the American College of Surgeons National Surgical Quality Improvement Program® (ACS NSQIP®) Pediatric database. Patient were assessed for characteristics associated with AEs, extended LOS (defined as more than 6 days), and hospital readmission using multivariate logistic regression. Individual AEs captured in the database were grouped into two categories, “any adverse event” (AAE) and “severe adverse events” (SAEs) for analysis. A total of 733 patients met inclusion criteria.
Results
Twenty-seven patients (3.7%) had AAE and 19 patients (2.6%) had SAEs. Both AAE and SAEs were associated with BMI-for-age ninety-fifth percentile or greater (AAE: odds ratio [OR], 3.31; 95% CI, 1.43–7.65; p = 0.005. SAE: OR, 3.46; 95% CI, 1.32–9.09; p = 0.012). Extended LOS occurred for 60 patients (8.2%) and was associated with greater than 13 levels instrumented (OR, 2.00; 95% CI, 1.11–3.61; p = 0.021) and operative time of 365 minutes or more (OR, 2.57; 95% CI, 1.39–4.76; p = 0.003). Readmission occurred for 11 patients (1.5%), most often for surgical site infection, and was associated with the occurrence of any complication during the initial hospital stay (OR, 180.44; 95% CI, 35.47–917.97; p < 0.001).
Conclusions
Further research on prevention and management of obesity and surgical site infections may reduce perioperative morbidity for patients with AIS undergoing PSF.
Level of Evidence
Level III, prognostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Adolescent idiopathic scoliosis (AIS) is a common disorder with an overall prevalence of 0.5% to 5.2%, according to the literature [22]. During the past few decades, an increased proportion of patients have undergone multilevel posterior spinal fusion (PSF) for correction of AIS [6]. These are large, invasive operations, and careful patient (and parent) counseling is important before performing surgery.
Currently, there is inadequate information regarding which patient factors are associated with the occurrence of adverse events (AEs), prolonged length of stay (LOS), and readmission after PSF for AIS. Such factors are not only important clinically and for setting appropriate patient expectations, but they also are closely tied to total hospital costs associated with the procedure [30]. The information we have on risk factors for AEs in patients undergoing PSF have been derived mainly from studies that either were limited by small sample size [21, 41, 44, 54], only studied one variable [2, 16, 25, 29, 31, 42, 44, 45, 50, 55], included patients with neuromuscular scoliosis and cerebral palsy [5, 19, 20, 24, 32, 43], used potentially flawed administratively coded or self-reported data [5, 7, 35, 51], or included adult patients with deformity [35].
We therefore sought to address some of these shortcomings in our knowledge by querying a large, high-quality, national database about patients undergoing PSF for AIS to determine the frequency of, and factors associated with: (1) AEs, (2) extended LOS, and (3) readmission after surgery.
Patients and Methods
We conducted a retrospective cohort study using the newly developed American College of Surgeons National Surgical Quality Improvement Program® (ACS NSQIP®) Pediatric database [1, 38], which is a high-quality, national dataset that uses trained clinical reviewers to prospectively collect data on pediatric surgical patients from 50 US hospitals (Appendix 1).
Following institutional review board approval, the ACS NSQIP® Pediatric database from 2012 was queried to identify patients 11 to 18 years old who underwent PSF for AIS. Patients initially were selected with the postoperative diagnosis of AIS (ICD-9 737.30). From the initially selected patients, only those with the primary Current Procedural Terminology (CPT) codes for posterior fusion for deformity (22800 [1–6 levels], 22802 [7–12 levels], and 22804 [13 or more levels]) were included in our study.
Patients undergoing anterior spinal fusion, revision surgery, other spinal procedures, and patients with previous evidence of infection were excluded from our analysis. In addition, patients with cerebral palsy, other neuromuscular disorders, an American Society of Anesthesiologists classification of 3 or greater (indicating severe systemic disease), or any comorbidity that would suggest nonidiopathic scoliosis were excluded from our study. For example, patients with a history of cardiac disease were excluded from the study, as congenital heart disease is strongly associated with development of nonidiopathic scoliosis [18]. Patients also were excluded if they had missing perioperative data. Of an initial 1181 patients identified by ICD-9 and CPT codes, 733 (62.1%) were included in our study.
The ACS NSQIP® Pediatric database collects numerous demographic and comorbidity variables for each patient, including age, sex, height, and weight. Patient BMI is calculated by height and weight and categorized according to CDC BMI-for-age charts [8]. Per CDC guidelines, pediatric patients with less than the fifth percentile BMI-for-age are categorized as underweight; patients with BMI-for-age greater than the eighty-fifth percentile are categorized as overweight; and patients with BMI-for-age greater than the ninety-fifth percentile are categorized as obese (Appendix 2) [3, 4, 14]. Of the comorbidities collected by the ACS NSQIP®, only a history of asthma occurred with sufficient frequency to be used for our analysis. CPT codes 22210, 22212, and 22214 were used to determine if an osteotomy was performed. Operative time was defined as the time in minutes from opening incision to end-of-wound closure. For analysis, operative time was dichotomized at one SD greater than the mean operative time.
Demographics of Study Population
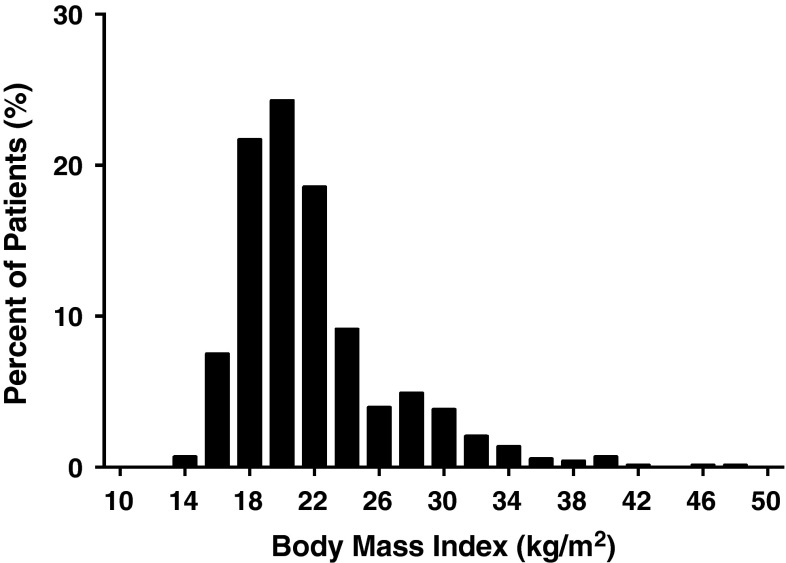
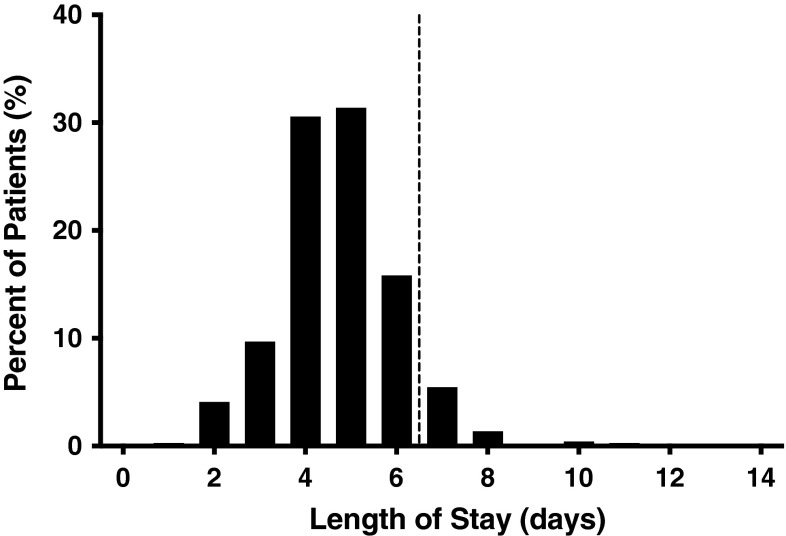
Of the 733 patients who met inclusion criteria, average age was 14.0 ± 1.7 years (mean ± SD). The cohort was 81.6% female (Table 1). The BMI distribution was right-skewed, with the majority of patients having a low-to-normal BMI (mean BMI of 21.8 ± 4.8 kg/m2), with a long tail of patients with greater BMI (Fig. 1). Other comorbidities with frequencies less than 1% are shown (Table 2). The average operative time was 275 ± 90 minutes (mean ± SD), and the average LOS was 5.0 ± 4.8 days (mean ± SD) (Fig. 2). Although the postoperative LOS for some patients was shorter than that typically seen at many institutions, certain centers have accelerated discharge pathways that allow patients to be discharged in as few as 2 days after surgery [13].
Table 1.
Study patient and operative characteristics
| Characteristic | Number | Percentage (%) |
|---|---|---|
| Overall | 733 | 100 |
| Age (years) | ||
| 11–13 | 302 | 41.2 |
| 14–15 | 258 | 35.2 |
| 16–18 | 173 | 23.6 |
| Female sex | 598 | 81.6 |
| BMI-for-age | ||
| < 5th percentile (underweight) | 14 | 1.9 |
| 5th–85th percentile | 495 | 67.5 |
| 85th–95th percentile (overweight) | 96 | 13.1 |
| ≥ 95th percentile (obese) | 128 | 17.5 |
| History of asthma | 41 | 5.6 |
| Number of levels fused | ||
| 1–6 | 39 | 5.3 |
| 7–12 | 542 | 73.9 |
| ≥ 13 | 152 | 20.7 |
| Osteotomy performed | 253 | 34.5 |
| Operative time ≥ 365 minutes | 108 | 14.7 |
Fig. 1.
Distribution of BMI for study patient population is shown.
Table 2.
Frequency of other comorbidities in the patient population
| Comorbidity | Number | Percentage |
|---|---|---|
| Additional comorbidities* | ||
| Steroid use | 3 | 0.4 |
| Bleeding disorder | 3 | 0.4 |
| Hematologic disorder | 3 | 0.4 |
| Diabetes | 1 | 0.1 |
| Premature birth | 1 | 0.1 |
| Immune disorder | 1 | 0.1 |
| Patients excluded† | ||
| Neuromuscular disorder | 195 | |
| ASA classification ≥ 3 | 94 | |
| Age younger than 11 years | 53 | |
| Incomplete/missing data | 51 | |
| Impaired cognitive status | 47 | |
| History of cardiac disease | 40 | |
| Seizure disorder | 7 | |
| Preexisting infection | 7 | |
| Malignancy | 5 | |
| Tracheostomy | 3 | |
| Cerebrovascular accident | 3 | |
| On ventilator | 2 | |
| Central nervous system tumor | 2 | |
| Organ transplant | 2 | |
| Oxygen support | 1 | |
| Marrow transplant | 1 | |
| Chemotherapy | 1 | |
ASA = American Society of Anesthesiologists
* Patients with these comorbidities were included in the study but the comorbidities were not analyzed separately because of their low incidence
†Patients with these comorbidities were excluded as these comorbidities suggested nonidiopathic scoliosis
Fig. 2.
Postoperative LOS* after PSF in patients with AIS is shown. *Extended LOS defined as > 90th percentile LOS or 6 days. Patients to the right of the dashed vertical line had extended LOS.
Adverse Events
The ACS NSQIP® Pediatric database tracks patients for the occurrence of individual AEs occurring during the first 30 postoperative days. A severe AE (SAE) was defined as: death, coma more than 24 hours, on ventilator more than 48 hours, stroke or cerebrovascular accident, thromboembolic event (pulmonary embolism or deep vein thrombosis), cardiac arrest requiring cardiopulmonary resuscitation, renal failure, sepsis, return to the operating room, wound dehiscence, deep surgical site infection, organ or space infection, nerve injury (spinal cord, nerve root, or peripheral nerve injury), or graft, prosthesis, or flap failure. A minor AE was defined as: superficial surgical site infection, urinary tract infection, pneumonia, and progressive renal insufficiency. Any AE (AAE) was defined as the occurrence of a severe or minor AE.
LOS
LOS is defined in the ACS NSQIP® Pediatric database as calendar days from patient surgery to hospital discharge. An extended LOS is defined as an LOS greater than the ninetieth percentile LOS. The ninetieth percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons, while still capturing patients with abnormally extended LOS. During the period of time covered by our database inquiry, an LOS greater than 6 days met the definition for “extended” LOS.
Readmission
The ACS NSQIP® Pediatric database also collects data on readmissions during the first 30 postoperative days. For our study, readmission was defined as positive when a patient had an unplanned readmission at least once after surgery.
Suspected reasons for readmission and the total days from the surgery to readmission also were collected. Suspected reasons for readmission are entered in the database as either a category (such as superficial surgical site infection) or specified by an ICD-9 diagnosis code. The number of days between discharge and readmission was calculated by subtracting each patient’s LOS from the days between surgery and readmission.
Statistical Analysis
Statistical analyses were conducted using Stata® version 11.2 (StataCorp LP, College Station, Texas, USA). Patient and operative characteristics were tested for association with the binary outcome variables AAE, SAE, extended LOS, and readmission using bivariate logistic regression (Appendix 3). Multivariate logistic regressions were performed for each outcome variable using a backward stepwise process that initially included all patient and operative variables, and sequentially excluded variables with the highest p value until only those with a p value less than 0.200 remained. Variables with a p value between 0.050 and 0.200 were left in the model to control for potential confounding but were not considered to be statistically significant. Only variables with a p value less than 0.05 were considered statistically significant. Extended LOS and the occurrence of any complication during the initial patient admission for PSF were assessed as independent variables in the readmission analysis because the information was available at the time of discharge and would be useful to include in a model that predicts odds of readmission. The fitness of the final logistic regression models was assessed using the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
AEs
Twenty-seven of 733 patients (3.7%) had AAEs and 19 of 733 (2.6%) had an SAE (Table 3). Nervous injury occurred in 3 patients (0.41% of all patients), and the rate of nervous injury in patients receiving an osteotomy (1 of 253 patients [0.40%]) was similar to the rate of nervous injury in patients who did not receive an osteotomy (2 of 480 patients [0.42%]). Fourteen of the 27 patients (51.8%) had AEs during initial hospital stay, and of the fourteen patients, seven (50.0%) had surgical site infections, four (28.6%) had urinary tract infection, two (14.3%) had nervous injury, two (14.3%) had wound dehiscence, and one (7.1%) had pneumonia. Using multivariate logistic regression, which controlled for the influence of likely confounding variables (Table 4), BMI-for-age in the 95th or greater percentile continued to be the only factor independently associated with the occurrence of any adverse event (odds ratio [OR]; 3.31; 95% CI, 1.43–7.65; p = 0.005) and severe adverse events (OR, 3.46; 95% CI, 1.32–9.09; p = 0.012).
Table 3.
Frequency of outcomes in the patient population
| Outcome | Number | Percentage (%) |
|---|---|---|
| Any adverse event | 27 | 3.7 |
| Serious adverse event | 19 | 2.6 |
| Death | 0 | 0.0 |
| Coma greater than 24 hours | 0 | 0.0 |
| Unplanned intubation | 0 | 0.0 |
| Stroke/cerebrovascular accident | 0 | 0.0 |
| Thromboembolic event (DVT/PE) | 0 | 0.0 |
| Cardiac arrest requiring CPR | 0 | 0.0 |
| Acute renal failure | 0 | 0.0 |
| Sepsis | 0 | 0.0 |
| Return to the operating room | 13 | 1.8 |
| Wound dehiscence | 4 | 0.6 |
| Deep surgical site infection | 4 | 0.6 |
| Organ space infection | 0 | 0.0 |
| Central line associated blood stream infection | 0 | 0.0 |
| Seizure | 0 | 0.0 |
| Nervous injury | 3 | 0.4 |
| Graft/prosthesis/flap failure | 0 | 0.0 |
| Minor adverse event | 12 | 1.6 |
| Superficial surgical site infection | 7 | 1.0 |
| Urinary tract infection | 5 | 0.7 |
| Pneumonia | 1 | 0.1 |
| Progressive renal insufficiency | 0 | 0.0 |
| Length of stay more than 6 days | 60 | 8.2 |
| Readmitted | 11 | 1.5 |
DVT = deep vein thrombosis; PE = pulmonary embolism; CPR = cardiopulmonary resuscitation
Table 4.
Results of multivariate analyses
| Outcome/associated factor | Odds ratio (95% CI) | p value |
|---|---|---|
| Any adverse event | ||
| BMI-for-age ≥ 95th percentile versus 5th–95th percentiles | 3.31 (1.43–7.65) | 0.005 |
| Serious adverse event | ||
| BMI-for-age ≥ 95th percentile versus 5th–95th percentiles | 3.46 (1.32–9.09) | 0.012 |
| Length of stay more than 6 days | ||
| ≥ 13 levels instrumented versus 7–12 levels | 2.00 (1.11–3.61) | 0.021 |
| Operative time ≥ 365 minutes | 2.57 (1.39–4.76) | 0.003 |
| Readmission | ||
| Any inpatient complication | 180.44 (35.47–917.97) | < 0.001 |
LOS
Sixty patients had extended LOS (defined as LOS greater than ninetieth percentile or more than 6 days in our cohort). In the multivariate model, 13 or more levels instrumented (OR, 2.00; 95% CI, 1.11–3.61; p = 0.021) and operative time of 365 minutes or more (OR, 2.57; 95% CI, 1.39–4.76; p = 0.003) were found to be independently associated with extended LOS (Table 4).
Readmission
Eleven of 733 patients (1.5%) were readmitted within 30 days of undergoing PSF. On multivariate analysis (Table 4), the occurrence of any inpatient complication was the only variable associated with readmission (OR, 180.44; 95% CI, 35.47–917.97; p < 0.001). The most common cause of readmission was surgical site infection (Table 5). Readmissions for surgical site infections occurred, on average (mean ± SD = 16.8 ± 3.0 days), approximately 2 weeks after discharge. Three of 11 patients (27%) who were readmitted did not have a suspected reason for readmission reported in the database. The average time from discharge to readmission was 10.1 ± 6.3 days (mean ± SD) (Table 5).
Table 5.
Reasons for readmission within 30 days
| Reason for readmission | Number | Percentage (%) | Days from discharge to readmission (mean ± SD) |
|---|---|---|---|
| Total patients readmitted | 11 | 100.0 | 10.1 ± 6.3 |
| Surgical site infection | 4 | 36.4 | 16.8 ± 3.0 |
| Superficial incisional surgical site infection (n = 2) | |||
| Deep incisional surgical site infection (n = 2) | |||
| Wound disruption | 2 | 18.2 | 10.0 ± 4.2 |
| Ileus/gastrointestinal complaints | 2 | 18.2 | 5.5 ± 3.5 |
| Unspecified | 3 | 27.3 | 4.3 ± 3.5 |
Each multivariate logistic regression model was subsequently assessed using the C statistic and the Hosmer-Lemeshow goodness-of-fit test. The C statistic of the final multivariate model for readmission (0.87) indicated a very good distinguishing capacity, while the distinguishing ability of each multivariate model of AAE, SAEs, and extended LOS was more moderate (C statistic range, 0.68–0.72). Hosmer-Lemeshow goodness-of-fit tests showed no evidence of a lack of fit for the multivariate models for any AE, SAE, extended LOS, or readmission, indicating that the predicted event rates from each model did not differ significantly from the observed event rates.
Discussion
Posterior fusion for patients with AIS is a common pediatric orthopaedic procedure, yet there is incomplete information regarding factors associated with short-term outcomes after the surgery [2, 5–7, 16, 19–22, 24, 29, 32, 35, 41–44, 50, 51, 54, 55]. We identified patient and operative characteristics associated with the occurrence of AEs, extended LOS, and readmission using multivariate logistic regression to control for potentially confounding variables. We found that obesity (BMI-for-age ≥ 95th percentile) was associated with increased rates of AAEs and SAEs; an increased number of levels instrumented and increased operative time were associated with LOS more than 6 days, and any inpatient complication was associated with readmission.
Our study has several limitations. First, there is lack of data for scoliosis-specific outcomes in the database, such as preoperative curve magnitude and complexity, pain, and scoliosis-specific outcome instruments. As such, our study was designed to analyze more general patient outcomes after undergoing PSF for AIS: the occurrence of AEs, extended LOS, and readmission. Operative time, number of levels fused, and the performance of osteotomies were included in multivariate analyses in order to control for curve magnitude and complexity. Additionally, occurrence of AEs and readmissions were captured only up to 30 days postoperatively (including after discharge) in the ACS NSQIP® Pediatric database. Although AEs occurring 30 days after PSF were not available, it is an improvement over other large databases that use administratively coded datasets, which only capture AEs occurring inpatient or those associated with a readmission. The risk factors for adverse outcomes identified in our study may be less applicable to long-term outcomes. Third, although our study contained a large number of patients, it represents only a proportion of the total number of patients with AIS treated surgically during the same time period of our study. Only 50 hospitals were included in the database and the ACS NSQIP® does not capture every patient. The ACS NSQIP® also does not record the number of surgeons or the percentage of all cases at each hospital included in the database. As the ACS NSQIP® includes a sample of cases at a limited number of pediatric hospitals, there may be selection bias. In addition, surgical technique may vary by surgeon, institution, and the number of procedures performed, but these variables were not available in the dataset. However, by using a large national database, surgeon- and institution-specific biases were thought to be minimized. Fourth, a limitation inherent to database research is the difficulty of identifying patients with a precise diagnosis and specific procedure from the dataset. In order to ensure that only patients with AIS who underwent PSF were included in our study, we had extensive inclusion and exclusion criteria based on CPT codes, ICD-9 codes, and patient characteristics. Of the initially identified patients, 37.9% were excluded, which could potentially introduce selection bias; however, excluded patients (Table 2) were those with a noted neuromuscular disorder, other characteristics suggestive of nonidiopathic scoliosis, or missing data. The risk of selection bias was therefore believed to be outweighed by the advantages of ensuring a homogenous population of patients with AIS.
In our study, the only factor found to be associated with AEs was obesity (BMI-for-age ≥ 95th percentile). Previous studies have had conflicting results regarding increased BMI in patients with AIS [16, 45]. Importantly, previous studies used BMI-for-age greater than the eighty-fifth percentile as a clinical cutoff and did not further separate patients with BMI-for-age greater than the ninety-fifth percentile, as was done in our study. In our study, only patients who had BMI-for-age greater than the ninety-fifth percentile had an increased frequency of AEs, whereas patients with BMI-for-age between the eighty-fifth and ninety-fifth percentiles did not. This finding suggests that BMI-for-age greater than the ninety-fifth percentile may be an important clinical cutoff, and future studies in pediatric orthopaedics should consider stratifying BMI percentiles as we have done. Our study also highlights the issue of childhood obesity, which is increasing in the United States and is associated with numerous health consequences [12]. However, despite increased attention given to prevention and treatment, childhood obesity remains a resistant problem [46]. Several weight-loss methods show promise, including outpatient or inpatient weight management programs, medication, and bariatric surgery, however, the long-term efficacy and safety of such interventions are debated [9, 34, 46, 56]. Our study highlights a clear need for future research regarding the prevention, perioperative management, and long-term treatment of obesity in pediatric patients, particularly in the AIS population.
Postoperative LOS greater than 6 days after PSF for patients with AIS was significantly associated with 13 or more levels instrumented and increased operative time. A greater number of levels instrumented and increased operative time likely indicate a more complex procedure that could be associated with a greater number of postoperative issues, such as pain management and prolonged rehabilitation. A greater number of levels fused also has been associated with increased blood loss and thus, need for a blood transfusion, which could prolong LOS [11, 17, 54]. There may be a benefit to reducing operative blood loss in order to reduce postoperative LOS. Several methods of reducing intraoperative blood loss show promise, including, tranexamic acid [26, 47, 53], use of a bipolar sealer [15, 28], and intrathecal morphine [23]. While minimally invasive procedures have been reported to reduce postoperative LOS and blood loss in patients treated with other spinal procedures [10, 36, 40, 48], minimally invasive surgery for AIS has not yet shown the same benefits [39]. These strategies, among others, for potentially reducing LOS in patients with AIS undergoing larger operations warrant further study in order to establish safety and efficacy.
Readmission was very strongly associated with the occurrence of any complication during the initial procedure or hospitalization. After adjusting for potentially confounding variables, a patient with any inpatient complication was readmitted nearly 200 times more often than a patient who did not have an inpatient complication. Inpatient complications may cause clinical sequelae that could predispose the patient to having additional complications develop after discharge. Surgical site infection was the most common inpatient complication and cause for readmission, underlining the need for improved strategies for preventing surgical site infections in patients with AIS undergoing PSF. Risk factors for surgical site infections in adult patients undergoing spine surgery are often related to medical comorbidities [2, 5, 37, 52]; however, due to the general lack of medical comorbidities in the AIS population, such infections are likely related to potentially modifiable surgical factors, including bacterial screening, skin preparation, antibiotic administration, and sterile technique [27, 33, 49]. Optimization of these factors could potentially reduce the rate of infection-related readmissions, however, further study is needed to determine the ideal perioperative routine [27, 33, 49].
In conclusion, obesity (BMI-for-age ≥ 95th percentile) was significantly associated with short-term AEs, reinforcing the urgent need for improved strategies to manage the challenging and growing problem of pediatric obesity. Larger and longer surgical procedures were found to be associated with a hospital stay of a week or more, and strategies for minimizing LOS may include reducing operative blood loss and the need for postoperative transfusion. The occurrence of a complication during the initial procedure or hospital stay was highly associated with patient readmission within 30 days, and inpatient complications and readmissions were most often due to surgical site infections. In the relatively healthy AIS population, optimizing surgeon-related factors, such as perioperative antibiotic administration and sterile technique, have the potential to significantly impact the frequency of inpatient complications and subsequent readmissions. The results of our study should be used for preoperative risk stratification, postoperative planning, and directing future research efforts in order to reduce short-term morbidity associated with PSF in patients with AIS.
Appendix 1. Characteristics of the ACS NSQIP® Pediatric Database
The American College of Surgeons National Surgical Quality Improvement Program® (ACS NSQIP®) database initially included only the adult population and subsequently was expanded to the pediatric population [38]. The first year data were released to participating institutions was 2012 [1]. The ACS NSQIP® Pediatric database captures data from a sample of pediatric surgery patients taken from 50 participating hospitals across the United States [1, 38]. In the ACS NSQIP® Pediatric database, 129 patient variables are collected to assess 30-day adjusted surgical outcomes. A trained Surgical Clinical Reviewer (SCR) prospectively identifies patients and captures data at each hospital using a variety of methods, including medical chart abstraction and patient interviews. The ACS NSQIP® additionally conducts Inter-Rater Reliability (IRR) audits of participating sites, and reports an inter-rater disagreement rate of approximately 2% for all assessed program variables. The SCR collects patient data in 8-day cycles, and is required to submit data from 40 of 46 8-day cycles (87.0%) in 1 year. Case selection and case mix are monitored by the program on a weekly basis to ensure appropriate sampling [1]. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time [38].
Appendix 2. BMI Percentiles* by Sex and Age
| Age (years) | BMI (kg/m2) | ||
|---|---|---|---|
| 5th percentile | 85th percentile | 95th percentile | |
| Men | |||
| 11 | 14.6 | 20.2 | 23.2 |
| 12 | 15.0 | 21.0 | 24.2 |
| 13 | 15.5 | 21.9 | 25.2 |
| 14 | 16.0 | 22.7 | 26.0 |
| 15 | 16.6 | 23.5 | 26.8 |
| 16 | 17.1 | 24.2 | 27.6 |
| 17 | 17.7 | 24.9 | 28.3 |
| Women | |||
| 11 | 14.4 | 20.9 | 24.1 |
| 12 | 14.8 | 21.7 | 25.3 |
| 13 | 15.3 | 22.6 | 26.3 |
| 14 | 15.8 | 23.3 | 27.3 |
| 15 | 16.3 | 24.0 | 28.1 |
| 16 | 16.8 | 24.7 | 28.9 |
| 17 | 17.2 | 25.2 | 29.6 |
* Percentiles per CDC guidelines.
Appendix 3. Results of Bivariate Analyses
| Outcome/associated factor | OR (95% CI) | p value |
|---|---|---|
| AAE | ||
| BMI-for-age ≥ 95th percentile versus 5th–95th percentile | 2.93 (1.31–6.56) | 0.009 |
| SAE | ||
| BMI-for-age ≥ 95th percentile versus 5th–95th percentile | 2.79 (1.08–7.24) | 0.035 |
| LOS > 6 days | ||
| ≥ 13 levels instrumented versus 7–12 levels | 2.45 (1.39–4.32) | < 0.001 |
| Operative time ≥ 365 minutes | 3.04 (1.69–5.47) | < 0.001 |
| Readmission | ||
| Any inpatient complication | 107.10 (27.04–424.12) | < 0.001 |
AAE = any adverse event; SAE = severe adverse event; LOS = length of stay.
Footnotes
One or more of the authors (BAB) has received funding from the National Institutes of Health. Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.American College of Surgeons. User Guide for the 2012 ACS NSQIP Pediatric Participant Use Data File. American College of Surgeons, 2013. Available at: http://www.pediatric.acsnsqip.org/acsNsqipDataPed/jsp/pdf/PEDS.ACS.NSQIP.PUF.UserGuide.2012.pdf. Accessed July 24, 2014.
- 2.Auerbach JD, Lonner BS, Antonacci MD, Kean KE. Perioperative outcomes and complications related to teaching residents and fellows in scoliosis surgery. Spine (Phila Pa 1976). 2008;33:1113–1118. doi: 10.1097/BRS.0b013e31816f69cf. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 4.Barsdorf AI, Sproule DM, Kaufmann P. Scoliosis surgery in children with neuromuscular disease: findings from the US National Inpatient Sample, 1997 to 2003. Arch Neurol. 2010;67:231–235. doi: 10.1001/archneurol.2009.296. [DOI] [PubMed] [Google Scholar]
- 5.Burnette JB, Ebramzadeh E, Lee JL, Galanti S, Hoffer MM. Incidence of inpatient surgeries in children and young adults with childhood orthopaedic diagnoses. J Pediatr Orthop. 2004;24:738–741. doi: 10.1097/01241398-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Basics About Childhood Obesity. 2012. Available at: http://www.cdc.gov/obesity/childhood/basics.html. Accessed July 24, 2014.
- 7.Centers for Disease Control and Prevention. Data Table of BMI-for-age Charts. 2001. Available at: http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm. Accessed July 24, 2014.
- 8.Coe JD, Arlet V, Donaldson W, Berven S, Hanson DS, Mudiyam R, Perra JH, Shaffrey CI. Complications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2006;31:345–349. doi: 10.1097/01.brs.0000197188.76369.13. [DOI] [PubMed] [Google Scholar]
- 9.De Miguel-Etayo P, Bueno G, Garagorri JM, Moreno LA. Interventions for treating obesity in children. World Rev Nutr Diet. 2013;108:98–106. doi: 10.1159/000351493. [DOI] [PubMed] [Google Scholar]
- 10.Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9:560–565. doi: 10.3171/SPI.2008.9.08142. [DOI] [PubMed] [Google Scholar]
- 11.Doi T, Harimaya K, Matsumoto Y, Taniguchi H, Iwamoto Y. Peri-operative blood loss and extent of fused vertebrae in surgery for adolescent idiopathic scoliosis. Fukuoka Igaku Zasshi. 2011;102:8–13. [PubMed] [Google Scholar]
- 12.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8:257–263. doi: 10.1007/s11832-014-0587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryar CD, Ogden CL. Prevalence of Underweight Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2007–2010 2012. Available at: http://www.cdc.gov/nchs/data/hestat/underweight_child_07_10/underweight_child_07_10.pdf. Accessed July 24, 2014.
- 15.Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33:700–706. doi: 10.1097/BPO.0b013e31829d5721. [DOI] [PubMed] [Google Scholar]
- 16.Hardesty CK, Poe-Kochert C, Son-Hing JP, Thompson GH. Obesity negatively affects spinal surgery in idiopathic scoliosis. Clin Orthop Relat Res. 2013;471:1230–1235. doi: 10.1007/s11999-012-2696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan N, Halanski M, Wincek J, Reischman D, Sanfilippo D, Rajasekaran S, Wells C, Tabert D, Kurt B, Mitchell D, Huntington J, Cassidy J. Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion. 2011;51:2133–2141. doi: 10.1111/j.1537-2995.2011.03175.x. [DOI] [PubMed] [Google Scholar]
- 18.Herrera-Soto JA, Vander Have KL, Barry-Lane P, Myers JL. Retrospective study on the development of spinal deformities following sternotomy for congenital heart disease. Spine (Phila Pa 1976). 2007;32:1998–2004. doi: 10.1097/BRS.0b013e318131b225. [DOI] [PubMed] [Google Scholar]
- 19.Hod-Feins R, Anekstein Y, Mirovsky Y, Barr J, Abu-Kishk I, Lahat E, Eshel G. Pediatric Scoliosis Surgery - the association between preoperative risk factors and postoperative complications with emphasis on cerebral palsy children. Neuropediatrics. 2007;38:239–243. doi: 10.1055/s-2008-1062715. [DOI] [PubMed] [Google Scholar]
- 20.Jain A, Njoku DB, Sponseller PD. Does patient diagnosis predict blood loss during posterior spinal fusion in children? Spine (Phila Pa 1976). 2012;37:1683–1687. doi: 10.1097/BRS.0b013e318254168f. [DOI] [PubMed] [Google Scholar]
- 21.Kadhim M, Spurrier E, Thacker D, Pizarro C, Mackenzie WG. Scoliosis surgery in children with congenital heart disease [Epub ahead of print]. Spine (Phila Pa 1976). 2013. [DOI] [PubMed]
- 22.Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013;7:3–9. doi: 10.1007/s11832-012-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesniak AB, Tremblay P, Dalens BJ, Aucoin M, Mercier P. Intrathecal morphine reduces blood loss during idiopathic scoliosis surgery: retrospective study of 256 pediatric cases. Paediatr Anaesth. 2013;23:265–270. doi: 10.1111/pan.12096. [DOI] [PubMed] [Google Scholar]
- 24.Lipton GE, Miller F, Dabney KW, Altiok H, Bachrach SJ. Factors predicting postoperative complications following spinal fusions in children with cerebral palsy. J Spinal Disord. 1999;12:197–205. [PubMed] [Google Scholar]
- 25.Lonner BS, Auerbach JD, Estreicher MB, Kean KE. Thoracic pedicle screw instrumentation: the learning curve and evolution in technique in the treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2009;34:2158–2164. doi: 10.1097/BRS.0b013e3181b4f7e8. [DOI] [PubMed] [Google Scholar]
- 26.Lykissas MG, Crawford AH, Chan G, Aronson LA, Al-Sayyad MJ. The effect of tranexamic acid in blood loss and transfusion volume in adolescent idiopathic scoliosis surgery: a single-surgeon experience. J Child Orthop. 2013;7:245–249. doi: 10.1007/s11832-013-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie WG, Matsumoto H, Williams BA, Corona J, Lee C, Cody SR, Covington L, Saiman L, Flynn JM, Skaggs DL, Roye DP, Jr, Vitale MG. Surgical site infection following spinal instrumentation for scoliosis: a multicenter analysis of rates, risk factors, and pathogens. J Bone Joint Surg Am. 2013;95:800–806. doi: 10.2106/JBJS.L.00010. [DOI] [PubMed] [Google Scholar]
- 28.Mankin KP, Moore CA, Miller LE, Block JE. Hemostasis with a bipolar sealer during surgical correction of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2012;25:259–263. doi: 10.1097/BSD.0b013e3182334ec5. [DOI] [PubMed] [Google Scholar]
- 29.Marks M, Petcharaporn M, Betz RR, Clements D, Lenke L, Newton PO. Outcomes of surgical treatment in male versus female adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976). 2007;32:544–549. doi: 10.1097/01.brs.0000256908.51822.6e. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy IM, Hostin RA, Ames CP, Kim HJ, Smith JP, Boachie-Adjei O, Schwab FJ, Klineberg EO, Shaffrey CI, Gupta MC, Polly DW; International Spine Study Group. Total hospital costs of surgical treatment for adult spinal deformity: an extended follow-up study [Epub ahead of print]. Spine J. 2014. [DOI] [PubMed]
- 31.Miyanji F, Slobogean GP, Samdani AF, Betz RR, Reilly CW, Slobogean BL, Newton PO. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization?: a multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94:809–813. doi: 10.2106/JBJS.J.01682. [DOI] [PubMed] [Google Scholar]
- 32.Murphy NA, Firth S, Jorgensen T, Young PC. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What’s the difference? J Pediatr Orthop. 2006;26:216–220. doi: 10.1097/01.bpo.0000206516.61706.6e. [DOI] [PubMed] [Google Scholar]
- 33.Myung KS, Glassman DM, Tolo VT, Skaggs DL. Simple steps to minimize spine infections in adolescent idiopathic scoliosis. J Pediatr Orthop. 2014;34:29–33. doi: 10.1097/BPO.0b013e31829b2d75. [DOI] [PubMed] [Google Scholar]
- 34.Oberbach A, Neuhaus J, Inge T, Kirsch K, Schlichting N, Bluher S, Kullnick Y, Kugler J, Baumann S, Till H. Bariatric surgery in severely obese adolescents improves major comorbidities including hyperuricemia. Metabolism. 2014;63:242–249. doi: 10.1016/j.metabol.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8:904–910. doi: 10.1016/j.spinee.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976). 2009;34:1385–1389. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 37.Pull ter Gunne AF, Hosman AJ, Cohen DB, Schuetz M, Habil D, van Laarhoven CJ, van Middendorp JJ. A methodological systematic review on surgical site infections following spinal surgery: part 1: risk factors. Spine (Phila Pa 1976). 2012;37:2017–2033. doi: 10.1097/BRS.0b013e31825bfca8. [DOI] [PubMed] [Google Scholar]
- 38.Saito JM, Chen LE, Hall BL, Kraemer K, Barnhart DC, Byrd C, Cohen ME, Fei C, Heiss KF, Huffman K, Ko CY, Latus M, Meara JG, Oldham KT, Raval MV, Richards KE, Shah RK, Sutton LC, Vinocur CD, Moss RL. Risk-adjusted hospital outcomes for children’s surgery. Pediatrics. 2013;132:e677–e688. doi: 10.1542/peds.2013-0867. [DOI] [PubMed] [Google Scholar]
- 39.Sarwahi V, Horn JJ, Kulkarni PM, Wollowick AL, Lo Y, Gambassi M, Amaral TD. Minimally invasive surgery in patients with adolescent idiopathic scoliosis: Is it better than the standard approach? A two year follow-up study [Epub ahead of print]. J Spinal Disord. Tech. 2014. [DOI] [PubMed]
- 40.Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop. 2009;33:1683–1688. doi: 10.1007/s00264-008-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan LQ, Skaggs DL, Lee C, Kissinger C, Myung KS. Intensive care unit versus hospital floor: a comparative study of postoperative management of patients with adolescent idiopathic scoliosis. J Bone Joint Surg. Am. 2013;95:e40. doi: 10.2106/JBJS.L.00467. [DOI] [PubMed] [Google Scholar]
- 42.Smith JT, Smith MS. Does a preoperative bowel preparation reduce bowel morbidity and length of stay after scoliosis surgery? A randomized prospective study. J Pediatr Orthop. 2013;33:e69–e71. doi: 10.1097/BPO.0b013e318296e032. [DOI] [PubMed] [Google Scholar]
- 43.Takaso M, Nakazawa T, Imura T, Okada T, Ueno M, Saito W, Takahashi K, Yamazaki M, Ohtori S. Surgical correction of spinal deformity in patients with congenital muscular dystrophy. J Orthop Science. 2010;15:493–501. doi: 10.1007/s00776-010-1486-9. [DOI] [PubMed] [Google Scholar]
- 44.Tarrant RC, Lynch S, Sheeran P, O’Loughlin PF, Harrington M, Moore DP, Kiely PJ. Low body mass index in adolescent idiopathic scoliosis: relationship with pre- and postsurgical factors. Spine (Phila Pa 1976). 2014;39:140–148. doi: 10.1097/BRS.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 45.Upasani VV, Caltoum C, Petcharaporn M, Bastrom T, Pawelek J, Marks M, Betz RR, Lenke LG, Newton PO. Does obesity affect surgical outcomes in adolescent idiopathic scoliosis? Spine (Phila Pa 1976). 2008;33:295–300. doi: 10.1097/BRS.0b013e3181624573. [DOI] [PubMed] [Google Scholar]
- 46.van der Baan-Slootweg O, Benninga MA, Beelen A, van der Palen J, Tamminga-Smeulders C, Tijssen JG, van Aalderen WM. Inpatient treatment of children and adolescents with severe obesity in the Netherlands: A randomized clinical trial [Epub ahead of print]. JAMA Pediatr. 2014. [DOI] [PubMed]
- 47.Verma K, Errico T, Diefenbach C, Hoelscher C, Peters A, Dryer J, Huncke T, Boenigk K, Lonner BS. The relative efficacy of antifibrinolytics in adolescent idiopathic scoliosis: a prospective randomized trial. J Bone Joint Surg Am. 2014;96:e80. doi: 10.2106/JBJS.L.00008. [DOI] [PubMed] [Google Scholar]
- 48.Villavicencio AT, Burneikiene S, Bulsara KR, Thramann JJ. Perioperative complications in transforaminal lumbar interbody fusion versus anterior-posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord. 2006;19:92–97. doi: 10.1097/01.bsd.0000185277.14484.4e. [DOI] [PubMed] [Google Scholar]
- 49.Vitale MG, Riedel MD, Glotzbecker MP, Matsumoto H, Roye DP, Akbarnia BA, Anderson RC, Brockmeyer DL, Emans JB, Erickson M, Flynn JM, Lenke LG, Lewis SJ, Luhmann SJ, McLeod LM, Newton PO, Nyquist AC, Richards BS, 3rd, Shah SA, Skaggs DL, Smith JT, Sponseller PD, Sucato DJ, Zeller RD, Saiman L. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop. 2013;33:471–478. doi: 10.1097/BPO.0b013e3182840de2. [DOI] [PubMed] [Google Scholar]
- 50.Wazeka AN, DiMaio MF, Boachie-Adjei O. Outcome of pediatric patients with severe restrictive lung disease following reconstructive spine surgery. Spine (Phila Pa 1976). 2004;29:528–534; discussion 535. [DOI] [PubMed]
- 51.Woo JG, Zeller MH, Wilson K, Inge T. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatr. 2009;154:327–331. [DOI] [PMC free article] [PubMed]
- 52.Xing D, Ma JX, Ma XL, Song DH, Wang J, Chen Y, Yang Y, Zhu SW, Ma BY, Feng R. A methodological, systematic review of evidence-based independent risk factors for surgical site infections after spinal surgery. Eur Spine J. 2013;22:605–615. [DOI] [PMC free article] [PubMed]
- 53.Yagi M, Hasegawa J, Nagoshi N, Iizuka S, Kaneko S, Fukuda K, Takemitsu M, Shioda M, Machida M. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976). 2012;37:E1336–E1342. [DOI] [PubMed]
- 54.Yu X, Xiao H, Wang R, Huang Y. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine (Phila Pa 1976). 2013;38:350–355. [DOI] [PubMed]
- 55.Yuan N, Fraire JA, Margetis MM, Skaggs DL, Tolo VT, Keens TG. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine (Phila Pa 1976). 2005;30:2182–2185. [DOI] [PubMed]
- 56.Zitsman JL, Inge TH, Reichard KW, Browne AF, Harmon CM, Michalsky MP. Pediatric and adolescent obesity: management, options for surgery, and outcomes. J Pediatr Surg. 2014;49:491–494. [DOI] [PubMed]